Abstract
There is considerable controversy about the origins of sex differences in cognitive abilities, particularly the male superiority in spatial abilities. We studied effects of early androgens on spatial and mechanical abilities in adolescents and young adults with congenital adrenal hyperplasia (CAH). On tests of 3D mental rotations, geography, and mechanical knowledge, females with CAH scored higher than their unaffected sisters, and males with CAH scored lower than their unaffected brothers. Exploratory regression analyses suggest that androgens affect spatial ability in females directly and through male-typed activity interests. Findings indicate that early androgens influence spatial and mechanical abilities, and that androgen effects on abilities may occur in part through effects on sex-typed activity interests.
Keywords: spatial ability, mental rotation, mechanical ability, geography, cognition, sex typing, gender typing, sex differences, androgens, sex hormones, congenital adrenal hyperplasia
Considerable interest surrounds the nature, origins, and consequences of sex differences in human cognitive abilities. There are no sex differences in overall intellectual ability, but there are differences in the pattern of cognitive abilities. Compared to the other sex, boys and men have better spatial, mathematical, and mechanical skills, whereas girls and women are more skilled in verbal fluency, writing, perceptual speed, and verbal memory (for reviews see Blakemore, Berenbaum, & Liben, 2009; Halpern, 2012).1
The largest cognitive sex difference is in spatial ability, with males outperforming females in most aspects, but the size of the difference varying across abilities. The largest spatial sex difference is in mental rotation, especially rotation of objects in three dimensions. This sex difference is apparent beginning in infancy (Moore & Johnson, 2008; Quinn & Liben, 2008), with boys and men having better ability than girls and women; the size of the difference increases slightly from childhood to adulthood (Geiser, Lehmann, & Eid, 2008).
Moderate to large differences are also seen in other spatial skills, with boys and men superior to girls and women (Blakemore et al., 2009; Halpern, 2012). This includes spatial perception, the ability to identify spatial relations with respect to one’s body in relation to external space, or to identify the true vertical or horizontal. There are also differences in abilities related to navigating in the real world, typically referred to as “wayfinding”; some of the sex differences result from differences in strategy, with males relying on cardinal directions and females relying on landmarks (Lawton, 2010). A large sex disparity is seen in National Geography Bee winners (despite equal participation from boys and girls); the sex ratio increases at each level of competition, so that in many years, all 10 finalists are boys (Liben, 1995). One spatial domain shows a moderate sex difference in the other direction: females are better than males in memory for spatial location (Voyer, Postma, Brake, & Imperato-McGinley, 2007).
The sexes also differ in other cognitive abilities. Males are better than females in mathematical and mechanical abilities. The latter has not been well-studied. Sex differences in mathematical abilities are the subject of considerable debate with respect to the size, origins, consequences, and universality of the difference (Else-Quest, Hyde, & Linn, 2010; Guiso, Monte, Sapienza, & Zingales, 2008; Lindberg, Hyde, Petersen, & Linn, 2010). Females are superior to males in many verbal abilities, memory, and processing speed, but most of the differences are smaller than the differences in spatial and mechanical abilities (Halpern, 2012)
Sex differences in spatial and related abilities have figured prominently in discussions of sex differences in occupational choice. Women’s lower spatial ability (compared to men’s) has been suggested to contribute to their underrepresentation in science, technology, engineering, and mathematics (STEM) careers (reviewed in Ceci & Williams, 2007, 2010).
Hypothesized Causes of Sex Differences in Spatial Ability
Work from multiple perspectives suggests that sex differences in spatial ability arise from multiple sources. We briefly describe the evidence regarding socialization and genetic effects, and focus on hormonal influences.
Socialization Effects on Spatial Ability
Several types of evidence suggest that spatial abilities are influenced by social experiences. Sex differences in spatial ability have been seen to depend on socioeconomic status (SES), with differences apparent in children from middle and high SES backgrounds, but not in children from low SES backgrounds (Levine, Vasilyeva, Lourenco, Newcombe, & Huttenlocher, 2005). SES effects were suggested to result in part from access to experiences that facilitate spatial ability.
The experiences most often suggested to contribute to spatial ability include play with boys’ toys (e.g., construction sets, videogames) and engagement in boy-typed activities (e.g., sports) that encourage manipulation and exploration of the environment (e.g., Baenninger & Newcombe, 1989; Connor & Serbin, 1977). The link between spatial ability and aspects of sex-typed activities is weak-to-moderate (e.g., Newcombe, Bandura, & Taylor, 1983), with some variability and inconsistency that likely reflects methodological and conceptual issues (Baenninger & Newcombe, 1989; Voyer, Nolan, & Voyer, 2000). Nevertheless, correlations are not evidence of causation: engagement in boy-typed activities might enhance spatial ability or instead reflect that ability, that is, children with high spatial ability might be attracted to toys that allow manipulation and exploration, or a third factor (such as early hormones or gendered socialization) may influence both of them. Some longitudinal data suggest that the causal path is from abilities to activities rather than the reverse (Newcombe & Dubas, 1992).
It is, therefore, important to note direct experimental evidence that spatial ability can be enhanced by experience. In particular, spatial ability can be improved through practice and training, with generalization beyond training stimuli. For example, playing an action video game was seen to improve both spatial attention and mental rotation ability (Feng, Spence, & Pratt, 2007). Training benefits both sexes, with women sometimes benefiting more than men, so that training may eliminate a sex difference (Lawton, 2010).
Finally, stereotypes that emphasize women’s cognitive inferiority appear to impair their performance. This has been demonstrated in experimental studies of both math and spatial abilities, in which test-taking conditions are manipulated to emphasize or de-emphasize cognitive sex differences and their malleability. Women who were told that sex differences in math have genetic causes performed worse on math tests than those who were told that the differences have experiential causes (Dar-Nimrod & Heine, 2006). Women who were told that men outperform women on spatial tasks performed worse on a mental rotations test than women who received neutral information, and the poorer performance of the group given negative stereotypes appeared to reflect increased emotional load (Wraga, Helt, Jacobs, & Sullivan, 2007). The effect of stereotype information on spatial sex differences has also been seen in judgments of line orientation (Campbell & Collaer, 2009).
Sex Chromosome Influences on Spatial Ability
Sex-related genetic and physiological factors also contribute to sex differences in spatial ability. Studies of individuals with sex chromosome anomalies show that genes on the X chromosome influence spatial ability (Ross, Roeltgen, & Zinn, 2006). There is little evidence regarding effects of genes on the Y chromosome, although evidence from rodents increasingly shows that such genes play an active role in behavioral sexual differentiation (e.g., Arnold & Chen, 2009; De Vries et al., 2002). The minimal human evidence suggests no behavioral or cognitive effects of genes on the Y chromosome (Hines, Ahmed, & Hughes, 2003; Imperato-McGinley, Pichardo, Gautier, Voyer, & Bryden, 1991; Masica, Money, Ehrhardt, & Lewis, 1969), but this has not been well studied.
Sex Hormone Influences on Spatial Ability
There is also considerable work on sex hormone effects on spatial ability, as also discussed in other papers in this special section. Most studies concern effects of circulating levels of testosterone and estradiol, although there is some evidence about early hormones, particularly androgens.
Evidence on cognitive effects of circulating sex hormones (activational effects) comes from studies of natural variations in hormones across individuals and within individuals (e.g., in association with the menstrual cycle) and effects of hormone replacement (in association with aging or surgical removal of the ovaries). Findings are complex (for reviews, see Hampson, 2007; Maki & Sundermann, 2009), but generally suggest that spatial ability is facilitated by testosterone in the moderate range (levels that are high for females and low for males) and verbal memory is facilitated by estradiol, especially in young postmenopausal women. Nevertheless, correlations between cognition and hormones may be difficult to interpret (e.g., circulating hormones may actually reflect prenatal hormones) and are not always found (e.g., Puts et al., 2010), probably due to both methodological factors (e.g., statistical power, variations in tests used), and factors that modify the effects of both hormones (e.g., diet) and cognition (e.g., experiences). It is important to note that studies with even relatively large samples do not have sufficient power to see what are likely to be small effects. For example, even with 170 participants, power is still less than .80 to see a correlation as large as .2 with a two-tailed Type I error of .05; power is further reduced when several measures are examined.
