Summary
T helper 17 (Th17) cells specifically transcribe the Il17 and Il17f genes, which are localized in the same chromosome region, but the underlying mechanism is unclear. Here, we report a cis element that we previously named conserved noncoding sequence 2 (CNS2), physically interacted with both Il17 and Il17f gene promoters and was sufficient for regulating their selective transcription in Th17 cells. Targeted deletion of CNS2 resulted in impaired retinoic acid-related orphan receptor gamma (RORγ)-driven IL-17 expression in vitro. CNS2-deficient T cells also produced substantially decreased amounts of IL-17F. These cytokine defects were associated with defective chromatin remodeling in the Il17-Il17f gene locus, possibly due to effects on CNS2-mediated recruitment of histone modifying enzymes p300 and JmjC domain-containing protein 3 (JMJD3). CNS2-deficient animals were also shown to be resistant to experimental autoimmune encephalomyelitis (EAE). Our results thus suggest that CNS2 is sufficient and necessary for Il17 and optimal Il17f gene transcription in Th17 cells.
Introduction
In contrast to the effector CD4+ T helper 1 (Th1) and Th2 cells which produce interferon (IFN)-γ and interleukin (IL)-4, respectively, Th17 cells uniquely produce two related effector cytokine IL-17 and IL-17F, and these cytokines have been shown to be important in immunity against bacteria and fungi (Korn et al., 2009). However, because of the powerful pro-inflammatory activities conferred by IL-17 and IL-17F, Th17 cells also potently induce or maintain tissue inflammation and can cause many inflammatory and autoimmune diseases, including multiple sclerosis (MS), asthma, rheumatoid arthritis (RA), and inflammatory bowl diseases (IBD) (Dong, 2008; Korn et al., 2009). Therefore, the transcriptional regulation of the Il17 and Il17f genes has attracted much attention recently.
The Il17 and Il17f genes are encoded at the same chromosomal locus. They are separated by ~43.9 kb in mice and are transcribed in opposite directions. Th17 cell differentiation requires IL-6 and transforming growth factor (TGF)-β (Dong, 2008; Korn et al., 2009), and IL-1 is also important in regulating early Th17 cell differentiation (Chung et al., 2009). These cytokines, together with T cell receptor signaling, lead to activation of signal transducer and activator of transcription 3 (STAT3), interferon regulatory factor 4 (IRF4), retinoic acid-related orphan receptor (ROR)γt, RORα, Runt-related transcription factor 1 (RUNX1), B-cell-activating transcription factor (Batf) and IkappaBζ (IκBζ) (Chen et al., 2006; Ivanov et al., 2006; Okamoto et al., 2010; Schraml et al., 2009; Yang et al., 2008b; Zhang et al., 2008). Among these transacting factors, RORγt was identified as the “master regulator” in Th17 cells, and is both necessary and sufficient for IL-17 and IL-17F expression (Ivanov et al., 2006). RORα plays redundant and synergistic functions with RORγt (Yang et al., 2008b). Despite these studies on trans-acting factors, their target cis-acting elements in driving selective Th17-specific cytokine expression are poorly understood.
Cis-acting regulatory elements are often conserved across species (Meireles-Filho and Stark, 2009), and have been shown to be crucial in controlling T cell differentiation. In Th2 cells, the effector cytokine genes are controlled by a conserved non-coding sequence (CNS) 1 located between the Il4 and Il13 genes, a locus control region (LCR) between the Il13 and Il5 genes, an intronic enhancer HS2 within the Il4 gene, and several other cis-elements (Lee et al., 2001; Solymar et al., 2002; Tanaka et al., 2010). Targeted deletion of these cis-elements impairs the expression of Th2 cytokines in vitro and in vivo (Loots et al., 2000; Lee et al., 2005; Tanaka et al., 2010). In regulatory T (Treg) cells, the stability, frequency and tissue or organ-specific development of (forkhead box P3) FOXP3+ T cells are controlled by three different non-coding sequences CNS1–3 (Zheng et al. 2010). The expression of IFN-γ in Th1 cells may be controlled predominantly by a T-bet-dependent enhancer CNS-22, a conserved sequence located 22 kb upstream of the IFN-γ gene, based on studies using a transgenic model (Hatton et al., 2006). In T cell development, one important feature of cis-acting regulatory elements is that they undergo permissive chromatin remodeling in a lineage-specific manner thereby allowing the binding of various trans-acting factors for selective gene transcription. Additionally, the distal cis-regulatory elements may interact with the cytokine gene promoters by forming a 3-dimentional loop, which therefore provides a platform capable of initiating gene transcription by bringing together transacting factors to a close spatial proximity, such as the cis-regulatory loop formed at the Th2 cytokine gene locus (Spilianakis and Flavell, 2004).
