Abstract
Transcranial magnetic stimulation (TMS) is a neuropsychiatric tool that can serve as a useful method to better understand the neurobiology of cognitive function, behavior, and emotional processing. The purpose of this paper is to examine the utility of TMS as a means of measuring neocortical function in neuropsychiatric disorders in general, and schizophrenia in particular, for the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative. When incorporating TMS paradigms in research studies, methodological considerations include technical aspects of TMS, cohort selection and confounding factors, and subject safety. Available evidence suggests benefits of TMS alone or in combination with neurophysiologic and neuroimaging methods, including positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), functional MRI (fMRI), functional near infrared spectroscopy (fNIRS), magnetoencephalography (MEG), and electroencephalography (EEG), to explore neocortical function. With the multiple TMS techniques including single-pulse, paired-pulse, paired associative stimulation, and repetitive TMS and theta burst stimulation, combined with neurophysiologic and neuroimaging methods, there exists a plethora of TMS experimental paradigms to modulate different neocortical physiologic processes. Specifically, TMS can measure cortical excitability, intracortical inhibitory and excitatory mechanisms, and local and network cortical plasticity. Coupled with functional and electrophysiological modalities, TMS can provide insight into the mechanisms underlying healthy neurodevelopment and aging, as well as neuropsychiatric pathology. Thus, TMS could be a useful tool in the CNTRICS armamentarium of biomarker methods. Future investigations are warranted to optimize TMS methodologies for this purpose.
Keywords: Transcranial magnetic stimulation, TMS, schizophrenia, CNTRICS, cortical function, biological marker
Introduction
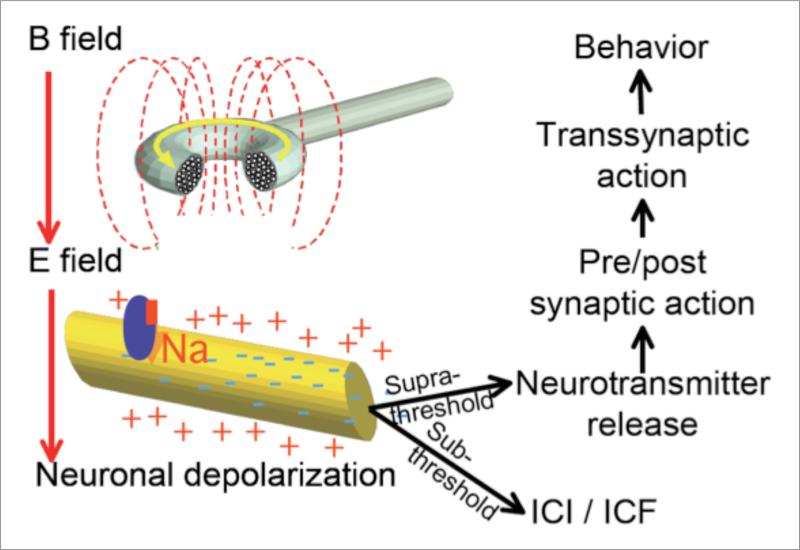
Transcranial magnetic stimulation (TMS) is a neuroscientific tool that can be used to explore and better understand neocortical function and treat psychiatric symptomatology (1, 2). It involves the generation of a magnetic field through the use of an electromagnetic coil connected to a TMS device. The generated magnetic field induces an electrical current in the brain. Depending on the characteristics of stimulation (e.g., intensity, timing in relation to ongoing brain activity, pulse shape), TMS can induce neuronal depolarization, intracortical inhibition or facilitation, or the releasing of endogenous neurotransmitters resulting in transsynaptic action (3) (Fig. 1).
Figure 1.
Mechanism of action of transcranial magnetic stimulation (TMS). TMS uses magnetic fields that enter cortical tissue and with varied intensities can result in neuronal depolarization, intracortical inhibition or facilitation, or the releasing of endogenous neurotransmitters that results in transsynaptic action.
The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative included TMS in its second meeting (4) due to its potential, when used alone or in combination with other methodologies, to address basic, clinical, and translational science questions. As TMS is a noninvasive technique that provides ubiquitous means to measure brain function in an efficient and safe manner, it can serve CNTRICS in its goals to enhance the information gathered from neurocognitive measures (CNTRICS phase I) (5, 6) through the development of reliable and valid biomarkers.
Pioneering work of Hoffman and colleagues (7) and Cohen and colleagues (8) have led to a multitude of TMS neurotherapeutic trials for the treatment of auditory verbal hallucinations and negative symptoms of schizophrenia, respectively. Though there is current meta-analytic evidence that TMS is efficacious for the treatment of positive (9, 10) and, to a lesser extent, negative (10, 11) symptomatology in schizophrenia, full discussion of such application is beyond the scope of this paper. Stanford et al. (12) provide a comprehensive review of the therapeutic benefits of TMS for patients with schizophrenia. The purpose of this paper is to examine the utility of TMS as a means of measuring neocortical function in neuropsychiatric disorders in general, and schizophrenia in particular.
Methodological considerations for TMS
There are many methodological considerations when including TMS in research studies to investigate neocortical function and develop biomarkers of disease and disease progression. These include technical aspects of TMS, cohort selection and confounding factors, and subject safety. This is comprehensively detailed in the online supplementary material.
Combination of TMS and other neuroimaging modalities
Combining TMS with electrophysiological and neuroimaging modalities facilitates the generation of comprehensive neurophysiologic data and collaborative research between diverse fields of expertise, such as cognitive neuroscience and neuropsychiatry. Neuroimaging methods that have been successfully combined with TMS include positron emission tomography, single photon emission computed tomography, functional magnetic resonance imaging (fMRI), functional near infrared spectroscopy, magnetoencephalography (MEG), and electroencephalography (EEG). Most of these modalities produce reliable and valid data, though caution must be taken when using fMRI, EEG, and MEG as they may produce artifacts when combined with TMS due to their measurement of cortical functions with electromagnetic spectrums. Additionally, specific safety concerns apply to the combination of TMS with other brain imaging modalities. For example, it may be possible for EEG electrodes to become heated due to TMS-induced Eddy currents and result in scalp burn, unless appropriate electrode materials (e.g., plastic) or shapes (e.g., slotted) are utilized (13).
