Abstract
A major feature of focal hand dystonia (FHD) pathophysiology is the loss of inhibition. One inhibitory process, surround inhibition, for which the cortical mechanisms are still unknown, is abnormal in FHD. Since the ventral premotor cortex (PMv) plays a key role in the sensorimotor processing involved in shaping finger movements and has many projections onto the primary motor cortex (M1), we hypothesized that the PMv-M1 connections might play a role in surround inhibition. A paired-pulse transcranial magnetic stimulation (TMS) paradigm was used in order to evaluate and compare the PMv-M1 interactions during different phases (rest, preparation and execution) of an index finger movement in FHD patients and controls. A sub-threshold conditioning pulse (80% resting motor threshold) was applied to the PMv 6 ms before M1 stimulation. Right abductor pollicis brevis, a surround muscle, was the target muscle. In healthy controls, the results show that PMv stimulation induced an ipsilateral ventral premotor-motor inhibition at rest. This cortico-cortical interaction changed into an early facilitation (100 ms before movement onset) and turned back to inhibition 50 ms later. In FHD patients, this PMv-M1 interaction and its modulation were absent. Our results show that although the ipsilateral ventral premotor-motor inhibition does not play a key role in the genesis of surround inhibition, PMv has a dynamic influence on M1 excitability during the early steps of motor execution. The impaired cortico-cortical interactions observed in FHD patients might contribute, at least in part, to the abnormal motor command.
Keywords: TMS, Motor Cortex, ventral premotor cortex, surround inhibition, movement disorders
Introduction
A major feature in the pathophysiology of focal hand dystonia (FHD) is the lack of inhibition at the cortical, subcortical, and spinal levels likely due to GABAergic dysfunction (Hallett, 2010). Impairment of intracortical circuits has been demonstrated in FHD, and this may be either an intrinsic abnormality or secondary to striatal dysfunction (Peller et al., 2006). In particular, surround inhibition (SI), which represents the suppression of excitability in the area surrounding an activated neural network in order to focus and select neuronal responses (Sohn & Hallett, 2004b), is impaired in FHD (Sohn & Hallett, 2004a). The lack of SI might explain, at least in part, the excessive antagonist and accessory muscle activation in FHD patients. (van der Kamp et al., 1989).
The mechanisms responsible for SI are still unknown. No intracortical inhibitory circuit located in or projecting to the primary motor cortex (M1) has been identified as a source of SI (Beck & Hallett, 2011). Since it starts during movement preparation, SI could result from connections between M1 and premotor areas involved in hand motor control. Accordingly, Beck and colleagues investigated the potential role of the dorsal premotor cortex in the generation of SI. Indeed, dorsal premotor cortex plays an important role in movement selection (Rushworth et al., 2003) and some imaging studies have shown an impairment of dorsal premotor cortex activation in right-sided focal hand dystonia (Ceballos-Baumann et al., 1997a; Ceballos-Baumann & Brooks, 1998; Ibanez et al., 1999). However, the results demonstrated that the ipsilateral dorsal premotor-motor inhibition was not involved in the genesis of SI (Beck et al., 2009a).
The ventral premotor cortex (PMv) plays a key role in fine finger and hand movements. PMv neurons specialize in sensorimotor transformations and are actively involved in hand posture during grasping. PMv is also responsible for fingertips positions and elaborates the appropriate pattern of activation of intrinsic hand muscles (Davare et al., 2006). Positron emission tomography (PET) studies have shown abnormal activation pattern in PMv and PMd in FHD (Ceballos-Baumann et al., 1997b; Ibanez et al., 1999). These studies showed a dysfunction of premotor cortical network as well as a dysfunction of premotor cortex-basal ganglia circuits. Using TMS, it has been demonstrated that PMv has an inhibitory influence on M1 at rest in healthy subjects (Davare et al., 2008). This PMv-M1 interaction is muscle specific and modulated during different phases of grasp preparation and execution (Davare et al., 2008).
The aims of this study were to evaluate the PMv-M1 interactions during different phases of an index finger movement using a paired-pulse TMS paradigm, and to compare these interactions between FHD patients and healthy volunteers. We hypothesized that the ipsilateral ventral premotor-motor inhibition would be involved in the physiology of surround inhibition and impaired in FHD.
Materials and Methods
Population
Eighteen patients with FHD (mean age 57.9 ± 6.4 years, 14 male) and 18 healthy volunteers (mean age 55.7 ± 11.4 years, 11 male) participated in the study (see Table 1). FHD patients had unilateral, right hand, symptoms. One patient was left-handed but had symptoms in his right hand (musician's dystonia, guitar player). Participants had no history of psychiatric disorders, neurosurgery or metal or electronic implants. Most patients had been treated with local injections of botulinum toxin type A in the affected hand and forearm muscles. For each patient, the last injection had been given at least 3 months prior to the recordings (table 1).
Table 1. Patients' demographics.
| Gender | Age | Type of cramp | Duration (years) | Botulinum toxin/ last injection |
|---|---|---|---|---|
| M | 54 | MC | 5 | Yes / 3 months |
| F | 57 | WC | 23 | No |
| M | 62 | WC | 11 | Yes / 18 months |
| M | 61 | WC | 15 | No |
| M | 57 | MC | 4 | Yes / 4 years |
| F | 54 | WC | 11 | No |
| F | 63 | WC | 2 | No |
| M | 59 | MC | 7 | Yes / 6 months |
| M | 60 | WC | 19 | Yes / 4 months |
| M | 57 | WC | 16 | Yes / 3 years |
| M | 58 | MC | 14 | Yes / 2 years |
| M | 58 | MC | 8 | No |
| M | 56 | WC | 9 | Yes/ 3 months |
| F | 48 | WC | 5 | No |
| M | 75 | WC | 18 | Yes / 2 years |
| M | 47 | MC | 16 | Yes / 3 months |
| M | 51 | WC | 15 | Yes/ 6 months |
| M | 65 | WC | 41 | No |
MC: Musician cramp, WC: writer cramp.
