Abstract
Background
Ara h 2 and Ara h 6, co-purified together in a 13-25 kD fraction (Ara h 2/6; 20 kD fraction) on gel filtration chromatography, account for the majority of effector activity in a crude peanut extract (CPE) when assayed with RBL SX-38 cells sensitized with IgE from human peanut allergic sera.
Objectives
To determine if Ara h 2/6 are the primary peanut allergens responsible for allergic reactions in vivo and to determine if Ara h 2/6 would be sufficient to prevent allergic reactions to a complete CPE.
Methods
An oral sensitization mouse model of peanut allergy was used to assess the activity of Ara h 2/6 (20 kD) and CPE without the 20 kD fraction (CPE w/o 20 kD) for allergic provocation challenge and immunotherapy. The activity of these preparations was also tested in an assay of histamine release from human basophils in whole blood.
Results
Compared to mice challenged with control CPE, mice challenged with CPE w/o 20 kD experienced reduced symptoms (p<0.05) and a smaller decrease in body temperature (p<0.01). Results with the basophil histamine release assay corroborated these findings (p<0.01). The mouse model was also used to administer Ara h 2/6 (20 kD) in an immunotherapy protocol, in which peanut-allergic mice treated with the 20 kD fraction experienced significantly reduced symptoms, changes in body temperature, and mast cell protease (MMCP-1) release compared to placebo (p<0.01 for all parameters). Importantly, immunotherapy with the 20 kD fraction was just as effective as treatment with CPE, whereas CPE w/o 20 kD was significantly less effective for higher dose peanut challenges.
Conclusions and Clinical Relevance
Ara h 2/6 are the most potent peanut allergens in vivo and can be used to desensitize peanut-allergic mice. These results have potential implications for clinical research in the areas of diagnosis and immunotherapy for peanut allergy.
Keywords: Food allergy, peanut allergy, Ara h 2, Ara h 6, desensitization, immunotherapy, human basophil assay, mouse model
Introduction
Peanut allergy is a public health concern that affects approximately 1% of the population of the United States [1, 2]. Allergic reactions to peanut can be severe and several reports demonstrate that peanuts can induce fatal anaphylactic reactions [3-5]. Eleven proteins from the peanut, Arachis hypogaea, are documented allergens [6], including Ara h 1 [7], Ara h 2 [8], and Ara h 3 [9], which have been characterized as major allergens [10]. The peanut 2S albumin, Ara h 2, and its homolog, Ara h 6, have been extensively characterized as allergens [11-13]. Ara h 2 and Ara h 6 are 59% homologous, are reported to cross-react, and are considerably more resistant to protease degradation by pepsin and trypsin than either Ara h 1 or Ara h 3, a property that may contribute to their allergenicity [14, 15].
The ability to bind IgE, while a requisite for defining a protein as an allergen, does not necessarily mean that the protein will be able to effectively cross-link IgE molecules on mast cells or basophils to trigger degranulation and subsequent histamine release (i.e. effector function) [16, 17]. Using assays that measure cross-linking of IgE/FcεRI receptor complexes, Ara h 2 has been found to be more potent than Ara h 1 or Ara h 3 in triggering basophil histamine release and producing positive skin prick tests [11, 14]. We have presented evidence that 80-90% of the effector activity of CPE is found in a chromatographic fraction that contains proteins of 13-25 kD (contained in a peak of 13-25 kD called the 20 kD fraction) by chromatography [18]. This fraction consists predominantly (>97%) of variants of Ara h 2 and Ara h 6, does not contain detectable levels of Ara h 1 or Ara h 3, and contains <1% of other known peanut allergens [18]. Similarly, removal of both Ara h 2 and Ara h 6 (but not either individually) by immunodepletion from CPE, leads to a significant diminution of effector activity [19].
The C3H/HeJ mouse model of peanut allergy has been used extensively in pre-clinical studies of food allergy. Similar to findings with humans, these mice develop strong IgE responses against Ara h 1, Ara h 2, Ara h 3, and Ara h 6 and develop anaphylaxis following exposure to peanut proteins [20, 21]. Additionally, immunotherapy in this mouse model has been conducted previously and outcomes have provided important preliminary data regarding the effectiveness of translational approaches for the treatment of peanut allergy [22, 23]. These findings establish this model as a solid platform for testing in vivo the hypotheses that the peanut 2S albumin allergens account for the majority of the effector activity of CPE and that treatment with Ara h 2/6 would effectively desensitize peanut-allergic mice.
In the present studies, Ara h 2 and Ara h 6 were depleted from peanut protein extracts and these depleted preparations were then used to challenge peanut-sensitized mice. In addition, human whole blood basophil histamine release assays were conducted to further define the role of these peanut allergens. Finally, immunotherapy with Ara h 2/6 (20 kD), CPE, and CPE w/o 20 kD was performed to evaluate Ara h 2/6 (20 kD) as a possible therapeutic approach for peanut allergy.
Materials and Methods
Crude peanut extracts and chromatography
Crude peanut extracts (CPE) were prepared and characterized as previously described [18]. Gel filtration of CPE was carried out and fractions were recombined in proportional amounts with and without the 15-25 kD fraction (now called the 20 kD fraction) as previously described [18]. The CPE recombined consists of all fractions from the gel filtration effluent, recombined each in proportion to the size of the fraction. The CPE recombined w/o 20 kD consists of all fractions from the gel filtration effluent, recombined each in proportion to the size of the fraction, except the 20 kD fraction was excluded. CPE recombined has biologic activity indistinguishable from unfractionated CPE [18].
Electrophoresis and IgE immunoblots
One dimensional gel electrophoresis were performed as previously described with rabbit polyclonal anti-peptide antibodies (all at 1 mg/ml) to the following peptides and used at the noted dilution: Ara h 1 (SPEKEDQEEENG); 1:100,000), Ara h 2 (DRRDPYSPSPYDRR; 1:100,000), and Ara h 6 (RRERGRQGDSSS; 1:50,000 (custom preparations; YenZym Antibodies, South San Francisco, CA) [19, 24].