To characterize the mechanism underlying lineage-specific expression of IL-17 and IL-17F, we previously identified a total of eight conserved non-coding (CNS) sites in the Il17-Il17f locus by sequence comparison and found at least several of them, including CNS2, were associated with hyperacetylated histone H3, a marker of permissive chromatin structure (Wilson et al., 2009), in Th17 cells (Akimzhanov et al., 2007). The lineage-specific chromatin remodeling of CNS2 and the Il17-Il17f locus was recently confirmed by global mapping histone modifications in different T-helper cells (Wei et al., 2009). Interestingly, we observed that CNS2 functioned to promote the activation of Il17 gene promoter possibly through binding to ROR factors (Yang et al., 2008b). In addition, CNS2 may also interact with other Th17-regulating transcription factors (Okamoto et al., 2010; Schraml et al., 2009; Zhang et al., 2008). These in vitro data together suggest that CNS2 may be important in regulating IL-17 expression in vivo. In this study, we generated CNS2-deficient mice by homologous recombination. With these mice, the role of CNS2 and the underlying mechanism in regulating IL-17 and IL-17F expression and Th17 cell differentiation was evaluated in both in vitro and in vivo systems.
RESULTS
CNS2 regulated the lineage-specific transcription of both Il17 and Il17f genes
To test whether CNS2 functions as a lineage-specific regulatory element, we first assessed whether it was bound by p300, because p300 binding has recently shown to predict enhancer elements in the genome (Visel et al., 2009). We thus performed a chromatin immunoprecipitation (ChIP) assay using an antibody to p300. CNS2 was bound by p300 specifically in Th17 but not in Th1 cells whereas the IFN-γ promoter (IFNγp) interacted with p300 only in Th1 cells (Figure S1a).
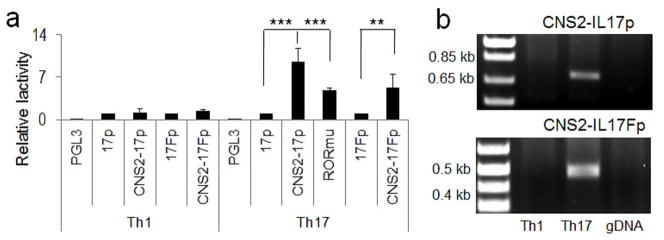
The regulation of IL-17 by CNS2 has been suggested by luciferase reporter assays in EL4 and Jurkat cells (Yang et al., 2008b; Zhang et al., 2008). To further test if CNS2 dictates Th17-specific transcription of the Il17 and Il17f genes, we developed a luciferase reporter assay in primary T cells. Wheras the Il17 or Il17f gene promoter did not exhibit substantially increased activation in Th17 compared to Th1 cells, addition of CNS2 increased the Il17 promoter activity by approximately 9.5 fold in Th17 but not Th1 cells (Figure 1a). In addition, CNS2 also increased the cell type-specific Il17f promoter activity (Figure 1a). These results suggest that CNS2 is sufficient in driving lineage-specific transcription of the Il17 and Il17f genes. To examine whether the role of CNS2 is dependent on binding by ROR factors, the two ROR-binding sites (RORE) predicted in the CNS2 region were mutated, which consistently led to ~50% reduction of the promoter activity of the CNS2-Il17 reporter construct (Figure 1a), although it completely lost ROR-mediated transcription in EL4 cells (Figure S1b), excluding the presence of additional RORE sites in the CNS2 region.
Figure 1. CNS2 was sufficient to regulate lineage-specific Il17 and Il17f gene transcription.
a, The luciferase activities of the PGL3, IL-17 promoter (17p)-PGL3, IL-17F promoter (17Fp)-PGL3, CNS2-17p-PGL3 and CNS2-17Fp-PGL3 reporter constructs in Th1 and Th17 cells. In CNS2-17p-PGL3 vector, two putative ROR binding sites were mutated and the mutant reporter was designated as RORmu-PGL3 (Shown here are the combinational results from 2–3 biological replicas). b, The interaction of CNS2 with the Il17 and 17f promoters was determined in Th1 and Th17 cells by 3C analysis. C57BL/6J mouse genomic DNA was used as a negative control for PCR (Shown here is one of three representative results). (See also Figure S1).
