Abstract
Objective
We investigated the effect of various doses of vertical oscillation (vibration) on soleus H-reflex amplitude and post-activation depression in individuals with and without SCI. We also explored the acute effect of short-term limb vibration on skeletal muscle mRNA expression of genes associated with spinal plasticity.
Methods
Six healthy adults and five chronic complete SCI subjects received vibratory stimulation of their tibia over three different gravitational accelerations (0.3g, 0.6g, and 1.2g) at a fixed frequency (30 Hz). Soleus H-reflexes were measured before, during, and after vibration. Two additional chronic complete SCI subjects had soleus muscle biopsies 3 h following a single bout of vibration.
Results
H-reflex amplitude was depressed over 83% in both groups during vibration. This vibratory-induced inhibition lasted over 2 min in the control group, but not in the SCI group. Post-activation depression was modulated during the long-lasting vibratory inhibition. A single bout of mechanical oscillation altered mRNA expression from selected genes associated with synaptic plasticity.
Conclusions
Vibration of the lower leg inhibits the H-reflex amplitude, influences post-activation depression, and alters skeletal muscle mRNA expression of genes associated with synaptic plasticity.
Significance
Limb segment vibration may offer a long term method to reduce spinal reflex excitability after SCI.
Keywords: Mechanical oscillation, Post-activation depression, Spinal cord injury, Paralysis
1. Introduction
An understanding of spinal neuronal plasticity to environmental mechanical stimuli has been the focus of several intervention strategies for those with spinal cord injury (SCI). Mechanical oscillation (i.e. vibration) has been used to study neural and musculoskeletal adaptations in athletes and individuals with neuromuscular disorders including SCI (Torvinen et al., 2003; Verschueren et al., 2004; Ward et al., 2004; Iwamoto et al., 2005; Gilsanz et al., 2006). Early studies have shown that vibrating directly over a muscle tendon will produce inhibitory effects on the H-reflex (Hoffmann reflex) (De Gail et al., 1966; Lance, 1966; Lance et al., 1966; Fromm and Noth, 1976; Rymer and Hasan, 1981; Hultborn et al., 1987). The inhibitory effect induced by direct tendon vibration is attributed to increased presynaptic inhibition, the refractory state of Ia afferent fibers, neuronal transmitter depletion at Ia terminals, postsynaptic reciprocal and non-reciprocal Ia inhibition, and effects from cutaneous and other afferent receptors (Stein, 1995).
Recently, human studies have expanded their scope to include vibrating the whole body vertically and therefore altering gravitational load on the head and body segments simultaneously. Contrary to localized muscle tendon vibration, whole body vibration induces excitatory effects on homonymous alpha motor neurons, and increased synchronization of motor neuron activity (Cardinale and Bosco, 2003; Abercromby et al., 2007). Whole body vibration has been reported to have widespread effects on multiple tissues including increased muscle strength, increased bone density, and modulation of the central nervous system (Stein, 1995; Bosco et al., 2000; McCall et al., 2000; Naito et al., 2000; Cardinale and Bosco, 2003; Verschueren et al., 2004; Ward et al., 2004; Judex et al., 2005; Gilsanz et al., 2006; Zhi et al., 2008). During whole body vibration, the motor descending pathways, vestibular system, peripheral sensory systems, and visual inputs may influence the spinal reflex pathways (Iles and Pisini, 1992b).
We designed this experiment to deliver various doses of mechanical oscillation to a single limb segment (tibia) of humans in order to minimize supra spinal input (vestibular). We also studied individuals with no supra spinal pathways from complete SCI. These experiments offer two unique contributions that, to our knowledge, have not been studied in humans with and without SCI. First, we examined post-activation depression, a phenomenon known to be diminished with chronic SCI (Schindler-Ivens and Shields, 2000, 2004), and implicated as one primary mechanism contributing to spasticity after SCI (Grey et al., 2008). Second, we examined the acute effects of vibration on muscle genes known to be associated with retrograde modulation of synaptic plasticity (Nakajima et al., 1999; Jovanovic et al., 2000; Feng et al., 2002; Brock et al., 2004; Gomez-Pinilla et al., 2004; Hetman and Gozdz, 2004; Zelano et al., 2006; Rajalu et al., 2009; Martin et al., 2010).
The purposes of this study were to (1) examine the effects of single limb fixed frequency vibration on spinal neuronal excitability in individuals with and without SCI across various doses of acceleration, and (2) examine the effect of vibration on a family of genes related to synaptic plasticity in individuals with SCI. We expect that the single limb vibration will inhibit motor neuron excitability in both groups, will be independent of the presence of descending spinal pathways, but modulate according to the gravitational acceleration. Those with SCI will display less H-reflex depression compared to healthy adults and the limb segment vibration will modulate mRNA gene expression in muscle of individuals with SCI.
2. Methods
2.1. Subjects
Six healthy adults and seven individuals with sub-acute/chronic spinal cord injury (SCI) participated in the study (Table 1). In the SCI group, five SCI subjects were enrolled in the study for Aim 1 (H-reflex analysis) and two subjects were enrolled in the study for Aim 2 (muscle biopsy and mRNA analysis). The healthy controls have no acute or ongoing orthopedic, neuromuscular, or neurological deficits or disorders. For the SCI group, inclusion criteria were (1) complete SCI classified according to the American Spinal Injury Association (ASIA) Impairment Scale, (2) able to passively achieve the neutral position at the paretic ankle with the knee flexed to 90° in a seated position, (3) clinical evidence of involvement of upper motor neuron lesions, measured by the presence of at least mild to moderate resistance to stretch supporting the presence of hyper-reflexia. Exclusion criteria were (1) presence of pressure ulcers, (2) history of any other significant neurological, cardiac, vascular, pulmonary, orthopaedic, hematologic, infectious, immune, gastrointestinal, urogenital, integumentary, oncological, or endocrine diseases or disorders affecting neuromuscular function of the legs, (3) lesions involving lower motor neurons, (4) any medical conditions in which electrical stimulation is contraindicated such as pregnancy or the utilization of cardiac pacemaker or defibrillator. All subjects gave informed consent before participation and the study was approved by the University of Iowa Human Subjects Institutional Review Board.
Table 1.
Subject characteristics.
| Participant | Age (years) | Sex | Height (m) | Weight (kg) | NLI | ASIA Impairment Scale | Duration of injury (months) |
|---|---|---|---|---|---|---|---|
| SCI group | |||||||
| S1 | 30 | M | 1.96 | 66.1 | T8 | A | 38 |
| S2 | 26 | F | 1.68 | 49.9 | C6 | A | 15 |
| S3 | 27 | M | 1.75 | 99.9 | T10 | A | 21 |
| S4 | 21 | M | 1.83 | 81.7 | T7 | A | 3 |
| S5 | 45 | M | 1.75 | 84.9 | T4 | A | 18 |
| S6 | 30 | M | 1.85 | 77.2 | T6 | A | 49 |
| S7 | 39 | M | 1.83 | 104.4 | T10 | A | 25 |
| 31.1 ± 8.2 | 1.81 ± 0.09 | 80.6 ± 18.8 | 24.1 ± 15.2 | ||||
| Control group | |||||||
| C1 | 24 | F | 1.70 | 77.2 | – | – | – |
| C2 | 35 | M | 1.63 | 56.8 | – | – | – |
| C3 | 30 | M | 1.85 | 99.9 | – | – | – |
| C4 | 33 | F | 1.78 | 72.6 | – | – | – |
| C5 | 38 | F | 1.65 | 64.9 | – | – | – |
| C6 | 26 | M | 1.85 | 81.7 | – | – | – |
| 31.0 ± 5.4 | 1.74 ± 0.10 | 75.5 ± 14.9 | |||||
SCI: Spinal Cord Injury; NLI: Neurological Level of Injury; The NLI is defined as most caudal segment with normal motor and sensory function for classification of spinal cord injury. ASIA: American Spinal Injury Association. The severity of injury is quantified based on ASIA Impairment Scale: A = Complete; no motor or sensory function is preserved in the sacral segments S4–S5; B = Incomplete; sensory but not motor function is preserved below the neurological level and includes the sacral segments S4–S5; C = Incomplete; motor function is preserved below the neurological level, and more than half of key muscles below the neurological level have a muscle grade less than 3; D = Incomplete; motor function is preserved below the neurological level, and at least half of key muscles below the neurological level have a muscle grade of 3 or more. E = Normal; motor and sensory function are normal. The bottom row in each group represents group mean ± 1SD.