The study was approved by the Institutional Review Board (IRB) of the National Institute of Neurological Disorders and Stroke (NINDS). All participants gave their informed oral and written consent before the experiments in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and NINDS guidelines.
Experimental procedures
Participants were seated in a comfortable armchair with both arms resting on a pillow placed on their laps. Their right hand was supported on a small board, to which a force transducer was attached (Strain Measurement Devices, Inc, Meriden, CT, model S215 load cell). They rested their palm on the board, with the tip of their index finger on the force transducer.
- EMG
Electromyographic (EMG) activity of the right first dorsal interosseus (FDI) and abductor pollicis brevis (APB) was recorded in a belly-tendon montage using Ag-AgCl surface electrodes. Impedances were kept below 5 kΩ. EMG signals were collected using a Viking IV EMG machine (Nicolet Biomedical, Madison, Wisconsin), bandpass-filtered at 20-2000Hz. The amplified analog outputs from the Viking were digitized at 5 kHz using Labview software (National Instruments, Austin, Texas), and stored on a PC for off-line analysis.
- Motor task
The task, similar to one previously published (Beck et al., 2008; Beck et al., 2009a; Beck et al., 2009b; Beck et al., 2009c; Beck & Hallett, 2010), was a simple acoustic reaction time task. Subjects had to perform an index finger flexion in order to press on the force transducer in response to a tone. The acoustic signal lasted 200 ms. In this task, FDI participates as a synergist rather than as prime mover, but it has been shown that the modulation of the cortical excitability of synergists is similar to prime movers (Sohn & Hallett, 2004b).
In response to the tone, subjects had to press the transducer as fast as possible, using only 10% of their maximum voluntary contraction (MVC). MVC was defined as the averaged strength obtained after three trials during which subjects used their maximal strength to push on the transducer device. They were told to use only the strength of their index finger and not to contract other forearm and arm muscles. The force level was then individually adjusted to 10% of the MVC and displayed online as a target line on an oscilloscope placed on a table in front of them. The output of the force transducer was also displayed on the oscilloscope as direct online feedback. During the task, subjects had to maintain their contraction for approximately 1 s. Subjects practiced the task at the beginning of the experiment to attain a consistent motor performance.
Once the subjects showed consistent motor performance, four different phases of the movement preparation were assessed: rest, 100 ms before EMG onset in FDI (T100), 50 ms before EMG onset (T50) and at the time of the first peak of EMG in FDI (Tpeak). EMG onset and first peak were measured individually in an average of FDI EMG in ten consecutive trials (Fig. 1).
Figure 1. Experimental set-up.
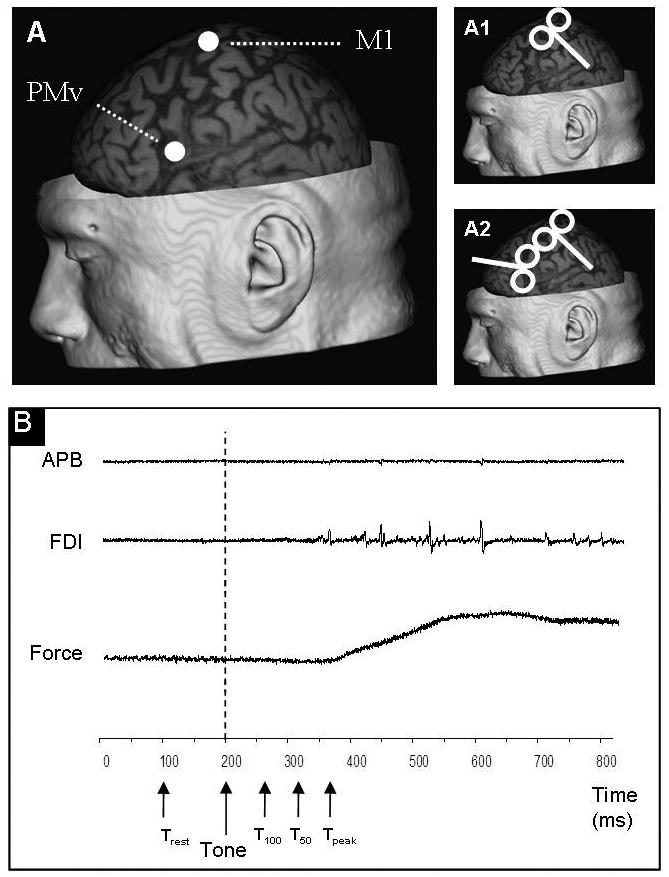
A shows an example of localization of M1 and PMv in one participant. M1 was defined as the stimulating point over which the greatest MEPAPB could be evoked. PMv was defined using a neuronagivation system (Brainsight, Rogue Research Inc., Rogue Resolutions Ltd, Cardiff, UK), and placed over the caudal portion of the pars opercularis of the inferior frontal gyrus. B: Acoustic, force and EMG signals are displayed in C. Single (part 1) or paired-pulse (part 2) TMS was applied at four different timing (rest, T100, T50 and Tpeak). Subjects had to respond as fast as possible to the tone by pressing the button, using 10% of their strength. APB muscle stayed at rest during the entire task. Subjects were maintaining the movement during approximately 1s and then came back to a relaxed position.