Less is known about cognitive effects of hormones during early development (organizational effects). Evidence from rodents shows that high levels of early sex hormones enhance spatial performance in females (Williams & Meck, 1991). The one study in nonhuman primates (Herman & Wallen, 2007) is not straightforward because the spatial task used (attending to and using landmarks to solve spatial problems) showed a female superiority. Nevertheless, the sex difference appears to be influenced by prenatal androgens: blocking androgen exposure in males (through flutamide administration to pregnant rhesus monkey mothers) improved one aspect of their performance.
Human work linking prenatal hormones to later cognitive abilities is not entirely consistent. There are several types of human studies, focusing on (a) abilities in individuals with disorders of sex development (DSDs), in which prenatal and/or neonatal hormone levels are sex-atypical in the presence of sex-typical rearing and gender identity, (b) links between prenatal amniotic hormones and later abilities, (c) abilities in relation to inferred hormone exposure, determined by somatic markers (such as digit ratio) or having an opposite-sex twin.
Most studies of DSDs involve girls and women with congenital adrenal hyperplasia (CAH), who are exposed to higher than normal (sex-atypical) androgen levels during early development but who are reared and identify as female. Females with CAH have been found to have higher spatial ability than their sisters in childhood, adolescence, and adulthood (Hampson, Rovet, & Altmann, 1998; Hines, Fane, et al., 2003; Mueller et al., 2008; Resnick, Berenbaum, Gottesman, & Bouchard, 1986), with a meta-analysis suggesting that the effect is small to moderate (Puts, McDaniel, Jordan, & Breedlove, 2008). But, females with CAH are not always seen to have enhanced spatial ability (Hines, Fane, et al., 2003; Malouf, Migeon, Carson, Petrucci, & Wisniewski, 2006; for review of other studies, see Berenbaum, 2001). Evidence is more consistent in showing females with CAH to be masculinized in other sex-typed characteristics, most prominently activity interests but also sexual orientation and personal-social attributes such as aggression and interest in babies (for reviews, see Blakemore et al., 2009; Hines, 2010).
Males with CAH have also been studied for comparison. Most studies in nonhuman animals show that excess androgens in males have inconsistent effects, sometimes further masculinizing, sometimes demasculinizing, and most often having no effects (reviewed in Becker, Breedlove, Crews, & McCarthy, 2002; Goy & McEwen, 1980). Males with CAH have been found to be similar to unaffected males (usually their brothers) in most ways, including sex-typed activity interests and personal-social attributes (reviewed in Blakemore et al., 2009). In contrast, males with CAH have been seen to have lower spatial ability than unaffected males (Hampson et al., 1998; Hines, Fane, et al., 2003); it is unclear whether such effects reflect androgens or complications from the disease that are more common in males than in females.
There are several methodological explanations for inconsistencies regarding spatial ability differences between females with and without CAH. The effect is not large, and most studies are underpowered; even with 50 participants per group (which is more than was used in any study), power is sufficient to detect a moderate difference at best. Tests used do not always show large sex differences in typical samples, further reducing power. Comparisons are often made to unrelated individuals, failing to provide sufficient matching on important background factors; in fact, in some studies, there were differences between females with and without CAH on IQ or on measures that do not show sex differences, confounding interpretation of spatial scores (for specific details and discussion, see Berenbaum, 2001). Some samples were heterogeneous in age, which is related to spatial ability in nonlinear ways: Sex differences in spatial ability can be difficult to detect before mid-adolescence unless special tests are used; spatial ability declines with age beginning in early adulthood. Further, given demonstrated social influences on spatial ability, it is possible that social moderators reduced effects, making it difficult to detect differences between females with and without CAH in small samples.
Some inconsistencies may also reflect complexities of androgen effects on cognitive abilities. Studies in other species, including primates, indicate that there are multiple sensitive periods for behavioral effects of hormones, even during early development (e.g., Goy, Bercovitch, & McBrair, 1988). If this happens in people, androgen may continue to have organizational effects into the neonatal period and perhaps beyond; this would result in spatial enhancement only in those females with CAH whose disease was not diagnosed or well-controlled immediately after birth.
In understanding the failures to find spatial enhancements in females with CAH, it is crucial to note that females with CAH have not been found to have significantly lower spatial ability than unaffected females. Such a difference would occur on occasion if there is truly no population difference between females with and without CAH.
Confirming evidence for early androgen effects on spatial ability comes from several other sources. The first concerns the counterpart of CAH: males with very low androgen levels reared as and identifying as males. Males with low early androgen levels due to idiopathic hypogonadotropic hypogonadism (IHH) were found to have lower spatial ability than controls (Hier & Crowley, 1982), although there has been a failure to replicate (Cappa et al., 1988); inconsistencies likely reflect low power with small samples. The second piece of converging evidence comes from the only published study of spatial ability in relation to amniotic hormones (Grimshaw, Sitarenios, & Finegan, 1995). Seven-year-old girls who had high levels of amniotic testosterone had better spatial ability than girls who had low levels. But this result is complicated by the fact that the difference was not found on accuracy, and was seen only on a measure of speed of rotation and only in girls who showed evidence of using a mental rotation strategy.
The third source of converging evidence for androgen effects on spatial ability comes from individuals with an opposite-sex twin, in which prenatal hormone levels are inferred. This experiment is based on studies in nonhuman animals showing that behavior and physiology are influenced by naturally occurring variations in hormones that result from an animal’s position in the uterus, particularly the sex of its littermates (Ryan & Vandenbergh, 2002). In particular, female rodents that develop between two males are less sex-typed than those that develop between two females. In three studies of human opposite-sex twins, females with a male co-twin were found to have higher spatial ability than females with a female co-twin (Cole-Harding, Morstad, & Wilson, 1988; Heil, Kavsek, Rolke, Beste, & Jansen, 2011; Vuoksimaa et al., 2010). But, prenatal hormone influences are confounded with postnatal socialization in these findings; females with a male co-twin are reared with a male sibling of the same age. Evidence against socialization effects comes from women with slightly older siblings: those with a brother did not have better spatial ability than those with a sister (Heil et al., 2011).
Finally, there are other studies of inferred hormones that focus on somatic biomarkers. The most widely studied marker is the ratio of the second to the fourth finger (digit ratio, 2D:4D) which shows a sex difference. Digit ratio is related to effective prenatal androgen exposure, but the link is relatively modest, making it a very weak marker of individual differences in prenatal androgens (Berenbaum, Bryk, Nowak, Quigley, & Moffat, 2009). It is therefore difficult to know how to interpret data indicating that 2D:4D is generally not related to spatial ability (Puts et al., 2008).
Thus, evidence from multiple sources converges to suggest that spatial ability is influenced by early androgen exposure. Evidence that sex differences in spatial ability appear very early in life (3–5 months of age) (Moore & Johnson, 2008; Quinn & Liben, 2008) is also consistent with (although does not prove) the importance of early hormones. Nevertheless, the evidence is less compelling than that for androgen effects on other sex-typed characteristics, especially activity interests and sexual orientation (for reviews, see Berenbaum & Beltz, 2011; Blakemore et al., 2009; Hines, 2010) and conclusions about early androgen effects on spatial ability are sometimes dismissed because of the inconsistencies (Hines, 2010; Hines, Fane, et al., 2003; Valla & Ceci, 2011). Furthermore, most studies have used a limited group of spatial tests (focused on two- and three-dimensional mental rotations), and none have considered “real-world” abilities that show large sex differences, including geographical knowledge and mechanical knowledge.