Interestingly, we found that CNS2, when fused to the Ifng or Il4 promoters, robustly induced their transcription in Th17 cells, and even in EL4 or 293T cells (Figures S1c-S1e), indicating CNS2 can function independent of Il17 or Il17f gene promoter to regulate Th17-specific transcription. Taken together, these results suggest that CNS2 is sufficient in mediating lineage-specific transcription of Il17 and Il17f genes possibly by serving as an enhancer element.
CNS2 interacted with both the Il17 and Il17f promoters in Th17 cells
CNS2 is located ~2 kb upstream of the Il17 gene promoter and ~56.5 kb away from the Il17f gene in mice (Akimzhanov et al., 2007). To investigate the mechanism underlying this long-distance regulation, we performed a chromosome conformation capture (3C) assay – a powerful technology that can detect long-range intra- or inter-chromosomal associations between distantly related DNA elements (Tolhuis et al., 2002). In this technique, DNA-protein complexes are covalently cross-linked by formaldehyde or paraformaldehyde. Following restriction enzyme digestion, DNA fragments crosslinked together in the same complex can be preferentially ligated together when diluted to a very low concentration, which can be detected by PCR amplification. In Th17 but not Th1 cells, we found that the 5′-end of CNS2 can be directly ligated to the 3′-end of Il17p based on the size of PCR product (Figure 1b). Moreover, a strong association of CNS2 with the Il17fp was also observed (Figure 1b). The cell-type specific association with the Il17 and Il17f promoters further suggests an important role of CNS2 in regulating lineage-specific Il17 and Il17f gene transcription.
CNS2 was required for IL-17 and optimal IL-17F expression in Th17 cells generated in vitro
The importance of CNS2 in the control of IL-17 and IL-17F gene expression has thus far been highlighted by luciferase reporter assays and 3C analysis. To further test the function of CNS2 genetically, CNS2-deficient mice were generated by homologous recombination and the neomycin-selecting gene was subsequently removed by loxp-cre system to avoid its influence over transcription of endogenous genes (Figure S2a). The deletion of CNS2 in mice was confirmed by PCR (Figure S2b).
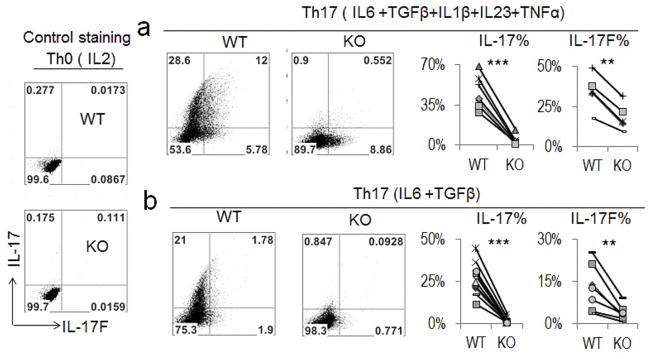
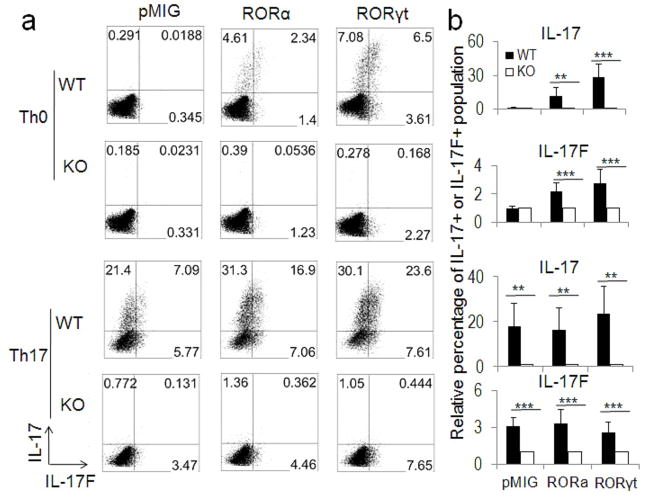
Naïve CD4+ T cells were isolated from both the CNS2 wild-type (WT) and CNS2-deficient mice and polarized into various effector Th cells. The gene expression pattern of these effector T cells was then analyzed by different assays including intracellular staining, real-time RT-PCR or ELISA (enzyme-linked immunosorbent assay). CNS2-deficient T cells expressed less than 10% IL-17 and less than 30–55% IL-17F compared with the WT cells under different Th17 conditions (Figure 2 and Figures S2c–S2d), although they displayed normal Th1, Th2, and iTreg cell differentiation (Figure S2e). The expression of other Th17-related genes, including ROR factors and IL-21, was not affected in CNS2-deficient cells (Figure S2c). These data indicate that CNS2 functions downstream of RORs in mediating the Il17 and Il17F gene transcription. To ascertain this, ROR factors were retrovirally overexpressed in both WT and CNS2-deficient T cells cultured under Th0 and Th17 conditions. Remarkably, overexpression of ROR factors even under Th17-polarized condition could not lead to any noticeable expression of IL-17 in CNS2-deficient cells (Figures 3a and 3b). In addition, a strong reduction in IL-17F expression was also observed in CNS2-deficient cells (Figures 3a and 3b). This result suggests that CNS2 is required for the function of ROR factors in mediating IL-17 expression in Th17 cells.