2.2. Experimental paradigm
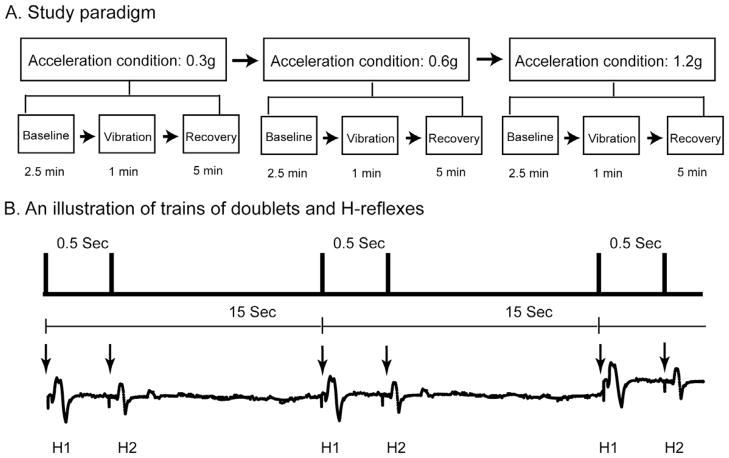
The study paradigm for Aim 1 consisted of three different acceleration conditions during a single test session: low (0.3g), moderate (0.6g), and high (1.2g) accelerations (Fig. 1A). The vibration frequency was always set to 30 Hz because of the known osteogenic capacity of this frequency. The amplitude of vertical oscillations on the lower leg was varied by using three specific g forces. The order of three acceleration conditions assigned to each subject was fixed progressing from low (0.3), to moderate (0.6), and then to high (1.2). Previous work on whole body vibration revealed that different H-reflex recovery times indeed exist between healthy adults and people with chronic complete/incomplete SCI (Armstrong et al., 2008; Sayenko et al., 2010). Importantly, based on our pilot work, we found that the H-reflex for both healthy and SCI groups fully returned to the baseline values after 2.5 min post limb segment vibration. Thus, each vibration g force session was separated by 5 min to allow a complete washout of any effect. Each g force condition involved a Baseline, Vibration, and Recovery phase. Paired-pulse electrical stimulations (doublets), with inter-pulse intervals of 500 ms, were delivered every 15 s to elicit H-reflexes across all phases (Fig. 1B). During the Baseline phase, ten doublets were delivered over 3 min to generate H-reflexes while there was no vibration. After the Baseline phase, vertical oscillation stimuli were applied on the lower leg segment for 1 min (Vibration phase) followed by a 5-min rest period (Recovery phase). There were 4 doublets delivered during each Vibration phase and 20 doublets delivered during each Recovery phase. The very first doublet was always delivered within 3 s of the start of each phase. Each subject underwent three acceleration conditions where 102 doublets were delivered in total: 30 doublets before vibration stimuli (10 doublets in each Baseline phase), 12 doublets during vibration stimuli (4 doublets in each Vibration phase), and 60 doublets after vibration (20 doublets in each Recovery phase).
Fig. 1.
Illustrations of study paradigm (A) and electrical stimulation protocol (B). Three acceleration conditions (0.3g, 0.6g, and 1.2g) were assigned to each subject in order. Each acceleration condition consisted of three consecutive phases: Baseline, Vibration, and Recovery phases, corresponding to the time duration before, during, and after vibration stimuli (A). A series of doublets (paired-pulse electrical stimulations with an inter-pulse interval of 0.5 s) was delivered every 15 s over three phases in each acceleration (upper panel, B). The bottom traces depicted soleus background muscle activity and H-reflexes elicited by doublets over time. Arrows indicated timing when electrical stimuli were delivered to the tibial nerve. Note the second H-reflex (H2) was suppressed compared to the first H-reflex (H1) elicited by any doublet due to the post-activation depression mechanism.
The study paradigm for Aim 2 involved a short-term vibration intervention session for one leg. Three hours after the intervention a soleus muscle biopsy was taken from the vibrated leg and the leg that did not receive vibration from two subjects. The vibration acceleration and frequency were always set to 0.6g and 30 Hz. In the vibration intervention session, four bouts of cyclic vibration were delivered to one leg and the opposite leg served as a control. Each bout consisted of 60 vibration cycles with each cycle lasting 10 s (5 s on/5 s off). Two minutes separated each bout.
2.3. Instrumentation
Bipolar Ag–AgCl surface electrodes (8 mm in diameter, with a fixed inter-electrode distance of 20 mm) were used to record H-reflexes and M waves from the soleus muscle of the right leg. Electromyographic (EMG) signals were on-site preamplified with a gain of 35. The EMG signals were then further amplified by a CS 67 differential amplifier (Therapeutics Unlimited, Iowa City, IA) with a gain range from 1000 to 10,000. The differential amplifier had an input impedance of 15 MΩ at 100 Hz, a frequency response of 15–1000 Hz, a common mode rejection ratio of 87 dB at 60 Hz, and a bandwidth of 20–4000 Hz.
A constant-current electrical stimulator (Digitimer Ltd., Welwyn Garden City, Herts, UK) with a range from 50 μA to 1A and a total output capacity of 400 V was used to deliver square waves with a pulse width of 1000 μs. The stimulator was triggered by a digital pulse from a data acquisition board (Metrabyte DAS 16F, Keithley Instruments Inc., Cleveland, OH, USA) housed in a microcomputer and controlled by custom software. Through custom programming, the stimulator delivered paired-pulse electrical stimulations (doublets) with inter-pulse intervals of 500 ms every 15 s. Stimulation was administered via a single surface probe secured over the tibial nerve in the right popliteal fossa. The cathode was positioned through a dispersive electrode placed over top of the femoral condyles.
A vibration servo-control system (Ling Dynamic Systems Ltd., Royston, Herts, UK), equipped with a V721 shaker, a PA1000 linear amplifier, and a COMET controller, was used to precisely control frequency and amplitude of vertical oscillations over the lower leg. The vibration control system can generate accelerations up to 66.3g, induce continuous peak-to-peak displacement of 25.4 mm, and control a range of oscillatory frequencies from zero to 400 Hz with the accuracy level of 0.08 dB (testing at 1000 Hz sine waves). In this study, vertical peak-to-peak displacements of the lower leg segment introduced by the vibration control system for the 0.3g, 0.6g, and 1.2g acceleration conditions were 0.16, 0.33, and 0.66 mm, respectively.