Immunodepletion of Ara h 2 and Ara h 6
Purification of preimmune rabbit IgG and production of rabbit polyclonal anti-peptide antibodies to Ara h 2.02 capable of removing Ara h 2.01 and 2.02 from CPE and an antibody to Ara h 6 capable of removing Ara h 6 from CPE have been described [19, 24]. CPE passed over a column with pre-immune IgG is referred to as control CPE for these experiments and the CPE passed over a column with anti-Ara h 2 and anti-Ara h 6 IgG is referred to as Ara h 2/6 immunodepleted CPE [19].
Two-dimensional gel electrophoresis
Fifty μg of CPE recombined and CPE recombined w/o 20 kD were minimally labeled with Cy3 and Cy5 respectively (Amersham Biosciences/GE Healthcare; Piscataway, NJ). Cy3- and Cy5-labeling and processing was performed as previously described [24].
Mouse model of peanut allergy
Female C3H/HeJ mice, 5 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME) and were sensitized to peanut as previously described [20]. Briefly, mice were sensitized by oral gavage on days 1, 8, and 15 with 10 mg ground, roasted peanuts plus 20 μg cholera toxin (List Biological Laboratories, Inc.) and boosted on day 22 with 50 mg ground, roasted peanuts plus 20 μg cholera toxin. Mice were bled on day 36 to assay peanut-specific IgE. All mice had comparable levels of peanut-specific IgE (data not shown). All mouse procedures were conducted under specific pathogen-free conditions following standard guidelines for care and use and were approved by the Institutional Animal Care and Use Committee at Duke University and at the University of Colorado, Denver.
Specific IgE measurements
IgE levels were determined by ELISA as previously described [25]. All extracts were coated on Immulon 4 HBX plates at 20 μg/ml, except the 20 kD fraction and Ara h 1 were coated at 5 μg/ml.
Immunotherapy in mice
Two weeks post-sensitization, mice began a four-week course of immunotherapy. Mice were injected i.p. three times per week (i.e. Monday, Wednesday, and Friday) with increasing doses of CPE, 20 kD, CPE w/o 20 kD, or PBS as placebo. The dosing protocol was: week 1 – 10, 15, then 25 μg; week 2 – 50, 100, then 125 μg; weeks 3 and 4 – 150 μg given six times as the maintenance dose.
Allergic provocation challenges
We determined the optimal doses of CPE to induce strong, non-lethal reactions (data not shown). Challenges with CPE recombined and CPE recombined w/o 20 kD were given ip at doses of 300-500 μg as described in the text and figure legends. Mice underwent allergic provocation challenges 10 days after receiving the final immunotherapy injection. In experiments where a second, higher high dose challenge was administered, this was two weeks after the first challenge. Allergic symptom scores were assigned on a 0 to 5 scale with the following criteria: 0-no symptoms; 1-scratching, rubbing of face and snout; 2-puffy features around the eyes and face with reduction in normal activity; 3-respiratory distress and/or cyanosis of the tail and feet; 4-no movement following prodding; 5-death [20]. Body temperature was measured with a rectal thermal probe following challenge. Mouse mast cell protease-1 (MMCP-1) levels, a sensitive and reproducible measure of mast cell degranulation [26] measured via an ELISA kit (Moredun Scientific, Scotland, UK) from blood drawn 60 minutes following challenge.
Subjects and sera
This study was approved by the Institutional Review Boards of the University of Colorado Denver and Duke University. All subjects or their guardians signed informed consent and, for minors, assent. Individuals were selected on the basis of having high concentrations of specific IgE to peanuts. Four subjects with a strong clinical history of an immediate hypersensitivity reaction to peanuts and elevated anti-peanut IgE on ImmunoCap® assays were studied in a basophil histamine release assay: D63 (total IgE= 3,263 IU/ml, anti peanut IgE =79 kU/ml), D19 (total IgE=124 IU/ml, anti-peanut IgE = 39 kU/L), D74 (total IgE=194 IU/ml, anti-peanut IgE = 28.9 kU/L) and D86 (total IgE=174 IU/ml, anti-peanut IgE = 4.9 kU/L).
Basophil histamine release assay
Basophil histamine release was assayed essentially as previously reported using an inhibition ELISA kit as described by the manufacturer (Immunotech, Beckman Coulter, Brea, CA) except that all acylated samples were diluted with borate buffered saline (pH 8.0) to fall on the steep portion of the standard curve [24].
Statistical analyses
GraphPad Prism 5 (La Jolla, CA) was used to generate best fit lines for the dose response curves and for all statistical comparisons. Allergic symptom scores were compared using the Mann-Whitney test. Body temperatures and MMCP-1 levels were compared using the unpaired two-tailed t-test. The BHR data were compared using a two-tailed, paired t-test, comparing the extent of degranulation at the EC50 value with CPE. P-values less than 0.05 were considered to be significant.
Results
Ara h 2 and Ara h 6 are selectively removed from CPE via chromatography and immunodepletion
Removal of the peanut 2S albumin allergens, Ara h 2 and Ara h 6, by chromatography was highly effective (Fig. 1A). CPE recombined w/o 20 kD (lane 3) shows the complete absence of the 20 kD material containing Ara h 2 and Ara h 6 (lane 2) compared with CPE recombined (lane 4) or the original CPE (lanes 1 and 5). The Ara h 2/6 immunodepleted CPE (Fig. 1B) likewise shows the complete absence of Ara h 2 and Ara h 6 (lane 2 compared with lane 1). Ara h 2/6 immunodepleted CPE is different from CPE recombined w/o 20 kD in that these specific proteins were removed by immunodepletion rather than by gel filtration. In both preparations, Ara h 1 is not depleted (Fig. 1A and 1B).
Figure 1. Removal of Ara h 2 and Ara h 6 from CPE.