Figure 2. Impaired expression of IL-17 and IL-17F in vitro in the absence of CNS2.
Th17 cells were generated in vitro using naïve T cells from WT and CNS2-deficient (hereafter referred to as KO in the figure) mice under two Th17 conditions. The expression of IL-17 and IL-17F was determined by intracellular staining. Th0 cells were used as a staining control. Statistical analysis of the intracellular data is shown on the right. (See also Figure S2).
Figure 3. CNS2 was required for ROR-regulated Th17 cell differentiation.
a, WT and CNS2-deficient T cells cultured under Th0 and Th17 conditions were infected with retroviruses RORα-hCD2, RORγt-hCD2 or empty-hCD2 on day 1, and analyzed on day 4 (gated on hCD2+ cells). b, Statistics of the intracellular staining data from three independent retroviral assays. The percentage values from CNS2-deficient cells were set as 1 for comparison between biological replicates.
CNS2 controlled chromatin accessibility at the Il17-Il17f locus
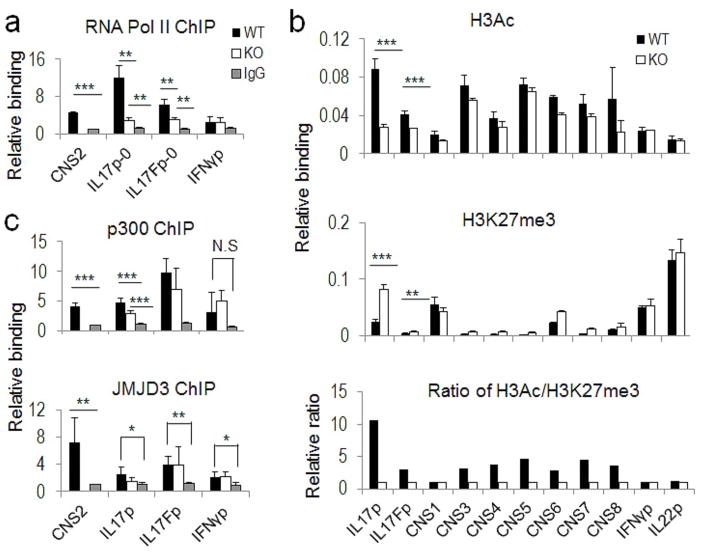
To understand the possible mechanism whereby CNS2 functions to regulate Il17 and Il17f gene transcription, we examined the binding of RNA polymerase II (Pol II) to the Il17p and Il17fp in WT and CNS2-deficient Th17 cells, and found that both CNS2 and the Il17 and 17f promoters interacted with Pol II in WT Th17 cells. Consistent with the findings that CNS2 was more important for Il17 than Il17f gene transcription (Figure 2 and Figures S2c–S2d), deletion of CNS2 greatly reduced the binding of RNA Pol II to the Il17 promoter whereas the binding to the Il17f promoter was only partially affected (Figure 4a).
Figure 4. CNS2 controlled the chromatin accessibility in the Il17-17f gene locus.
The ChIP assays were performed using WT and CNS2-deficient Th17 cells with different antibodies. The binding of antibodies to the Il17-17f gene locus was detected by realtime PCR. When indicated, the Ifng gene promoter (IFNγp) or I promoter (IL22p) or IgG ChIP signal in WT Th17 cellsl22 were used as negative controls for realtime PCR assays. a, Anti-RNA Pol II ChIP assay. b, Anti-H3Ac and anti-H3K27me3 ChIP assays. The results were normalized to the input control and the overall change of chromatin accessibility was evaluated by using the ratio of histone markers H3Ac/H3K27me3. Shown here is one of three representative results. c, Anti-p300 and anti-JMJD3 ChIP assays. The assay performed with anti-RNA Pol II, p300 or JMJD3 is shown as the combinational result from 2–3 independent experiments and the data from each replicate were normalized to the average IgG ChIP signals in WT and CNS2-deficient Th17 cells for comparison across biological replicates. (See also Table S1 and Figure S3).
