Abstract
Abnormalities of cortical representational maps and their plasticity have been described in dystonia. A common polymorphism for BDNF has been associated with abnormal cortical plasticity, and thus might contribute to pathogenesis of dystonia in some subjects. As a first step towards this suggestion, the current study examined the prevalence of this polymorphism. BDNF genotype was examined in 34 subjects with cervical dystonia, 54 age-matched healthy controls, and 53 subjects with a different movement disorder, Parkinson's disease. ApoE genotype, known to influence neurological outcome in some conditions, was also examined as a control. In subjects with cervical dystonia, the val66met polymorphism was approximately twice as prevalent when compared to either control group. This was not true of ApoE genotype, which was similarly distributed across subject groups. The current findings suggest that the BDNF val66met polymorphism might play a role in the pathogenesis of cervical dystonia in some subjects.
Keywords: dystonia, plasticity, genetics
The pathogenesis of dystonia remains incompletely understood. Prior studies have found that subjects with dystonia show abnormalities of cortical function, for example, abnormal organization of motor cortex representational maps [24], reduced sensorimotor cortex response to sensory stimulus [23], and abnormal response to an inhibitory motor cortex influence [6]. Together, these observations have led to the theory that dystonia might in part be due to pathological sensorimotor cortex plasticity [8, 12].
Recent studies suggest that cortical plasticity in humans is significantly modified in the presence of a single nucleotide polymorphism (val66met) in the gene for brain derived neurotrophic factor (BDNF). BDNF is the most abundant neurotrophin in the brain, and influences a wide range of brain events related to plasticity and repair [5]. Healthy subjects who have a copy of the val66met polymorphism in one or both alleles show several differences in brain structure and function, including abnormal motor cortex plasticity [3, 14]. In the U.S., this polymorphism is present in one allele in 27% of people (Val/Met), and in both alleles in 4.5% (Met/Met) [20].
Together, these observations suggest the possibility of a role for the val66met polymorphism in the genesis of dystonia. As a first step, the current study sought to determine whether the prevalence of the BDNF val66met polymorphism in subjects with cervical dystonia, the most common adult form of dystonia, differed as compared to (a) healthy controls or (b) a cohort of subjects with a different movement disorder, Parkinson's disease. As an additional control, the prevalence of the ApoE4 genotype was also evaluated across these subject groups.
Methods
Entry criteria were for dystonia subjects were a diagnosis of dystonia based on clinical assessment with no secondary cause; and for Parkinson's disease subjects, a diagnosis of Parkinson's disease based on clinical assessment with no secondary cause. Diagnosis was by a board certified neurologist who was fellowship trained and experienced in movement disorders. Healthy controls were recruited from patient's spouses and from the community via flyers, and had no history of any type of neurological diagnosis or symptoms. All subjects were required to have age > 18 years and be English speaking.
This study was approved by the UC Irvine Institutional Review Board. All subjects signed informed consent in accordance with this approval. They then underwent exam that included scoring on the Geriatric Depression Scale (15 Q version), as well as the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) [4] for subjects with cervical dystonia. A cheek swab was then used to obtain DNA that was processed for the BDNF val66met polymorphism, as described previously [14]. Subjects were classified as Val/Val if no copies of the polymorphism were present, Val/Met if one copy was present, and Met/Met if two copies were present. In all subjects, genotype for ApoE was also assessed [10]. In subjects with dystonia, genotype for DYT1 was additionally assessed [17].
Statistical analysis was performed using JMP 7.0.1 (SAS, Cary, NC). Non-parametric methods were used to compare continuous data (Wilcoxon rank sum test), and Chi square testing to compare categorical data, across subject groups. Each pair of subject groups was evaluated independently. All analyses were two-tailed and used alpha < 0.05. Because of the rarity of the Met/Met genotype, the primary analysis focused on the distribution of the Val/Met genotype across subject groups.
Results
A total of 141 subjects were enrolled, 34 with cervical dystonia, 54 age-matched healthy controls, and 53 subjects with Parkinson's disease. Note that none of the subjects with Parkinson's disease had a history of dystonia. The age of diagnosis for subjects with cervical dystonia was 48 ± 12 (mean ± SD) years. Fifteen subjects with cervical dystonia had a positive family history (including primary and secondary relatives) of neurological and psychiatric conditions, spanning a range of symptoms and diagnoses. The mean TWSTRS score total was 33 ± 14 (range 10-58, maximum score=87).