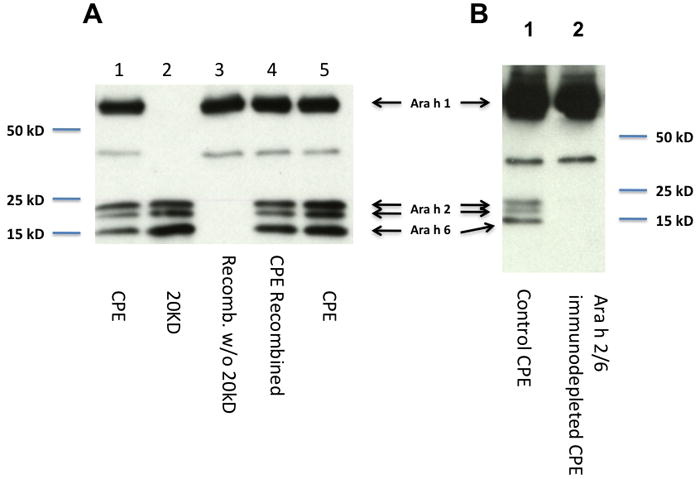
Removal by chromatography, A: Immunoblots of control CPE (1 μg, lanes 1 and 5), the 20 kD fraction (0.2 μg lane 2), fractionated CPE with proportional recombination of all fractions without (1 μg, CPE w/o 20 kD; lane 3) or the same material with inclusion of the fraction containing proteins of 20 kD (1 μg, CPE recombined; lane 4). Probes were rabbit anti-peptide antibodies to Ara h 1, 2, and 6. Removal by immunodepletion, B: Immunoblots of control CPE (lane 1), Ara h 2/6 immunodepleted CPE (lane 2). Control CPE was CPE passed over a column with affinity purified pre-immune IgG.
Ara h 2/6 are the predominant proteins in the 20 kD fraction
Fifty μg of CPE recombined (Fig. 2A) and CPE recombined w/o 20 kD (Fig. 2B) were minimally labeled with Cy3 (red) and Cy5 (green) CyDyes, respectively and run together. The superimposed images are shown (Fig. 2C). The identical spots appear in yellow (red + green) whereas the spots that are found in the CPE recombined but not in the CPE w/o 20 kD, that is those proteins that constitute the 20 kD fraction, are shown in red. Based on their mobility, the bright yellow spots at ~60 kD (Fig. 2C) are Ara h 1 (63 kD monomers) and the red spots in the 15-20 kD range (Fig. 2C) are Ara h 2 (17 and 19 kD) and Ara h 6 (15 kD) as previously identified by Porterfield et al. [18].
Figure 2.
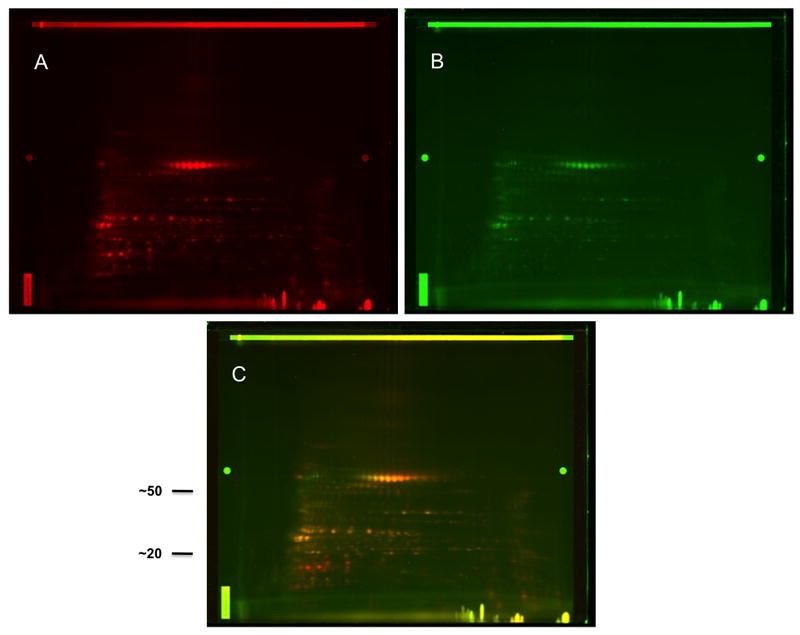
Characterization of the CPE recombined and CPE recombined w/o 20 kD. Fifty μg of CPE recombined (A) and 50 μg of CPE recombined w/o 20 kD (B) were minimally labeled with Cy3 (red) and Cy5 (green) CyDyes, respectively and run together. The superimposed images are shown (C) where identical spots appear in yellow. Approximate molecular weights (kD) are shown (C).
Peanut-sensitized mice have IgE responses against CPE, Ara h 1, and Ara h 2/6
Peanut-sensitized mice display robust IgE responses to all preparations used in these experiments. Serum from peanut-sensitized mice had similar amounts of IgE that bound to CPE, CPE passed over a column with pre-immune rabbit IgG (control CPE), Ara h 2/6 depleted CPE, CPE recombined, CPE recombined w/o 20 kD, 20 kD, and purified Ara h 1 (Fig. 3).
Figure 3. Specific IgE levels to various peanut preparations.
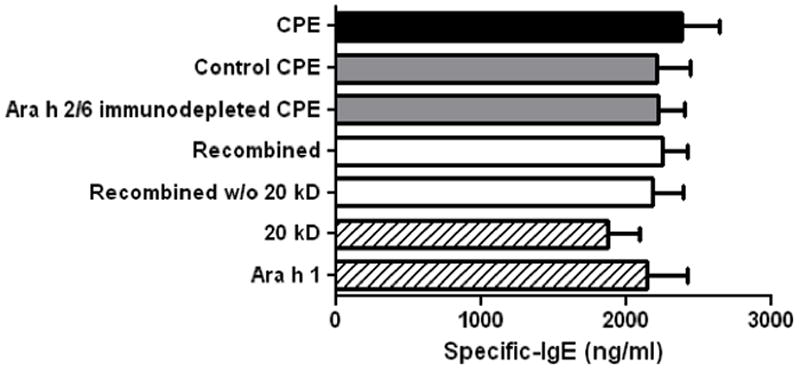
Peanut-sensitized mouse sera were used in an ELISA assay for specific IgE binding to: CPE (black bar); control CPE and Ara h 2/6 immunodepleted preparations (gray bars); recombined and recombined w/o 20 kD CPE (white bars); Ara h 1 and 20 kD (cross-hatched bars). Bars represent means with standard deviation.