2.4. Data collection
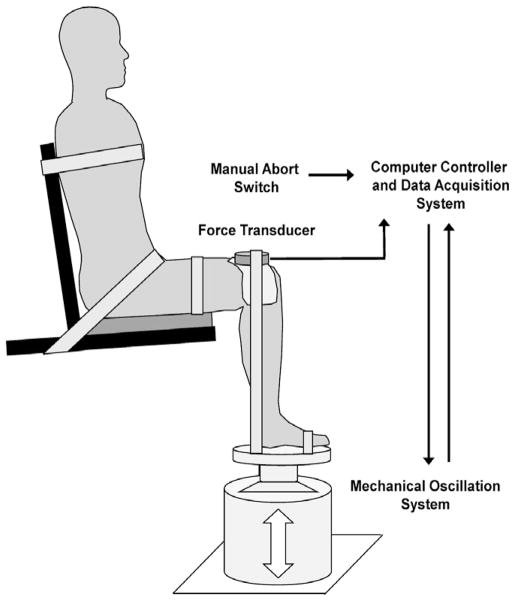
Subjects sat on a custom designed chair with the hip and knee flexed at 90° and the ankle at the neutral position (Fig. 2). The testing leg was secured by a Velcro strap around the thigh and the foot to maintain a fixed position on the platform attached to the vibration system. The foot placement was marked on the platform and was closely monitored through the entire testing session. Additional straps were used to stabilize the upper body. Subjects were instructed to relax and sit quietly throughout the entire experiment. The skin over the soleus muscle was abraded with sandpaper and cleaned with alcohol swabs. The recording EMG electrode was placed 2 cm lateral to the midline of the posterior calf and 2 cm distal to the lowest end of the palpable border of the gastrocnemius muscle. A reference electrode was placed on the anterior aspect of the tibia of the right leg.
Fig. 2.
Lateral view of the experimental setup. The subject was seated comfortably with her/his torso, pelvis, and right leg stabilized by Velcro straps. The right ankle was dorsiflexed to neutral and the knee was flexed 90°. The right foot was placed on the custom platform attached to the top of the mechanical oscillation system (the gray cylinder on the ground). A force transducer was placed on the top of right knee joint and aligned perpendicularly to the longitudinal axis of the lower leg to measure gravitational force introduced by the mechanical oscillation system. The mechanical oscillation device was controlled by a servo controller system that closely monitored real-time gravitational acceleration during testing. A manual abort switch was used to terminate the oscillation if necessary.
For soleus H-reflex measures, the tibial nerve was electrically activated via a stimulating electrode positioned in the popliteal fossa. The stimulating electrode was determined by the position that allowed H-reflexes to be elicited with a small or absent M wave. Next, a maximal H-reflex was generated and noted. A maximal M wave (M-max) was elicited and defined as the peak-to-peak value when the amplitude failed to increase with increasing stimulation current. To ensure that the tibial nerve was activated supra-maximally, the stimulation intensity was adjusted to 120% of the maximal current value, and the peak amplitude was verified. The stimulation intensity was adjusted to 25% of the M-max, which corresponded to ~50% of the maximal H-reflex amplitude for each subject. All data were recorded at 2000 Hz using a Datapac 2K2 system (RUN Technologies, Mission Viego, CA, USA) and time-synchronized with the vibration control system.
Muscle biopsies were obtained using a Temno biopsy needle (T1420, Cardinal Health, Dublin, OH) from the soleus muscle in both legs. Once the soleus muscle was located using ultrasound imaging, the biopsy site was then marked and injected with local anesthetic (lidocaine, 0.5%; w/v). In each subject, four samples were collected with multiple needle passes. The samples were processed by immersing them in Trizol reagent solution (Invitrogen, Paisley, UK) for RNA extraction. The transcriptional profiles of RNA were analyzed using microarray hybridization at the University of Iowa DNA Core Facility using a previously published protocol (Adams et al., 2011). Briefly, the microarray technique involved RNA isolation, amplification, and labeling. Arrays were scanned (3000 7G, Affymetrix, Santa Clara, CA) and recorded using Gene Chip operating software. Each gene expression was defined based on the logarithm base-2 hybridization (Exon 1.0 ST array) for both vibration-trained soleus and control soleus muscles from each subject.
2.5. Data analysis
All analyses were conducted using Datapac software. Offline, raw EMG signals were first zero offset and digitally filtered (fourth-order band-pass Butterworth) at 20–200 Hz prior to any advanced processing. In each doublet, paired H-reflexes 500-ms apart were first identified and were categorized as the primary H-reflex (H1) and secondary H-reflex (H2) in order to investigate the neural mechanism of post-activation depression (Fig. 1B). The peak-to-peak amplitude of each H-reflex was then computed and expressed as a percentage of M-max across all phases for each acceleration condition. To quantify post-activation depression of soleus H-reflexes, we calculated the depression ratio by dividing the amplitude difference between H1 and H2 to the average value of H1 amplitudes during the Baseline phase. A depression ratio of 0 indicating that no post-activation depression effect occurred; whereas a ratio of 1.0 indicated that the greatest depression occurred such that the decrement of H2 was equal to the baseline H1 amplitude. In order to compare the vibration effect on soleus H-reflex and depression ratios between groups, all variables were normalized to their baseline H1 averages. To depict H-reflex recovery over the course of time, we specifically compared H1 amplitudes across three discrete time points in the Recovery phase to their averages during Baseline and Vibration phases: 0-, 1-, 5-min after the cessation of vibration. All variables were averaged over all subjects to create group means for each acceleration condition.
We focused our mRNA study on five genetic transcriptions that are known to mediate synaptic plasticity in central nerve systems: glycine receptor alpha 3 subunit gene (GLRA3), metabotropic glutamate receptor subtype 7 (GRM7), synapsin III (SYN3), N-Cadherin (CDH2), and mitogen-activated protein kinase 3 (ERK1, also known as p44 MAPK). The expression of each gene was quantified as the logarithm base-2 and expressed as the ratio between the soleus that received the vibration and the control soleus that did not receive the vibration from the same subjects.
Preliminary statistical analyses showed that there were no significant differences in H-reflex measurements across the three acceleration conditions (0.3g, 0.6g, 1.2g) for both SCI and control groups. Accordingly, all dependent variables were averaged across all acceleration conditions within each group for subsequent statistical comparisons.
2.6. Statistical analysis
Statistical comparisons were made using SAS/STAT software (SAS, Cary, NC, USA). We used a two-way (group × time) mixed model ANOVA with repeated measures on one factor (time) to test for the effects of time (baseline, vibration, recovery) and group (SCI vs Control). When a significant interaction was present, post hoc analyses were performed using Tukey’s honest significant difference test. Paired-t test was used to compare mRNA expression between the vibration-trained soleus and the control soleus muscles. The level for statistical significance was set at P < 0.05.