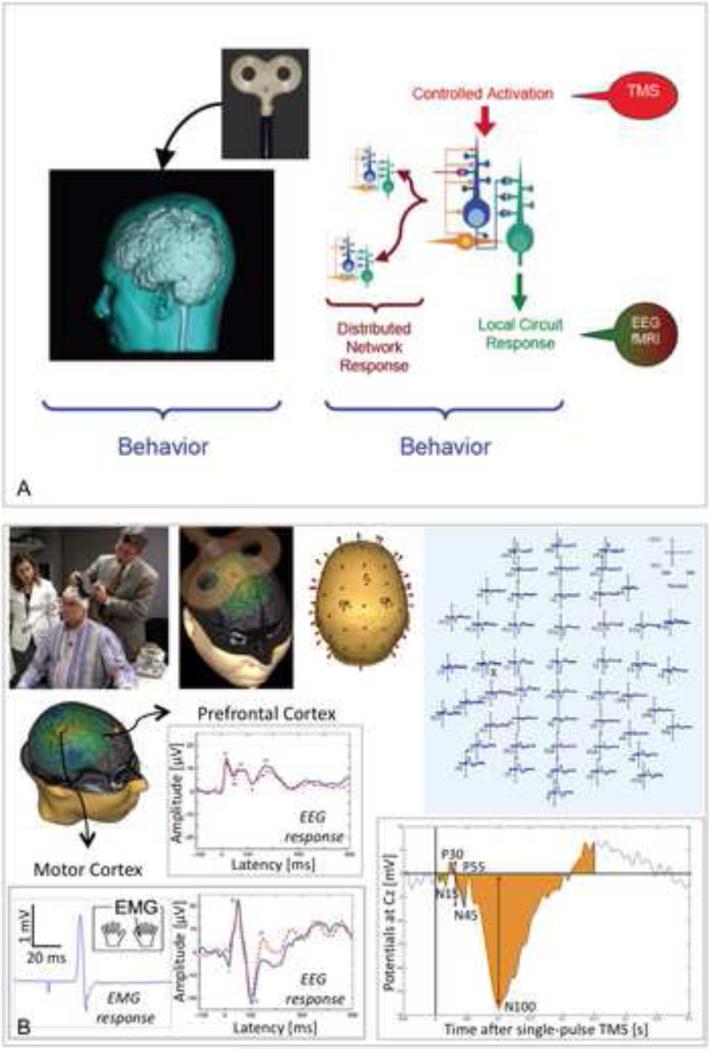
A particularly appealing aspect of combining TMS with other imaging techniques is that it becomes possible to obtain physiologic, objective measures of TMS effects, rather than behavioral performance on cognitive or motor tasks. This minimizes the impact of factors such as motivation, attention, or cognitive ability that restrict other diagnostic tests to higher functioning, adolescent or adult subjects. In particular, the combination of TMS with simultaneous EEG recording (14, 15) can offer exquisite temporal resolution with acceptable spatial resolution, concurrent information about effects on local and network brain activity, be applicable across the age-span in healthy subjects and patients, and be translated from preclinical to clinical models (16). Moreover, EEG provides a direct measure of neuronal activity and is capable of differentiating between inhibitory and facilitatory effects (17, 18). A noninvasive input (TMS) of known spatial and temporal characteristics can thus be applied to study local reactivity of the brain and interactions between different brain regions with directional and precise chronometric information. Furthermore, brain functional connectivity and inter-regional coordination can be directly estimated from EEG. Reliable TMS-EEG systems are now commercially available (e.g., Nexstim, Neuroscan) (Fig. 2).
Figure 2.
Combined transcranial magnetic stimulation (TMS) with electrophysiological and neuroimaging methods to develop biomarkers of disease. A: TMS can be safely combined with imaging techniques, such as functional magnetic resonance imaging (fMRI), or neocortical recording methods, such as electroencephalography (EEG), to comprehensively study behavior and develop useful biomarkers; B: The eXimia Neuronavigation Brain Stimulation system by Nexstim, and schematic plots of acquired TMS-EEG data on motor and prefrontal cortices.
TMS paradigms to measure cortical function in schizophrenia
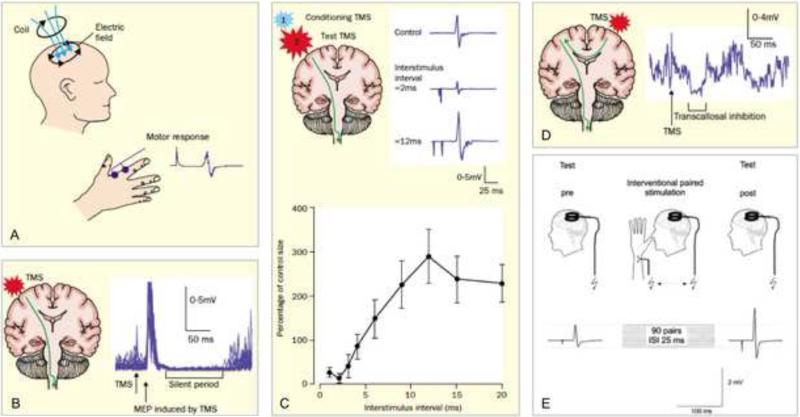
TMS paradigms, including single- and paired-pulse TMS, paired associative stimulation, and repetitive TMS, provide in vivo noninvasive indices of cortical excitability, intracortical excitation and inhibition, and cortical plasticity (Fig 3). These methods may be important in characterizing the neural pathology associated with schizophrenia and assessing the efficacy of therapeutic interventions. In the following paragraphs, we briefly explain each of these TMS protocols and the information they may provide by exemplifying with studies conducted in schizophrenia patients using these measures alone or in combination with other neuroimaging modalities.