Removal of Ara h 2 and Ara h 6 by gel filtration chromatography renders CPE significantly less potent for induction of allergic reactions in mice
Mice sensitized to peanut through the oral route were divided into groups based on peanut-specific IgE levels each of which had mean peanut-IgE of 2.2 μg/ml. In two separate experiments, mice challenged with 500 μg of CPE recombined had levels of symptoms and drops in temperature (Fig. 4) similar to those described for multiple other preparations of CPE (data not shown). Mice challenged with 500 μg of CPE recombined w/o 20 kD had significantly lower allergic responses (Fig 4). The difference in allergic symptoms was striking, with the CPE recombined (i.e. not depleted of any peanut allergens) producing strong reaction symptoms with a median of 3 (i.e. labored breathing and cyanosis), whereas the CPE recombined w/o 20 kD produced median symptom scores of zero (p<0.05). Mice challenged with CPE recombined also had significantly lower body temperatures at 30 (p<0.01) and 45 minutes (p<0.05) post-challenge than those challenged with CPE recombined w/o 20 kD.
Figure 4. Allergic provocation challenges in peanut-sensitized mice with CPE recombined and CPE recombined w/o 20 kD.
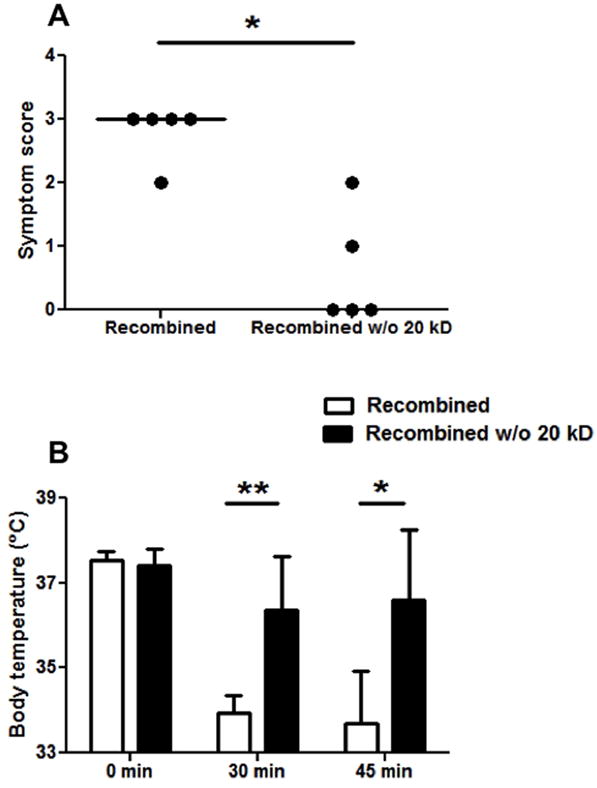
A: Symptom scores following challenge with CPE recombined or CPE recombined w/o 20 kD. B: Body temperatures at baseline, 30, and 45 minutes post-challenge. Closed circles represent individual mice; bars show means with standard deviation; * indicates p<0.05; ** indicates p<0.01. A second, independent experiment with 3 mice per group and several doses of these CPE preparations gave similar results.
In a complementary set of experiments, we challenged two separate sets of peanut-sensitized mice with control CPE and Ara h 2/6 immunodepleted CPE (n=5 in each group for each experiment). Due to the scarcity of this material, we challenged these mice with 22.5 μg iv. As in the mice challenged ip with recombined w/o Ara h 2/6, mice challenged with Ara h 2/6 immunodepleted CPE had highly significant decreases in allergic responses for all three challenge parameters (Fig. 5, A-C). As seen in the experiments with CPE recombined and CPE recombined w/o 20 kD (Fig. 4), the difference in allergic symptoms was striking, with the control CPE (i.e. not depleted of any peanut allergens) producing strong reaction symptoms with a median of 3 (i.e. labored breathing and cyanosis), whereas the Ara h 2/6 immunodepleted CPE produced median symptom scores of zero (p<0.01). Mice challenged with control CPE had significantly lower body temperatures at 30 (p<0.01) and 45 minutes (p<0.01) post-challenge. Additionally, a significantly lower level of MMCP-1 was found in mice challenged with Ara h 2/6 immunodepleted CPE compared with control CPE (p<0.01), indicating that significantly less mast cell degranulation had occurred.
Figure 5. Allergic provocation challenges in peanut-sensitized mice with Ara h 2/6 immunodepleted CPE.
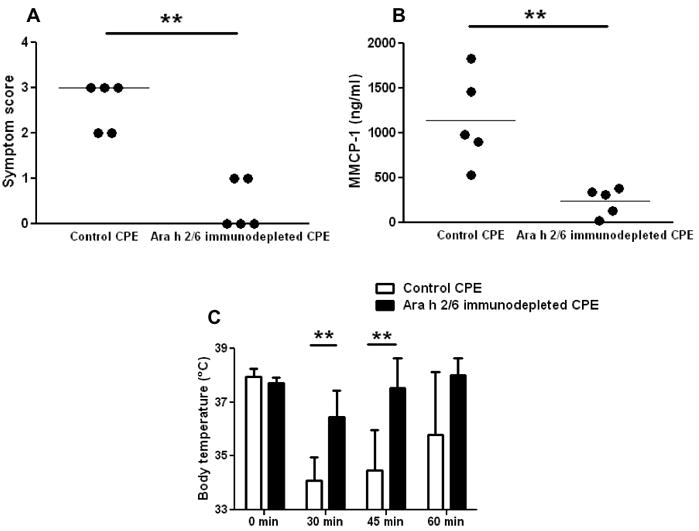
A: Symptom scores following challenge with control CPE and Ara h 2/6 immunodepleted CPE. B: Body temperatures at baseline, 30, 45, and 60 minutes post-challenge. C: MMCP-1 levels in sera of mice post-challenge. Closed circles represent individual mice; bars show means with standard deviation; ** indicates p<0.01. A second independent experiment with 5 mice per group gave similar results.
