Abstract
The gold standard technique for measuring gastric emptying is scintigraphy using radiolabeled test meals. Recently, a standardized radiolabeled solid meal has been proposed and adopted by many centers. There is still a need for alternative meals and several such meals with demonstrated radiolabel stability have been evaluated in small numbers of subjects. Updated radiation dosimetry associated with these meals has been calculated for adult males and adult females with normal GI transit as well as transit abnormalities.
INTRODUCTION
The use of radiolabeled test meals with scintigraphy is considered the gold standard for measurement of gastric emptying. It would be ideal if an individualized radiolabeled meal could be chosen to match the digestive responses which cause symptoms in an individual patient. In addition ideal properties of the radiolabeled meal should include a radiolabel which is nonabsorbable from the GI tract and does not bind to gastrointestinal mucosa. As many patients do not tolerate a solid meal the meal choice should allow for a physiologic solid or liquid, which ideally should be palatable to encourage the patient to ingest the entire meal. Furthermore, the meal should be adequately standardized in a large population to provide standard ranges for comparison with patient data.
SOLID MEALS
It is generally accepted that a radiolabeled solid meal is preferred as a test meal for gastric emptying studies, in order to provoke the fed state and full physiologic gastric response to a meal including the accommodation response, and to test both the grinding function and the emptying function of the stomach. Historically, many attempts to achieve a radiolabeled solid meal did not validate the stability of the radiolabel and resulted in use of test meals in which the radiolabel dissociated from the solid particles. The first test meal with validated labeling was in vivo 99mTc-SC chicken liver(1). Since then, many other meals have been employed (Table 1) but not all have been adequately tested for stability in gastric fluid. Evaluation in hydrochloric acid only (without proteolytic enzymes such as pepsin) can be misleading in that meals can be stable in acid but not in gastric fluid.
Table 1.
Examples of Acceptable Radiolabeled Foods
| SOLID MEALS | ref | ||
|---|---|---|---|
| 99mTc-SC | Liquid egg white | Cooked thoroughly after mixing | (2) |
| 99mTc-SC | Raw beaten whole egg | Cooked thoroughly after mixing | (37) |
| 99mTc-SC | Chicken liver in vivo | Inject into live chicken; cook liver | (1) |
| 99mTc-SC | Injected liver cubes | Inject liver, then cook thoroughly | (37) |
| 99mTc-SC | Pancake batter | Cooked thoroughly after mixing | (9) |
| 99mTc-SC | Milk | Acidify, heat, collect labeled cheese | (38) |
| 99mTc-SC | Cheddar cheese | Melt cheese with Tc-SC, cool | (6) |
| 111InCl2 | Raw egg or whites | Cooked thoroughly after mixing | (10) |
| 111In-oxine | Raw egg or whites | Cooked thoroughly after mixing | (14) |
| 67Ga-citrate | Raw egg or whites | Cooked thoroughly after mixing | (12) |
| SEMISOLID MEALS | |||
| 99mTc-SC | Liver pate | Mix, then cook thoroughly | (39) |
| 99mTc-SC | Instant oatmeal | Microwave after mixing | (7) |
| 99mTcO4− | Dowex 2-x8 resin | Mix with oatmeal | (40) |
| 99mTcO4−/Sn | Chelex resin | Mix with oatmeal | (41) |
| LIQUID NUTRIENT MEALS | |||
| 99mTc-SC | High caloric liquid | Mix well | (15) |
| (Ensure ®) | |||
Current Consensus Standardized meal: Low fat egg white sandwich labeled with Tc-SC
This meal has recently been recommended as an international standard test meal for gastric emptying studies(2). Normal gastric emptying values were established in a large multicenter trial(2). The meal has been endorsed as a standard meal by a working group of representative clinicians from both nuclear medicine and gastroenterology(3) and by practice guidelines by both the Society of Nuclear Medicine and American College of Radiology(4).
Composition and nutritional characteristics of the meal
The meal consists of 120 g (60 kcal) of liquid egg white (EggBeaters® or equivalent), equivalent to the volume of two large eggs, which is mixed with 99mTc-sulfur colloid (99mTc-SC) and cooked to a firm, rubbery consistency. This is served with two slices of white bread (120 kcal), 30 g strawberry jam, (75 kcal) and 120 ml of water. The nutritional composition of the meal is 72% carbohydrate, 24% protein, 2% fat and 2% fiber(2).
Considerations in preparing the meal to assure reproducible data
For valid gastric emptying studies of a solid meal, it is essential that the radiolabel remain associated with the particles of solid during exposure to gastric fluid. Evaluation of the 99mTc-SC egg white meal indicated that either microwave cooking or griddle cooking provides a satisfactory result, provided the egg white is cooked to a completely solid consistency(5). Interestingly, the use of scrambled fresh whole eggs labeled with 99mTc-SC, which was the practice in some nuclear medicine facilities prior to the adoption of the standard low-fat egg white meal, may lead to a less stable radiolabel when prepared by either microwave or griddle cooking; this was attributed to the presence of yolk which does not bind the sulfur colloid well especially with uneven heating which may result with microwave cooking. (5)
ALTERNATIVE RADIOLABELED MEALS
There is a need for alternatives to the low fat egg white sandwich in order to evaluate patients who may have egg or gluten allergy or who have another objection to eating the components of this standardized meal. No other meal has been fully standardized for this purpose, however.