Figure 3.
Schematic representation of TMS measures of motor cortical reactivity and plasticity. A: Single-pulse TMS and motor evoked potential; B: Cortical silent period; C: Paired-pulse TMS to assess intracortical inhibition and intracortical facilitation; D: Paired-pulse TMS to assess transcallosal inhibition; E: Paired associative stimulation [schematics A-D from (21); schematic E from (57)].
Single-pulse TMS
Single-pulse TMS (spTMS) can be used to spatially and temporally map behavior-related neurocircuitry to then test brain-behavior relationships. The temporal resolution is excellent as each pulse is less than 1 millisecond (ms), but its action can be substantially extended past the 1-ms timeframe (19). The spatial resolution is moderate, estimated to affect approximately 5 mm3 of brain cortex. However, because of the transsynaptic action of TMS, the manifested behavior or cognitive change could result from indirect stimulation of other synaptically connected cortical regions (20). Importantly, the direct magnetic field does not reach deeper, subcortical structures unless specially shaped coils (e.g., H-coil) are employed. Even then, selective stimulation of deep structures is currently not possible. Nevertheless, stimulation of subcortical regions of interest can be activated through transsynaptic action from stimulation of superficial cortical structures. A strength of TMS is that it can help establish causality while other functional imaging and physiology methods only provide correlational data. However, it is important to note that the absence of a TMS effect on a behavior due to stimulation of a select region does not, unequivocally, determine that the area in question is not involved in that particular behavior. Rather, the lack of response may be due to methodological challenges such as stimulation parameters, medication effects, or psychiatric pathology.
The spTMS technique can be used to measure motor threshold (MT) intensity to produce a motor response, determine the size of a motor evoked potential (MEP), and the duration of the silent period. When spTMS is applied to the motor cortex at appropriate stimulation intensity, MEPs can be elicited and recorded by surface electromyography (EMG) from contralateral extremity muscles. MT refers to the lowest TMS intensity necessary to evoke an MEP in the target muscle and is commonly defined as the minimum stimulus intensity required to elicit MEPs of more than 50 μV peak-to-peak amplitude in at least 50% of successive trials in resting target muscles (21). MTs and MEPs can evaluate motor cortical excitability. A recent study using this technique found that neuroleptic-naïve, first-episode schizophrenia patients showed significantly lower resting MT (RMT) relative to healthy controls (22). Davey et al. (23) used spTMS to assess effects of antipsychotic medications and found that while there was no difference between schizophrenia patients with or without medication on MT, there was a longer latency of maximum suppression in those on medication. This shows the potential of TMS to monitor the effects of antipsychotic pharmacotherapy.
In addition to MT, the cortical silent period (cSP) is another important variable stemming from spTMS with EMG monitoring. When an individual is instructed to maintain muscle contraction and a single suprathreshold TMS pulse is applied to the motor cortex contralateral to the target muscle, the EMG activity is arrested for a few hundred milliseconds after the MEP. This period of EMG suppression is referred to as a ‘silent period’ and is normally defined as the time from the end of the MEP to the return of voluntary EMG activity (21). Whereas spinal inhibition contributes to the early part of the SP (its first 50-75ms), the late part originates most likely in the motor cortex (24). Most of the SP is hypothesized to be due to inhibitory mechanisms at the motor cortex, likely mediated by gamma-hydroxybutyric acid (GABA) B (GABAB) receptors (21).
Recently, cSP deficits have been inversely associated with negative symptom severity in schizophrenia, suggesting alteration in GABAB-mediated neurotransmission (25). Wobrock et al. (26) demonstrated a significant prolongation of cSP in patients with first-episode schizophrenia with limited exposure to antipsychotic treatment, compared to healthy controls, potentially due to a compensatory increase in GABAergic neurotransmission, or to effects of medication. In fact, Liu et al. (25) showed that patients receiving clozapine had longer cSP whereas patients receiving other antipsychotics or unmedicated patients had shorter cSP. Shortening of the cSP was also reported by Eichhammer et al. (27) in drug-naïve, first-episode schizophrenia patients, thus indicating a dysfunctional GABAB-mediated inhibitory process, presumably within thalamo-cortical circuits.
Single-pulse TMS can also assess use-dependent plasticity. A commonly used paradigm involves training subjects to practice a specific kinematic movement (e.g., of the thumb) in the opposite direction to the kinematic movement induced by TMS. The effect of motor training on the TMS-induced direction of movement is then assessed. This provides a measure of motor cortical, use-dependent plasticity (28). Daskalakis et al. (29) found that schizophrenia patients, regardless of the presence of medication, showed deficits in use-dependent plasticity, which may be related to disruption in dopaminergic, GABA or N-methyl-D-aspartic acid (NMDA) neurotransmitter systems.
TMS coupled with EEG can be used to study excitability of cortical areas outside the motor cortex (16). TMS-evoked potentials (EPs) in the EEG can be induced using spTMS and effects evaluated interhemispherically within homologue regions (30) or within interconnected regions of extended networks (31). In a study by Ferrarelli et al. (32), 8- to 10-minute EEG sessions were recorded during spTMS over the right premotor cortex. Results suggested that schizophrenia patients relative to healthy controls, had reduced evoked gamma oscillations in the frontal cortex, thus reinforcing the existence of an intrinsic dysfunction of the frontal thalamo-cortical circuits, as well as glutamatergic (33, 34) and GABAergic (35-37) neurotransmission.