It is also important to consider the psychological mechanisms whereby early hormones influence spatial ability, particularly whether early androgens influence ability through their effects on childhood activities. Compelling evidence indicates that prenatal androgens increase interest in male-typed activities. Girls and women with CAH are more interested in and engaged with such activities than are unaffected females (including unaffected sisters). The differences are large, seen across the age range (childhood, adolescence, and adulthood), and found in studies using different measures and conducted in diverse countries (for reviews, see Blakemore et al., 2009; Hines, 2010). Converging evidence comes from other DSDs (reviewed in Blakemore et al., 2009) and from a recent study of typical children, in which testosterone in amniotic fluid was found to be positively associated with childhood interest in boy-typical toys and activities (Auyeung et al., 2009). But, it is currently unknown whether sex-typed activities mediate the effects of early androgens on spatial ability.
Present Study
Thus, there is need for more evidence regarding early androgen effects on spatial ability. In particular, it is important to obtain additional evidence from individuals with known androgen exposure, to understand the breadth of androgen effects on spatial and related abilities, and to examine the potential mediating role of sex-typed activities. We report here such evidence from individuals with CAH, using an array of tests, and linking abilities to activities. We focused on females with CAH, hypothesizing that they would have higher spatial scores than unaffected females; we also expected that their enhanced spatial ability would be mediated at least in part by their activity interests. Males with CAH were also studied as a comparison. Given previous work, we expected that males with CAH would have lower spatial ability than unaffected males. But, in light of their sex-typical activity interests, we expected that spatial ability would not be mediated by activity interests in males.
Method
Participants
Females and males with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency were studied as part of a larger project on androgen effects on psychological development. They were recruited from university-affiliated pediatric endocrinology clinics in the Midwestern United States and from a family support group, and compared to their unaffected siblings. Cognitive abilities were assessed during in-home test sessions. We report data on spatial and mechanical abilities, and vocabulary (an ability that does not show a sex difference and serves as a proxy for general intelligence, Sattler & Ryan, 2009), focusing on individuals aged 16 and older given difficulties in measuring some of these abilities in children. Data on abilities were available for 58 individuals: 19 females and 12 males with CAH, 13 sisters, 14 brothers. Participants ranged in age from 16 to 30 years (M = 20.26, SD = 2.99); groups did not differ in age, F(3, 54) = 1.78, p > .05. Because not all participants with CAH had a same-sex sibling, siblings of males and females with CAH were combined to form control groups.2 Participants represented a variety of socioeconomic backgrounds and most were Caucasian. Other findings from the larger study are summarized elsewhere (Berenbaum & Bryk, 2008; Blakemore et al., 2009).
Disease features of potential relevance to behavior were rated from medical records for most individuals with CAH. Most had the severe form of the disease, and thus are presumed to have been exposed to high levels of androgens during prenatal and early postnatal life (Speiser, 2001). With respect to type of CAH, the salt-wasting form was present in 15 females and 7 males and the non-salt-wasting form was present in 4 females and 4 males; data were missing for 1 male. All females with CAH had genital virilization at diagnosis, rated from medical records using the Prader (1954) scale, which ranges from 0 (typical female genitalia) through 6 (typical male genitalia); scores ranged from 1 to 4 (M = 2.50, SD = 1.41); data were unavailable for 3 girls. Given the relatively small sample and limited variability in indicators of degree of prenatal androgen exposure, we were unable to examine links between these indicators and abilities.
Cognitive Tests
Participants were administered tests of multiple aspects of spatial ability, mechanical knowledge, and vocabulary (as a control). All tests show acceptable psychometric properties and, except for vocabulary, all show sex differences that are at least moderate in size; for details, see the references listed for each test.
Mental Rotations Test
(Vandenberg & Kuse, 1978). This test measures mental rotation in three dimensions. Each item includes pictorial (two-dimensional) images of a three-dimensional target figure and four three-dimensional stimulus figures; the task is to choose the two stimulus figures that are rotations of the target figure in three-dimensional space. Participants are given ten minutes to complete 20 items. The score is the total number correct, which can range from 0 to 40.
Space Subtest, Test of Primary Mental Abilities
(Thurstone, 1963). This test measures mental rotation in two dimensions. Each item includes a two-dimensional target figure and five two-dimensional stimulus figures; the task is to determine which of the stimulus figures are rotations of the target figure in two-dimensional space. Participants are given seven minutes to complete 30 items. The number of correct items varies across the items, ranging from one to three. The score is the total number correct, which can range from 0 to 70. Data are missing for one participant, one female with CAH.
Water Level Test
(L. J. Harris, personal communication). This test measures spatial perception or judgment of horizontality. Each item shows four identically shaped bottles that contain water and are tilted at different angles, with a line drawn to indicate the level of the water; the task is to choose the single bottle showing the correct (horizontal) placement of the line. Participants have to complete 12 items, with no time limit. The score is the number correct, which can range from 0 to 12.
House Plans
(Kerns & Berenbaum, 1991). This test measures spatial visualization. The participant is presented with two two-dimensional pictorial views of a “house” designed from squares, an aerial view and a frontal view, and is asked to use blocks to construct the three-dimensional representation of the house. Each item is timed. There are seven “house plans”; one point is credited for each house built correctly. The score can range from 0 to 7. Data are missing for two participants, one male with CAH and one unaffected male.
Geography Test
(Snyder & Harris, 1996). This test measures geographical knowledge. The test consists of a map of the world with 16 locations numbered; the task is to match the 16 numbers with a list of 16 geographical locations. There is no time limit. The score is the number correct, which can range from 0 to 16.
Test of Mechanical Knowledge, Comprehensive Ability Battery
(Hakstian & Cattell, 1976). This test measures mechanical knowledge. Each item contains a question about a mechanical object or mechanical process; the task is to choose the correct answer out of five choices. Participants are given 6.5 minutes to answer 18 questions. The score is the number correct, which can range from 0 to 18.
Advanced Vocabulary, Educational Testing Service Kit of Factor-Referenced Cognitive Tests
(Ekstrom, French, & Harman, 1976). Vocabulary correlates highly with general intelligence (Sattler & Ryan, 2009). This test has two parts of 18 items each. Each item consists of a target word and five stimulus words; the task is to choose the correct definition from the choices. Participants are given four minutes for each part. The score is the total number correct, which can range from 0 to 36.
Sex-typed Activity Interests
Participants also completed a questionnaire about their interest in 60 leisure activities (hobbies), with each item rated on a 5-point scale (strongly dislike to strongly like) (Lippa, 1995, 2002). Interest in male-typed activities (hobbies) is reflected in scores on an average of 15 items (alpha = .84). Data are missing for three participants: one control female, one female with CAH, and one male with CAH.
Data Analysis Procedures
The main analyses involved group comparisons on spatial tests. Individuals with CAH were compared to their unaffected relatives with analyses of covariance (ANCOVA), with factors of sex and status (CAH, unaffected same-sex relatives), and covariates of age and vocabulary score, followed by post-hoc t-tests probing significant interactions between sex and status. Because the sample is relatively small, power was maximized by conducting separate univariate analyses for each cognitive test, with Type I error set at .10 and no correction for multiple comparisons; consequences of this decision are considered in the discussion.