Labeled cheese
It was recently proposed that cheddar cheese can be radiolabeled by melting the cheese together with 99mTc-SC followed by cooling to resolidify the cheese(6). The stability of this radiolabel was demonstrated to be >95% in freshly aspirated human gastric fluid, pH 2. This labeled food has been proposed as the basis for an appetizing meal for children who need to undergo gastric emptying tests. The caloric content and fat content of pizza with cheese or macaroni with cheese is similar to a scrambled whole egg test meal(6). However, standardized gastric emptying values are not available for meals based on labeled cheddar cheese, or for any solid meal in a pediatric population.
Meals prepared from a dry mix
Because of the convenience of preparing a test meal from dry ingredients which have a long shelf life and do not require refrigeration, some centers prefer test meals which are based on dry mixes that can be reconstituted and radiolabeled. There has been a resurgence in popularity of an oatmeal test meal for gastric emptying studies(7, 8). Reconstituted instant oatmeal provides a semi-solid meal rather than a solid meal, because it does not require trituration prior to emptying. It is convenient to prepare and palatable to a large number of subjects. One reported oatmeal test meal(7) labeled with 99mTc-SC has a caloric content of 100 kcal and contains 2 g of fat, 4 g of protein, and 19 g of carbohydrate. Some centers employ a meal consisting of pancakes which are prepared from a mix into which water and 99mTc-SC are incorporated prior to cooking on a griddle(9). This meal consisted of 273 kcal (8.6 g protein, 56.0 g carbohydrate, 2.9 g fat, 1.4 g fiber). Again however, standard values in a large subject population are not available for either of these meals. Both meals were evaluated for radiolabel stability, and appeared to be stable although they were tested in diluted acid (not gastric fluid). A recent evaluation(6) of 99mTc-SC-labeled instant oatmeal in human gastric fluid suggested that it is not adequately stable; however, it is not clear whether different methods of preparation of instant oatmeal had an effect (simple mixing with hot water(6) or microwave cooking after adding tracer(7)), or whether the technique of separating oatmeal particles from radiocolloid is really relevant if they empty together from the stomach.
Other radiolabels have been proposed for labeling test meals, in order to have a second radionuclide (such as 111In) for simultaneous measurement of liquid and solid meals, or of simultaneous assessment of gallbladder emptying (99mTc-hepatobiliary agent) with gastric emptying (alternative label on the meal). Some stable examples include eggs (scrambled whole eggs or egg whites) labeled with 111In-chloride(10), 111In-oxine(11) or 67Ga-citrate(12). One component of egg white is conalbumin, a protein which binds trivalent metal ions(13). Provided the radiometal is added to the egg white in a form that is not strongly chelated, the radiometal can become bound to the conalbumin. This means that 111In-DTPA would not be suitable for labeling eggs. It has been found that 111In-oxine, 111In-chloride or 67Ga-citrate mixed with beaten raw egg or egg white and followed by cooking were quite stable in gastric fluid(12, 14). The standard transit values that have been established for the 99mTc-SC low-fat egg sandwich meal(2) should be valid when the liquid egg white is labeled with one of these alternative radionuclides, as long as the other components of the meal remain the same.
It should be noted that 99mTc-SC is approved by the FDA for oral administration, whereas most other radiopharmaceuticals including 111In radiopharmaceuticals are not. Any alternative route of administration meal therefore must be listed on the user’s radioactive materials license. Unless the GI tract absorption/adsorption for a new radionuclide has been established, that must be studied and documented. Every new radiolabeled solid meal must be evaluated for stability in gastric fluid in vitro. Standard values for emptying in normal subjects must also be established. Alteration of the nutrient composition of an established meal will probably require revision of normal values for emptying parameters.
At present, there is no convenient solid or semisolid meal which is truly gluten-free and vegan, unless special gluten-free instant oatmeal is used.