Additionally, spTMS or short trains of TMS during real-time EEG or other brain imaging methods can be used to activate a given cortical region and assess the distributed effects on the basis of transsynaptic cortico-cortical and cortico-subcortical effects. Though this approach has yet to be applied to schizophrenia, the potential seems most appealing. Accumulating evidence from dual coil TMS, combined TMS/EEG, and combined TMS/functional neuroimaging experiments suggests that TMS modulates neuronal activity beyond the site of stimulation, impacting a distributed network of brain regions (38-40). Comparison of such distributed effects of spTMS in patients with schizophrenia or at-risk individuals, compared with healthy controls, may yield valuable insights into alterations in functional brain circuitry.
Paired-pulse TMS
Paired-pulse TMS (ppTMS) involves the use of two TMS pulses—a test pulse and a conditioning pulse—to examine intracortical inhibition and facilitation, which might be in vivo measures of GABAergic and NMDA activity, respectively. The ppTMS is a reliable and valid technique that can track changes in response to interventions. Furthermore, the combination of ppTMS and EEG allows for the assessment of intracortical inhibitory and excitatory phenomena in non-motor cortical regions and functionally connected neuronal circuits. However, the neurobiological underpinnings and reliability of combined ppTMS and EEG are less well studied.
Paired-pulse TMS enables the study of cortical excitation/inhibition (E/I) balance. There are three distinct ppTMS approaches [for review, (21)]:
In short interval intracortical inhibition (SICI), TMS-EPs are obtained in response to a subthreshold conditioning stimulus followed by suprathreshold stimulation to the same cortical region. When these pulses are given with an interstimulus interval (ISI) of 1-6 ms, this results in a relative suppression of the evoked response to the second pulse as compared to a single pulse at the same intensity. It is hypothesized that the first subthreshold pulse activates low-threshold inhibitory circuits (via inhibitory post-synaptic potentials; IPSP) resulting in a suppression of the response to the second, suprathreshold pulse. Moreover, as GABAA agonists increase SICI, it is hypothesized that SICI is GABAA-dependent (41).
With long interval intracortical inhibition (LICI), both TMS pulses are delivered at supratheshold intensities with an ISI of 50-200ms. There is strong evidence that LICI is mediated by long-lasting GABAB-dependent IPSPs and activation of pre-synaptic GABAB receptors on inhibitory interneurons (42).
In intracortical facilitation (ICF), the amplitude of a suprathreshold test TMS-evoked potential can be enhanced if it is preceded by a subthreshold, conditioning pulse applied 10-25 ms earlier. ICF is believed to result from the net facilitation of inhibitory and excitatory mechanisms mediated by GABAA and NMDA receptors, respectively. Glutamatergic cortical interneurons are likely to be involved in ICF, since ICF is reduced by NMDA-antagonists such as dextromethorphan (43).
Deficits in TMS measures of cortical inhibition (44) have been reported in schizophrenia [e.g., (45-47)]. Reduced SICI was found to be associated with positive symptom severity (whereas negative symptoms appear to be inversely associated with cSP) (25, 45), and has been observed in first-episode schizophrenia patients (26, 48). Significant deficits in cerebellar inhibition in schizophrenia patients compared with healthy controls have also been reported suggesting that an abnormality in the cerebellum or disrupted cerebellar-thalamic-cortical connectivity may mediate disorganized thought processes and psychosis (49). Antipsychotic medications have been found to alter TMS measures of CI. Liu et al. (25) observed in a relatively small sample of patients with schizophrenia treated either with clozapine, olanzapine/quetiapine, or risperidone that only clozapine was associated with decreased SICI (and longer cSP). This finding indicates that different antipsychotic medications may have differential effects on the mechanisms underlying schizophrenia symptomatology and, thus, that CI measures could serve as a biomarker for those changes. In agreement, Fitzgerald et al. (50) found that olanzapine and risperidone confer different effects on RMT and CI suggesting that each may uniquely alter inhibitory mechanisms. A question stemming from those studies relates to whether there is a dose-dependent relationship between antipsychotic medication and TMS measures of CI. For instance, Daskalakis et al. (51) found that single-dose administration of antipsychotic medications (haloperidol and olanzapine) did not affect CI in healthy controls. Regardless, TMS can serve to study single- or repeated-dose administration of pharmacotherapy to understand the effects on neurotransmitter systems in schizophrenia patients undergoing medication management.
Koch et al. (52) also used ppTMS to investigate ipsilateral parieto-motor connectivity in schizophrenia patients. They found that, compared to healthy subjects in whom a conditioning subthreshold TMS pulse applied over the posterior parietal cortex was able to increase the excitability of the ipsilateral motor cortex, medicated and unmedicated patients with schizophrenia failed to show any facilitatory parieto-motor interaction, thus suggesting a cortico-cortical dysconnection in schizophrenia.
Transcallosal inhibition (TCI) and facilitation (TCF) can also be measured with ppTMS. This method involves stimulation of the contralateral motor cortex several milliseconds prior to stimulation of the ipsilateral motor cortex (41), inhibiting or enhancing the size of the MEP produced by ipsilateral stimulation as a function of the interval between them. Hoy et al. (53) showed that schizophrenia patients exhibited significantly less TCI than controls, but found no difference in TCF. The lack of TCI in 25% of healthy relatives of schizophrenia patients has also been reported (54). However, the cause of these TCI alterations are likely attributable to alterations in intracortical inhibition mechanisms, rather than deficits in corpus callosum connections, as the latency of TCI was not altered in patients with schizophrenia (55).
It is possible to combine ppTMS with EEG to study cortical inhibition in regions closely related to the pathophysiology of schizophrenia. Specifically, when examining the effects of GABAergic inhibitory neurotransmission on gamma-oscillations, thought to be generated through the execution of higher order cognitive tasks (e.g., working memory) in the DLPFC, it has been shown that schizophrenia patients have significant deficits of inhibition of gamma oscillations compared to healthy subjects and patients with bipolar disorder (56).