Demographics were overall similar across the three groups (Table). Subjects with dystonia showed no significant differences in age, gender, ethnicity, or handedness as compared to healthy controls. However, subjects with dystonia had Geriatric Depression Scale scores (2.8 ± 2.6) that were higher than scores among healthy controls (1.2 ± 1.8, p < 0.002), though this difference was of little clinical significance given that a score > 5 points is suggestive, and > 10 diagnostic, of depression. Subjects with dystonia showed no significant differences in ethnicity, handedness, or Geriatric Depression Scale as compared to subjects with Parkinson's disease. However, mean age was slightly lower in subjects with dystonia (59 ± 13 vs. 65 ± 10, p < 0.04) and gender distributions were not matched (dystonia, 24F/10M; Parkinson's disease 19F/34 M; p < 0.002).
Table.
| Cervical dystonia | Healthy controls | Parkinson's disease | |
|---|---|---|---|
| n | 34 | 54 | 53 |
| Gender (M/F) | 10/24 | ||
| Ethnicity Caucasian/Hispanic/Asian/Other | 33/1/0/0 | 51/0/3/0 | 48/2/1/2 |
| Age (years) | 59 ± 13 | 55 ± 15 | 65 ± 10 |
| Handedness # right-handed / # left-handed | 32/2 | 52/2 | 49/4 |
Values for age are mean ± SD. Subjects with dystonia showed no significant differences compared to healthy controls. Subjects with dystonia had lower age (p < 0.04) and different gender distributions (p < 0.002) as compared to subjects with Parkinson's disease.
Both BDNF and ApoE were in Hardy-Weinberg equilibrium. None of the subjects with dystonia was positive for the DYT1 gene mutation.
When comparing subjects with dystonia to those with Parkinson's disease, the distribution of BDNF genotypes varied (p < 0.007), but the distribution of ApoE did not (p > 0.5). The primary analysis was that the proportion of subjects with the Val/Met BDNF genotype was significantly different (dystonia, 50%; Parkinson's disease, 19%; p<0.003, see Figure). The proportion of subjects with ApoE4 did not differ (p > 0.75). Excluding the one subject with Parkinson's disease whose BDNF genotype was Met/Met had minimal effect on these results, as there was still a difference in the proportion of subjects with the Val/Met genotype (p < 0.003) but not the ApoE (p > 0.75).
Figure. Distribution of genotypes according to diagnosis.
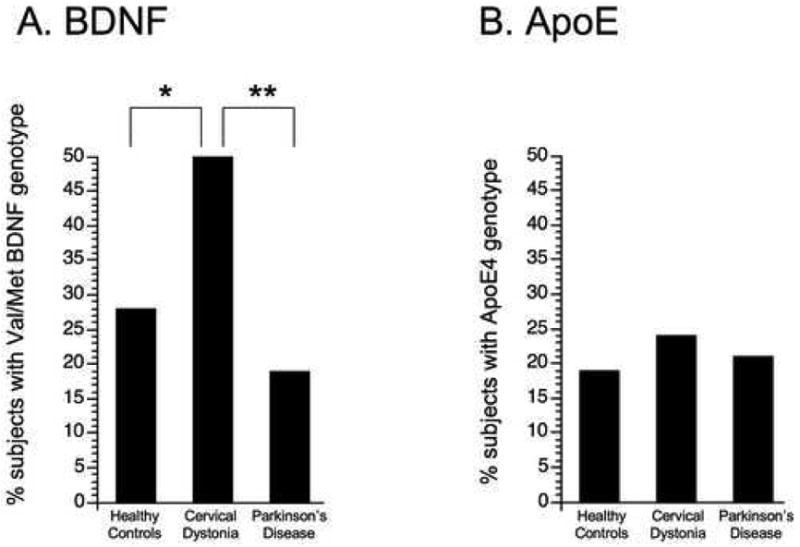
The prevalence of two different genotypes is presented. (A) The proportion of subjects with the BDNF Val/Met genotype differed significantly, being higher in subjects with cervical dystonia (17 of 34 subjects) as compared to either healthy controls (15 of 54) or to subjects with Parkinson's disease (10 of 53). (B) The proportion of subjects with the ApoE4 genotype did not differ across groups (dystonia, 8 of 34 subjects; controls, 10 of 54; Parkinson's, 11 of 53). *p<0.05; **p<0.005
When comparing subjects with dystonia to healthy controls, the distribution of BDNF genotypes varied (p < 0.04), but the distribution of ApoE did not (p > 0.6). The primary analysis was that the proportion of subjects with the Val/Met genotype was significantly different (dystonia, 50%; healthy controls, 28%; p<0.04, see Figure). The proportion of subjects with ApoE4 did not differ (p > 0.55). Excluding the three healthy controls whose BDNF genotype was Met/Met had minimal effect on these results, as there was still a difference in the proportion of subjects with the Val/Met genotype (p < 0.056) but not the ApoE4 genotype (p > 0.65).