3. Results
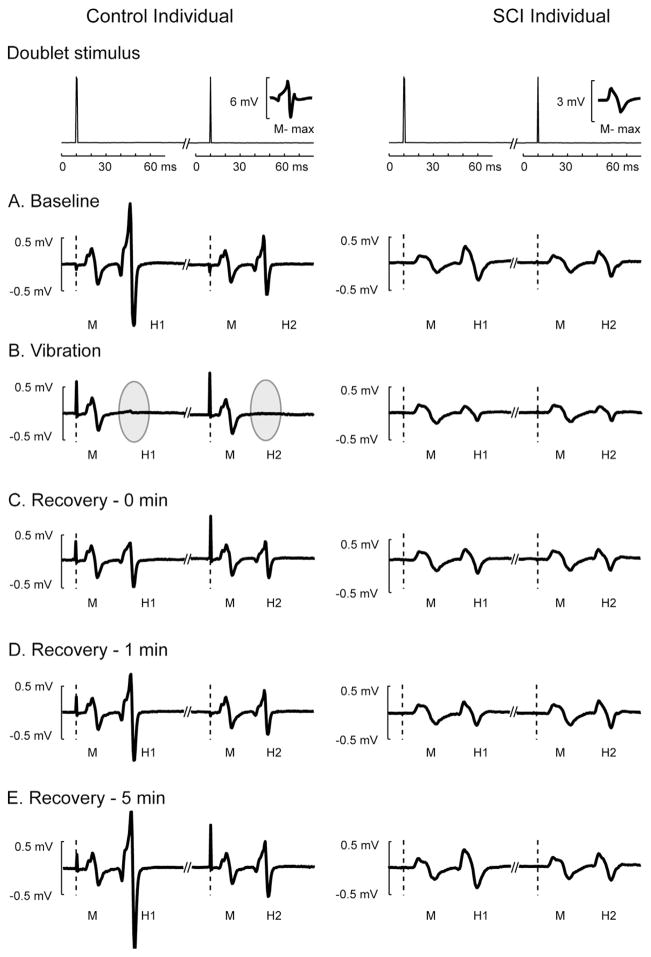
An example of the individual H reflexes elicited by doublet stimulation is depicted in Fig. 3 from a subject with chronic SCI and a healthy control. Because of muscle atrophy, the SCI subjects consistently showed a smaller M-max compared with the control subjects, resulting in relatively smaller H-reflex amplitudes (Fig. 3A). During vibration, the H-reflexes were suppressed in both SCI and control subjects (Fig. 3B). Furthermore, this depression was extensive in the control subject (gray areas, Fig. 3B), despite a stable M-wave, supporting that stimulation conditions remained stable. Also evident is that the H-reflex was not yet fully recovered to its baseline level at 1 min post-vibration in both SCI and control subjects (Fig. 3C–E) and the recovery rate for the SCI subjects was relatively faster than the control subjects.
Fig. 3.
M-waves and H-reflexes from a typical SCI (right) and control (left) participants across different phases during a 0.3g acceleration condition. Top panels show time series data of paired electrical pulses (doublet) in which voltage signals were plotted 10 ms before and 60 ms after each electrical stimulus was delivered. The amplitude of maximal M-wave (M-max) was overlaid on the bottom right in each top panel for the comparison between the control and SCI individuals. Dashed vertical lines indicate the time when each electrical stimulus was delivered for each doublet. M-waves (M) and H-reflexes (H1 and H2) elicited by doublets were depicted across baseline phase (A), vibration phase (B), and three time points during recovery phase (C–E). Note H-reflexes were greatly suppressed during vibration stimuli in the control subject (gray ellipses).
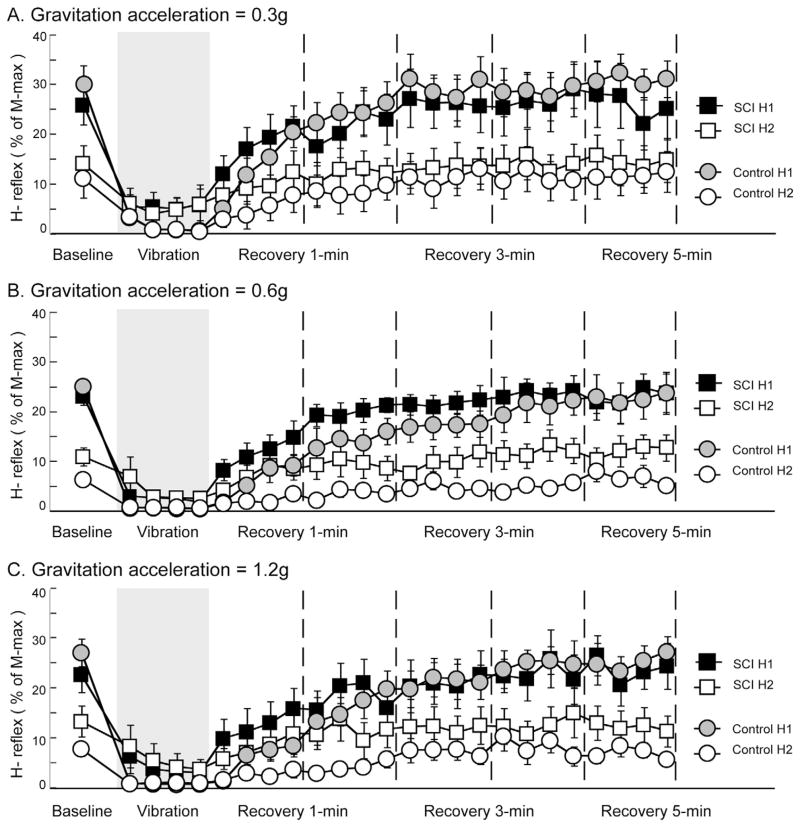
Average H-reflex data for both groups during three acceleration conditions is shown in Fig. 4. There is a clear trend demonstrating that vibration induced an inhibitory effect on the soleus H-reflex across all acceleration conditions for both SCI and control groups. During baseline, H1 amplitudes were similar between groups across three acceleration conditions, which ranged from 22.6% to 30.1% of M-max. In contrast, H-reflex depression ratios in the control group at baseline were greater than the SCI group across three acceleration conditions, given by a combination of higher H1 and lower H2 amplitudes (Fig. 4A–C). When vibration stimulation was administrated, H-reflexes were suppressed in both SCI and control groups. In addition, a consistent pattern was observed in all three acceleration conditions: the extent of depression was greater in the control group compared to SCI group. After vibration was terminated (recovery phase), H-reflexes were gradually returned to the baseline levels in both groups across three acceleration conditions. In particular, during the first minute of recovery following vibration, average H-reflex amplitudes in the SCI group were consistently higher than the control group across all acceleration conditions, indicating a relatively faster recovery rate.
Fig. 4.
Average H-reflexes across different phases during different acceleration conditions. Each data point represents the peak-to-peak amplitude of H-reflex, expressed as the percent of the maximal amplitude of the M-wave across 0.3g (A), 0.6g (B), and 1.2g (C) acceleration conditions. During baseline phase, data points were averaged across all available trials within each subject and then averaged across subjects in each group. Data points during vibration and recovery phases were averaged over all subjects in each group on a trial-by-trial basis. Gray areas indicate the time when the vibration stimuli were applied on the lower leg segment. Dashed vertical lines delineate 1-min intervals after the cessation of vibration stimuli. Error bars, ±1 SEM.