Paired associative stimulation
Paired associative stimulation (PAS) involves the pairing of an electric stimulus to the peripheral median nerve with a TMS pulse over the contralateral motor cortex (57). This TMS protocol is used to study plasticity within the sensorimotor system based on the principle of spike timing-dependent plasticity. It is thought that long-term potentiation (LTP)- and depression (LTD)-like plasticity is reflected by the change in MEPs, registered by EMG, after PAS as compared to before. If the pairs of pulses are delivered at an ISI of 10ms (PAS10), suppression of MEPs is observed, an index LTD-like plasticity (58). If the pairs of pulses are delivered at an ISI of 25ms (PAS25), there is facilitation of MEPs post-PAS for a given time period, which indexes LTP-like plasticity (57, 58).
Using the PAS25 paradigm, Frantseva et al. (59) demonstrated that schizophrenia patients, compared to healthy subjects, showed deficits in MEP facilitation indicating disrupted LTP-like plasticity, which appeared to be associated with impaired motor skill learning. This example highlights the utility of the PAS-TMS paradigm to assess synaptic plasticity within the motor system [for review, (60)], and its safe application in schizophrenia patients.
Repetitive TMS
The application of repeated TMS pulses at a specific rate or frequency is referred to as repetitive TMS (rTMS). Trains of rTMS, at various stimulation frequencies and patterns (e.g., duration, ISIs, pulses per train, intensity), can result in synaptic and transsynaptic action, thus inducing a lasting modification of activity in the targeted brain region that outlasts the effects of the stimulation itself. This technique of TMS can be used to modulate cortical plasticity and track dynamic changes in reactivity. For example, Fitzgerald et al. (61) showed reduced plastic brain responses in medicated and unmedicated patients with schizophrenia. Specifically, cortical excitability, as assessed by MT levels, was not reduced in both groups of patients in response to a single 15-min train of 1-Hz rTMS applied to the motor cortex, as compared to a healthy control group. In contrast, significant differences were seen between the patients’ and the control groups in response to rTMS for MEP size and cSP duration.
Repetitive TMS holds promise as a potential enhancer of cortical function related to cognition in schizophrenia. However, to our knowledge, no studies have specifically addressed TMS-induced cognitive enhancement. An appealing aspect of such an application is that it might be combined with other interventions (e.g., computer-based cognitive training) to achieve synergistic potentiating effects. Other forms of non-invasive brain stimulation (e.g., transcranial direct current stimulation), might offer alternatives for such an application. Potentially useful investigations of enhancement of cortical function directly related to cognition for schizophrenia can be envisioned based on studies in healthy volunteers. For instance, Barr et al. (62) recently provided evidence of enhanced gamma-oscillatory activity elicited by performance on the N-back task after a single, 20 Hz rTMS session applied bilaterally to the DLPFC in healthy participants. Indeed, active rTMS significantly increased gamma oscillatory activity compared with baseline and sham stimulation, causing the greatest change in frontal gamma oscillatory activity in the N-back conditions with the greatest cognitive demand. Yet, this effect was not shown to improve working memory performance. Eventually, repeated, daily rTMS sessions might be needed to produce changes in plasticity [e.g., (63)] and lasting effects on cognition. On the other hand, Plewnia et al. (64) showed that synchronous, bifocal rTMS can induce an increase of interregional EEG coherence, which may eventually have behavioral consequences. In this context, it is worth noting again that rTMS has been applied in the treatment of positive and negative schizophrenia symptomatology [for review, (9-12)]. Furthermore, Mittrach and colleagues (65) have assessed the tolerability and safety of 10 Hz rTMS over the left DLPFC (a TMS paradigm commonly used to treat negative symptoms) with regard to cognitive function, in a sham-controlled trial, and found no deterioration of cognitive function due to rTMS treatment. In fact, their study pointed to the usefulness of considering baseline cognitive status to optimize treatment efficacy, as inferior performance in certain neuropsychological aspects before treatment seemed to predict a better response to active rTMS. One of the limitations of rTMS, however, is that the underlying neurophysiologic effect is uncertain and likely complex, with the modulatory effects not relying solely on cortical synaptic efficacy changes (66).
A unique rTMS protocol, known as theta burst stimulation (TBS), is capable of more specifically assessing cortical LTP-like and LTD-like plasticity by introducing a train of high-frequency stimulation and then evaluating the cortical/cortico-spinal response to spTMS (e.g., through EEG or EMG) for a period of time following the plasticity-inducing train (67). Intermittent or continuous TBS protocols (iTBS, cTBS) have been shown to change cortical activity that lasts well beyond the duration of the TMS application, and a time-course consistent with that found with LTP and LTD in preclinical models [for review, (60, 68)]. Furthermore, iTBS and cTBS appear to modulate the glutamatergic and GABAergic systems (69, 70).
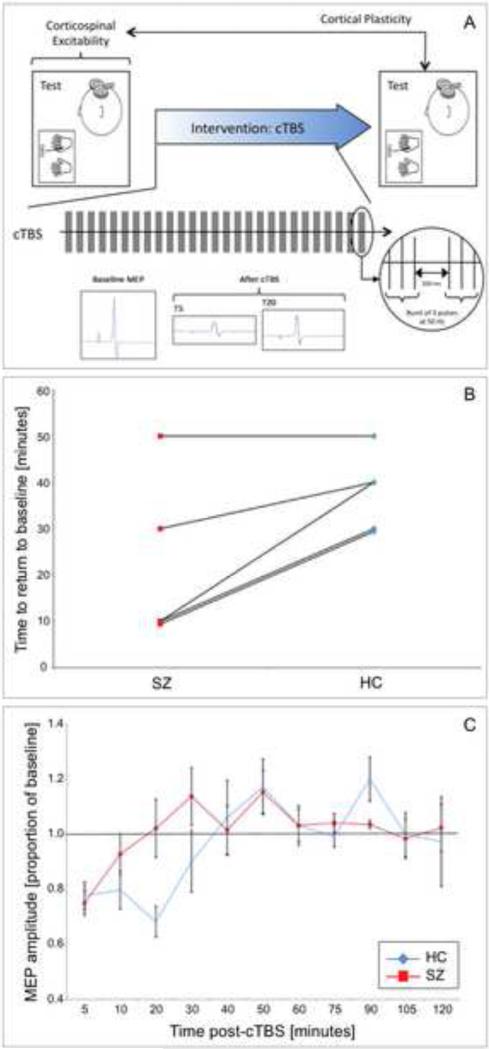
As previously discussed, TMS-EPs can be registered by EMG when targeting the motor cortex, or by EEG or fMRI when stimulating non-motor cortical regions. After iTBS or cTBS, EPs can thus assess the efficiency of LTP- and LTD-like phenomena, which in turn provides a valuable biomarker for local and network cortical plasticity. Recent preliminary data collected in Pascual-Leone's lab (unpublished data; Fig. 4) demonstrated the feasibility of using TBS to measure cortical plasticity noninvasively in newly diagnosed, early-course, antipsychotic-naïve individuals with schizophrenia. Results showed that, on average, patients had 42% reduced duration of cTBS-induced after-effects, as compared to age- and gender-matched healthy controls, thus suggesting that corticomotor plasticity mechanisms are already abnormally reduced in very early stages of schizophrenia.