LIQUID MEALS
Nutrient drink meal
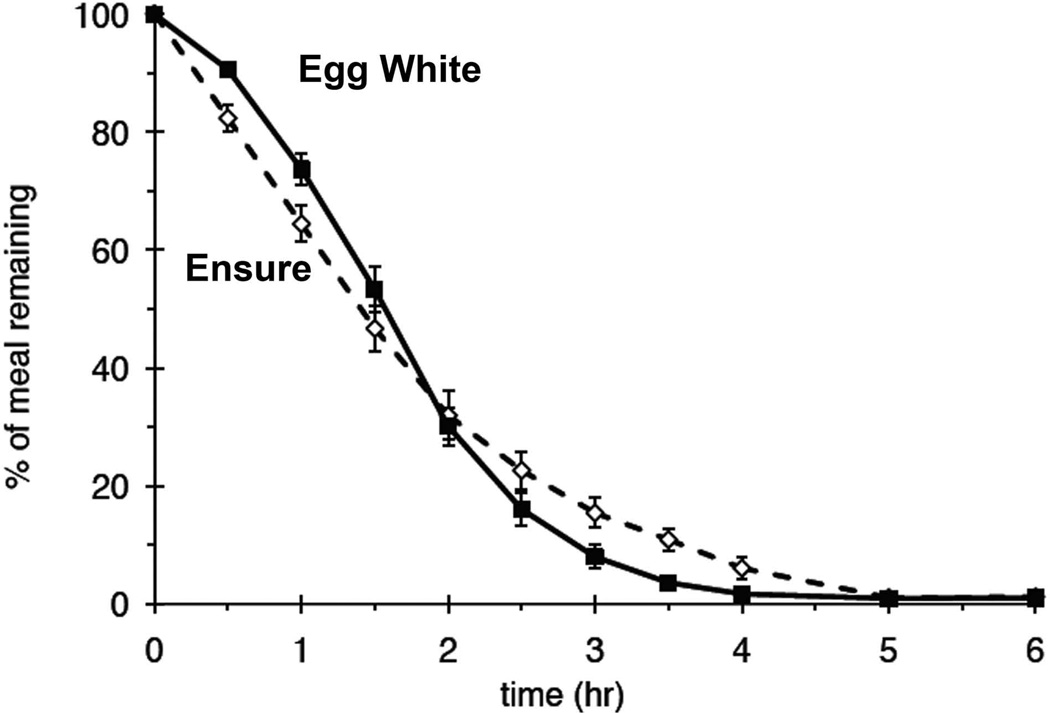
In order to use a convenient meal which is readily available and avoids egg and gluten and is suitable for vegans, a nutrient drink has been proposed. A high caloric, high fat nutrient meal, such as Ensure®, mixed with radiolabel, has been anecdotally used for evaluation of gastroparesis using gastric emptying scintigraphy when patients are unable to tolerate the usual egg sandwich meal. The radiotracer 99mTc-SC is simply mixed with 237 ml of Ensure Plus® which contains 350 kcal (28% fat, 15% protein, 57% carbohydrate). This meal has recently been evaluated to characterize gastric emptying parameters in normal subjects and to directly compare the emptying curves with the low fat egg sandwich meal(15). In 20 healthy volunteers, the T ½ of the nutrient liquid meal was similar to the low-fat egg white sandwich meal (1.41 ± 0.11 vs 1.52 ± 0.08 hr, respectively), but the shapes of the emptying curves were different (Figure 1). When individual time points were evaluated, there was less gastric retention of the nutrient liquid meal at early times and greater gastric retention at later times, compared with the low-fat egg white sandwich meal. The 2 hr and 4 hr retention values are very similar to those currently in use for the recent consensus recommended liquid egg-white sandwhich meal (normal at 2 hr low-fat egg white sandwich < 60% retention vs 2 hr Ensure <50% and 4 hr low-fat egg white sandwich < 10% vs 4 hr Ensure <10%). The meal does not require trituration in order to empty, but may empty slowly because of its high fat content. Normal values in a larger number of subjects are needed in order for this meal to become useful in standard evaluation of dyspepsia in patients.
Figure 1.
Comparison of a nutrient liquid meal (Ensure Plus) and current consensus egg-white sandwhich meal. The mean ± sem values for percentage retention over time for the egg meal are shown (filled squares, solid line) vs the Ensure meal (dashed line). The data are for 20 subjects each studied twice, once with each meal. The liquid meal has slightly faster early gastric emptying due to lack of any need for trituration which occurs with solids, but has similar retention at 2 hours. There is slightly increased retention between 2–4 hours likely due to its higher fat and caloric content.
Non-nutrient liquid meals
(water) have also been labeled with 99mTc-SC, 99mTc-DTPA or 111In-DTPA(16). If the non-nutrient liquid is given on a separate occasion from a 99mTc-labeled solid meal, 99mTc-SC can be used as the radiotracer in the water. In some cases it may be desirable to perform the liquid-only emptying study during a 30 minute period prior to administering the solid meal for a solid emptying study. In this case, one would use 111In-DTPA for the water and 99mTc in the solid meal(17).
GASTRIC EMPTYING STUDIES USING OTHER TECHNIQUES
Because of concerns about radiation exposure, other methods of assessing gastric emptying have been proposed which do not require radioactive materials or scintigraphy.
Breath tests have been developed in which a meal containing 13C-labeled digestible substrate is metabolized to release 13CO2 which is excreted in the breath. The collected breath samples can be analyzed by mass spectrometry to quantify the nonradioactive 13C (18). The results of these tests depend not just on gastric emptying, but also on intact absorption and metabolic function. Breath tests give an estimate of global gastric emptying, but are not able to assess the regional (antral vs fundal) gastric function that scintigraphy is able to provide. The methods of analysis of the data are not standardized.