Exploratory analyses were conducted to examine whether early androgen effects on spatial ability were due to their effects on gendered activity interests. First, an analysis of variance (ANOVA) with factors of sex and status were used to examine group differences in male-typed activity interests. Next, linear regression analyses were conducted to examine whether male-typed activity interests mediated early androgen effects on spatial ability, and whether the mediation reflects the beginning of a cascade of androgen effects on abilities. Tests of mediation required that four criteria be met (Baron & Kenny, 1986): (1) status (CAH versus unaffected) predicted male-typed activity interests; (2) male-typed activity interests predicted spatial ability; (3) status predicted spatial ability; (4) controlling for male-typed activity interests, status was a significantly reduced predictor of spatial ability, determined by Sobel’s test. Cascade effects were tested in a regression, examining whether male-typed interests not only mediated prenatal androgen effects on spatial ability but also provided incremental unique prediction (beyond variation accounted for by status) (Dodge et al., 2009). In order to increase confidence in inferences about the direction of effects, alternative mediation analyses were also conducted, reversing the order of spatial ability and male-typed activity interests, that is, testing whether spatial abilities mediated early androgen effects on male-typed activity interests. Age and vocabulary score were included as covariates in the mediation and cascade analyses.
Results
Group Comparisons
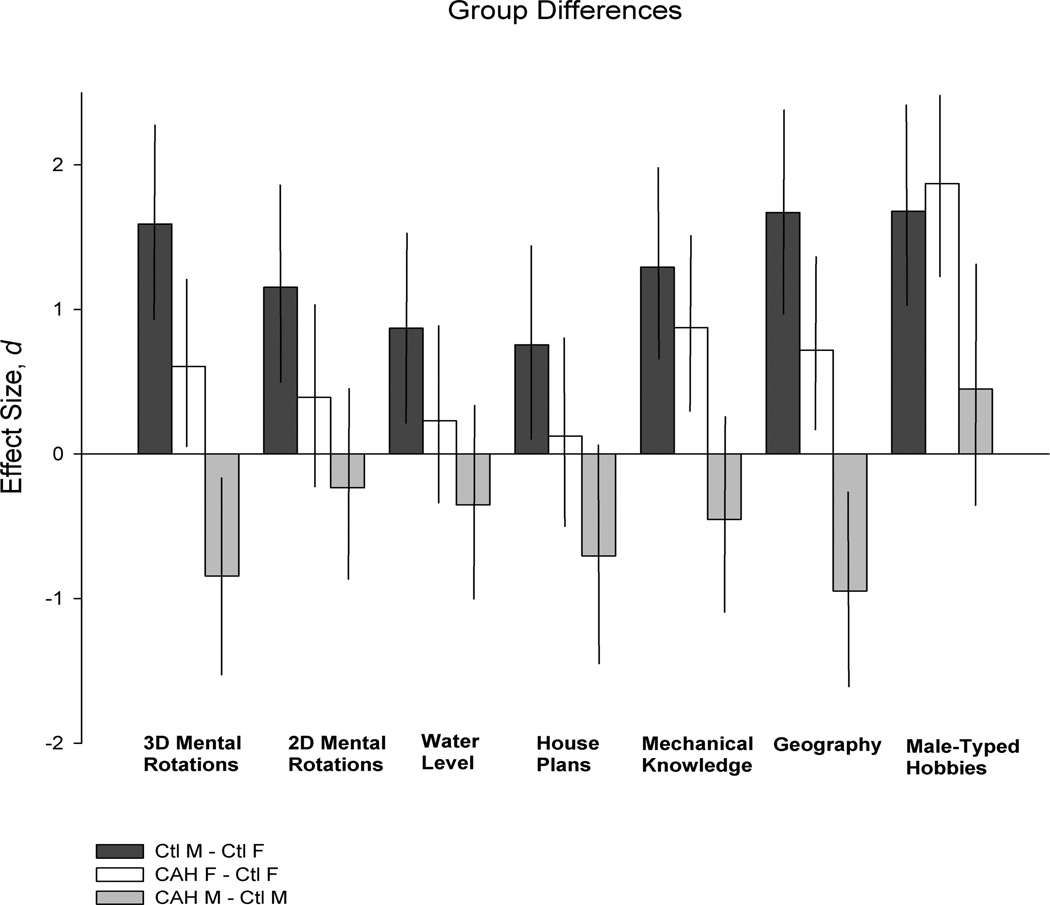
Scores for females and males with and without CAH on all cognitive tests and male-typed activity interests are shown in Table 1. Results of statistical testing, shown in Table 2, revealed significant effects of sex on all spatial tests except house plans3, no main effects of status (CAH vs. unaffected) on any spatial test, and significant interactions between sex and status on tests of 3D mental rotations, geography, and mechanical knowledge; there were no significant effects on vocabulary. There were significant effects of the covariates: age positively affected geography, and vocabulary positively affected 2D mental rotations, water level, house plans, geography, and mechanical knowledge. Post-hoc t-tests of the interactions revealed that females with CAH had better spatial abilities than unaffected sisters (scoring significantly higher on 3D mental rotations, geography, and mechanical knowledge), whereas males with CAH had worse abilities than unaffected brothers (scoring significantly lower on 3D mental rotations and geography). Statistical testing of male-typed activity interests showed significant effects of sex, status, and their interaction; post-hoc t-tests revealed that females with CAH had significantly more male-typed hobbies than unaffected sisters, but males with CAH were not significantly different than unaffected brothers.
Table 1.
Participants’ Scores on Cognitive Tests and Male-typed Activity Interests: Means (and Standard Deviations in Parentheses)
| Measure (followed by possible range of scores) |
Unaffected Females N = 12–13 |
Females with CAH N = 18–19 |
Unaffected Males N = 13–14 |
Males with CAH N = 11–12 |
|---|---|---|---|---|
| Age | 19.95 (3.01) |
21.33 (2.17) |
18.99 (2.33) |
20.40 (4.26) |
| Vocabulary (possible scores: 0–36) |
12.77 (4.46) |
14.21 (5.12) |
15.07 (6.94) |
11.92 (4.03) |
| 3D Mental Rotations (possible scores: 0–40) |
27.31 (6.55) |
30.68 (4.58) |
35.93 (4.29) |
30.67 (8.19) |
| 2D Mental Rotations (possible scores: 0–70) |
41.15 (9.02) |
45.28 (12.00) |
52.57 (10.77) |
49.75 (13.30) |
| Water Level (possible scores: 0–12) |
5.92 (3.52) |
6.74 (3.57) |
8.93 (3.38) |
7.67 (3.80) |
| House Plans (possible scores: 0–7) |
5.92 (0.76) |
6.05 (1.27) |
6.46 (0.66) |
5.55 (1.92) |
| Geography (possible scores: 0–16) |
11.38 (3.31) |
13.79 (3.39) |
15.36 (1.45) |
13.50 (2.47) |
| Mechanical Knowledge (possible scores: 0–18) |
6.31 (2.93) |
8.95 (3.10) |
10.21 (3.12) |
8.67 (3.73) |
| Male-typed Hobbies (possible scores: 1–5) |
2.67 (0.52) |
3.50 (0.37) |
3.72 (0.73) |
3.93 (0.23) |
Note. See text for information on missing data.
Table 2.