To assess whether the chromatin accessibility at the Il17-17f gene locus may be altered by the absence of CNS2, a ChIP assay was performed using antibodies against acetyl histone H3 (H3Ac) and K27-tri-methylated histone (H3K27me3) in Th17 cells. In the Il17 promoter region, permissive histone marker H3Ac was significantly reduced in CNS2-deficient cells along with increased presence of repressive histone marker H3K27me3 (Wilson et al., 2009) (Figure 4b). In addition, chromatin remodeling was also affected, though to a lesser extent, in the Il17f promoter region and other CNS sites (Figure 4b). The results here thus suggest that chromatin remodeling of the Il17-Il17f locus is at least partially dependent on CNS2. Moreover, the extent of the altered chromatin structures caused by CNS2 deficiency at the respective promoter regions was consistent with the level of cytokine expression.
To further understand how CNS2 regulates chromatin accessibility, we examined the binding of several histone remodeling enzymes to CNS2 and to the Il17-17f promoter regions, and found that CNS2 interacted with p300 and JmjC domain-containing protein 3 (JMJD3) in Th17 cells (Figure 4c). p300 contains histone acetyltransferases (HAT) activity and mediates permissive histone acetylation (Wang et al., 2008); JMJD3 has been identified as a histone demethylase that specifically removes repressive histone marker H3K27me3 (Agger et al., 2007; De Santa et al., 2007; Xiang et al., 2007). Considering CNS2 association with both the Il17 and 17f promoters as shown by our 3C analysis, binding of these histone remodeling enzymes to CNS2 may increase their localization near the promoters and therefore increase locus-specific enzymatic activity. Interestingly, deletion of CNS2 significantly decreased the binding of p300 to the Il17 promoter but less to the Il17f promoter in Th17 cells (Figure 4c).
Previous ChIP and electrophoretic mobility shift (EMS) assays suggest that RORγt physically interacted with CNS2 (Yang et al., 2008b; Zhang et al., 2008). In our retroviral overexpression assays, we found that CNS2 was functionally required for ROR-dependent IL-17 expression in Th17 cells (Figure 3). To further understand the mechanism underlying CNS2-dependent IL-17 transcription, we investigated the role of RORγ in chromatin activation of the Il17-17f gene locus. We found the Il17 promoter showed a hallmark of “closed” chromatin structure in RORγ-deficient Th17 cells characterized by greatly reduced H3Ac and increased H3K27me3 (Figure S3). However, the Il17fp region was only mildly affected by the deletion of RORγ (Figure S3). The RORγt-deficient Th17 cells thus displayed a similar, although not identical, phonotype to that of CNS2-deficient Th17 cells (Figure 4b and Figure S3).
Our results altogether support a model that RORγt (and RORa) binds to CNS2 and initiates chromatin remodeling at Il17-17f locus, in which CNS2 provides a necessary binding site for RORγt to activate the transcription of the Il17 gene.
CNS2 differentially regulated IL-17 and IL-17F production in different types of immune cells in vivo
IL-17 and IL-17F can be expressed not only by Th17 cells but also by innate or innate-like immune cells, such as γδ T and lymphoid tissue inducer (LTi) or LTi-like cells (Cua and Tato, 2010). We first examined memory CD4+ T cells and found that CNS2 deficiency impacted IL-17 and IL-17F expression in a similar manner as in Th17 cells generated in vitro (Figure 5 and Figure S4). Th17 cells are also abundant in gut-associated lymphoid tissues and are suggested to play an important role in mucosal immunity or immune homeostasis in the gut (Cua and Tato, 2010; Ivanov et al., 2006). We thus isolated laminal propria (LP) cells and intestinal intraepithelial lymphocytes (IELs) from the intestinal tissues of healthy WT and CNS2-deficient mice for intracellular staining analysis. To our surprise, CNS2 was required for optimal expression of IL-17 protein but not for IL-17F, which even showed increased expression in CNS2-deficient cells (Figure S5a).