Figure 4.
Results from study of plasticity mechanisms in five early-onset, first-episode, antipsychotic-naïve schizophrenia patients. A: Summary of individual results: on the x axis, red dots represent the schizophrenia (SCZ) patients and blue dots the age-matched healthy controls (HC); values on the Y axis represent the time to return to baseline levels following continuous theta burst stimulation (cTBS). All subjects in the schizophrenia group show shorter duration of the modulatory effects of cTBS on cortical reactivity than their matched controls, with the exception of one patient whose effects returned to baseline at the same time as his matched control; B: Average baseline-corrected MEP amplitude for the schizophrenia group (in red) and control group (in blue), at all time-points assessed (5 to 120 minutes post-cTBS). In both graphs, error bars indicate standard error of the mean for each time point. Values are represented as proportion of baseline amplitude with a line at 1.0 representing baseline amplitude.
Therapeutically, TBS has also been used in schizophrenia. Recent evidence suggests that iTBS applied over the cerebellar vermis is safe and may improve mood and cognition (71). Alternatively, cTBS applied to the temporo-parietal cortex has been shown to reduce or suppress auditory verbal hallucinations in single cases (72-74). For instance, left-sided cTBS was shown to reduce a patient's long-term persistent auditory hallucinations, which was accompanied by overall improved performance in neuropsychological measures (73). Similarly, long-term bilateral application of cTBS to temporo-parietal cortical areas, in a patient with a 22-year history of paranoid schizophrenia, resulted in complete remission of chronic, continuous, distressing voices, with maintenance of the effect at 3-month follow-up, as well as a salient improvement in general psychopathology and global function (74). Sham-controlled clinical trials are nonetheless needed to ascertain the efficacy of TBS in schizophrenia. Overall, different patterns of TBS and conventional rTMS protocols differentially modulate the activity of inhibitory cortical systems and protein expression (75, 76). Eventually, some TMS protocols may prove to be more efficacious than others in modulating human cortical excitability and plasticity, and, particularly, the cellular mechanisms underlying schizophrenia symptoms.
Future directions to use TMS to develop biomarkers for schizophrenia
TMS can assess and modulate different physiologic processes in the brain. Specifically, TMS can measure cortical excitability, intracortical inhibitory and excitatory mechanisms, and local and network cortical plasticity. Furthermore, if coupled with functional and electrophysiological modalities, TMS can provide valuable insights into the processes of the brain in health and disease. In particular, TMS may elucidate the mechanisms underlying healthy neurodevelopment and aging, as well as neuropsychiatric pathology.
If one builds on a conceptual paradigm in which changes in E/I balance and brain plasticity are the ultimate result of the interaction between genes and environment, then measuring those changes may provide extraordinary insight into how the brain may initiate and compensate for pathology. If so, defining and measuring characteristic ‘TMS-related endophenotypes’ for distinct neuropsychiatric disorders may thus provide valuable biomarkers of disease. For instance, if the specific pattern of findings from Pascual-Leone's lab and others [e.g., (59)] prove to be specific to schizophrenia, diminished cortical plasticity might be a biological marker for schizophrenia, and a progressive reduction of plasticity during developmental years may even be a predictor of disease, as those results were obtained in first-episode, early-onset, unmedicated schizophrenia patients. Moreover, early-life deficits in the mechanisms of LTP/LTD, which are considered molecular correlates of learning and memory (and can be induced by TBS), may originate and underlie the impairments in higher cognitive functioning observed in schizophrenia patients not only at the time of first episode (77) but even long before illness onset (78). Ultimately, abnormal changes in plasticity mechanisms may be the proximal cause of schizophrenia.
Furthermore, TMS—using TBS protocols coupled with EEG, for instance—can measure the efficiency of plasticity mechanisms in a given cortical region and network dynamics in functionally connected regions (16). Of critical interest is the assessment of plasticity dynamics of the prefrontal circuitry for its involvement in working memory and abstraction abilities, profoundly disrupted in schizophrenia [e.g., (79)], and of the prefrontal-temporal limbic network, involved in verbal episodic memory and disrupted in both schizophrenia patients and their relatives [e.g., (80, 81)]. Thus, defining biomarkers and predictors of schizophrenia, and other neuropsychiatric disorders, based on this TMS methodology appears to be a promising strategy for future research and diagnostics in schizophrenia, and ultimately for novel plasticity-based interventions.