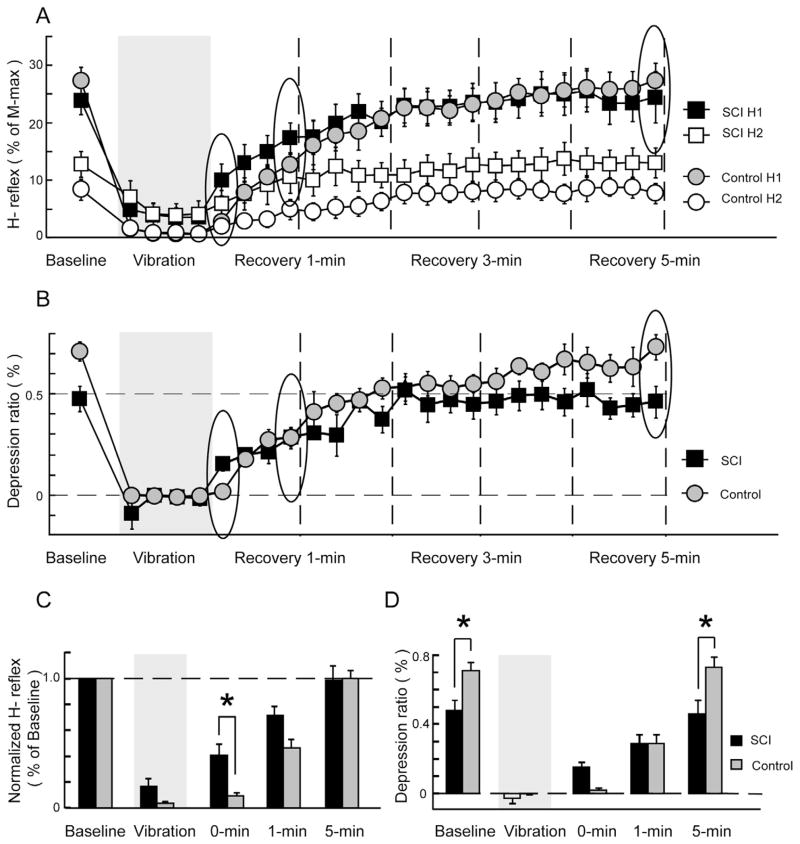
Similar depression effects and recovery patterns were also observed when pooling all acceleration conditions together, yielding overall averages across conditions (Fig. 5). In comparison with the SCI group, the control group showed greater H-reflex depression during the vibration phase and slower recovery rate during the first minute of recovery (Fig. 5A). Due to extensive, long-lasting inhibitory effects induced by vibration, the H-reflex depression ratio in both groups was close to 0 during vibration and remained at relatively lower levels compared to the baseline ratios for more than 1 min (Fig. 5B). Notably, depression ratios in the control group were comparable to the SCI group during the first 3 min post-vibration.
Fig. 5.
Average H-reflexes and depression ratios across all acceleration conditions. Each data point represents the H-reflex, expressed as the percent of the maximal amplitude of the M-wave (A). The depression ratio was calculated by dividing the amplitude difference between paired H-reflexes (i.e. H1 and H2) to its average baseline H1 amplitude (B). During baseline phase, data points were averaged across all available trials and across three acceleration conditions within each subject, and were then averaged over all subjects in each group. Data points during vibration and recovery phases were averaged across three acceleration condition and over all subjects in each group on a trial-by-trial basis. Gray areas indicate the time when the vibration stimuli were applied on the lower leg segment. Dashed vertical lines delineate 1-min intervals after the cessation of vibration stimuli. Five key time points were identified for statistical comparisons: baseline (averaged across all available trials), vibration (averaged across all available trials), and data points at 0, 1, and 5 min during recovery phase (indicated by circles in (A) and (B), respectively). Average H reflexes were normalized to the baseline average across five time points (C). The depression ratios across five key time points were shown in (D). Error bars, ±1 SEM. Asterisks indicate significant post hoc differences between groups.
By comparing over five key time points, there were significant main effects of group (P = 0.038) and time (P < 0.0001), and a group × time interaction effect (P = 0.008) in H-reflex amplitudes (Fig. 5C). Both SCI and control groups showed a significant decrease in H-reflex amplitude (H1) during vibration as compared to their baseline values (post hoc, both P < 0.0001). Although there is a clear trend showing that vibration-induced H-reflex depression is greater in the control group, H-reflex amplitude failed to demonstrate significant group differences due to a floor effect (post hoc, P = 0.88; Fig. 5C). During the recovery phase, H1 amplitudes in both groups remained significantly smaller than their baseline values at 0 min (post hoc, both P < 0.0001) and 1 min (post hoc, SCI: P = 0.017; control: P < 0.0001) after the cessation of vibration, indicating long-lasting inhibitory effects induced by this vibration protocol. Interestingly, the SCI group had a significantly higher H1 amplitude than the control group at 0-min post-vibration (post hoc, P = 0.024), suggesting faster recovery in the SCI group. However, H1 amplitudes showed no differences between groups at 1 and 5 min post-vibration (post hoc, P = 0.14 and P = 1.0, respectively).
The depression ratio also showed a group × time interaction effect (P = 0.0001) and a significant main effect of time (P < 0.0001), but a borderline main effect of group (P = 0.078) when comparing across five key time points (Fig. 5D). During baseline, the depression ratios in the control group were significantly higher than the SCI group (post hoc, P = 0.036). As expected, both SCI and control groups showed a significant decrease in depression ratio during vibration as compared to their baseline values (post hoc, both P < 0.0001) due to substantial reductions in H1 amplitudes. During the recovery phase, the depression ratios remained at lower values for both groups and showed indifferences between groups at 0-min (post hoc, P = 0.59) and 1-min post-vibration (post hoc, P = 1.0). Finally, the depression ratios for both groups returned to their baseline levels and showed significant group differences again (post hoc, P = 0.009) at 5 min post-vibration.
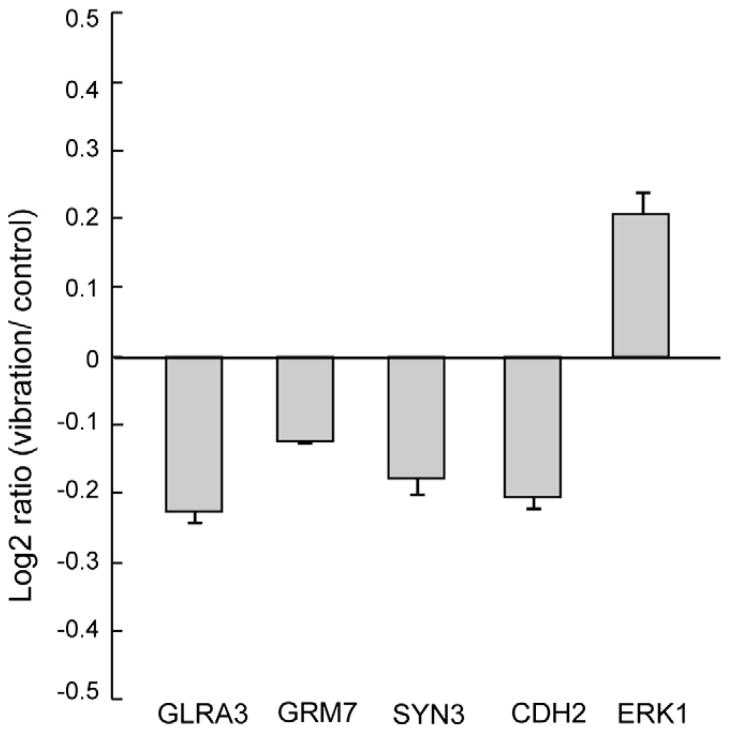
Average log2 ratios of mRNA expression in the vibration-trained soleus to the control soleus muscle 3 h after a short-term vibration intervention are shown in Fig. 6. The acute effects of limb segment vibration on soleus mRNA expression showed significant decreases in the log2 ratios of GLRA3, GRM7, SYN3, and CDH2 (P = 0.021, 0.005, 0.036, and 0.023, respectively), whereas a significant increase in the log2 ratio of ERK1 (P = 0.046).