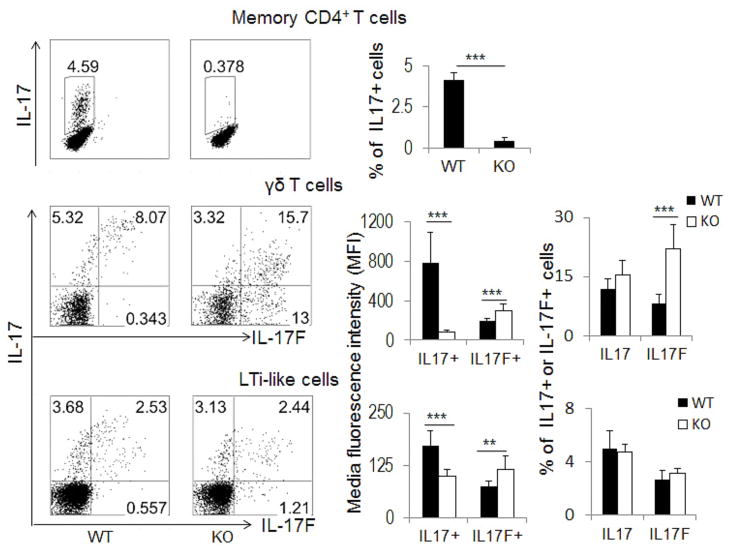
Figure 5. Regulation of IL-17 or IL-17F expression by CNS2 in unchallenged mice.
The expression of IL-17 in memory CD4+ T cells (gated on CD4+CD62LloCD44hiCD25−) (WT=4, KO=3) or IL-17 and IL-17F in γδ T cells (gated on γδ+ TCR) (WT=4, KO=4) and LTi-like cells (gated on CD4+ CD3−) (WT=5, KO=4) was analyzed by intracellular staining after ex vivo restimulation. The staining data were shown in the left panel, and the statistical data of media fluorescence intensity (MFI) and intracellular staining (for γδ T and LTi-like cells) or intracellular staining data alone (for memory CD4+ T cells) were shown in the corresponding right panel as mean ± SD (standard deviation). All the experiments were repeated 2–3 times with consistent results. (See also Figure S4).
To determine whether CNS2 also regulates the expression of IL-17 and IL-17F in innate immune cells, γδ T and LTi-like cells were isolated from the spleens in healthy CNS2-deficient mice and their WT control mice and then analyzed for Th17-related gene expression after ex vivo restimulation. In contrast to Th17 cells, CNS2 did not greatly alter the frequency of IL-17+ expressing cells in either γδ T or LTi-like cells (Figure 5). Instead, deletion of CNS2 led to substantially reduced per-cell protein level of IL-17 as judged by media fluorescence intensity (MFI) of IL-17 staining, with similar effects at the mRNA level (Figure 5 and Figure S4). In addition, we found that CNS2 was no longer required for IL-17F expression in both γδ T and LTi-like cells and deletion of CNS2 even led to its increased expression (Figure 5 and Figure S4). These results suggest that CNS2 differentially controls IL-17 and IL-17F expression in not only Th17 cells but also between adaptive and innate immune cells.
CNS2-deficient mice were resistant to experimental autoimmune encephalomyelitis (EAE)
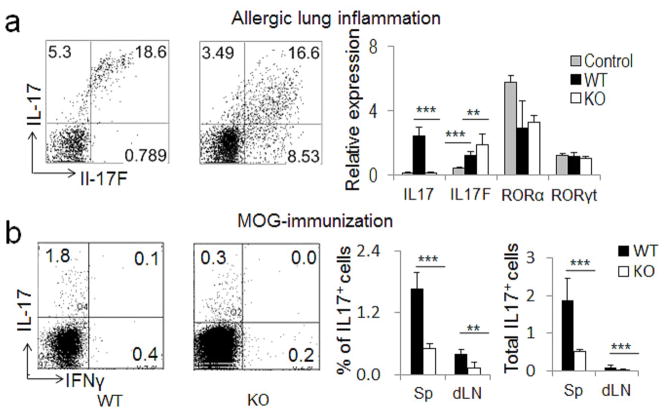
To further understand the function of CNS2 during in vivo immune responses, we first examined the role of CNS2 in an allergic lung inflammation model by intranasally challenging mice with an allergenic fungal proteinase (FAP) derived from Aspergillus oryzae as described (Kiss et al., 2007; Yang et al., 2008a). As expected, IL-17F expression was not impaired in lung tissues and lung γδ T cells, and was even increased in the absence of CNS2 (Figure 6a and Figures S5b–S5c). Accordingly, we observed more neutrophils infiltrating the brondchoalveolar lavage (BAL) of CNS2-deficient mice (Figure S5d).