CPE depleted of Ara h 2 and Ara h 6 dramatically reduces the ability to release histamine in whole blood basophil histamine release assays
Previously we reported that removal of Ara h 2 and Ara h 6 from CPE by gel filtration chromatography dramatically reduces the effector activity of CPE in an in vitro RBL SX-38 assay using IgE from peanut allergic subjects [18]. In the present study, whole blood from four peanut allergic subjects was exposed to various doses of CPE w/o 20 kD or to CPE (Fig. 6). As found with the RBL SX-38 cell assay, degranulation (measured as release of histamine) was consistently less (p<0.01) when basophils are stimulated with the preparation deficient in Ara h 2 and Ara h 6.
Figure 6. Effect of removing Ara h 2 and Ara h 6 in a basophil histamine release assay.
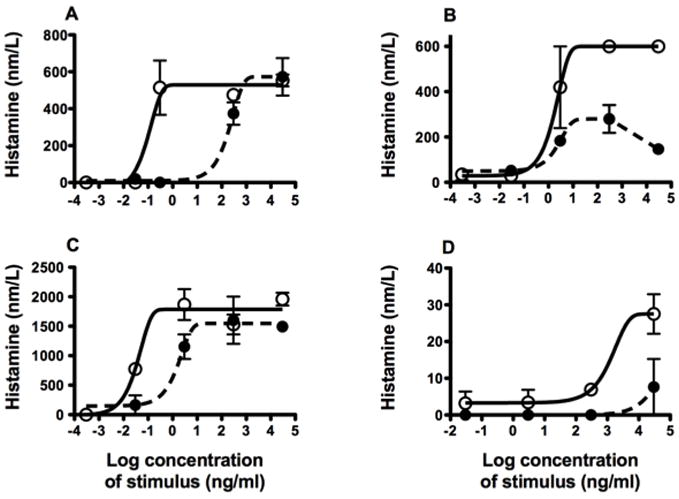
Whole blood was stimulated with CPE (open circles) or CPE recombined w/o 20 kD (closed circles) and histamine release was measured. Subjects: A) D63, B) D19, C) D74, and D) D86. Each subject was studied once; each data point is the mean of duplicates. At the dose giving half-maximal for CPE, the CPE w/o 20 kD gave 16±11% (mean±SEM) of that response (n=4; p<0.01).
Immunotherapy with Ara h 2/6 is sufficient to prevent allergic reactions to CPE challenge
In that the 20 kD fraction appeared to be very potent in inducing anaphylaxis, it was important to learn if this material can desensitize peanut allergic mice. In a pilot experiment, we observed that the CPE recombined w/o 20 kD was not as effective a desensitizing agent as CPE (p<0.05; data not shown). For this reason, another separate group of mice were sensitized orally to whole roasted peanuts and subsequently underwent immunotherapy for four weeks with CPE, 20 kD, CPE recombined w/o 20 kD, or placebo. These mice were then challenged ip with 300 μg of CPE. As seen in Fig. 7, desensitization with CPE, 20 kD, and CPE recombined w/o 20 kD all provided significantly decreased allergic symptoms and body temperature decreases compared with placebo treatment. In this experiment, placebo treated mice reacted strongly, with a median symptom score of four, the mice treated with CPE w/o 20 kD had a median score of 2 (p<0.05) and both the CPE and 20 kD treated mice had median scores of zero (p<0.01). Mice desensitized with any of the three preparations experienced almost no decrease in body temperature, whereas placebo treated mice experienced an approximate 6°C drop in temperature by 45 minutes (p<0.01). MMCP-1 release was also significantly higher in placebo mice relative to all three immunotherapy groups (p<0.01) indicating that mast cell degranulation was blunted by immunotherapy. Although desensitization with CPE recombined w/o 20 kD resulted in significant protection, this effect appeared to be less than what was seen with either CPE or the 20 kD fraction with a somewhat higher, but not statistically significant, median symptom score (2 vs 0 and 2 vs 0; p=0.054 and 0.097, respectively) and a somewhat lower but not statistically significant body temperature (36.4 vs 37.5 and 36.4 vs 37.4; p=0.107 and 0.073, respectively).
Figure 7. Allergic provocation challenge with 300 μg CPE in peanut-sensitized mice following immunotherpy with CPE, recombined w/o 20 kD, 20 kD fraction, or placebo.
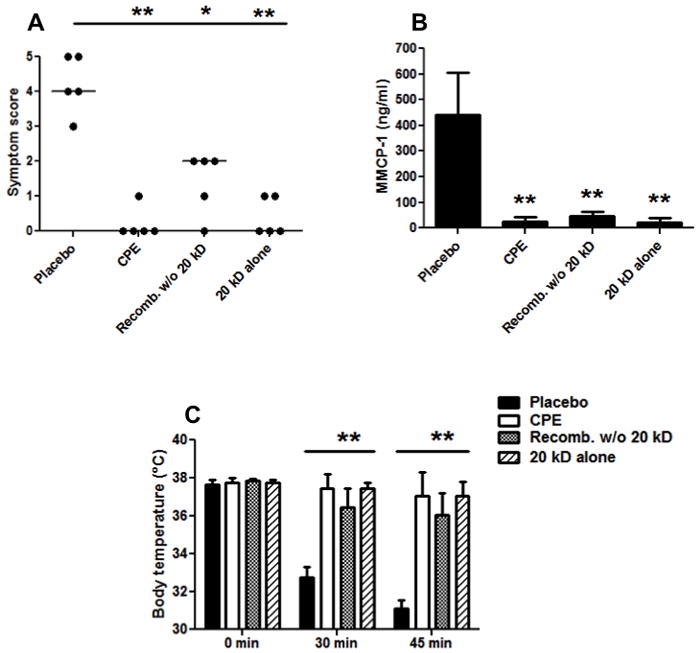
A: Symptom scores following CPE challenge in the four immunotherapy groups. B: Body temperatures at baseline, 30, and 45 minutes post-challenge. C: MMCP-1 levels in sera samples post-challenge. Five mice were in each treatment group. Closed circles represent individual mice; bars show means with standard deviation; * indicates p<0.05; ** indicates p<0.01.