Results of Statistical Testing of Group Differences
| Covariates | Sex Effect | Status Effect | Sex *Status | Post-hoc tests | |
|---|---|---|---|---|---|
| Vocabulary | not applicable | F (1,54)=.00 | F (1,54)=.37 | F (1,54)=2.65 | |
| 3D Mental Rotations | Age F(1,52)=.07 Vocabulary F(1,52)=1.37 |
F(1,52)=6.99* | F (1,52)=.17 | F(1,52)=5.82* | F CAH>Ctl, t(30)=1.72* M CAH<Ctl, t(24)=2.10* |
| 2D Mental Rotations | Age F(1,51)=.02 Vocabulary F(1,51)=4.24* |
F(1,51)=6.64* | F (1,51)=.18 | F (1,51)=.49 | |
| Water Level | Age F(1,52)=.30 Vocabulary F(1,52)=2.80+ |
F(1,52)=4.67* | F(1,52)=.05 | F(1,52)=.51 | |
| House Plans | Age F(1,50)=.39 Vocabulary F(1,50)=4.92* |
F(1,50)=.01 | F(1,50)=1.33 | F(1,50)=1.25 | |
| Mechanical Knowledge | Age F(1,52)=.05 Vocabulary F(1,52)=7.39** |
F(1,52)=4.65* | F(1,52)=.85 | F(1,52)=3.65+ | F CAH>Ctl, t(30)=2.42* |
| Geography | Age F(1,52)=8.18** Vocabulary F(1,52)=4.76* |
F(1,52)= 10.03** |
F(1,52)=.01 | F(1,52)=6.85* | F CAH>Ctl, t(30)=1.99* M CAH<Ctl, t(24)=2.38* |
| Male-typed Hobbies | Age F(1,49)=.53 Vocabulary F(1,49)=7.06* |
F(1,49)= 30.47*** |
F(1,49)= 11.43** |
F(1,49)=8.41** | F CAH>Ctl, t(28)=5.14*** |
Note. F: female; M: male; CAH: individuals with CAH, Ctl: unaffected same-sex relatives
Effects significant with F-test (ANCOVA) or one-tailed t-test (post-hoc comparisons),
p ≤ .10,
p ≤ .05,
p ≤ .01,
p ≤ .001
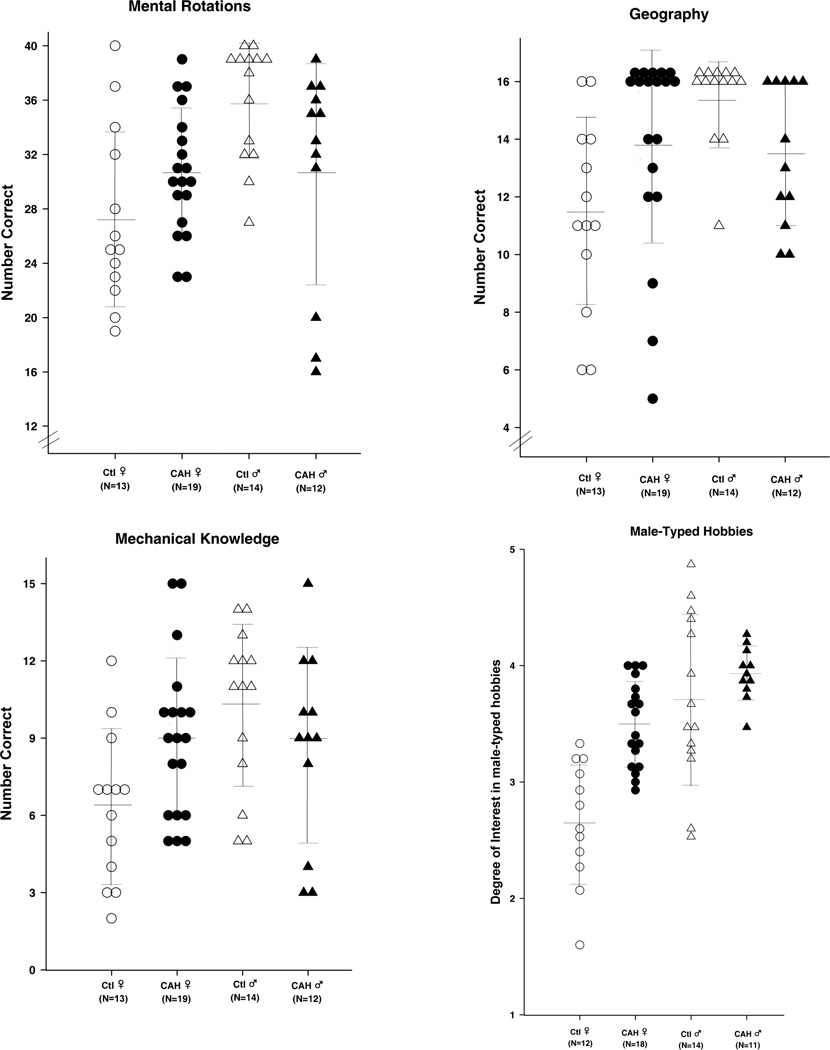
Group differences are generally moderate to large in size, as shown in Figure 1, along with confidence intervals around the mean differences. The nature of the differences on the tests that show significant androgen effects is illustrated in Figure 2: scores of individuals in each group are plotted for the three cognitive tests that differentiated those with and without CAH, and on male-typed hobbies that differentiated females with and without CAH.
Figure 1.
Summary of group differences on measures of cognition and male-typed activity interests. Bars represent effect size, d; lines represent 90% confidence intervals.
Figure 2.
Individuals’ scores on measures of cognition and male-typed activity interests. Ctl ♀: unaffected females; CAH ♀: females with CAH; Ctl ♂: unaffected males; CAH ♂: males with CAH. Lines represent group means and standard deviations. Circles represent individual female participants; triangles represent individual male participants. Open symbols are unaffected controls; black symbols are individuals with CAH. See the text for reports of statistical tests of group differences.
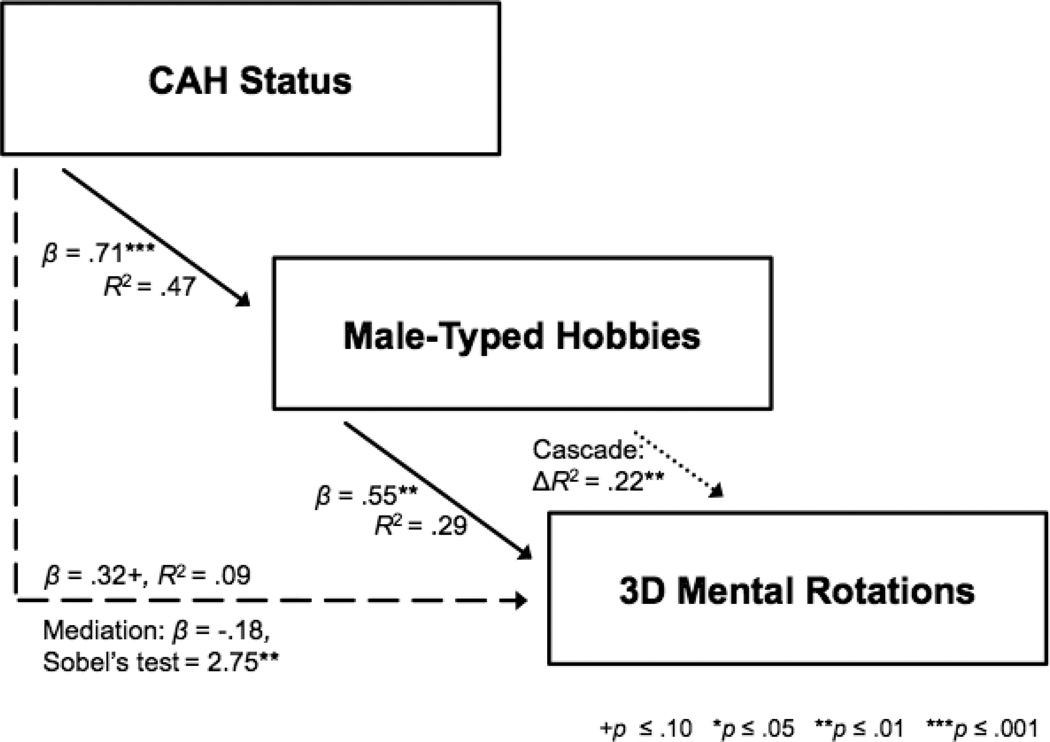
Mediation and Cascade Effects
Exploratory analyses were conducted to determine if early androgens affect spatial ability directly or indirectly through male-typed activity interests. These analyses were only conducted in females, because males with CAH did not differ from unaffected males in activity interests, so such interests cannot account for lower spatial ability. As shown in Figure 3, there was a cascade of early androgen effects (indexed by status) on 3D mental rotation ability when age and vocabulary score were controlled. The four criteria of statistical mediation were met (Baron & Kenny, 1986): (1) status predicted male-typed hobbies; (2) male-typed hobbies predicted 3D mental rotation ability; (3) status predicted 3D mental rotation ability; (4) controlling for male-typed hobbies, the predictive power of status on 3D mental rotation ability was significantly reduced according to Sobel’s test. Further, there was evidence of cascade effects: male-typed hobbies significantly provided incremental prediction of 3D mental rotation when status was controlled. Thus, male-typed hobbies partially mediated the effect of early androgen on 3D mental rotations and provided incremental prediction (that is, male-typed hobbies continued to be a significant predictor of 3D mental rotations after controlling for status). Results for mechanical knowledge showed mediation, but not cascade effects, that is, male-typed hobbies mediated mechanical knowledge, but did not provide incremental prediction beyond status. Results for geography showed no mediation effects, so cascade effects were not tested. Results for reverse mediation were not significant: none of the spatial abilities mediated early androgen effects on male-typed activity interests.