Figure 6. Regulation of IL-17 or 17F by CNS2 in innate and adaptive immune responses.
a, Age-matched WT and CNS2-deficient mice (n=5 or 6) were intranasally challenged with FAP twice on day 1 and then analyzed on day 2 for IL-17 and IL-17F expression in lung γδ T cells (left: intracellular staining data) or in the lung tissues (right: realtime RT-PCR data). b, Age-matched WT and CNS2-deficient mice (n=5) were immunized with MOG35–55 peptides on day 1 and then analyzed on day 8 for IL-17 and IFN-γ production. Left: intracellular staining data from MOG35–55 restimulated splenocytes. Middle: statistics of IL-17 staining data in spleens (Sp) or draining lymph nodes (dLN). Right: statistics of the total number of IL-17+ cells in Sp or dLN. (See also Figure S5).
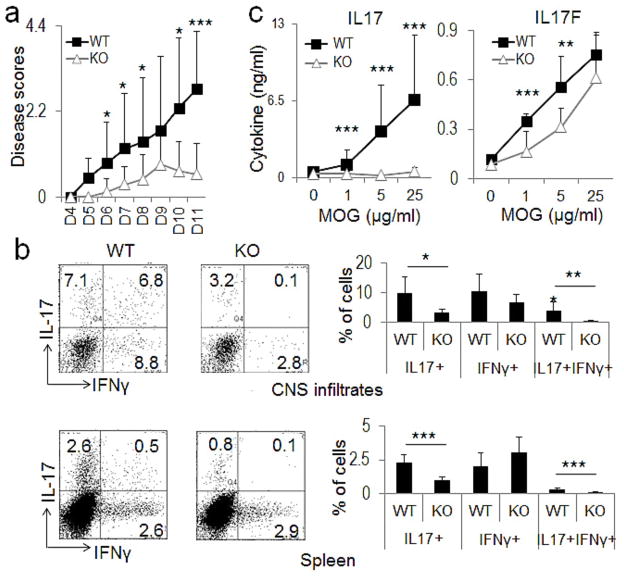
To understand the role of CNS2 in Th17 generation in vivo, we immunized mice with MOG35–55 peptide, and found that the number and frequency of MOG-specific IL-17+ Th17 cells were greatly reduced in both the spleens and draining lymph nodes of CNS2-deficient mice (Figure 6b). To further test the contribution of CNS2 in the development of Th17-dependent autoimmune diseases, both WT and CNS2-deficient mice were subjected to myelin oligodendrocyte glycoprotein (MOG) peptide-induced EAE – an autoimmune mouse disease model that closely resembles human multiple sclerosis (Baxter, 2007). The severity of EAE diseases was significantly reduced in CNS2-deficient mice (Figure 7a). Within the 11-day period after second MOG immunization, only one of the six CNS2-deficient mice reached score 2. However, 5 of the 6 WT mice reached scores 2.5 or above. In addition, the disease onset was also delayed in CNS2-deficient mice (Figure 7a). These results are consistent with the phenotype of EAE diseases in IL-17-deficient mice (Komiyama et al., 2006; Yang et al., 2008a). Interestingly, 2 of the 4 diseased CNS2-deficient mice showed signs of recovery on day 9 after second immunization and one of them was fully recovered on day 10 (data not shown). However, none of the 5 diseased WT mice showed any signs of recovery. The phenotype of early recovery from EAE was also observed in IL-17F-deficient mice (Komiyama et al., 2006; Yang et al., 2008a).
Figure 7. CNS2-deficient mice were resistant to EAE.
a, WT and CNS2-deficient mice were immunized twice with MOG35–55. Mean clinical scores are shown versus days after second MOG immunization. Shown here is the combinational result of two EAE experiments (the total number of mice used: WT = 12, KO = 11). b, The IL-17 and IFN-γ level was determined in the infiltrates of central nervous system (CNS) and the splenocytes of MOG35–55-immunized mice. Left: intracellular staining; Right: statistic of the staining data. c, IL-17 and IL-17F expression in the splenocytes of immunized mice was determined by ELISA after restimulation using MOG35–55 peptide. A total of 6 WT and 5–6 KO mice were analyzed in Figures 7b and 7c. All the data were shown as mean ± SD. (See also Figure S6).
We then examined the expression of IL-17 and IL-17F in EAE mice. There was ~3 fold reduction in the percentages of IL-17+ cells in the central nervous system and ~2.3 fold reduction in the spleens of CNS2-deficient mice when compared with WT mice (Figure 7b). A dramatic reduction of IL-17 secretion was also observed by ELISA assays in the splenocytes of CNS2-deficient mice in EAE experiments (Figure 7c). Consistent with the in vitro differentiation results, IL-17F expression was also partially reduced as determined by ELISA assays (Fig. 7c) or by intracellular staining in CNS2-deficient mice (Figure S6). Interestingly, although the percentages of IFN-γ+ cells were not significantly affected by the deletion of CNS2, the IL-17+ and IFN-γ+ double positive cells were completely absent in CNS2-deficient mice (Figure 7b). These data pinpoint the importance of CNS2 in regulating the expression of both IL-17 and IL-17F in vivo and Th17-related autoimmune diseases.