This led us to further evaluate the potency of the 20 kD fraction and the CPE recombined w/o 20 kD material as desensitizing agents by administering a second, higher dose challenge of CPE (450 μg) to the desensitized mice described in Fig. 7 (Fig. 8). Our Institutional Animal Care and Use Committee protocol does not allow us to challenge mice with doses that we expect to cause death. For this reason we made the decision to forego the high dose challenge in the placebo-desensitized mice due to the ethical concern that they would have a fatal reaction, as well as the fact that the comparison we needed at this juncture was among the 3 immunotherapy reagents. The high dose challenge clearly demonstrated that immunotherapy with either CPE or 20 kD offers significant protection from challenge with this high dose of CPE whereas immunotherapy with the CPE recombined w/o 20 kD material did not. In this experiment, the CPE and 20 kD immunotherapy groups have median symptom scores of zero, whereas recombined w/o 20 kD had strong allergic reactions with median score of three (p<0.01). Likewise, body temperatures were significantly higher in CPE and 20 kD treated groups, relative to recombined w/o 20 kD (p<0.01). Additionally, serum MMCP-1 was highly increased in the recombined w/o 20 kD immunotherapy group relative to CPE and 20 kD immunotherapy groups (p<0.01). As stated in the figure legend, the major findings from the immunotherapy experiments were replicated in separate experiments one conducted at Duke University and the second at the University of Colorado, Denver. These findings demonstrate that the CPE and 20 kD immunotherapy groups were well protected from anaphylactic reactions on challenge with whole CPE as measured by body temperature and symptom scores (p<0.01; data not shown).
Figure 8. Allergic provocation challenge with 450 μg CPE in peanut-sensitized mice following immunotherapy with CPE, recombined w/o 20 kD, or 20 kD fraction.
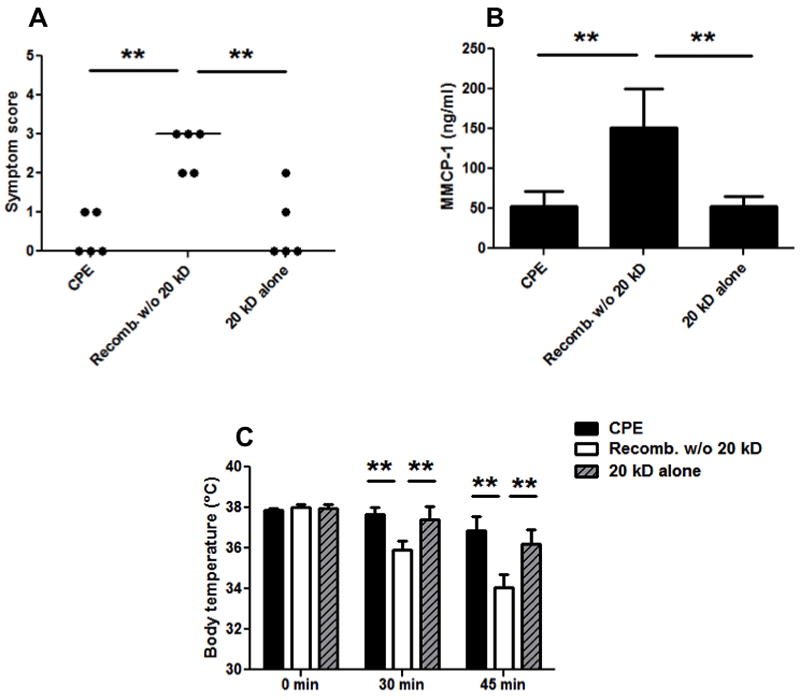
A: Symptom scores following CPE challenge in mice given immunotherapy. B: Body temperatures at baseline, 30, and 45 minutes post-challenge. C: MMCP-1 levels in sera samples post-challenge. These are the same mice challenged in Fig. 7 except the mice desensitized with placebo were excluded from challenge. Closed circles represent individual mice; bars show means with standard deviation; ** indicates p<0.01. A second independent experiment measuring symptoms and temperature changes with 3-5 mice in each group gave similar results.
Discussion
Identifying the most important allergens from allergenic sources has been a priority for allergy researchers for many years as this knowledge can contribute to the usefulness of diagnostic assays as well as to the success of immunotherapy strategies [27]. The most common approach has been to measure the ability of allergens to bind human IgE in immunoblots and/or ELISAs but IgE binding in itself does not mean that an allergen will cause a reaction in vivo and even if it does cause a reaction, these assays do not predict the potency of that allergen.
The C3H/HeJ mouse model of peanut allergy is a commonly used model in which to test novel allergen preparations [22, 23, 28, 29]. This model system has some limitations in that the mice have defective signaling via TLR4 and must be treated with cholera toxin to elicit an IgE response to peanuts. Nonetheless, the C3H/HeJ mouse strain develops IgE anti-Ara h 1, Ara h 2, Ara h 3, and Ara h 6 mimicking naturally occurring human peanut allergy [21] and is very susceptible to anaphylaxis that can be measured both subjectively (symptoms) and objectively (body temperature and degranulation of mast cells). This makes it an ideal model in which to test the hypothesis that Ara h 2 and Ara h 6 represent the majority of effector activity in CPE. In this manuscript, we verified that mice sensitized to peanut according to our protocol produce IgE anti-Ara h 1 and anti-Ara h 2/6 (Fig. 3).