Transabdominal 2-D ultrasound has been proposed as a method for assessing gastric emptying(19). This technique measures gastric volume based on stomach cross-sectional area and can selectively evaluate antral motility(20). Recently, 3-D ultrasound has been proposed as an improved method which enables assessment of intragastric meal distribution as with scintigraphy(21). Liquid meals have primarily been used, but recent studies have also used pasta meals(22).
Recent improvements in MRI which enable higher spatial resolution and more rapid acquisition have enabled imaging of the gastrointestinal tract for emptying studies(23). The images are three-dimensional and the assessment of emptying is based on changes in volume. If a gadolinium-containing meal such as pudding mixed with Gd-DTPA is used, gastric volume can be corrected for secretions to estimate the emptying of the meal(24). MRI uses expensive technology which is in high demand for other types of exams. To enable a rapid test, measurements have been made only through 30 minutes and extrapolated to calculate the later T1/2(24). Furthermore, in most centers the patient must be supine for the exam and this is not reflective of most patients’ body position while eating or after meal completion. MRI may be particularly useful in assessing gastric accommodation response.
DOSIMETRY FOR RADIOLABELED MEALS
One of the main issues raised about scintigraphic determination of gastric emptying is radiation exposure. This section will address this important concern. Previous estimates of radiation exposure from gastric emptying scintigraphy reported radiation doses only for adult males, not adult females or children. Currently, radiation exposure is reported as Effective Dose Equivalent (EDE), which is a whole-body weighted average of doses delivered to various body organs and tissues. The previous publications did not report the EDE for radiolabeled meals.
Radiation dosimetry for radionuclides ingested orally was previously based on an assessment of the physiology of the GI tract by Eve(25) and incorporated into ICRP 30 in 1979(26). The transit times utilized in these models were based on observations of barium paste rather than physiologic meals. The transit times for previous models are shown in Table 2.
Table 2.
Transit time assumed for modeling segments of the GI tract
| Portion of the GI tract | time (hr) | time (hr) | time (hr) | time (hr) |
|---|---|---|---|---|
| (Reference) | (25) | (27) | (29) | (this article) |
| Stomach | ||||
| Liquid | 1 | 0.38 | 0.5 | 0.35 |
| Semisolid | 0.44 | |||
| Solid | 1 | 1.47 | 1 | 1.42 |
| Small intestine | 4 | 2.8 | 4* | 4 |
| Upper large intestine | 13 | 10 | - | 8 |
| Ascending colon | - | - | 8.4 | |
| Transverse colon | - | - | 7.3 | |
| Lower large intestine | 24 | 17 | 15.9 | 18 |
| (Descending/rectosigmoid) | ||||
Upon filling the terminal ileum, 50% of activity is transferred to ascending colon in a bolus. Thirty minutes later, the remaining small intestinal activity is transferred to the ascending colon as a bolus.(29)
The radiation dosimetry for radiolabeled solid and liquid meals was summarized in 1983 (27). The reported dosimetry included solid meals labeled with 99mTc-SC, 99mTc-ovalbumin or 111In-colloid, and non-nutrient liquids labeled with 99mTc-SC, 111In-DTPA or 113mIn-DTPA. Sulfur colloid currently is the only radiopharmaceutical used in GI studies which is FDA approved for oral administration, but the dosimetry for sulfur colloid when orally administered as meals of various compositions (e.g., solid vs semisolid vs liquid) is not reported in the package insert.
The dosimetry published in 1983 used a catenary compartment model (28) consisting of four compartments: stomach, small intestine, upper large intestine, and lower large intestine. In order to simplify calculations, first-order kinetics were assumed for the transit from each compartment. The transit times were obtained from transit data in 20 normal subjects: T1/2 for stomach 0.38h for liquid, 1.47h for solid; small intestine 2.8h; upper large intestine 10h; lower large intestine 17h. The radiation absorbed doses were reported for only GI tract, gallbladder wall and gonads.
In 1992, a new model (RIDIC model) of gastrointestinal transit was proposed(29), which assumed more complex patterns of transit (a combination of linear, exponential and bolus transit). A unique feature of this model was the modeling of transit from small intestine (terminal ileum) to ascending colon: transit was assumed to occur in two boluses 30 minutes apart. Further, this model divided the colon into three segments, in which the segments ascending colon and transverse colon correspond to the upper large intestine of the previous models and the third segment was the descending/rectosigmoid colon (equivalent to lower large intestine in the earlier models). In comparison with the earlier ICRP 30 model, stomach residence times for solids were essentially unchanged, but small intestinal residence times increased and colon residence times decreased. The RIDIC model was used to consider age- and gender-related differences in GI transit.