Note that when comparing subjects with Parkinson's disease to healthy controls, neither the distribution of BDNF genotypes (p > 0.25) nor ApoE (p > 0.65) varied significantly. The proportion of subjects with the Val/Met genotype (p > 0.25) or ApoE4 genotype (p > 0.75) also did not differ.
A sub-analysis found that the number of copies of ApoE2, ApoE3, or ApoE4 did not differ in any of these comparisons.
Among subjects with dystonia, BDNF genotype was not significantly related to age of onset, duration of symptoms, gender, race, handedness, positive family history, Geriatric Depression Scale score, TWSTRS total score, or value for any of the TWSTRS subscores. The distributions of BDNF and ApoE genotypes were not related (p>0.5).
Discussion
A number of abnormalities of cortical function have been described in dystonia, particularly those related to cortical representational maps and their plasticity [1, 6, 15, 21, 23, 24]. A common polymorphism for BDNF, val66met, has been associated with abnormal cortical plasticity [3, 14]. The current study explored whether this polymorphism might be related to the pathogenesis of dystonia by examining its prevalence. In subjects with cervical dystonia, the Val/Met genotype was approximately twice as prevalent when compared to either healthy controls or to subjects with a different movement disorder, Parkinson's disease. This was not true for the ApoE4 genotype, which was similarly distributed across subject groups.
These findings suggest the possibility that the val66met polymorphism might play a role in the genesis of dystonia in some subjects. The mechanism of such an effect remains unclear. The main effect of this polymorphism on BDNF function is to reduce activity-dependent secretion [7], which is associated with reduced cortical plasticity [3, 14] and a dampening of cortical processes related to long term potentiation [3]. This is in contrast with findings in dystonia, where cortical plasticity is exaggerated [1, 6, 19, 21]. How might this BDNF genotype therefore contribute to the dystonia phenotype? One possible explanation is that the polymorphism might be a source of altered gain within sensorimotor loops, with reduced responsiveness in one node resulting in increased plasticity within a second node [19]. For example, key effects of the val66met polymorphism might be manifested subcortically; indeed, it has been suggested that the abnormality in dystonia might be primarily in the basal ganglia, with cortical findings in this setting thus reflecting a reaction to striatal events such as sensory gating [8, 12]. Alternatively, the impaired activity-dependent BDNF secretion associated with the polymorphism might blur the temporal resolution of either sensory processing [22] or cortical inhibition [2, 21], each of which is suspected to contribute to the pathogenesis of dystonia.
One weakness of the study is that anxiety was not measured. Anxiety is a common psychiatric comorbidity in cervical dystonia [25], and has also been associated with the presence of the val66met polymorphism [9], and is thus a covariate of potential interest to be measured in future studies. Although the prevalence of this polymorphism among subjects with Parkinson's disease has not been reported to be abnormal, some (though not all) studies suggest that the polymorphism influences onset age [11, 13], suggesting the utility of enrolling a different movement disorder as controls in future studies, possibly including other forms of dystonia.
A recent report from Martino et al [16] found that the distribution of BDNF genotypes among subjects with various forms of dystonia did not differ as compared to healthy controls. This remained true even when only the subgroup of subjects with cervical dystonia was analyzed. The reason for the discrepancy in results between this report and the current one is not immediately apparent. One possible explanation lies in the differing ethnicities of study enrollees, as BDNF genotypes are substantially different in Italy as compared to the US. [20]
Dystonia has been suggested to be a condition whereby homeostatic mechanisms that regulate cortical plasticity become overwhelmed [8, 18]. Hallett recently suggested that a genetic cause is likely in dystonia given that the abnormality appears to be an endophenotype. The current study found that the prevalence of the BDNF val66met polymorphism, known to modulate cortical plasticity, is significantly increased in subjects with cervical dystonia, and so supports this hypothesis. Given that half of the current cohort of subjects with dystonia did not have the BDNF polymorphism, future studies might explore other genes that influence regulation of brain plasticity, with attention to ethnicity, and might also examine the interaction of this genotype with behavior and experience.