Fig. 6.
Changes in gene expression induced by segment limb vibration. Each data point represents the log2 ratio, expressed as the logarithm base-2 of the ratio between the vibration-trained soleus and the control soleus muscles. GLRA3: glycine receptor alpha 3 subunit gene; GRM7: metabotropic glutamate receptor subtype 7; SYN3: synapsin III; CDH2: N-Cadherin; ERK1: mitogen-activated protein kinase 3 (also known as p44 MAPK).
4. Discussion
In this study, we investigated the effects of segmental limb vibration on spinal neuronal excitability in individuals with and without SCI. The results showed that: (1) segmental vibration resulted in H-reflex depression in both SCI and healthy control groups, (2) the SCI group showed rapid H-reflex recovery within the first 2 min after vibration whereas H-reflex in the control group appeared to remain at a depressed state for more than 2 min, and (3) the post-activation depression ratio for the SCI group was similar to the control group over 3 min post-vibration. The findings suggest that vibration-induced changes in spinal excitability are likely attributed to inhibitory modulations from multiple sources including presynaptic, postsynaptic, disynaptic, and post-activation depression mechanisms.
A substantial, immediate decrease in H-reflex amplitude in the presence of segmental vibration supports our first hypothesis that the segmental limb vibration can effectively inhibit the H-reflex in both groups. This vibratory-induced H-reflex depression is consistent with findings from earlier studies showing that directly vibrating over the arm or leg muscle tendons results in H-reflex depression (De Gail et al., 1966; Lance et al., 1966; Gillies et al., 1969; Fromm and Noth, 1976; Rymer and Hasan, 1981; Martin et al., 1986; Hultborn et al., 1987). Several underlying mechanisms responsible for the reduction of the H-reflex after prolonged tendon vibration include (1) increased discharge threshold by group Ia afferents, (2) enhanced presynaptic inhibition at Ia terminals, and (3) transmitter depletion at Ia synapses (Curtis and Eccles, 1960; Hayward et al., 1986; Hultborn et al., 1987; Stein, 1995). It is possible that the segmental vibration protocol from this study is analogous to the classic study paradigms of presynaptic inhibition in which a conditioning stimulus (i.e. vibration stimulus) is delivered before the test stimulus for the H-reflex measure. Importantly, segmental limb vibration can be seen as a form of conditioning stimulus, similar to tendon vibration, which enhances classic presynaptic inhibition mechanisms at Ia terminals derived from other leg muscles, including agonist and synergistic muscles around the ankle and knee. In addition to those mechanisms proposed above, we also believe that oscillating the whole lower leg synchronously vibrates antagonistic muscles, and in turn, may induce a massive postsynaptic inhibition to the soleus muscle. Martin et al. (1986) previously showed that the soleus/gastrocnemius H-reflex was inhibited when tendon vibration stimuli were applied to both agonist (i.e. soleus/gastrocnemius) and antagonist (tibialis anterior) muscles simultaneously as compared to vibrating each muscle tendon in isolation. Taken together, these mechanisms likely contribute to the long-lasting post-vibration inhibition of the soleus H-reflex observed in both SCI and healthy individuals. This massive inhibition provides a framework to perhaps influence spinal cord circuitry adaptations, like collateral sprouting, which is known to occur from the acute to the chronic state after SCI. It is also well known that the extent of spasticity after SCI does not peak until several months after the injury, supporting a “reorganization” of spinal circuitry.
Vibration-induced H-reflex inhibition across three different acceleration conditions is also consistent with findings from an earlier study that showed maximal inhibition of the H-reflex between 5 and 30 Hz (Martin et al., 1984). This significant inhibition observed during segmental vibration was attributed to the sum of presynaptic and postsynaptic inhibitory mechanisms derived from continuous, homonymous activation of the muscle spindles within agonist and antagonist muscles (Martin et al., 1984, 1986). Importantly, the current study further advances the effects of segmental vibration on a group of subjects with complete SCI adding that descending drive is not necessary to induce a similar spinal inhibitory effect. In the absence of supraspinal drive (i.e. cortical or subcortical origins), we found that individuals with complete SCI demonstrate significant depression of soleus H-reflexes during vibration as compared to their H-reflex amplitudes before vibration.
Our findings of vibration-induced inhibition of the H-reflex and recovery are consistent with two previous whole-body vibration studies (Armstrong et al., 2008; Sayenko et al., 2010), but not consistent with one recent report (McBride et al., 2010) that showed no change in H-reflex amplitude after whole-body vibration. An important distinction between our single limb segment vibration study and other whole body vibration studies was the consistent recovery response of the H-reflex by 1-min among healthy young adults. Whole body vibration showed a widespread H-reflex recovery time in the healthy subjects from 1, 3, 7, 15, and up to 30 min in previous studies (Armstrong et al., 2008; Sayenko et al., 2010). The variation of H-reflex recovery was even greater in the SCI group after whole-body vibration (Sayenko et al., 2010). This important distinction likely demonstrates the added complexity associated with various other sensory/motor inputs (descending drive, vestibular, visual, joint receptors, and skin receptors), and slight methodological differences among studies. For example, there may be fatiguing effects (Armstrong et al., 2008), various degrees of post-activation depression based on inter-stimulus intervals (Sayenko et al., 2010), various degrees of descending drive when studying individuals with incomplete SCI (Sayenko et al., 2010), and various levels of vestibular modulation (Iles and Pisini, 1992a,b; Abercromby et al., 2007). The finding that body position (supine to upright) facilitates the H-reflex in healthy adults but has no effect in labyrinthine-defective patients supports the importance of vestibular system influence on motor neuron excitability (Aiello et al., 1983). Importantly, the vestibular system influences multiple spinal systems including Ia afferent reciprocal, non-reciprocal, and presynaptic inhibitory pathways (Iles and Pisini, 1992a,b). The unique finding from our study is that, in the absence of those secondary influences, the H-reflex response to isolated limb segment oscillation is robust and internally consistent.
We also speculate that the vibratory-induced inhibition is attributed to a post-activation depression (homosynaptic inhibition) mechanism, particularly during the recovery phase. The evidence that supports this conclusion is that the post-activation depression ratios for both groups stayed at lower values over the first 2 min of the recovery phase. The post-activation depression ratio is known as an indicator of efficacy of monosynaptic transmission, which is measured by the depression of the H-reflex amplitude following the conditioning stimulus (Crone and Nielsen, 1989). Before vibration, the H-reflex depression ratio for the SCI group was significantly lower than the non-SCI group. This reduction of post-activation depression is a known phenomenon in individuals after SCI, resulting from adaptive changes of inter-neurons secondary to the loss of supra spinal control (Mailis and Ashby, 1990; Calancie et al., 1993; Schindler-Ivens and Shields, 2000). During vibration, the depression ratios for both groups were close to zero, and therefore failed to show any group differences. A decrease in depression ratio during vibration may directly result from maximal attenuation of H1 amplitude induced by segmental vibration. However, if 1-min of segmental vibration is analogous to a train of conditioned stimuli, homosynaptic depression at the Ia terminals would likely occur during the segmental limb vibration. Thus, depression ratios were expected to stay at relatively lower values as compared to pre-vibration values for more than a few seconds after termination of the segmental vibration. Indeed, we did observe that the depression ratios stayed at similarly lower levels for both groups over the first 2 min in the recovery phase. The indifference in the depression ratios during the early recovery phase suggests synaptic efficiency of neurotransmitter release from Ia afferents was equally compromised in both SCI and able bodied subjects after 1-min of vibration. Findings of long lasting depression of synaptic transmission were consistent with earlier studies demonstrating that depression of the soleus H-reflex was observed for more than 10 s following the conditioning stimulus (i.e. passive ankle dorsiflexion) (Hultborn et al., 1996). The depression ratios progressively returned to the baseline levels and showed a significant difference between groups at 5 min post-vibration, similar to the pre vibratory condition. A provocative question is whether chronic exposure of paralyzed limbs, from the acute to the chronic state, will modulate the reorganization of the spinal circuitry to prevent the loss of post-activation depression which normally occurs in chronic SCI (Schindler-Ivens and Shields, 2000). This may be important because the loss of post-activation potentiation has recently been associated with stiffness in spastic muscles of those with SCI and multiple sclerosis (Grey et al., 2008).