Figure 3.
Cascade model for females depicting a continuum of early androgen effects on male-typed activity interests and spatial ability (controlling for age and vocabulary score). Solid arrows represent simple effects. The dashed arrow represents mediation; statistics are presented for the analysis before and after (with Sobel’s tests) controlling for the mediating variable. The dotted arrow represents the prediction of incremental variation after controlling for the previous predictor.
Discussion
Our results – showing higher scores on 3D mental rotations, geography, and mechanical knowledge for females with CAH compared to their unaffected sisters – confirm that spatial abilities are enhanced by exposure to high levels of androgens during early life, and extend the nature of androgen influences to “real world” abilities: geography and mechanical knowledge. The confidence intervals show why effects have not always been seen in other studies, and urge caution in arguing against cognitive effects of early androgens on the basis of data from small samples.
Preliminary evidence from females with CAH also suggests a cascade of androgen effects on spatial ability. Regression results indicate that androgens influence spatial ability directly and, for some aspects, through male-typed activity interests; interests mediated androgen effects on both 3D mental rotations and mechanical knowledge, and further independently contributed to 3D mental rotations. An important question concerns the direction of the effects depicted in the cascade model. An alternative model testing reverse causation (status → 3D mental rotations → male-typed hobbies) did not fit the data. Further, the pattern of effect sizes for the differences between females with and without CAH (male-typed hobbies > 3D mental rotations) is consistent with the original model. Nevertheless, these results should be considered preliminary and serve as a guide for further longitudinal studies that would allow a full test of the hypothesized cascade (Dodge et al., 2009).
Findings in males with CAH showing reduced spatial abilities – lower scores on 3D mental rotations and geography – compared to unaffected male relatives replicate and extend previous studies (Hampson et al., 1998; Hines, Fane, et al., 2003), but the source of the differences remains unknown. Lower ability relative to controls could result from several different mechanisms. First, low spatial ability might result from lower-than-typical levels of androgen during early life (prenatal and neonatal periods) owing to negative feedback from the boys’ own testes (Pang, et al., 1980; Pang, Levine, Chow, Faiman, & New, 1979). This explanation would best be tested by prospective studies examining neonatal hormone levels in relation to later spatial abilities. Second, low spatial ability could reflect adverse consequences of the disease, such as salt wasting episodes or hypoglycemia (as suggested by data that spatial abilities are more likely than verbal abilities to be affected in individuals with diabetes, Naguib, Kulinskaya, Lomax, & Garralda, 2009). Boys may be more likely than girls to have these adverse consequences because they are less likely to be detected clinically (owing to no obvious physical signs) before they experience these events. We did not have good enough medical information to test this possibility. It is important to note that low spatial abilities occur in the presence of male-typical activity interests, indicating that such activities are not sufficient for male-typical spatial abilities.
Limitations
There are some limitations that should be considered in interpreting our results. First, CAH is not a perfect experiment; individuals with CAH differ from unaffected relatives in ways other than early androgens, e.g., abnormalities in cortisol, ambiguous genitalia in females. Nevertheless, early androgens are the most likely explanation for the results because the enhanced ability of females with CAH was restricted to spatial and mechanical abilities. Further, androgen effects on spatial ability have been seen in other samples of females with CAH and in other types of experiments, as described above.
Second, there is uncertainty about the exact timing of early androgen effects, that is, whether they reflect organizational changes during the prenatal or early postnatal periods. Given the evidence from other species for multiple sensitive periods for behavioral effects of early hormones, it is possible that spatial ability – more than any other sex-typed characteristic – is influenced by early postnatal androgens. This would explain both the variation in females with CAH (only some of whom continue to have elevated androgens beyond the prenatal period) and the lower scores in males with CAH (who appear to have subnormal androgen levels during early postnatal life, Pang et al., 1979).
Third, we used lenient criteria for statistical errors. It is difficult to obtain cognitive data on large samples of individuals with CAH, given its relatively low incidence (1 in 15,000 live births, Speiser, 2001), and the need for in-person testing; this is particularly challenging in a large, geographically-mobile country like the United States. Thus, we had statistical power to see only relatively large effects. Further, the trade-off between Type I and Type II errors led to the decision to use a Type I error rate of .10, and no correction for multiple comparisons. Therefore, we emphasize that differences were found on tests that show large sex differences, and not on vocabulary, and in confirmation of other results. The main limitation of the small sample was in testing mediating and cascading effects, so these results should be considered preliminary.
Fourth, not all spatial tests showed expected effects. One test (house plans) did not show a significant sex difference when tested via ANOVA, although there was a significant difference between unaffected males and females with a t-test. Two tests (2D mental rotations and water level) that showed a sex difference did not show differences between individuals with and without CAH. But, group differences were all in the expected directions (see Figure 1), and statistical power is the likely explanation for our inability to see what are probably not large effects. This is supported by findings that significant effects of androgens were found on the tests that showed the largest sex differences.
Fifth, we were unable to examine abilities on which females typically score higher than males, such as perceptual speed and verbal memory. Sex differences in these abilities are not large, and we did not have enough statistical power to examine them.
Mechanisms of Effects
Our findings that females with CAH have better geographical and mechanical knowledge than unaffected sisters make clear that androgens affect abilities through the social environment. The interesting questions then become about the ways in which spatial ability develops from joint influences of biology and social experiences. Consider two possible paths. First, androgens might create a predisposition to have experiences that facilitate spatial ability; this would reflect a correlation between hormones and the social environment (parallel to a genotype-environment correlation, Scarr & McCartney, 1983). It is clear from many studies that prenatal androgens influence the likelihood of engaging in activities that appear to facilitate the development of spatial abilities. Our preliminary regression analyses suggest that abilities are, in fact, enhanced in part through this path, but longitudinal data are necessary to test fully that model. Second, androgens might facilitate learning of spatial skills, that is, individuals with high early androgens (females with CAH and males) would be more likely than typical females to learn about the spatial world when exposed to spatial-enhancing experiences; this would reflect an interaction between hormones and the social environment (parallel to a genotype-environment interaction, Scarr & McCartney, 1983). Existing training studies do not provide compelling support for this idea, because training appears to benefit the sexes equally, or perhaps even to benefit those with low androgen levels (women) more than those with high androgen levels (men). But, the situation might differ in childhood when abilities are developing, and on tests that do not show ceiling effects (which is often a problem in training studies). It would be interesting to provide training to girls with and without CAH to see if the former benefit more than the latter. This question can also be studied in typical children, by examining the effects of practice on boys versus girls at varying ages and in children who vary in their environmental experiences, e.g., as a function of SES.