- TMS
Magnetic stimulation was delivered using two custom-made figure-of-8 coils with an inner loop diameter of 35 mm connected to two high-power Magstim 200 stimulators (Magstim Company Ltd, Whitland, Dyfed, UK). Stimulations were applied over the point that evoked the largest motor evoked potential (MEP) in the contralateral APB (“motor hotspot”). MEPs were measured over the APB and the FDI, but only one motor hotspot was tested (APB hotspot). MEP size was determined by averaging peak-to-peak amplitudes. The coil used to stimulate the motor hotspot was held tangentially to the scalp, at a 45° angle from the anteroposterior axis and with the handle pointing posterolaterally (Fig. 1, A1). Resting motor threshold of the APB (RMTAPB) was measured for each subject and defined as the lowest intensity that induced a 50 μV peak-to peak amplitude MEP in at least 5 out of 10 trials. The second coil was positioned over the left PMv, with the handle pointing forward to induce a current directed anterioposteriorly (Fig. 1, A2). Neuronavigation (Brainsight, Rogue Research Inc., Rogue Resolutions Ltd, Cardiff, UK) was used for precise positioning of the coil over the PMv. Magnetic Resonance Imaging (MRI) data specific to each participant were used to ensure correct placement of the coil, which was placed over the caudal portion of the pars opercularis of the inferior frontal gyrus (Davare et al., 2006). Each individual MRI was normalized, a posteriori, onto the Montreal Neurological Institute (MNI) brain template using the same software. PMv stimulation coordinates were then expressed with respect to the MNI standard space. The mean normalized MNI coordinates of the PMv stimulation sites were (-59.0±2.5, -2.1±9.8, 7.6±4.9) in controls and (-60.4±3.8, -1.5±8.0, 9.5±4.0) in FHD (x, y, z; mean ± SD in mm). These two mean coordinates belong to BA6 according to the Talairach atlas (see Fig.1). This confirmed that the conditioning coil was targeting the PMv in both groups. The positions of the two coils were marked on a tight fitting cap to ensure proper coil placement throughout the experiment.
The experiment was conducted in two parts (part 1 and part 2). Part 1 aimed at assessing SI. Single TMS pulses were delivered over the motor hotspot at an intensity of 140% RMTAPB in four different conditions, in a random order: at rest, T100, T50, Tpeak and a condition in which no stimulation was given. In order to be able to randomize the order of the different phases, rest stimulation was given 100 ms before the acoustic tone (Fig. 1, B). Two blocks of 45 stimuli were recorded, resulting in 18 MEPs for each condition.
Part 2 consisted of a paired-pulse paradigm designed to assess the effect of a conditioning stimulation over PMv on the excitability of M1. The conditioning stimulus was applied at 80% RMTAPB at an interval interstimulus (ISI) of 6 ms (Davare et al., 2008). The test stimulus was applied over the motor hotspot at an intensity set to evoke an MEP of 1mV over the APB, at rest. Due to spatial interference of the two coils, the conditioning coil was placed directly on the skull, while the test pulse coil over the motor hotspot was slightly elevated. Four separate paired-pulse blocks were conducted for each subject: at rest, with the test pulse stimulating M1 at T100, with the test pulse at T50 and with the test pulse at Tpeak. Thirty stimuli were applied for each of the 4 blocks (15 conditioned and 15 un-conditioned stimuli).
During TMS recording, EMG from ABP was monitored. APB is not involved in the task and therefore remained relaxed throughout the entire experiment. Trials in which there was background EMG > 0.02mV in APB, assessed as root mean square over 50 ms prior to MEP onset in each phase, were rejected.
Statistical analysis
RMTAPB, reaction times (RTs) and MEP sizes at rest in APB and FDI were compared between groups using an independent samples t-test. In each group, rest MEPAPB and rest MEPFDI were compared using an independent samples t-test. The x, y and z coordinates of the PMv location were compared, between groups, using a Mann-Whitney test.
Statistical analyses of MEP amplitudes obtained in part 1 and part 2 were done using a repeated analysis of variance. Since the data were not Gaussian, our analyses used Conover's free distribution method, a non-parametric ANOVA based on ranks (Conover & Iman, 1982). Two factors were used: GROUP (2 levels: FHD and controls) and PHASE (4 levels: rest, T100, T50, Tpeak). If a main effect was observed at the 0.05 level, contrasts were calculated. If a significant interaction was found between the two factors, Mann-Whitney tests were performed to compare, between groups, MEP sizes for each phase. In part 2, the interaction between PMv and M1 during the different phases of motor preparation was expressed as a ratio between conditioned and test MEPs (unconditioned), in percent: MEPcond / MEPtest * 100. This ratio was used in the Conover analysis. If a significant interaction was found between the two factors, a non-parametric one-way analysis of variance (Friedman test) was performed to attest for significant differences between phases, in each group. If a significant main effect was found, Mann-Whitney tests were performed to compare MEP sizes for each phase between the two groups.
Independently, and for each group, the effect of the premotor-motor interaction on MEPs was assessed using a non-parametric Wilcoxon test comparing test MEPs to conditioned MEPs, for each phase. This analysis was made to assess whether premotor stimulation had an inhibitory or facilitatory influence on the MEPs, in each group and for each phase.
In order to define whether musician cramp (MC) and writer cramp (WC) patients displayed the same results, we performed a sub-group analysis. First, Wilcoxon tests were used in each group to detect any significant effect of PMv stimulation on the test MEP amplitude, for each phase. Then, in order to determine whether those two subgroups behaved differently, a Conover analysis was performed on the PMv-M1 interactions results. Our subgroups were made of 6 MC and 12 WC. In order to balance the power of our test, we compared the 6 MC with 6 WC who were age and gender matched to the patients.
Lastly, and in order to test the relationship between surround inhibition and PMv-M1 interaction, we performed a non parametric Spearman correlation analysis between the amount of SI and the PMv-M1 interactions at T1, T2 and T3, in our two populations.