While the post-activation depression appeared to play an important role in the findings of this study, we do not believe that this mechanism was solely responsible for the long-lasting vibratory-induced inhibition of the soleus H-reflex in the recovery phase. We suggest that some supraspinal (able-bodied) and spinal interneuronal pathways could be involved in H-reflex recovery post-vibration, at least in the non-SCI subjects (Rossi et al., 1988). The evidence to support this conclusion is that we showed that the rates of H-reflex recovery were different between SCI and control groups whereas the post-activation depression ratios were at comparable levels between the two groups. It was clear that the depression ratio was significantly lower in the SCI group prior to vibration (as is well known) as compared to the control group, but was indifferent between groups over the first 3 min after vibration. If post-activation depression mechanisms play a major role in mediating H-reflex recovery, we suggest that a more consistent trend showing relatively smaller depression ratios in concordance with a rapid H-reflex recovery should be observed in the SCI group during recovery. Taken together, it appears that differences in H-reflex recovery between SCI and healthy control groups are attributed to modulation from multiple CNS levels.
The results of this study did not support our second hypothesis that the change in H-reflex amplitude during vibration is linearly correlated with the degree of gravitational acceleration. A previous study showed the level of H-reflex inhibition as a function of intensity and frequency of vibration (Martin et al., 1984). At constant acceleration, the inhibition decreases as the frequency increases; while at a fixed frequency, the inhibition increases with acceleration. Indeed, we expected that the greatest H-reflex depression would appear at the 1.2g acceleration condition. The rationale for this hypothesis was that the high acceleration would induce larger peak-to-peak displacement of the agonist and antagonist limb muscle masses which potentially causes a longer stretch duration of the muscle spindle. We believe that the segmental vibration protocol has already produced a floor effect of H-reflex inhibition in the acceleration condition at only 0.3g. Therefore, during the acceleration conditions of 0.6g and 1.2g, no additional reduction of H-reflex amplitude was produced. Indeed, during vibration, H-reflex amplitudes were maximally suppressed in the healthy control group (average H1 = 0.96% of M-max) as well as in the SCI group (average H1 = 4.03% of M-max) across all three acceleration conditions (Fig. 5C). As a result of the floor effect, we failed to demonstrate any acceleration group differences during vibration. We did observe a consistent trend across all three acceleration conditions that the H-reflex was suppressed less in individuals with complete SCI during vibration. It has been suggested that reduced H-reflex depression is associated with a greater loss of presynaptic inhibition from central drive and impaired inhibitory modulation within spinal neuronal circuits after CNS lesions (Mailis and Ashby, 1990; Calancie et al., 1993; Schindler-Ivens and Shields, 2000).
A novel component of this study was that we targeted five genes known to have retrograde influences on genes mediating synaptic plasticity in the central nervous system. While this purpose was purely exploratory, it revealed that mechanical oscillation, in the absence of muscle contraction, modulated key genes known to regulate synaptic plasticity when compared to the same persons limb that did not receive vibratory input. In particular, our data showed that ERK1, extracellular regulated kinase 1, was up regulated 3 h after a short-term vibration intervention. It has been suggested that ERK1/ERK2 (p44/p42 MAPK) plays an important role in cell proliferation and survival, neuronal differentiation and plasticity (Hetman and Xia, 2000; Pearson et al., 2001; Hetman and Gozdz, 2004). Importantly, ERK1/2 transduces anti-apoptotic signaling in neurons and mediates neuroprotective activity of extracellular factors, including neurotrophins (Xia et al., 1995; Meyer-Franke et al., 1998; Bonni et al., 1999; Nakazawa et al., 2002; Hetman and Gozdz, 2004). We also observed that other gene transcriptions that mediate inhibitory synaptic transmissions were down regulated 3 h after a single bout of limb vibration. Importantly, the changes in mRNA expression were all relative to the control limb that did not receive mechanical oscillation and also different from normal healthy controls (unpublished data).
It is also possible that different genes have different response times to vibration stimuli and were time-sensitive for levels of expression (Wang et al., 2010); the expression levels may reach peak values earlier and may be already decayed when performing the muscle biopsy 3 h after vibration. Thus, repeatedly sampling over different times after limb segment vibration may help to gain a better understanding of dynamic changes in gene transcription (Gomez-Pinilla et al., 2004; Ying et al., 2005; Wang et al., 2010; Dupont et al., 2011). Although brain-derived neurotrophin factor (BDNF), nerve growth factor (NGF), neurotrophins-3 (NT-3), and other trophic factors did not show significant increases in expression levels at 3 h after a single short-term limb segmental vibration, it is not known if repeated training bouts would ultimately change the regulation of BDNF or NGF. Although these findings are exploratory, they raise the possibility that repetitive use of mechanical oscillation may induce changes that regulate the loss of post-activation depression that occurs several months after SCI. Future studies are necessary to understand if regular mechanical oscillation influences velocity dependent change in stiffness (spasticity), post-activation depression, and specific transmitters under molecular control within the muscle.
5. Conclusion
This study demonstrated that mechanical oscillation of a single paralyzed limb segment modulates the amplitude of the H-reflex and influences the degree of post-activation depression in healthy and those with complete SCI. H-reflex depression is likely caused by the sum of modulations via multiple sources within the spinal neuronal circuitry including presynaptic inhibition, postsynaptic inhibition, and post-activation depression mechanisms, but does not require supra spinal drive. These mechanisms may ultimately contribute to the velocity dependent increase in stiffness (spasticity) commonly observed in those with SCI. In addition, specific gene signatures, known to mediate synaptic plasticity, were modulated by the mechanical oscillation. Future studies, designed to determine the long-term effects of mechanical oscillation on the neuro-musculoskeletal system, are necessary to develop methods to enhance the health of people with SCI.
HIGHLIGHTS.
Vertical limb oscillations (vibration) resulted in a predominant inhibition of H-reflex and post-activation depression mechanism in both SCI and healthy control groups.
Microarray analysis revealed that a short-term limb segment vibration had significant effects on genes associated with synaptic plasticity.
These findings highlight the possibility that long-term vibration training may promote spinal neuronal plasticity in individuals with chronic SCI.
Acknowledgments
This study was supported in part by awards to R.K.S. from the National Institutes of Health (R01HD062507), The U.S. Department of Veterans Affairs, and the Craig H. Neilsen Foundation. We thank engineer Jason Wu, MS, for his help with the feedback controlled vibration system.