More recently, the ICRP published a report containing a revised Human Alimentary Tract Model (HATM) for computing doses to the GI tract(30), to replace the ICRP 30 model and to take into account new information about gut transit and radiation sensitivity of various segments of the GI tract. The HATM also included modifications to allow for consideration of retention in the oral cavity, exposure to the esophagus, and estimates of dosimetry in children. In this model, the entire colon is treated as a single segment. The model was designed to allow consideration of various ingested radionuclides, but did not specifically consider radiolabeled test meals which are nonabsorbable. Although they recognized that the RIDIC model took into account some specific differences in movement of meals in different segments of the GI tract, the ICRP felt that first-order kinetics for each segment gives an adequate estimate of the movement for purposes of dosimetry calculation.
The dose calculations reported here are based on the catenary compartment model(28) as used by Siegel et al(27) and uses exclusively first-order kinetics as recommended in HATM(30). In addition to the target organs, the EDE is reported. The normal transit times for stomach were assumed to be 1.42 h for solids(2), 0.44 h for semisolid (oatmeal)(7) and 0.35 h for liquids; normal transit half-times for small intestine, upper large intestine and lower large intestine were assumed to be 4 h, 10 h and 18 h, respectively(29, 31). Physical decay of the radiolabel was also considered in the model. It was assumed that the meal is instantaneously deposited in the stomach, transits in one direction through the GI tract, and that no absorption into the bloodstream occurs. After calculation of the cumulated activities (uCi-hr, 133.2 MBq-s = 1 uCi-hr) in each segment of the GI tract, these four source organs were used to compute the dose to all the target organs in the body according to the RADAR method (RAdiation Dose Assessment Resource, www.doseinfo-radar.com). Phantoms for each radionuclide for adult male and adult female were used to calculate dose to each organ. Weighting factors from ICRP 103 (32) were used to compute EDE. The results are reported in Table 3. Detailed organ doses for the 99mTc-SC-labeled low-fat egg white sandwich meal are given in Table 4 for adult males and adult females with normal transit times.
Table 3.
Dosimetry for orally administered test meals in gastric emptying studies
| Meal | Amount | Condition | Highest | Dose | EDE |
|---|---|---|---|---|---|
| Organ | mrem (mSv) | mrem (mSv) | |||
| SOLID (e.g., low-fat egg sandwich) | |||||
| 99mTc-egg | 500 uCi | adult male, normal transit | ULI | 166 (1.66) | 35 (0.35) |
| 99mTc-egg | 500 uCi | adult male, gastroparesis* | S | 313 (3.13) | 51 (0.51) |
| 99mTc-egg | 500 uCi | adult male, constipation§ | ULI | 239 (2.39) | 35 (0.35) |
| 99mTc-egg | 500 uCi | adult female, normal transit | ULI | 194 (1.94) | 45 (0.45) |
| 99mTc-egg | 500 uCi | adult female, gastroparesis* | S | 368 (3.68) | 62 (0.62) |
| 99mTc-egg | 500 uCi | adult female, constipation§ | ULI | 279 (2.79) | 46 (0.46) |
| 111In-egg | 250 uCi | adult male, normal transit | LLI | 1871 (18.71) | 217 (2.17) |
| 111In-egg | 250 uCi | adult male, constipation§ | LLI | 3019 (30.19) | 452 (4.52) |
| 111In-egg | 250 uCi | adult female, normal transit | LLI | 2110 (21.1) | 289 (2.89) |
| 111In-egg | 250 uCi | adult female, constipation§ | LLI | 3414 (34.14) | 603 (6.03) |
| 67Ga-egg | 500 uCi | adult male, normal transit | ULI | 3013 (30.12) | 306 (3.06) |
| 67Ga-egg | 500 uCi | adult female, normal transit | ULI | 3290 (32.90) | 373 (3.73) |
| SEMISOLID (e.g., oatmeal) | |||||
| 99mTc-oats | 500 uCi | adult male, normal transit | ULI | 189 (1.89) | 30 (0.30) |
| 99mTc-oats | 500 uCi | adult female, normal transit | ULI | 221 (2.21) | 40 (0.40) |
| LIQUID (non-nutrient, e.g., water) | |||||
| 99mTc-SC | 500 uCi | adult male, normal transit | ULI | 191 (1.91) | 30 (0.30) |
| 99mTc-SC | 500 uCi | adult female, normal transit | ULI | 224 (2.24) | 40 (0.40) |
| 111In-DTPA | 125 uCi | adult male, normal transit | LLI | 950 (9.5) | 102 (1.02) |
| 111In-DTPA | 125 uCi | adult female, normal transit | LLI | 1057 (10.57) | 114 (1.14) |
Abbreviations: S = stomach, ULI = upper large intestine, LLI= lower large intestine
Normal transit times: S = 1.42h (solid), 0.44h (semisolid), 0.35h (liquid); SI = 4h; ULI = 8 h; LLI = 18 h
gastroparesis = stomach T ½ = 10 h
constipation transit times: ULI = 48h, LLI = 72h
Table 4.