Statistical analyses were performed using PASW Statistics 18.0 (SPSS inc., Chicago, USA).
Results
All the data are displayed in Table 2 and 3.
Table 2. RTs, RMTs and baseline MEPs amplitude.
| RT (mean±SD) | RMT (mean±SD) | MEP rest (mean± SD) | ||
|---|---|---|---|---|
| APB | FDI | |||
| HV | 161 ± 36 ms | 51.4 ± 8.5 | 2.5 ± 1.5 mV | 3.9 ± 2.1 mV |
| FHD | 168 ± 32 ms | 48.6 ± 7.6 | 2.2 ± 1.3 mV | 5.0 ± 3.3 mV |
HV: Healthy Volunteer, FHD: Focal Hand Dystonia; RT: Reaction Time; RMT: Resting Motor Threshold (expressed in percentage of stimulator output), MEP: Motor Evoked Potential.
Table 3. Surround inhibition and ventral premotor-motor interactions.
| Single pulse: MEP size in mV (median [range]) | Paired-pulse: percentage of test MEP (median [range]) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | T100 | T50 | Tpeak | Rest | T100 | T50 | Tpeak | ||
| APB | HV | 1.88 [0.69-5.42] | 1.82 [0.69-5.07] | 1.78 [0.73-5.28] | 1.59 [0.61-5.4] | 89.8 [35.3-115.4] | 112.6 [83.9-168.8] | 91.7 [25.4-109.2] | 97.7 [69.9-143.5] |
| FHD | 1.66 [0.65-4.63] | 1.84 [0.58-4.86] | 1.88 [0.73-4.7] | 1.94 [0.55-6.1] | 92.8 [75.6-120.7] | 100.4 [72-117] | 96.6 [71.8-135.3] | 96.8 [88-139.9] | |
|
| |||||||||
| FDI | HV | 3,47 [1,61-10,41] | 3,61 [1,57-9,98] | 3,82 [1,76-10,45] | 4,65 [2,06-8,42] | 99,47 [85,25-129,37] | 97,62 [77,66-123,96] | 98,93 [88,07-138,61] | 101,39 [84,99-120,62] |
| FHD | 4,13 [0,38-10,79] | 3,91 [0,38-10,7] | 5,1 [0,33-13,61] | 6,21 [0,56-14,11] | 108,78 [77,47-132,45] | 97,56 [88,56-102,31] | 107,47 [88,55-118,94] | 99,05 [76,26-111,56] | |
Table 3 shows MEPAPB and MEPFDI amplitudes during single and paired-pulse stimulations at the different timings (rest, T100, T50 and Tpeak). HV: Healthy Volunteer, FHD: Focal Hand Dystonia. A significant decrease in MEPAPB amplitude in HV is observed, during single pulses TMS, demonstrating a significant surround inhibition. A central excitation is demonstrated in both groups by a significant increase of MEPFDI amplitude No surround inhibition is found in patients. Paired-pulse TMS induced a significant premotor-motor inhibition at rest and at T50 in HV and a significant facilitation at T100. No such influence is observed in patients. * P<0.05.
RTs did not differ between groups (p=0.535). RMTAPB (table 2) was not significantly different (p=0.31) between FHD patients and controls (respectively 48.6 ± 7.6 and 51.4 ± 8.5 % of stimulator output). Rest MEPs over APB and FDI (table 2) were not significantly different between groups (p= 0.5 for APB and p= 0.25 for FDI). However, in each group, MEPAPB were smaller than MEPFDI (p=0.022 in controls and p=0.002 in patients). The x, y and z coordinates did not differ between groups (p> 0.05).
The Conover analysis of single pulse TMS on MEPAPB (part 1, Fig. 2, A) showed a significant GROUP effect (p=0.002), a significant GROUPxPHASE interaction (p=0.003), and no significant PHASE effect (p=0.974), probably due to the significant interaction. Mann-Whitney tests demonstrated significant group differences at T50 (p=0.007) and Tpeak (p=0.001). Indeed, Wilcoxon tests showed that MEPAPB were significantly inhibited at T50 (p=0.035) and Tpeak (p=0.006) in controls only, reflecting significant surround inhibition (table 3).
Figure 2. Effect of movement preparation and movement onset on the amplitude of MEPAPB and MEPFDI.
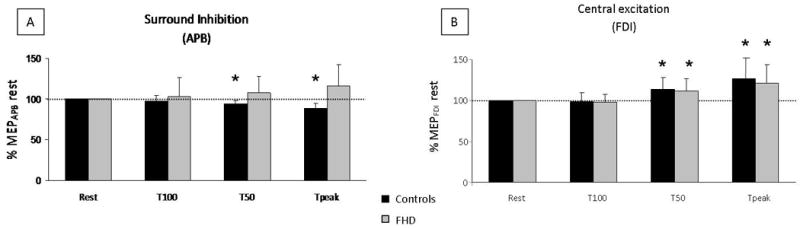
MEPs are expressed as percentage of rest MEPs in the four different conditions (rest, T100, T50, Tpeak), in controls (black) and patients (grey), during single-pulse TMS. A: A significant decrease of MEPAPB was observed in controls at T50 and Tpeak (surround inhibition) while patients exhibited no significant surround inhibition. * p< 0.05. B: Significant MEPFDI amplitude increase was found in both groups at T50 and Tpeak (central excitation).