In a broader perspective, it seems possible to define novel or support existent endophenotypes of schizophrenia using sophisticated investigations with TMS. Neurophysiologic and neurocognitive endophenotypes selected by the Consortium on the Genetics of Schizophrenia (COGS) (82) included measures of inhibitory deficits (P50 suppression, prepulse inhibition, and oculomotor, saccadic control) and of various cognitive impairments (e.g., continuous performance tests for sustained attention, letter-number span for working memory). All of these have shown significant associations with functional status and outcome. It is easily envisioned how TMS measures of cortical excitability and plasticity are potential candidates to add to such list. Importantly, and consistent with criteria emphasized by Braff et al. (82), measures of TMS can be (a) “state-independent” (i.e., impairments in cortical inhibition and plasticity do not seem to be due to medications, as they were found in drug-naïve patients; such impairments are observed regardless of illness state, as deficits seem to be present already at first-episode), and might be (b) altered also in “close non-affected biological relatives” (e.g., cortical inhibition deficits in non-psychotic first-degree relatives of schizophrenia patients have been reported). To date, no TMS studies on affected first-degree relatives (e.g., affected twins or affected offspring of schizophrenia parents) have been reported, and would certainly be desirable. Unquestionably, in order to apply a TMS-driven “endophenotype strategy” to schizophrenia, objectively and reliably, large samples of patients and genetic high-risk subjects are crucial, and multisite collaborations would be most advantageous.
Conclusion
A substantial body of evidence has been generated to support the use of TMS as a neuroscientific probe of cortical function as well as an intervention for the treatment of psychiatric symptomatology. TMS would be a powerful tool in the CNTRICS armamentarium of biomarker methods, particularly if combined with other electrophysiological and neuroimaging methods, and in the advancement of current knowledge on the underlying mechanisms and neurocircuitry of cortical and cognitive function and behavior in schizophrenia. Future investigations are warranted to optimize TMS methodologies for this purpose.
Supplementary Material
Acknowledgements
Work on this review was supported by grants from the National Center for Research Resources: Harvard-Thorndike General Clinical Research Center at BIDMC (NCRR MO1 RR01032) and Harvard Clinical and Translational Science Center (UL1 RR025758), NIH grant K24 RR018875 and K23 MH087739, and a grant from the Nancy Lurie Marks Family Foundation to APL. SM has received research support from the National Institutes of Health (NIH), National Center for Research Resources (NCRR), and the National Alliance for Research on Schizophrenia and Depression (NARSAD). CF was supported by a post-doctoral grant from the Foundation for Science and Technology, Portugal (SFRH/BPD/66846/2009), co-funded by the European Social Fund. LO was supported by NIH fellowship F32MH080493 and 1KL2RR025757-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
APL serves on the scientific advisory board of Starlab, Neuronix, Nexstim, and Neosync. SHL has received research funding from the National Institutes of Health (NIH), the National Alliance for Research on Schizophrenia and Depression (NARSAD), DARPA, Stanley Medical Research Foundation, American Federation for Aging Research/Beeson Scholars Program, Neuronetics, Cyberonics, Advanced Neuromodulation Systems, and Brainsway. She has received equipment support from Magstim and Magventures. She formerly chaired a Data Safety and Monitoring Board for a study sponsored by Northstar Neuroscience. All other authors disclose having no biomedical financial interests or potential conflicts of interest.
References
- 1.Lisanby SH, Luber B, Perera T, Sackeim HA. Transcranial magnetic stimulation: applications in basic neuroscience and neuropsychopharmacology. Int J Neuropsychopharmacol. 2000;3:259–273. doi: 10.1017/S1461145700002005. [DOI] [PubMed] [Google Scholar]
- 2.Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006;157:315–329. doi: 10.1016/s0079-6123(06)57019-0. [DOI] [PubMed] [Google Scholar]
- 3.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 4.Carter CS, Barch DM, Bullmore E, the CNTRICS Executive Committee Cognitive Neuroscience Treatment Research to Improve Cognition (CNTRICS) II: Developing Imaging Biomarkers to Enhance Treatment Development for Schizophrenia and Related Disorders. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol Psychiatry. 1999;46:130–132. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen E, Bernardo M, Masana J, Arrufat FJ, Navarro V, Valls S, et al. Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67:129–130. doi: 10.1136/jnnp.67.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleman A, Sommer IE, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68:416–421. doi: 10.4088/jcp.v68n0310. [DOI] [PubMed] [Google Scholar]
- 10.Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71:411–418. doi: 10.4088/JCP.08r04808yel. [DOI] [PubMed] [Google Scholar]
- 12.Stanford AD, Sharif Z, Corcoran C, Urban N, Malaspina D, Lisanby SH. rTMS strategies for the study and treatment of schizophrenia: a review. Int J Neuropsychopharmacol. 2008;11:563–576. doi: 10.1017/S1461145707008309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clin Neurophysiol. 2006;117:1870–1875. doi: 10.1016/j.clinph.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Thut G, Pascual-Leone A. Integrating TMS with EEG: How and what for? Brain Topogr. 2010;22:215–218. doi: 10.1007/s10548-009-0128-z. [DOI] [PubMed] [Google Scholar]
- 17.Komssi S, Kahkonen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp. 2004;21:154–164. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology. 2008;33:2860–2869. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- 19.Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 22.Eichhammer P, Wiegand R, Kharraz A, Langguth B, Binder H, Hajak G. Cortical excitability in neuroleptic-naive first-episode schizophrenic patients. Schizophr Res. 2004;67:253–259. doi: 10.1016/S0920-9964(03)00223-8. [DOI] [PubMed] [Google Scholar]
- 23.Davey NJ, Puri BK, Lewis HS, Lewis SW, Ellaway PH. Effects of antipsychotic medication on electromyographic responses to transcranial magnetic stimulation of the motor cortex in schizophrenia. J Neurol Neurosurg Psychiatry. 1997;63:468–473. doi: 10.1136/jnnp.63.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziemann U. Pharmacology of TMS measures. In: Wassermann EMZU, Walsh V, Paus T, Lisanby S, editors. The Oxford Handbook of Transcranial Stimulation. Oxford University Press Inc.; New York: 2008. pp. 135–151. [Google Scholar]
- 25.Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biol Psychiatry. 2009;65:503–509. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Wobrock T, Schneider-Axmann T, Retz W, Rosler M, Kadovic D, Falkai P, et al. Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry. 2009;42:194–201. doi: 10.1055/s-0029-1224137. [DOI] [PubMed] [Google Scholar]