It is also important to consider the neural mechanisms that mediate the cognitive effects of early androgens. Work examining sex differences in the neural processing of spatial ability suggests that men and women recruit some similar and some sex-specific brain regions to complete spatial tasks. Generally, both sexes engage parietal regions as well as hippocampal, premotor, and extrastriate areas, but women tend to engage frontal regions to a greater degree than men, and men tend to engage hippocampal and other temporal regions more than women (Grön, Wunderlich, Spitzer, Tomczak, & Riepe, 2000; Halari et al., 2006; Hugdahl, Thomsen, & Ersland, 2006; Weiss et al., 2003). There are no studies examining the ways in which such sex differences are influenced by early hormones, and data from individuals with CAH would be very informative.
Conclusions and Future Directions
This study replicates and extends work to show that sex-typed spatial and mechanical abilities are influenced by high levels of androgens present during early life. Preliminary findings suggest that these enhanced abilities are facilitated in part by androgen influences on male-typed activity interests, and that interests initiate a chain of events further facilitating ability. But, androgens appear to have smaller effects on spatial and mechanical abilities than on other sex-typed characteristics. Key questions now concern the neural substrates of androgen effects, and the social environmental factors that mediate and moderate them. It is time to move beyond asking whether early sex hormones influence human cognition to asking how they do so.
Acknowledgments
This research was supported by grant HD19644 from the National Institute of Child Health and Human Development. We thank the following people who contributed to the research reported here: Drs. Stephen Duck, Deborah Edidin, Erica Eugster, Reema Habiby, David Klein, Songya Pang, Bernard Silverman, Kumud Sane, Neil White, and David Wyatt facilitated recruitment of participants; Dr. Stephen Duck rated medical records; Dr. Lauren Harris provided some test materials; Lori Alegnani collected and processed data; Brad Fesi participated in initial data analyses and presentation of results; Lauretta Brennan and undergraduate research assistants processed data. We are very grateful to the patients and their families for their participation in the study.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Consistent with others, we describe sex differences in terms of effect size, d (Cohen, 1988): small (d ~ .2, 85% overlap in distributions of males and females), moderate (d ~ .5, 67% overlap, probably noticeable), and large (d ~ .8, 53% overlap, and very noticeable).
There were not enough same-sex sibling pairs (CAH, control) to conduct matched-pairs analyses.
Comparison of unaffected males and females with a one-tailed t-test revealed a significant sex difference in house plans (t(24)=1.93, p < .05).
Some of the data reported in this paper were presented at a meeting of the Society for Behavioral Neuroendocrinology.
Contributor Information
Sheri A. Berenbaum, Departments of Psychology and Pediatrics, The Pennsylvania State University
Kristina L. Korman Bryk, Department of Psychology, The Pennsylvania State University
Adriene M. Beltz, Department of Psychology, The Pennsylvania State University
References
- Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Frontiers in Neuroendocrinology. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, et al. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenninger M, Newcombe N. The role of experience in spatial test performance: A meta-anaylsis. Sex Roles. 1989;20:327–344. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. 2nd ed. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Berenbaum SA. Cognitive function in congenital adrenal hyperplasia. Endocrinology and Metabolism Clinics of North America. 2001;30:173–192. doi: 10.1016/s0889-8529(08)70025-2. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KK. Gender and occupational outcomes: Longitudinal assessments of individual, social, and cultural influences. In: Watt HMG, Eccles JS, editors. Biological contributors to gendered occupational outcome: Prenatal androgen effects on predictors of outcome. Washington, DC: American Psychological Association; 2008. pp. 235–264. [Google Scholar]
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150:5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore JEO, Berenbaum SA, Liben LS. Gender development. New York: Psychology Press; 2009. [Google Scholar]
- Campbell SM, Collaer ML. Stereotype threat and gender differences in performance on a novel visuospatial task. Psychology of Women Quarterly. 2009;33:437–444. [Google Scholar]
- Cappa SF, Guariglia C, Papagno C, Pizzamiglio L, Vallar G, Zoccolotti P, et al. Patterns of lateralization and performance levels for verbal and spatial tasks in congenital androgen deficiency. Behavioural Brain Research. 1988;31:177–183. doi: 10.1016/0166-4328(88)90021-6. [DOI] [PubMed] [Google Scholar]
- Ceci SJ, Williams WM. Sex differences in math-intensive fields. Current Directions in Psychological Science. 2010;19:275–279. doi: 10.1177/0963721410383241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci SJ, Williams WM, editors. Why aren't more women in science? Washington, DC: APA Books; 2007. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Academic Press; 1988. [Google Scholar]
- Cole-Harding S, Morstad AL, Wilson JR. Spatial ability in members of opposite-sex twin pairs (Abstract) Behavior Genetics. 1988;18:710. [Google Scholar]
- Connor JM, Serbin LA. Behaviorally based masculine and feminine activity-preference scales for preschoolers: Correlates with other classroom behaviors and cognitive tests. Child Development. 1977;48:1411–1416. [Google Scholar]
- Dar-Nimrod I, Heine SJ. Exposure to scientific theories affects women's math performance. Science. 2006;314:435. doi: 10.1126/science.1131100. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. Journal of Neuroscience. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE. A dynamic cascade model of the development of substance-use onset. Monographs of the Society for Research in Child Development. 2009;74(3):1–120. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH. Kit of factor-referenced cognitive tests. Princeton: Educational Testing Service; 1976. [Google Scholar]
- Else-Quest NM, Hyde JS, Linn MC. Cross-national patterns of gender differences in mathematics: A meta-analysis. Psychological Bulletin. 2010;136:103–127. doi: 10.1037/a0018053. [DOI] [PubMed] [Google Scholar]
- Feng J, Spence I, Pratt J. Playing an action video game reduces gender differences in spatial cognition. Psychological Science. 2007;18:850–855. doi: 10.1111/j.1467-9280.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- Geiser C, Lehmann W, Eid M. A note on sex differences in mental rotation in different age groups. Intelligence. 2008;36:556–563. [Google Scholar]
- Goy RW, Bercovitch FB, McBrair MC. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Hormones and Behavior. 1988;22:552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual differentiation of the brain. Cambridge: MIT Press; 1980. [Google Scholar]
- Grimshaw GM, Sitarenios G, Finegan JA. Mental rotation at 7 years: Relations with prenatal testosterone levels and spatial play experience. Brain and Cognition. 1995;29:85–100. doi: 10.1006/brcg.1995.1269. [DOI] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: Gender-different neural networks as substrate of performance. Nature Neuroscience. 2000;3:404–408. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- Guiso L, Monte F, Sapienza P, Zingales L. Culture, gender, and math. Science. 2008;320:1164–1165. doi: 10.1126/science.1154094. [DOI] [PubMed] [Google Scholar]
- Hakstian AR, Cattell RB. Manual for the Comprehensive Ability Battery. Champaign: Institute for Personality and Ability Testing; 1976. [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Experimental Brain Research. 2006;169:1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex differences in cognitive abilities. 4th ed. New York: Psychology Press; 2012. [Google Scholar]
- Hampson E. Sex differences in the brain: From genes to behavior. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman J, Young E, editors. Endocrine contributions to sex differences in visuospatial perception and cognition. New York: Oxford University Press; 2007. [Google Scholar]