The CPE used to challenge mice in these experiments was selectively depleted of both Ara h 2 and Ara h 6 either by size-exclusion chromatography or by immunodepletion. These two methodologically different approaches produce peanut extract preparations selectively depleted of Ara h 2 and 6 without affecting the level of Ara h 1 (See Fig. 1A and 1B, Fig. 2, and [18, 19]). The gel filtration method can produce milligram quantities of material, while the immunodepletion method produces much less material, is much more labor intensive to produce, and is more costly. In theory, the immunodepletion method should only remove Ara h 2 and 6, whereas the gel filtration method removes other proteins (e.g. Ara h 7), although these are very minor in abundance (see Porterfield et al. [18]). Data presented here (Fig. 1, 3, 4, and 5) demonstrate that these two different methods can successfully remove Ara h 2 and 6 and give comparable results in the mouse challenge model. The gel filtration method for removal of Ara h 2/6 was used for the immunotherapy studies, since the required amount of material would be very costly to produce by the immunodepletion methodology.
Peanut-sensitized mice challenged with CPE depleted of Ara h 2/6 by chromatography (CPE recombined w/o 20 kD) experienced minimal allergic responses on challenge (p<0.05; Fig. 4). In contrast, control, recombined CPE caused severe reaction symptoms and large decreases in body temperature similar to that seen in previous studies of non-manipulated CPE. Similar results were seen when mice were challenged with CPE depleted of Ara h 2 and Ara h 6 by immunodepletion (p<0.01; Fig. 5). Challenges with CPE depleted by each of these two approaches were performed twice resulting in 4 experiments demonstrating the importance of Ara h 2 and Ara h 6. We conclude that Ara h 2 and Ara h 6 are the major elicitors of anaphylaxis in this murine model of peanut allergy.
The basophil histamine release assay using whole blood from peanut allergic subjects is another model system to study cross-linking of IgE in an allergen-specific fashion. The basophil histamine release (BHR) assays were performed for 4 peanut allergic patients (Fig. 6). The BHR assay uses whole blood from patients. It is similar to the RBL assay in that it depends upon the patient’s IgE but is different in that patient-specific basophils are naturally sensitized with endogenous IgE. The results with the BHR assay demonstrate a significant effect of removing Ara h 2/6 (p<0.01), findings similar to those previously shown for the RBL assay [18].
Taken together these murine and BHR data confirm our previous findings using RBL SX-38 cells sensitized with IgE from peanut allergic subjects that the majority of the effector activity of CPE is attributable to Ara h 2 and Ara h 6 [18, 19]. Demonstration that this 20 kD fraction consisting almost entirely of Ara h 2 and Ara h 6 accounts for the majority of the allergic effector potential of CPE has important implications for both diagnostic and therapeutic purposes. In terms of diagnosing peanut allergy, it can reasonably be hypothesized that Ara h 2- and Ara h 6-specific IgE levels may be more meaningful than Ara h 1- or Ara h 3-specific IgE since Ara h 2 and Ara h 6 contain the majority of effector activity, as we have demonstrated here. Indeed, a recent study using recombinant peanut allergens concluded that Ara h 2-specific IgE is a better biomarker than IgE specific for either Ara h 1, Ara h 3, or Ara h 8 for predicting truly peanut allergic versus peanut sensitized but tolerant subjects [30].
Since Ara h 2/6 are the major elicitors of anaphylaxis in our model systems, we hypothesized that using these two peanut allergens together as immunotherapy would provide significant protection from allergic reactions during peanut challenges in mice. Immunotherapy with 20 kD produced an equivalent level of desensitization as CPE immunotherapy, which were both significantly better than CPE w/o 20 kD (p<0.01; Fig. 8). The finding that CPE w/o 20 kD has significant immunomodulatory activity may be due to the importance of secondary allergens such as Ara h 1 and Ara h 3 or may be due to small fragments of Ara h 2 and/or 6 that are likely present in this preparation. Taken together, these findings have important clinical implications, in that immunotherapy for peanut allergy may only need to contain the primary elicitors of reactions, i.e. Ara h 2/6. Furthermore, immunologic changes observed in oral and sublingual immunotherapy for Ara h 2/6-specific responses may be more relevant than those observed for CPE.
In conclusion, we have further advanced our previous reports that Ara h 2 and Ara h 6 are responsible for the majority of effector activity of CPE by demonstrating this phenomenon in vivo using a peanut allergy mouse model. Additionally, we have reported the novel finding that using Ara h 2/6 in immunotherapy produces an equivalent level of desensitization as using CPE containing all the major peanut allergens. These results have potential diagnostic and therapeutic importance.
Acknowledgments
The authors thank our study coordinator, Darcy G. Schlichting and our study subjects for their participation. Funding: RO1-AI052164 and an ARRA supplemental grant (for AI052164) from the National Institute of Allergy and Infectious Diseases to Dr. Dreskin. Dr. Kulis was supported by an NRSA training grant (#F32AI084332) from the National Institutes of Allergy and Infectious Diseases.