- 27.Eichhammer P, Langguth B, Zowe M, Kleinjung T, Jacob P, Sand P, et al. [GABA-B-associated neuropsychiatric disorders]. Psychiatr Prax. 2004;31(Suppl 1):S44–46. doi: 10.1055/s-2004-828429. [DOI] [PubMed] [Google Scholar]
- 28.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 29.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–385. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- 30.Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, et al. The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry. 2010;68:825–831. doi: 10.1016/j.biopsych.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Driver J, Blankenburg F, Bestmann S, Ruff CC. New approaches to the study of human brain networks underlying spatial attention and related processes. Exp Brain Res. 2010;206:153–162. doi: 10.1007/s00221-010-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 34.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 38.Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex. 2009;45:1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreri F, Pasqualetti P, Maatta S, Ponzo D, Ferrarelli F, Tononi G, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2011;54:90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 41.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 43.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 44.Nauczyciel C, Hellier P, Morandi X, Blestel S, Drapier D, Ferre JC, et al. Assessment of standard coil positioning in transcranial magnetic stimulation in depression. Psychiatry Res. 2010 doi: 10.1016/j.psychres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res. 2002;114:11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald PB, Brown TL, Marston NA, Oxley TJ, de Castella A, Daskalakis ZJ, et al. A transcranial magnetic stimulation study of abnormal cortical inhibition in schizophrenia. Psychiatry Res. 2003;118:197–207. doi: 10.1016/s0165-1781(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 48.Wobrock T, Schneider M, Kadovic D, Schneider-Axmann T, Ecker UK, Retz W, et al. Reduced cortical inhibition in first-episode schizophrenia. Schizophr Res. 2008;105:252–261. doi: 10.1016/j.schres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: a preliminary study. Am J Psychiatry. 2005;162:1203–1205. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of the effects of olanzapine and risperidone on motor cortical excitability in patients with schizophrenia. Psychopharmacology (Berl) 2002;162:74–81. doi: 10.1007/s00213-002-1068-4. [DOI] [PubMed] [Google Scholar]
- 51.Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Effect of antipsychotics on cortical inhibition using transcranial magnetic stimulation. Psychopharmacology (Berl) 2003;170:255–262. doi: 10.1007/s00213-003-1548-1. [DOI] [PubMed] [Google Scholar]
- 52.Koch G, Ribolsi M, Mori F, Sacchetti L, Codeca C, Rubino IA, et al. Connectivity between posterior parietal cortex and ipsilateral motor cortex is altered in schizophrenia. Biol Psychiatry. 2008;64:815–819. doi: 10.1016/j.biopsych.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 53.Hoy KE, Georgiou-Karistianis N, Laycock R, Fitzgerald PB. A transcranial magnetic stimulation study of transcallosal inhibition and facilitation in schizophrenia. J Clin Neurosci. 2008;15:863–867. doi: 10.1016/j.jocn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Saka MC, Atbasoglu EC, Ozguven HD, Sener HO, Ozay E. Cortical inhibition in first-degree relatives of schizophrenic patients assessed with transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2005;8:595–599. doi: 10.1017/S1461145705005456. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkarni J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophr Res. 2002;56:199–209. doi: 10.1016/s0920-9964(01)00222-5. [DOI] [PubMed] [Google Scholar]
- 56.Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, et al. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain. 2010;133:1505–1514. doi: 10.1093/brain/awq046. [DOI] [PubMed] [Google Scholar]
- 57.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 58.Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- 59.Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- 60.Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control. 2009;13:442–453. doi: 10.1123/mcj.13.4.442. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 62.Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2009;34:2359–2367. doi: 10.1038/npp.2009.79. [DOI] [PubMed] [Google Scholar]
- 63.Cohen DA, Freitas C, Tormos JM, Oberman L, Eldaief M, Pascual-Leone A. Enhancing plasticity through repeated rTMS sessions: the benefits of a night of sleep. Clin Neurophysiol. 2010;121:2159–2164. doi: 10.1016/j.clinph.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plewnia C, Rilk AJ, Soekadar SR, Arfeller C, Huber HS, Sauseng P, et al. Enhancement of long-range EEG coherence by synchronous bifocal transcranial magnetic stimulation. Eur J Neurosci. 2008;27:1577–1583. doi: 10.1111/j.1460-9568.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- 65.Mittrach M, Thunker J, Winterer G, Agelink MW, Regenbrecht G, Arends M, et al. The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry. 2010;43:110–117. doi: 10.1055/s-0029-1242824. [DOI] [PubMed] [Google Scholar]
- 66.Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 68.Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22:294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- 69.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, et al. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poulet E, Brunelin J, Ben Makhlouf W, D'Amato T, Saoud M. A case report of cTBS for the treatment of auditory hallucinations in a patient with schizophrenia. Brain Stimul. 2009;2:118–119. doi: 10.1016/j.brs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Sidhoumi D, Braha S, Bouaziz N, Brunelin J, Benadhira R, Januel D. Evaluation of the therapeutic effect of theta burst stimulation on drug-resistant auditory hallucinations in a schizophrenic patient and its impact on cognitive function and neuronal excitability: a case study. Clin Neurophysiol. 2010;121:802. doi: 10.1016/j.clinph.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 74.Eberle MC, Wildgruber D, Wasserka B, Fallgatter AJ, Plewnia C. Relief from chronic intractable auditory hallucinations after long-term bilateral theta burst stimulation. Am J Psychiatry. 2010;167:1410. doi: 10.1176/appi.ajp.2010.10070988. [DOI] [PubMed] [Google Scholar]
- 75.Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011;31:1193–1203. doi: 10.1523/JNEUROSCI.1379-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009 doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- 77.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 78.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 79.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 80.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 81.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.