Acknowledgments
This study was supported by grant M01 RR000827-29 from the UC Irvine General Clinical Research Centers Program of the National Center for Research Resources, and by R01 NS058755 from NINDS, both of the National Institutes of Health; and by the UC Irvine Parkinson's Disease and Movement Disorders Fund. The authors wish to thank Mrs. Nanda Gourkar for her coordination of the U.C. Irvine Movement Disorders Clinic, and Dr. Cathy M. Stinear, University of Auckland, for helpful discussions. We are also grateful for the assistance of Mrs. Martha Murphy, coordinator of the San Diego Dystonia Medical Research Foundation Support Group, and Mrs. Kim Hough, coordinator of the Rancho Mirage Dystonia Medical Research Foundation Support Group, each of whom played a valuable role in subject recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumer T, Demiralay C, Hidding U, Bikmullina R, Helmich RC, Wunderlich S, Rothwell J, Liepert J, Siebner HR, Munchau A. Abnormal plasticity of the sensorimotor cortex to slow repetitive transcranial magnetic stimulation in patients with writer's cramp. Mov Disord. 2007;22:81–90. doi: 10.1002/mds.21219. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. 2008;28:10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consky E, Lang A. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M, editors. Therapy with Botulinum Toxin. Marcel Dekker, Inc; New York: 1994. pp. 211–237. [Google Scholar]
- 5.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 6.Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–2186. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- 7.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 8.Hallett M. Dystonia: a sensory and motor disorder of short latency inhibition. Annals of neurology. 2009;66:125–127. doi: 10.1002/ana.21762. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K. BDNF variant linked to anxiety-related behaviors. Bioessays. 2007;29:116–119. doi: 10.1002/bies.20534. [DOI] [PubMed] [Google Scholar]
- 10.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 11.Hong CJ, Liu HC, Liu TY, Lin CH, Cheng CY, Tsai SJ. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms in Parkinson's disease and age of onset. Neuroscience letters. 2003;353:75–77. doi: 10.1016/j.neulet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Kaji R. Basal ganglia as a sensory gating devise for motor control. J Med Invest. 2001;48:142–146. [PubMed] [Google Scholar]
- 13.Karamohamed S, Latourelle JC, Racette BA, Perlmutter JS, Wooten GF, Lew M, Klein C, Shill H, Golbe LI, Mark MH, Guttman M, Nicholson G, Wilk JB, Saint-Hilaire M, DeStefano AL, Prakash R, Tobin S, Williamson J, Suchowersky O, Labell N, Growdon BN, Singer C, Watts R, Goldwurm S, Pezzoli G, Baker KB, Giroux ML, Pramstaller PP, Burn DJ, Chinnery P, Sherman S, Vieregge P, Litvan I, Gusella JF, Myers RH, Parsian A. BDNF genetic variants are associated with onset age of familial Parkinson disease: GenePD Study. Neurology. 2005;65:1823–1825. doi: 10.1212/01.wnl.0000187075.81589.fd. [DOI] [PubMed] [Google Scholar]
- 14.Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 15.Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. NeuroImage. 2004;21:904–913. doi: 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Martino D, Muglia M, Abbruzzese G, Berardelli A, Girlanda P, Liguori M, Livrea P, Quattrone A, Roselli F, Sprovieri T, Valente EM, Defazio G. Brain-derived neurotrophic factor and risk for primary adult-onset cranial-cervical dystonia. Eur J Neurol. 2009;16:949–952. doi: 10.1111/j.1468-1331.2009.02633.x. [DOI] [PubMed] [Google Scholar]
- 17.Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 18.Roze E, Soumare A, Pironneau I, Sangla S, de Cock VC, Teixeira A, Astorquiza A, Bonnet C, Bleton JP, Vidailhet M, Elbaz A. Case-control study of writer's cramp. Brain. 2009;132:756–764. doi: 10.1093/brain/awn363. [DOI] [PubMed] [Google Scholar]
- 19.Sanger T, Merzenich M. Computational model of the role of sensory disorganization in focal task-specific dystonia. Journal of neurophysiology. 2000;84:2458–2464. doi: 10.1152/jn.2000.84.5.2458. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- 21.Stinear CM, Byblow WD. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. Hum Mov Sci. 2004;23:527–538. doi: 10.1016/j.humov.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Tamura Y, Ueki Y, Lin P, Vorbach S, Mima T, Kakigi R, Hallett M. Disordered plasticity in the primary somatosensory cortex in focal hand dystonia. Brain. 2009 doi: 10.1093/brain/awn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tempel LW, Perlmutter JS. Abnormal vibration-induced cerebral blood flow responses in idiopathic dystonia. Brain. 1990;113(Pt 3):691–707. doi: 10.1093/brain/113.3.691. [DOI] [PubMed] [Google Scholar]
- 24.Thickbroom GW, Byrnes ML, Stell R, Mastaglia FL. Reversible reorganisation of the motor cortical representation of the hand in cervical dystonia. Mov Disord. 2003;18:395–402. doi: 10.1002/mds.10383. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel T, Schnider P, Wimmer A, Steinhoff N, Moraru E, Auff E. Psychiatric comorbidity in patients with spasmodic torticollis. J Psychosom Res. 1998;44:687–690. doi: 10.1016/s0022-3999(97)00229-8. [DOI] [PubMed] [Google Scholar]