Regarding MEPFDI sizes (evoked by stimulation of the APB hotspot), the Conover analysis demonstrated a significant PHASE effect (p<0.001) (Fig. 2, B). There were no significant GROUP or GROUPxPHASE interactions (p=0.427 and p=0.888, respectively). Contrast analyses revealed that in both groups MEP amplitudes at T50 and Tpeak were significantly different from the other conditions (p< 0.001 in each case). Wilcoxon tests showed a significant increase in MEPFDI at T50 in controls (p=0.003) and in patients (p=0.004). This increase of MEP size was maximal at movement onset (p=0.001 in both groups) and reflected a process that could be qualified as central excitation.
Regarding the premotor-motor interactions (Fig. 3, table 3), Conover's analysis of MEPAPB indicated a significant PHASE effect (p=0.006), a significant PHASExGROUP interaction (p=0.029) and no significant GROUP effect (p=0.615). Friedman's test indicated a significant main effect of PHASE in controls (p=0.001) and no significant main effect in FHD patients (p=0.737). The Mann-Whitney tests showed significant differences between the two groups at T100 (p=0.01), and T50 (p=0.04). At T100, PMv stimulation significantly enhanced MEP sizes in controls (p=0.025) but not in patients. At T50, a significant premotor-motor inhibition was observed in controls (p=0.001) and not in patients. In the patient group, no significant influence of PMv stimulation on MEPAPB size was found either at rest, or during the different phases of motor execution. A significant premotor-motor inhibition was observed in controls at rest (p=0.011). Although this inhibition was absent in patients, there was no significant difference between the 2 groups at rest (p=0.48). Analyses of MEPFDI revealed an absence of modulation of MEPFDI amplitude following PMv stimulation, either at rest or during movement, in both groups. The Conover analyses showed no PHASE effect (p=0.086), no GROUP effect (p=0.853) and no GROUP × PHASE interaction (p=0.645).
Figure 3. Premotor-motor interactions.
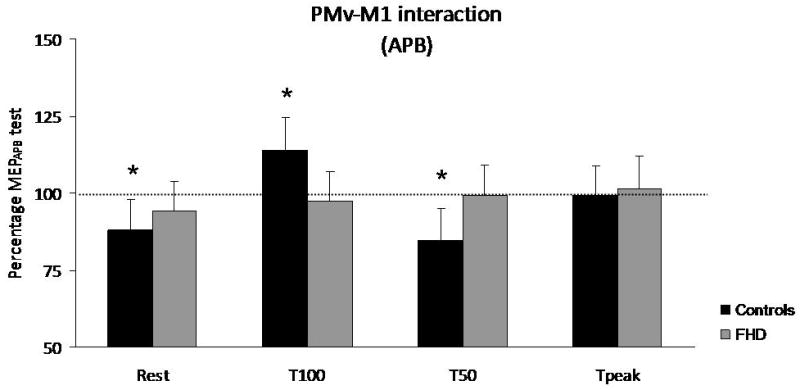
Premotor-motor interactions are expressed as percentage of MEPAPB test in controls (black) and patients (grey) during paired-pulse TMS. No influence of PMv over M1 was constantly found in patients. Controls showed a significant premotor-motor inhibition at rest and at T50, while a significant facilitation was observed at T100. * p<0.05.
The subgroup analyses showed that for the two subgroups, PMv exerted no significant influence over M1 (similar to the whole group analysis). The Conover test indicated that there was no significant differences between those two sub-groups of patients (p=1.00). Correlation analyses did not show any signification association between the amount of SI and the PMv-M1 interactions, suggesting an independence of the two phenomena.
Discussion
Our results showed that PMv exerted a modulatory influence on M1 at rest and during movement preparation and that this influence was absent in patients. We confirmed that PMv inhibited M1 at rest in controls and that this inhibition was muscle specific. Moreover, contrary to our hypothesis, we showed that this inhibition was not enhanced during movement initiation, indicating that the ipsilateral ventral premotor-motor inhibition does not play a key role in SI in normal subjects.
Surround inhibition – Central excitation
In accordance with the literature, we showed that healthy volunteers presented with surround inhibition (regarding the APB muscle) before and at movement onset and that this surround inhibition was absent in patients (Sohn & Hallett, 2004a, 2004b; Beck et al., 2008, 2009a, 2009b, 2009c). In parallel to this inhibition, the excitability of the synergist muscle cortical representation was increased before and at movement onset in controls as well as in FHD patients without significant differences between the two groups, as previously reported (Beck et al., 2008). Indeed, we showed that MEPFDI were significantly enhanced at T50 and Tpeak. This preserved central excitation, in line with the literature, shows that the corticospinal excitability of the synergist muscle is not impaired in FHD patients. Together with this finding, we did not observe any differences in RTs between patients and controls (Stinear & Byblow, 2005; Beck et al., 2008, 2009a, 2009b). Although RTs as well as the central excitation were not impaired in patients, it is noteworthy that some EEG studies have demonstrated an abnormal motor preparation in FHD patients. Abnormally reduced event-related desynchronization (ERD) or Bereitschaftspotential (BP) have been reported in FHD patients, preceding voluntary, self-paced movements (Deuschl et al., 1995; Ikeda et al., 1996; Yazawa et al., 1999; Toro et al., 2000). ERD and BP reflect the activation of premotor and motor areas involved in movement preparation and execution. Abnormal ERD or BP suggests an impairment of premotor and/or motor cortex activation during self-paced movement preparation. These complementary EEG-TMS data suggest that although the excitability of the synergist muscle representation over M1 is preserved in FHD patients, the premotor-motor interactions preceding voluntary movement are impaired.