Abbreviations
- 20 kD fraction
gel filtration chromatography fraction containing Ara h 2 and Ara h 6
- Ara h2/6
Ara h 2 plus Ara h 6 as found in the 20 kD fraction
- Ara h 2/6 immunodepleted CPE
CPE after removing Ara h 2 and Ara h 6 by immunodepletion
- BHR
basophil histamine release
- CPE
Crude peanut extract
- CPE recombined (also, control CPE)
CPE with 20 kD fraction
- CPE recombined w/o 20 kD (also, CPE w/o 20 kD)
CPE excluding the 20 kD fraction
- GFC
gel filtration chromatography
- MMCP-1
mouse mast cell protease 1
Footnotes
Disclosures
M. Kulis has received research support from NIH NIAID.
A. W. Burks is a consultant for ActoGeniX NV, Intelliject, McNeil Nutritionals, Novartis, and Schering Plough; is a minority stockholder of Allertein and MastCells Inc; is on the advisory board for Dannon Co Probiotics; is on the expert panel for Nutricia; has received research support from the NIH, the Food Allergy & Anaphylaxis Network, and the Wallace Research Foundation; has provided expert testimony on the topic of food allergy; is on the Medical Board of Directors for FAAN; is a Dermatological Allergy Committee member for ACAAI; is a study section member for NIH Hypersensitivity, Autoimmunity and Immune-mediated Disease; and is on the reviewer board of the Journal of Allergy & Clinical Immunology and the US Food and Drug Administration.
S. C. Dreskin has received research support from NIH NIAID, from HAL Allergy, Inc., and from the Food Allergy and Anaphylaxis Network (FAAAN); is on the Board of Directors of the American Board of Allergy and Immunology and the Aspen Allergy Conference; is a consultant for the Clinical Immunization and Safety Assessment (CISA) Network, administered by America’s Health Insurance Plans (AHIP); is a member of the Medical Expert Panel, Department of Health and Human Services, Division of Vaccine Injury Compensation; and is a consultant for Array BioPharma, Inc., Genentech, Inc., and Novartis, Inc.
References
- 1.Burks AW. Peanut allergy. Lancet. 2008;371:1538–1546. doi: 10.1016/S0140-6736(08)60659-5. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 5.Yunginger JW, Squillace DL, Jones RT, Helm RM. Fatal anaphylactic reactions induced by peanuts. Allergy Proc. 1989;10:249–253. doi: 10.2500/108854189778959993. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt H, Gelhaus C, Latendorf T, Nebendahl M, Petersen A, Krause S, Leippe M, Becker WM, Janssen O. 2-D DIGE analysis of the proteome of extracts from peanut variants reveals striking differences in major allergen contents. Proteomics. 2009;9:3507–3521. doi: 10.1002/pmic.200800938. [DOI] [PubMed] [Google Scholar]
- 7.Burks AW, Williams LW, Helm RM, Connaughton C, Cockrell G, O’Brien T. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol. 1991;88:172–179. doi: 10.1016/0091-6749(91)90325-i. [DOI] [PubMed] [Google Scholar]
- 8.Burks AW, Williams LW, Connaughton C, Cockrell G, O’Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90:962–969. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 9.Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, Bannon GA. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest. 1999;103:535–542. doi: 10.1172/JCI5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks W, Sampson HA, Bannon GA. Peanut allergens. Allergy. 1998;53:725–730. doi: 10.1111/j.1398-9995.1998.tb03967.x. [DOI] [PubMed] [Google Scholar]
- 11.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34:583–590. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer GW, Dibbern DA, Burks AW, Bannon GA, Bock SA, Porterfield HS, McDermott RA, Dreskin SC. Comparative Potency of Ara h 1 and Ara h 2 in Immunochemical and Functional Assays of Allergenicity. Clinical Immunology. 2005;115:301–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Moreno FJ, Clemente A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem J. 2008;2:16–28. doi: 10.2174/1874091X00802010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppelman SJ, de Jong GA, Laaper-Ertmann M, Peeters KA, Knulst AC, Hefle SL, Knol EF. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. 2005;35:490–497. doi: 10.1111/j.1365-2222.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 15.Koppelman SJ, Hefle SL, Taylor SL, de Jong GA. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion-resistant peptides. Mol Nutr Food Res. 2010;54:1711–1721. doi: 10.1002/mnfr.201000011. [DOI] [PubMed] [Google Scholar]
- 16.Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol Rev. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 17.Knol EF. Requirements for effective IgE cross-linking on mast cells and basophils. Mol Nutr Food Res. 2006;50:620–624. doi: 10.1002/mnfr.200500272. [DOI] [PubMed] [Google Scholar]
- 18.Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Zhang Y, Wang Q, Moutsoglou D, Ruiz G, Yen SE, Dreskin SC. Analysis of the Effector Activity of Ara h 2 and Ara h 6 by Selective Depletion from a Crude Peanut Extract. Journal of Immunological Methods. 2011 doi: 10.1016/j.jim.2011.06.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, Stanley JS, Burks AW, Bannon GA, Sampson HA. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk F, Hartgring S, Koppelman SJ, Pieters R, Knippels LM. Mixed antibody and T cell responses to peanut and the peanut allergens Ara h 1, Ara h 2, Ara h 3 and Ara h 6 in an oral sensitization model. Clin Exp Allergy. 2004;34:1422–1428. doi: 10.1111/j.1365-2222.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- 22.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, Sampson HA. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112:159–167. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123:443–451. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 24.McDermott RA, Porterfield HS, El-Mezayan R, Schlichting D, Hansen KC, Duncan MW, Solomon B, Redzic J, Simpson M, Dreskin SC. Contribution of Ara h 2 to peanut-specific immunoglobulin E-mediated, cell activation. Clinical & Experimental Allergy. 2007;37:752–763. doi: 10.1111/j.1365-2222.2007.02701.x. [DOI] [PubMed] [Google Scholar]
- 25.Kulis M, Pons L, Burks AW. In vivo and T cell cross-reactivity between walnut, cashew and peanut. Int Arch Allergy Immunol. 2009;148:109–117. doi: 10.1159/000155741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pemberton AD, Wright SH, Knight PA, Miller HR. Anaphylactic release of mucosal mast cell granule proteases: role of serpins in the differential clearance of mouse mast cell proteases-1 and -2. J Immunol. 2006;176:899–904. doi: 10.4049/jimmunol.176.2.899. [DOI] [PubMed] [Google Scholar]
- 27.Valenta R, Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007;119:826–830. doi: 10.1016/j.jaci.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170:3289–3295. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, Goldfarb J, Sampson HA, Li XM. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]