Estimated Absorbed Radiation Doses for 99mTc-Low Fat Egg White Sandwich Meal, mrem/0.5 mCi (mSv/18.5 MBq) NORMAL TRANSIT
| Organ | Adult Male | Adult Female | ||
|---|---|---|---|---|
| Upper Lg Intestine wall | 165.8 | (1.66) | 194.4 | (1.94) |
| Lower Lg Intestine wall | 127.2 | (1.27) | 142.9 | (1.43) |
| Sm Intestine wall | 107.8 | (1.08) | 133.4 | (1.33) |
| Stomach wall | 103.9 | (1.04) | 123.4 | (1.23) |
| Ovaries | - | - | 52.7 | (0.53) |
| Uterus | - | - | 34.7 | (0.35) |
| Testes | 2.1 | (0.02) | - | - |
| Gallbladder Wall | 24.3 | (0.24) | 31.3 | (0.31) |
| Pancreas | 19.3 | (0.19) | 24.4 | (0.24) |
| Spleen | 12.8 | (0.13) | 15.1 | (0.15) |
| Urinary Bladder Wall | 11.3 | (0.11) | 14.9 | (0.15) |
| Kidneys | 11.1 | (0.11) | 13.4 | (0.13) |
| Red Marrow | 8.3 | (0.08) | 10.0 | (0.10) |
| Adrenals | 6.2 | (0.06) | 8.0 | (0.08) |
| Brain | 0.0 | (0.00) | 0.0 | (0.00) |
| Breasts | 0.9 | (0.01) | 1.2 | (0.01) |
| Heart wall | 3.5 | (0.04) | 4.8 | (0.05) |
| Liver | 7.0 | (0.07) | 9.4 | (0.09) |
| Lungs | 1.8 | (0.02) | 2.7 | (0.03) |
| Muscle | 6.0 | (0.06) | 7.5 | (0.08) |
| Bone Surf | 7.6 | (0.08) | 9.6 | (0.10) |
| Skin | 1.8 | (0.02) | 2.2 | (0.02) |
| Esophagus | 0.6 | (0.01) | 0.9 | (0.01) |
| Thyroid | 0.1 | (0.00) | 0.1 | (0.00) |
| Salivary Glands | 0.0 | (0.0) | 0.0 | (0.00) |
Since the primary interest in performing GI transit studies is to evaluate patients with suspected alterations in transit times of food, it seems reasonable to estimate radiation doses in conditions in which transit is abnormal. For example, if gastric emptying of a 99mTc-labeled meal is significantly delayed from a normal half-emptying time of 1.42 hours to 10 hours, the model predicts that this will increase the radiation dose to the stomach by a factor of approximately two (Table 3). The EDE in this case is increased by about 50%. A situation of constipation was also modeled. Based on a study using 131I-fiber(31), normal subjects had upper and lower large intestine transit times of 8 and 18 hr, and constipated patients had upper and lower large intestine transit times of 48 and 72 hr, respectively. With a 99mTc-labeled solid meal, modeling this extent of constipation increased the dose to the upper large intestine (ULI) by about 44%, but did not significantly affect the EDE. When the longer-lived 111In is used for the solid meal label, however, this degree of constipation increased the dose to the lower large intestine (LLI) by about 61% and more than doubled the EDE (Table 3).
In this modeling, the same transit times were used for adult males and females, and the only differences were in the phantoms which take into account the different organ sizes and distances between organs. It is well known that transit times differ between normal males and females, between individuals of different ages, and between premenopausal women in different phases of the menstrual cycle(33–36). These differences are expected to affect primarily the transit times of the stomach and the colon(29), and should have effects on radiation dose similar to those modeled in the gastroparesis and constipation examples, except that the changes would be less pronouced than in the extreme examples modeled here.
There is a need to estimate radiation dosimetry in children who receive radiolabeled meals for gastric emptying studies. Unfortunately, there is a lack of information on normal transit times in children due to a reluctance to expose normal children to radiation for purely research purposes. Future studies should attempt to gather data from children who are undergoing gastric emptying studies as part of their care, to establish ranges of typical transit times and permit estimation of radiation dosimetry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meyer JH, MacGregor IL, Gueller R, Martin P, Cavalieri R. 99mTc-tagged chicken liver as a marker of solid food in the human stomach. American Journal of Digestive Diseases. 1976;21:296–304. doi: 10.1007/BF01071842. [DOI] [PubMed] [Google Scholar]
- 2.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EMM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Rydén J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ, Jr, Ziessman HA. Consensus recommendations for gastric emptying scintigraphy: A joint report for the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 4.ACR-SNM-SPR PRACTICE GUIDELINE FOR THE PERFORMANCE OF GASTROINTESTINAL SCINTIGRAPHY. 2010 wwwacrorg/SecondaryMainMenuCategories/quality_safety/guidelines/nuc_med/gi_scint igraphyaspx.