Impaired cortico-cortical interactions in FHD
Our results showed a lack of RT, RMT and rest MEP differences between patients and controls. This implies that any group differences observed in this study could not be explained by a change of motor threshold or a different RT in FHD patients. In the current study, we confirmed previous reports indicating that PMv has an inhibitory influence on M1 at rest in healthy subjects (Davare et al., 2008). This ipsilateral ventral premotor-motor inhibition might depend on GABA-a interneurons. Indeed, it has previously been shown in monkeys that injection of bicuculline (a GABA-a antagonist) in the premotor cortex (dorsal and ventral) provoked co-contractions of agonists and antagonists (Matsumura et al., 1991). The effects provoked by bicuculline injection in the premotor cortex were not as severe as the ones observed after M1 injection, but they shared the same time-course. Kurata and Hoffmann (1994) confirmed the GABA-a dependency of PMv neurons by injecting Muscimol (a GABA-a agonist) in the PMv. They observed a decrease of movement (wrist flexion or extension) amplitude and velocity. Although PMv has some direct projections to the spinal cord (Dum & Strick, 1991; He et al., 1993; Luppino et al., 1999; Dum & Strick, 2005) it has strong output onto the hand representation of M1 (Cerri et al., 2003; Shimazu et al., 2004). Shimazu and colleagues (2004) showed that, in monkeys, stimulation of F5 (the equivalent of the human PMv) can facilitate the corticospinal volley from M1 and that this effect can be abolished by a reversible inactivation of M1. The interstimulus interval of 6 ms between the CS and the TS in our experiment suggests that the cortico-cortical pathway between PMv and M1 might be a direct oligosynaptic connection (Shimazu et al, 2004).
The lack of ipsilateral ventral premotor-motor inhibition at rest in FHD patients (Fig. 3) is coherent with the pathophysiology of the disease and more particularly with the hypothesis of a dysfunction in GABA-a transmission. Indeed, many studies conducted on dystonic animal models have demonstrated alterations in GABA levels (Messer & Gordon, 1979; Loscher & Horstermann, 1992) or in GABA receptors density and affinity in different brain regions (Beales et al., 1990; Nobrega et al., 1995; Pratt et al., 1995; Gilbert et al., 2006; Alterman & Snyder, 2007). In FHD patients, a magnetic resonance spectroscopy study showed a decreased GABA level in sensorimotor cortex and lentiform nuclei contralateral to the affected hand (Levy & Hallett, 2002). This result however could not be reproduced in a larger population (Herath et al., 2010). Recently, a PET study conducted on patients presenting with primary dystonia showed a significant reduction in GABA-a receptor expression and affinity in premotor and primary motor cortex, primary and secondary somatosensory cortex and cingulate gyrus (Garibotto et al., 2011). The involvement of the PMv in FHD has also been suggested by several neuroimaging studies. Positron Emission Topography (PET) studies have shown abnormal functioning of PMv either toward an increase of activity (Ceballos-Baumann et al., 1997b) or toward a decrease of activity (Ibanez et al., 1999). These two results differed most probably because of the different patient selection and different tasks involved. Ibanez and colleagues studied cerebral activity during different tasks and showed a decreased activity in the left PMv during writing (1999). This result and the impaired functional interaction between PMv and M1 in our study suggest that PMv plays an important role in the generation of the abnormal motor command in FHD.
Abnormal balance between excitation and inhibition
Our results show that the ipsilateral ventral premotor-motor inhibition was modulated during the different phases of motor execution in healthy subjects. During the early stages of movement preparation, the inhibition turned into facilitation. This result is concordant with previous studies showing that the premotor-motor interactions differ according to the movements and muscles involved (Ceballos-Baumann et al., 1997b; Ibanez et al., 1999). One could hypothesize that this early premotor-motor facilitation reflects a general facilitatory influence of PMv on M1 during the early stages of motor execution. First, the excitability of the muscles located in the movement area would increase, then, along with the adjustment of the motor plan, the premotor-motor facilitation would turn into an inhibition if the muscles are not to be involved in the action. Indeed, the inhibition was restored 50 ms prior to movement and was abolished at the onset of movement. These findings suggest that ipsilateral ventral premotor-motor inhibition may help select the movement. In contrast, the absence of increased inhibition at movement onset, when SI is at its maximum (Sohn and Hallett, 2004a, 2004b; Beck et al, 2008), indicates that this ipsilateral ventral premotor-motor inhibition is not the main generator of SI. We can thus hypothesize that the premotor-motor inhibition might be complementary and different from surround inhibition. This might constitute an early step in movement selection since it starts and evolves before movement onset and disappears before the start of the movement.
Our results show a lack of premotor-motor inhibition and premotor-motor facilitation in FHD patients. In patients, PMv had no significant influence on M1 either at rest or during the early steps of motor execution. This shows that excitatory cortico-cortical connections are also impaired in FHD, which is consistent with a previous finding showing an abnormal facilitation instead of long afferent inhibition in FHD following median nerve stimulation (Abbruzzese et al., 2001). Although the major cortical and subcortical neurotransmission deficiency in FHD involves GABA-network, these results illustrate that excitatory circuits might also be impaired in patients and that the balance between inhibition and excitation is abnormal. The lack of premotor-motor inhibition suggests that the abnormal cortical hyperexcitability observed in FHD patients affects also the early steps of movement preparation, and not solely surround inhibition. It has also been demonstrated that the premotor-motor interactions are very sensitive to ISIs and stimulus intensity (Civardi et al., 2001; Davare et al., 2008, 2009). It is thus possible that the PMv-M1 interactions might be shifted towards different components (latencies, activation threshold) in FHD patients. Since our study focused on investigating the role of the premotor-motor interactions in surround inhibition at various phases of movement, the experiment even with one ISI took about 2 hours. Hence, we could not test more ISIs. We decided to test the ISI that exerted the most efficient premotor-motor influence (6 ms), as shown by Davare and colleagues (2008). In order to fully define the importance of the impairment of the premotor-motor interactions in FHD patients, more ISIs should be tested in future studies.