References
- Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Palosski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39:1642–50. doi: 10.1249/mss.0b013e318093f551. [DOI] [PubMed] [Google Scholar]
- Adams CM, Suneja M, Dudley-Javoroski S, Shields RK. Altered mRNA expression after long-term soleus electrical stimulation training in humans with paralysis. Muscle Nerve. 2011;43(1):65–75. doi: 10.1002/mus.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello I, Rosati G, Serra G, Tugnoli V, Manca M. Static vestibulospinal influences in relation to different body tilts in man. Exp Neurol. 1983;79:18–26. doi: 10.1016/0014-4886(83)90375-8. [DOI] [PubMed] [Google Scholar]
- Armstrong WJ, Nestle HN, Grinnell DC, Cole LD, Van Gilder EL, Warren GS, et al. The acute effect of whole-body vibration on the Hoffmann reflex. J Strength Cond Res. 2008;22:471–6. doi: 10.1519/JSC.0b013e3181660605. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, et al. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81:449–54. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- Brock JH, Elste A, Huntley GW. Distribution and injury-induced plasticity of cadherins in relationship to identified synaptic circuitry in adult rat spinal cord. J Neurosci. 2004;24:8806–17. doi: 10.1523/JNEUROSCI.2726-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–86. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31:3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Eccles JC. Synaptic action during and after repetitive stimulation. J Physiol. 1960;150:374–98. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966;29:1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Stevens L, Cochon L, Falempin M, Bastide B, Canu MH. ERK is involved in the reorganization of somatosensory cortical maps in adult rats submitted to hindlimb unloading. PLoS One. 2011;6:e17564. doi: 10.1371/journal.pone.0017564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chi P, Blanpied TA, Xu Y, Magarinos AM, Ferreira A, et al. Regulation of neurotransmitter release by synapsin III. J Neurosci. 2002;22:4372–80. doi: 10.1523/JNEUROSCI.22-11-04372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C, Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976;256:117–36. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies JD, Lance JW, Neilson PD, Tassinari CA. Presynaptic inhibition of the monosynaptic reflex by vibration. J Physiol. 1969;205:329–39. doi: 10.1113/jphysiol.1969.sp008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Hodgson J, Edgerton VR. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J Neurophysiol. 2004;92:3423–32. doi: 10.1152/jn.00432.2004. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M, et al. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–97. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Nielsen RP, Heckman CJ, Hutton RS. Tendon vibration-induced inhibition of human and cat triceps surae group I reflexes: evidence of selective Ib afferent fiber activation. Exp Neurol. 1986;94:333–47. doi: 10.1016/0014-4886(86)90107-x. [DOI] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271:2050–5. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Warsz) 2000;60:531–45. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–62. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;389:757–72. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. J Physiol. 1992a;455:425–46. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol. 1992b;455:407–24. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Sato Y, Uzawa M. Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in postmenopausal osteoporotic women treated with alendronate. Aging Clin Exp Res. 2005;17:157–63. doi: 10.1007/BF03324589. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–9. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, et al. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem. 2005;94:982–94. doi: 10.1002/jcb.20363. [DOI] [PubMed] [Google Scholar]
- Lance JW. The reflex effects of muscle vibration. Proc Aust Assoc Neurol. 1966;4:49–56. [PubMed] [Google Scholar]
- Lance JW, Degail P, Neilson PD. Tonic and phasic spinal cord mechanisms in man. J Neurol Neurosurg Psychiatry. 1966;29:535–44. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailis A, Ashby P. Alterations in group Ia projections to motoneurons following spinal lesions in humans. J Neurophysiol. 1990;64:637–47. doi: 10.1152/jn.1990.64.2.637. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Gauthier GM. Spinal reflex alterations as a function of intensity and frequency of vibration applied to the feet of seated subjects. Aviat Space Environ Med. 1984;55:8–12. [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Gauthier GM. Inhibitory effects of combined agonist and antagonist muscle vibration on H-reflex in man. Aviat Space Environ Med. 1986;57:681–7. [PubMed] [Google Scholar]
- Martin R, Durroux T, Ciruela F, Torres M, Pin JP, Sanchez-Prieto J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J Biol Chem. 2010;285:17907–17. doi: 10.1074/jbc.M109.080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JM, Nuzzo JL, Dayne AM, Israetel MA, Nieman DC, Triplett NT. Effect of an acute bout of whole body vibration exercise on muscle force output and motor neuron excitability. J Strength Cond Res. 2010;24:184–9. doi: 10.1519/JSC.0b013e31819b79cf. [DOI] [PubMed] [Google Scholar]
- McCall GE, Grindeland RE, Roy RR, Edgerton VR. Muscle afferent activity modulates bioassayable growth hormone in human plasma. J Appl Physiol. 2000;89:1137–41. doi: 10.1152/jappl.2000.89.3.1137. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–93. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol. 2000;83:1701–9. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J Biol Chem. 1999;274:27573–7. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002;43:3319–26. [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Rajalu M, Muller UC, Caley A, Harvey RJ, Poisbeau P. Plasticity of synaptic inhibition in mouse spinal cord lamina II neurons during early postnatal development and after inactivation of the glycine receptor alpha3 subunit gene. Eur J Neurosci. 2009;30:2284–92. doi: 10.1111/j.1460-9568.2009.07018.x. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R, Schieppati M. The H reflex recovery curve reinvestigated: low-intensity conditioning stimulation and nerve compression disclose differential effects of presumed group Ia fibres in man. Hum Neurobiol. 1988;6:281–8. [PubMed] [Google Scholar]
- Rymer WZ, Hasan Z. Prolonged time course for vibratory suppression of stretch reflex in the decerebrate cat. Exp Brain Res. 1981;44:101–12. doi: 10.1007/BF00238754. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Masani K, Alizadeh-Meghrazi M, Popovic MR, Craven BC. Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci Lett. 2010;482:66–70. doi: 10.1016/j.neulet.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–41. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler-Ivens SM, Shields RK. Soleus H-reflex recruitment is not altered in persons with chronic spinal cord injury. Arch Phys Med Rehabil. 2004;85:840–7. doi: 10.1016/j.apmr.2003.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–44. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Torvinen S, Kannus P, Sievanen H, Jarvinen TA, Pasanen M, Kontulainen S, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18:876–84. doi: 10.1359/jbmr.2003.18.5.876. [DOI] [PubMed] [Google Scholar]
- Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Wang GJ, Ho ML, Wang YH, Yeh ML, Chen CH. Low-magnitude vertical vibration enhances myotube formation in C2C12 myoblasts. J Appl Physiol. 2010;109:840–8. doi: 10.1152/japplphysiol.00115.2010. [DOI] [PubMed] [Google Scholar]
- Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK–p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–9. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Zelano J, Wallquist W, Hailer NP, Cullheim S. Expression of nectin-1, nectin-3, Ncadherin, and NCAM in spinal motoneurons after sciatic nerve transection. Exp Neurol. 2006;201:461–9. doi: 10.1016/j.expneurol.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Zhi J, Xu G, Rubin CT, Hadjiargyrou M. The lipogenic gene spot 14 is activated in bone by disuse yet remains unaffected by a mechanical signal anabolic to the skeleton. Calcif Tissue Int. 2008;82:148–54. doi: 10.1007/s00223-007-9100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]