- Hampson E, Rovet JF, Altmann D. Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Developmental Neuropsychology. 1998;14:299–320. [Google Scholar]
- Heil M, Kavsek M, Rolke B, Beste C, Jansen P. Mental rotation in female fraternal twins: Evidence for intra-uterine hormone transfer? Biological Psychology. 2011:90–93. doi: 10.1016/j.biopsycho.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Herman RA, Wallen K. Cognitive performance in rhesus monkeys varies by sex and prenatal androgen exposure. Hormones and Behavior. 2007;51:496–507. doi: 10.1016/j.yhbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier DB, Crowley WF. Spatial ability in androgen-deficient men. New England Journal of Medicine. 1982;306:1202–1205. doi: 10.1056/NEJM198205203062003. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends in Cognitive Sciences. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Ahmed F, Hughes IA. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Archives of Sexual Behavior. 2003;32:93–101. doi: 10.1023/a:1022492106974. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: Targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Thomsen T, Ersland L. Sex differences in visuo-spatial processing: An fMRI study of mental rotation. Neuropsychologia. 2006;44:1575–1583. doi: 10.1016/j.neuropsychologia.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J, Pichardo M, Gautier T, Voyer D, Bryden MP. Cognitive abilities in androgen-insensitive subjects: Comparison with control males and females from the same kindred. Clinical Endocrinology. 1991;34:341–347. doi: 10.1111/j.1365-2265.1991.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Berenbaum SA. Sex differences in spatial ability in children. Behavior Genetics. 1991;21:383–396. doi: 10.1007/BF01065974. [DOI] [PubMed] [Google Scholar]
- Lawton CA. Gender, spatial abilities, and wayfinding. In: Chrisler JC, McCreary DR, editors. Handbook of gender research in psychology, Vol 1: Gender research in general and experimental psychology. New York: Springer Science + Business Media; 2010. pp. 317–341. [Google Scholar]
- Levine SC, Vasilyeva M, Lourenco SF, Newcombe NS, Huttenlocher J. Socioeconomic status modifies the sex difference in spatial skill. Psychological Science. 2005;16:841–845. doi: 10.1111/j.1467-9280.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- Liben LS. Psychology meets geography: Exploring the gender gap on the national geography bee. Psychological Science Agenda. 1995;8:8–9. [Google Scholar]
- Lindberg SM, Hyde JS, Petersen JL, Linn MC. New trends in gender and mathematics performance: A meta-analysis. Psychological Bulletin. 2010;136:1123–1135. doi: 10.1037/a0021276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa R. Do sex differences define gender-related individual differences within the sexes? Evidence from three studies. Personality and Social Psychology Bulletin. 1995;21:349–355. [Google Scholar]
- Lippa R. Gender-related traits of heterosexual and homosexual men and women. Archives of Sexual Behavior. 2002;31:83–98. doi: 10.1023/a:1014035302843. [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann E. Hormone therapy and cognitive function. Human Reproduction Update. 2009;15:667–681. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, Wisniewski AB. Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hormone Research. 2006;65:142–150. doi: 10.1159/000091793. [DOI] [PubMed] [Google Scholar]
- Masica DN, Money J, Ehrhardt AA, Lewis VG. IQ fetal sex hormones and cognitive patterns: Studies in the testicular feminizing syndrome of androgen insensitivity. John Hopkins Medical Journal. 1969;124:34–43. [PubMed] [Google Scholar]
- Moore DS, Johnson SP. Mental rotation in human infants: A sex difference. Psychological Science. 2008;19:1063–1066. doi: 10.1111/j.1467-9280.2008.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes--a meta-analysis. Journal of Pediatric Psychology. 2009;34:271–282. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- Newcombe N, Bandura MM, Taylor DG. Sex differences in spatial ability and spatial activities. Sex Roles. 1983;9:377–386. [Google Scholar]
- Newcombe N, Dubas JS. A longitudinal study of predictors of spatial ability in adolescent females. Child Development. 1992;63:37–46. [PubMed] [Google Scholar]
- Pang S, Levine LS, Cederqvist LL, Fuentes M, Riccardi VM, Holcombe JH, et al. Amniotic fluid concentrations of delta 5 and delta 4 steroids in fetuses with congenital adrenal hyperplasia due to 21 hydroxylase deficiency and in anencephalic fetuses. Journal of Clinical Endocrinology and Metabolism. 1980;51:223–229. doi: 10.1210/jcem-51-2-223. [DOI] [PubMed] [Google Scholar]
- Pang S, Levine LS, Chow DM, Faiman C, New MI. Serum androgen concentrations in neonates and young infants with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clinical Endocrinology. 1979;11:575–584. doi: 10.1111/j.1365-2265.1979.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Prader A. Der genitalbefund beim pseudohermaphroditismus femininus des kongenitalen adrenogenitalen syndroms. Helvetica Paediatrica Acta. 1954;3:231–248. [PubMed] [Google Scholar]
- Puts DA, Cardenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Hormones and Behavior. 2010;58:282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, Breedlove NJ. Spatial ability and prenatal androgens: Meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Archives of Sexual Behavior. 2008;37:100–111. doi: 10.1007/s10508-007-9271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PC, Liben LS. A sex difference in mental rotation in young infants. Psychological Science. 2008;19:1067–1070. doi: 10.1111/j.1467-9280.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman II, Bouchard TJ. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Developmental Psychology. 1986;22:191–198. [Google Scholar]
- Ross J, Roeltgen D, Zinn A. Cognition and the sex chromosomes: Studies in Turner Syndrome. Hormone Research. 2006;65:47–56. doi: 10.1159/000090698. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neuroscience and Biobehavioral Reviews. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Sattler JM, Ryan JJ. Assessment with the WAIS-IV. San Diego: Jerome, M. Sattler, Publisher, Inc.; 2009. [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ. Where in the world am I? Sex and handedness differences in knowledge of geography. Perceptual and Motor Skills. 1996;82:1379–1385. doi: 10.2466/pms.1996.82.3c.1379. [DOI] [PubMed] [Google Scholar]
- Speiser PW. Congenital adrenal hyperplasia. Endocrinology and Metabolism Clinics of North America. 2001;30:i–244. doi: 10.1016/s0889-8529(08)70018-5. [DOI] [PubMed] [Google Scholar]
- Thurstone T. Primary Mental Abilities Tests. Chicago: Science Research Associates; 1963. [Google Scholar]
- Valla JM, Ceci SJ. Can sex differences in science be tied to the long reach of prenatal hormones? Brain organization theory, digit ratio (2D/4D), and sex differences in preferences and cognition. Perspectives on Psychological Science. 2011;6:134–146. doi: 10.1177/174569161140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations: A group test of three-dimensional spatial visualization. Perceptual and Motor Skills. 1978;47:599–605. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D, Nolan CL, Voyer S. The relation between experience and spatial performance in men and women. Sex Roles. 2000;43:891–915. [Google Scholar]
- Voyer D, Postma A, Brake B, Imperato-McGinley J. Gender differences in object location memory: A meta-analysis. Psychonomic Bulletin and Review. 2007;14:23–38. doi: 10.3758/bf03194024. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E, Kaprio J, Kremen WS, Hokkanen L, Viken RJ, Tuulio-Henriksson A, et al. Having a male co-twin masculinizes mental rotation performance in females. Psychological Science. 2010;21:1069–1071. doi: 10.1177/0956797610376075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Siedentopf CM, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, et al. Sex differences in brain activation pattern during a visuospatial cognitive task: A functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters. 2003;344:169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Wraga M, Helt M, Jacobs E, Sullivan K. Neural basis of stereotype-induced shifts in women's mental rotation performance. Social Cognitive and Affective Neuroscience. 2007;2:12–19. doi: 10.1093/scan/nsl041. [DOI] [PMC free article] [PubMed] [Google Scholar]