- 5.Knight LC, Kantor S, Doma S, Parkman HP, Maurer AH. Egg labeling methods for gastric emptying scintigraphy are not equivalent in producing a stable solid meal. J Nucl Med. 2007;48(11):1897–1900. doi: 10.2967/jnumed.107.044636. [DOI] [PubMed] [Google Scholar]
- 6.Drubach LA, Kourmouzi V, Fahey FH. Cheese is a reliable alternative meal for solid-phase gastric emptying study. Nucl Med Commun. 2010;31(5):430–433. doi: 10.1097/MNM.0b013e328333d2f0. [DOI] [PubMed] [Google Scholar]
- 7.Klingensmith WC, 3rd, Rhea KL, Wainwright EA, Hopper OW. The gastric emptying study with oatmeal: reference range and reproducibility as a function of age and sex. J Nucl Med Technol. 2010;38(4):186–190. doi: 10.2967/jnmt.110.077065. [DOI] [PubMed] [Google Scholar]
- 8.Maloy TA, Herrera M, Zimmer M, Spies SM. Optimal radiolabelling methods for Tc99m Sulfur Colloid oatmeal products for gastric emptying. Society of Nuclear Medicine 58th Annual Meeting; June 6, 2011; San Antonio, TX. 2011. p. 2334. [Google Scholar]
- 9.Schwartz JG, Green GM, Guan D, McMahan CA, Phillips WT. Rapid gastric emptying of a solid pancake meal in Type II diabetic patients. Diabetes Care. 1996;19(5):468–471. doi: 10.2337/diacare.19.5.468. [DOI] [PubMed] [Google Scholar]
- 10.Poitras P, Picard M, Déry R, Giguere A, Picard D, Morais J, Plourde V, Boivin M. Evaluation of gastric emptying function in clinical practice. Digestive Diseases and Sciences. 1997;42:2183–2189. doi: 10.1023/a:1018877512302. [DOI] [PubMed] [Google Scholar]
- 11.Simonian HP, Maurer AH, Knight LC, Kantor S, Kontos D, Megalooikonomou V, Fisher RS, Parkman HP. Simultaneous assessment of gastric accommodation and emptying: Studies with liquid and solid meals. J Nucl Med. 2004;45:1155–1160. [PubMed] [Google Scholar]
- 12.Jurgens MJ, Drane WE, Vogel SB. Dual-radionuclide simultaneous biliary and gastric scintigraphy to depict surgical treatment of bile reflux. Radiology. 2003;229:283–287. doi: 10.1148/radiol.2291020661. [DOI] [PubMed] [Google Scholar]
- 13.Gurd FRN, Wilcox PE. Complex formation between metallic cations and proteins, peptides, and amino acids. Advances in Protein Chemistry. 1956;11:311–427. doi: 10.1016/s0065-3233(08)60424-6. [DOI] [PubMed] [Google Scholar]
- 14.Maurer AH, Knight LC, Siegel JA, Scopinaro F, Perri JA, Krevsky B, Fisher RS, Malmud LS. Use of a new In-111-labeled solid meal for confirmation of the lag phase of gastric emptying. Radiology. 1986;161(P):135. [Google Scholar]
- 15.Sachdava P, Kantor S, Knight LC, Maurer AH, Fisher RS, Parkman HP. Use of a high caloric liquid meal (Ensure Plus) as a alternative meal for gastric emptying scintigraphy. Gastroenterology. 2010;138(5 Suppl 1):S715–S716. doi: 10.1007/s10620-013-2665-2. [DOI] [PubMed] [Google Scholar]
- 16.Ziessman HA, Okolo PI, Mullin GI, Chander A. Liquid gastric emptying is often abnormal when solid emptying is normal. J Clin Gastroenterol. 2009;(43):639–643. doi: 10.1097/mcg.0b013e318181b42f. [DOI] [PubMed] [Google Scholar]
- 17.Ziessman HA, Chander A, Clarke JO, Ramos A, Wahl RL. The added diagnostic value of liquid gastric emptying compared with solid emptying alone. J Nucl Med. 2009;50:726–731. doi: 10.2967/jnumed.108.059790. [DOI] [PubMed] [Google Scholar]
- 18.Bluck LJC. Recent advances in the interpretation of the 13C octanoate breath test for gastric emptying. J Breath Res. 2009;3:1–8. doi: 10.1088/1752-7155/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 19.Bateman DN, Whittingham TA. Measurement of gastric emptying by real-time ultrasound. Gut. 1982;23:524–527. doi: 10.1136/gut.23.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut. 1996;38:816–821. doi: 10.1136/gut.38.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens JE, Gilja OH, Gentilcore D, Hausken T, Horowitz M, Jones KL. Measurement of gastric emptying of a high-nutrient liquid by 3D ultrasonography in diabetic gastroparesis. Neurogastroenterol Motil. 2011;23(3):220–225. e113–e114. doi: 10.1111/j.1365-2982.2010.01630.x. [DOI] [PubMed] [Google Scholar]