Looking at the synergistic muscle, the current study shows that MEP amplitudes in FDI are not modulated by stimulation of PMv. This is most probably due to the fact that PMv-M1 interactions are muscle specific (Davare et al., 2009) and are extremely sensitive to the parameters of stimulation. Indeed, small variations of the CS intensity influence greatly the outcome (Civardi et al., 2001). Since the stimulation intensities used in the current study were adjusted to RMTAPB, we cannot make clear conclusions about the effects of the paired-stimulations over the FDI. Indeed, although FDI and APB hotspots and RMT are very close to each other, we showed that at rest, MEPFDI were higher than MEPAPB in both groups. This difference is most likely explained by a difference in input-output curve. Thus, a stimulation set at 80% RMTAPB might correspond to approximately 90% RMTFDI. It is then reasonable to expect significant differences in results between FDI and APB, since it has been demonstrated that a stimulation at 90% AMTFDI over the dorsal premotor cortex could inhibit M1 while a stimulation set at 80% or 100% AMTFDI had no effect on M1 (Civardi et al., 2001). As a consequence, we can only conclude about significant premotor-motor interactions regarding the APB muscle, a surrounding muscle, not involved in the task. Although the APB is not recruited during this task, it is most probable that this latter muscle might be under the influence of the PMv. Indeed, it has been shown that the PMv exerts an important role in hand posture, fingertips position, and elaborates the appropriate pattern of activation of intrinsic hand muscles (Ceballos-Baumann et al., 1997b; Ibanez et al., 1999; Davare et al., 2006). It has also been described that the PMv plays a relevant role in visually-cued finger movements (Pollok et al., 2009; Ruspantini et al., 2011). PMv might thus play a key role in finger positioning in our task. FHD patients suffer from an abnormal activation pattern of the hand muscles during writing or music playing, with abnormal overflow of agonist and antagonist muscles (van der Kamp, 1989). We can thus hypothesize that the muscular adjustment usually exerted by the PMv over M1 before movement onset is impaired in FHD patients, explaining the abnormal PMv-M1 interactions regarding the APB muscle.
It seems unlikely that the premotor-motor facilitation observed in controls at T100 is due to the tone processing. In this simple acoustic reaction time task, we were expecting a facilitation of the synergist muscle (FDI) starting 100 ms after the tone presentation, as it has been reported in previous studies (Starr et al., 1988; Pascual-Leone et al., 1992; Leocani et al., 2000). Our results confirmed this expectation. In the current experiment, reaction times were approximately 160 ms, which indicates that T50 was approximately 110 ms after the tone presentation; and during the single-pulse TMS paradigm, MEPFDI were significantly enhanced at T50 and Tpeak, in both groups. We did not observe an early facilitation of the synergist muscle (FDI) similar to that reported by Leocani and colleagues (2000). Moreover, many studies based on auditory evoked potentials recordings identified cortical potentials over the fronto-central areas 200-300 ms after the stimulus onset. In our study, T100 stimulation occurred in average 60 ms after the tone presentation, it is very unlikely that the premotor-motor facilitation that we observed was due to the influence of the tone processing on the motor and premotor areas.
One limitation regarding the interpretation of our results could arise from the issue as to whether the involvement of the PMv might be expected in a simple reaction time task of index finger pressing. However, recent neuroimaging studies have demonstrated the activation of the PMv during unilateral hand or finger tapping tasks (Horenstein et al., 2009; Pollok et al., 2009), and thus corroborate previous data reported in monkeys (Matsumura et al., 1991; Kurata & Hoffman, 1994). Since PMv is highly involved in shaping hand movements (Davare et al., 2009) and constitutes a key component of visuomotor transformation for hand posture, it is reasonable to hypothesize that the PMv is involved in the finger-pressing reaction-time task used in this study. The current results obtained using the paired-pulse paradigm prove indeed the involvement of the PMv.
In conclusion, this study highlights the importance of the PMv-M1 interactions in the generation of the hand motor command. PMv-M1 interactions are both excitatory and inhibitory in nature. The inhibitory effects do not seem to contribute to the genesis of SI. Further experimentations are needed in order to define clearly the nature of these cortico-cortical interactions as well as their exact role in the abnormal hand posture observed in FHD patients.
Table 4. Correlation coefficients between the amount of surround inhibition and PMv-M1 interactions.
| T100 | T50 | Tpeak | |
|---|---|---|---|
| HV | -0,098 (p=0,699) | -0,234 (p=0,349) | 0,309 (p=0,213) |
| FHD | 0,079 (p=0,754) | 0,313 (p=0,206) | 0,018 (p=0,945) |
Table 4 shows the correlations data between surround inhibition and PMv-M1 interactions at T100, T50 and Tpeak, in healthy volunteers (HV) and patients (FHD). Correlations were considered significant if p≤ 0.05. No significant correlations were observed between the two phenomena, in both populations.
Acknowledgments
This work was supported by the NINDS Intramural Research Program. Elise Houdayer was funded by the Fyssen Foundation.