- 22.Russo F, Clemente C, Linsalata M, Chiloiro M, Orlando A, Marconi E, Chimienti G, Riezzo G. Effects of a diet with inulin-enriched pasta on gut peptides and gastric emptying rates in healthy young volunteers. Eur J Nutr. 2011;50(4):271–277. doi: 10.1007/s00394-010-0135-6. [DOI] [PubMed] [Google Scholar]
- 23.Schwizer W, Fox M, Steingötter A. Non-invasive investigation of gastrointestinal functions with magnetic resonance imaging: towards an "ideal" investigation of gastrointestinal function. Gut. 2003;52 Suppl IV doi: 10.1136/gut.52.suppl_4.iv34. iv34-iv9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone SF, Tanganelli I, Capodivento S, Ricci V, Volterrani L. Magnetic resonance imaging in the evaluation of the gastric emptying and antral motion: feasibility and reproducibility of a fast not invasive technique. Eur J Radiol. 2010;75(2):212–214. doi: 10.1016/j.ejrad.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Eve IS. A review of the physiology of the gastrointestinal tract in relation to radiation doses from radioactive materials. Health Physics. 1966;12:131–161. doi: 10.1097/00004032-196602000-00002. [DOI] [PubMed] [Google Scholar]
- 26.ICRP. Dosimetric model for the gastrointestinal tract. Annals of the ICRP, Publication 30. 1979:30–34. ;2(3/4, ICRP Publ. 30, Part I) [Google Scholar]
- 27.Siegel JA, Wu RK, Knight LC, Zelac RE, Stern HS, Malmud LS. Radiation dose estimates for oral agents used in upper gastrointestinal disease. J Nucl Med. 1983;24:835–837. [PubMed] [Google Scholar]
- 28.Bernard SR, Hayes RL. Dose to various segments of the gastrointestinal tract. In: Cloutier RJ, Edwards CL, Snyder WS, editors. Medical Radionuclides: Radiation Dose and Effects. Oak Ridge, TN: USAEC Division of Technical Information Extension; 1970. pp. 295–314. [Google Scholar]
- 29.Stubbs JB. Results from a new mathematical model of gastrointestinal transit that incorporates age and gender-dependent physiological parameters. Radiation Protection Dosimetry. 1992;41:63–69. [Google Scholar]
- 30.ICRP. ICRP Publication 100: Human alimentary tract model for radiological protection: International Commision on Radiological Protection. 2004 doi: 10.1016/j.icrp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.McLean RG, Smart RC, Gastton-Perry D, Barbagallo S, Baker J, Lyons NR, Bruck CE, King DW, Lubowski DZ, Talley NA. Colon transit scintigraphy in health and constipation using oral Iodine-131-cellulose. J Nucl Med. 1990;31:985–989. [PubMed] [Google Scholar]
- 32.ICRP. ICRP 103. The 2007 recommendations of the International Commission on Radiological Protection. Annals of the ICRP. 2007 [Google Scholar]
- 33.Datz FL, Christian PE, Moore JG. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204–1207. [PubMed] [Google Scholar]
- 34.Gill RC, Murphy PD, Hooper HR, Bowes KL, Kingma YJ. Effect of the menstrual cycle on gastric emptying. Digestion. 1987;36:168–174. doi: 10.1159/000199414. [DOI] [PubMed] [Google Scholar]
- 35.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92:968–975. [PubMed] [Google Scholar]
- 36.Moore JG, Tweedy C, Christian PE, Datz FL. Effect of age on gastric emptying of liquid-solid meals in man. Digestive Diseases and Sciences. 1983;28:340–344. doi: 10.1007/BF01324951. [DOI] [PubMed] [Google Scholar]
- 37.Malmud LS, Fisher RS, Knight LC, Rock E. Scintigraphic evaluation of gastric emptying. Seminars in Nuclear Medicine. 1982;12:116–125. doi: 10.1016/s0001-2998(82)80003-2. [DOI] [PubMed] [Google Scholar]
- 38.Norris FS. Method of technetium-99m tagging of cottage cheese for gastrointestinal motility studies. J Nucl Med. 1983;24:99. [Google Scholar]
- 39.Christian PE, Moore JG, Datz FL. Comparison of Tc-99m labeled liver and liver paté as markers for solid-phase gastric emptying. J Nucl Med. 1984;25:364–366. [PubMed] [Google Scholar]
- 40.Theodorakis MC, Groutas WC, Whitlock TW, Tran K. Tc-99m-labeled polystyrene and cellulose macromolecules: Agents for gastrointestinal scintigraphy. J Nucl Med. 1982;23:693–697. [PubMed] [Google Scholar]
- 41.Wirth N, Swanson D, Shapiro B, Nakajo M, Coffey JL, Eckhauser F, Owyang C. A conveniently prepared Tc-99m resin for semisolid gastric emptying studies. J Nucl Med. 1983;24:511–514. [PubMed] [Google Scholar]