Abbreviations
- APB
Abductor Pollicis Brevis
- FDH
Focal hand Dystonia
- FDI
First Dorsal Interosseus
- MEP
Motor Evoked Potential
- PMv
Ventral Premotor Cortex
- RMT
Resting Motor Threshold
- SI
Surround Inhibition
- TMS
Transcranial Magnetic Stimulation
References
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Alterman RL, Snyder BJ. Deep brain stimulation for torsion dystonia. Acta Neurochir Suppl. 2007;97:191–199. doi: 10.1007/978-3-211-33081-4_21. [DOI] [PubMed] [Google Scholar]
- Beales M, Lorden JF, Walz E, Oltmans GA. Quantitative autoradiography reveals selective changes in cerebellar GABA receptors of the rat mutant dystonic. J Neurosci. 1990;10:1874–1885. doi: 10.1523/JNEUROSCI.10-06-01874.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition in the motor system. Exp Brain Res. 2011;210:165–172. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Houdayer E, Richardson SP, Hallett M. The role of inhibition from the left dorsal premotor cortex in right-sided focal hand dystonia. Brain Stimul. 2009a;2:208–214. doi: 10.1016/j.brs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. 2008;28:10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. 2009b;107:1513–1518. doi: 10.1152/japplphysiol.91580.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci. 2009c;29:1634–1640. doi: 10.1111/j.1460-9568.2009.06710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Brooks DJ. Activation positron emission tomography scanning in dystonia. Adv Neurol. 1998;78:135–152. [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain : a journal of neurology. 1997a;120(Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain. 1997b;120(Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–1057. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Toro C, Matsumoto J, Hallett M. Movement-related cortical potentials in writer's cramp. Ann Neurol. 1995;38:862–868. doi: 10.1002/ana.410380606. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Romito LM, Elia AE, Soliveri P, Panzacchi A, Carpinelli A, Tinazzi M, Albanese A, Perani D. In vivo evidence for GABA(A) receptor changes in the sensorimotor system in primary dystonia. Mov Disord. 2011;26:852–857. doi: 10.1002/mds.23553. [DOI] [PubMed] [Google Scholar]
- Gilbert SL, Zhang L, Forster ML, Anderson JR, Iwase T, Soliven B, Donahue LR, Sweet HO, Bronson RT, Davisson MT, Wollmann RL, Lahn BT. Trak1 mutation disrupts GABA(A) receptor homeostasis in hypertonic mice. Nat Genet. 2006;38:245–250. doi: 10.1038/ng1715. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath P, Gallea C, van der Veen JW, Horovitz SG, Hallett M. In Vivo Neurochemistry of Primary Focal Hand Dystonia: A Magnetic Resonance Spectroscopic Neurometabolite Profiling Study at 3T. Movement Disorders. 2010;25:2800–2808. doi: 10.1002/mds.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein C, Lowe MJ, Koenig KA, Phillips MD. Comparison of unilateral and bilateral complex finger tapping-related activation in premotor and primary motor cortex. Hum Brain Mapp. 2009;30:1397–1412. doi: 10.1002/hbm.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer's cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H, Kaji R, Terada K, Nagamine T, Honda M, Hamano T, Kimura J. Abnormal sensorimotor integration in writer's cramp: study of contingent negative variation. Mov Disord. 1996;11:683–690. doi: 10.1002/mds.870110614. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol. 1994;71:1151–1164. doi: 10.1152/jn.1994.71.3.1151. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol. 2002;51:93–101. [PubMed] [Google Scholar]
- Loscher W, Horstermann D. Abnormalities in amino acid neurotransmitters in discrete brain regions of genetically dystonic hamsters. J Neurochem. 1992;59:689–694. doi: 10.1111/j.1471-4159.1992.tb09423.x. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J Neurophysiol. 1991;65:1542–1553. doi: 10.1152/jn.1991.65.6.1542. [DOI] [PubMed] [Google Scholar]
- Messer A, Gordon D. Changes in whole tissue biosynthesis of gamma-amino butyric acid (GABA) in basal ganglia of the dystonia (dtAlb) mouse. Life Sci. 1979;25:2217–2221. doi: 10.1016/0024-3205(79)90095-x. [DOI] [PubMed] [Google Scholar]
- Nobrega JN, Richter A, Burnham WM, Loscher W. Alterations in the brain GABAA/benzodiazepine receptor-chloride ionophore complex in a genetic model of paroxysmal dystonia: a quantitative autoradiographic analysis. Neuroscience. 1995;64:229–239. doi: 10.1016/0306-4522(94)00334-2. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Peller M, Zeuner KE, Munchau A, Quartarone A, Weiss M, Knutzen A, Hallett M, Deuschl G, Siebner HR. The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer's cramp. Brain. 2006;129:2697–2708. doi: 10.1093/brain/awl181. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Butz M, Schnitzler A. Modality specific functional interaction in sensorimotor synchronization. Hum Brain Mapp. 2009;30:1783–1790. doi: 10.1002/hbm.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt GD, Richter A, Mohler H, Loscher W. Regionally selective and age-dependent alterations in benzodiazepine receptor binding in the genetically dystonic hamster. J Neurochem. 1995;64:2153–2158. doi: 10.1046/j.1471-4159.1995.64052153.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20 1:S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Ruspantini I, Mäki H, Korhonen R, D'Ausilio A, Ilmoniemi RJ. The functional role of the ventral premotor cortex in a visually paced finger tapping task: a TMS study. Behav Brain Res. 2011;7:325–330. doi: 10.1016/j.bbr.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004a;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004b;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol. 1988;70:26–32. doi: 10.1016/0013-4694(88)90191-5. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Task-dependent modulation of silent period duration in focal hand dystonia. Mov Disord. 2005;20:1143–1151. doi: 10.1002/mds.20514. [DOI] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Hallett M. Movement-related electroencephalographic desynchronization in patients with hand cramps: evidence for motor cortical involvement in focal dystonia. Ann Neurol. 2000;47:456–461. [PubMed] [Google Scholar]
- van der Kamp W, Berardelli A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatry. 1989;52:1043–1049. doi: 10.1136/jnnp.52.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kaji R, Terada K, Nagamine T, Toma K, Kubori T, Kimura J, Shibasaki H. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain. 1999;122:1357–1366. doi: 10.1093/brain/122.7.1357. [DOI] [PubMed] [Google Scholar]