DISCUSSION
In previous studies, we have identified a total of eight conserved non-coding sequences (CNS1–8) located in the Il17-17f locus which spread over 120 kb in the genome. Among these CNS sites, CNS2 is suggested to regulate IL-17 gene expression (Okamoto et al.; Schraml et al., 2009; Yang et al., 2008b; Zhang et al., 2008). However, the involvement of other CNS elements in regulating Th17 genes is also implicated. For example, CNS3-5 all undergo hyperacetylation in Th17 cells and are shown to interact with Batf – a transcription factor that is crucial for Th17 cell differentiation (Schraml et al., 2009; Akimzhanov et al., 2007). Moreover, there are over 6 DNase I hypersensitivity sites located in the Il17-17f gene locus other than CNS2 in Th17 cells (Mukasa et al. 2010). However, none of these cis elements have been examined in terms of their function in CD4+ T cells in vitro and in vivo. In this study, we characterized the function of a cis-acting element, CNS2. By using luciferase reporter gene assays in primary T cells, we demonstrate that CNS2 functions in a cell type-specific manner in driving not only the Il17 but also the Il17f gene transcription. Moreover, CNS2 induced transcription in Th17 even when fused to Il4 or Ifng gene promoters, supporting that CNS2 but not the Il17 and Il17f gene promoters dictates Th17-specific transcription.
To examine the function of CNS2 in vivo, we generated CNS2-deficient mice and found that CNS2 controlled the expression of IL-17 in Th17 cells generated both in vitro and in vivo, as well as in memory CD4+ T cells. However, the expression of IL-17F was only partially dependent on CNS2, as deficiency of CNS2 led to ~45–70% reduction of IL-17F expression in in vitro and in vivo generated Th17 cells. In contrast, in γδ T and LTi-like cells, CNS2 mainly controlled the per-cell protein expression of IL-17 but not the frequency of IL-17+ population. The IL-17F expression, however, may be even increased in innate immune cells in CNS2-deficient mice. One exception to our findings is that the Th17 (CD4+IL17+) cells present in the gut, which showed a similar CNS2-dependent regulatory pattern to innate immune cells in terms of IL-17 or IL-17F expression. These gut-resident Th17 cells are shown to constitutively express RORγt and readily produce IL-17 upon stimulation (Ivanov et al., 2006), and might represent a distinct Th17 population.
To understand the mechanism underlying the regulation of IL-17 and IL-17F by CNS2, we performed a 3C analysis and noticed that CNS2 can directly interact with the Il17 and Il17f gene promoters only in Th17 cells. The looping of both Il17 and Il17f genes together to CNS2 provides a physical basis for how these two genes are coordinately expressed in Th17 cells, whereas the lineage-specific looping suggests that this particular chromosomal configuration forms during Th17 cell differentiation and it may involve lineage-specific transcription factors. This is in contrast to Th2 cells in which the Th2 LCR interacts with the cytokine gene promoters before the commitment of CD4+ T cells to Th2 lineage, or even exists in other T cell lineages (Spilianakis and Flavell, 2004). This difference may explain that fact that a basal level of IL-4, but not IL-17, is detectable in naïve CD4+ T cells upon stimulation (Nakamura et al., 1997). This study therefore provides a distinct looping mechanism for the function of trans-acting factors in regulating Th17 gene transcription.
Chromatin remodeling in CD4+ T cells has been extensively studied due to its essential role in controlling helper T cell development and lineage plasticity. However, the involving cis-acting elements remain poorly understood in Th17 cells. Our study revealed an essential role of RORγt in mediating permissive histone remodeling of the Il17-17f gene locus in Th17 cells. RORγt critically controlled the permissive histone marker H3Ac at CNS2 and the Il17 promoter region in Th17 cells, as well as the repressive histone marker H3K27me3, and to a less extent at the Il17fp region. This result indicates an essential role of RORγt in the chromatin remodeling at CNS2. CNS2 then functions by controlling the chromatin structure at the Il17 or Il17f promoter region. Retroviral overexpression assays showed that CNS2 was indispensible for the function of RORs in mediating IL-17 expression. Taken together, these results suggest that the interaction between CNS2 and RORγt may play an important role in activation of the transcription at the Il17-17f gene locus.
Interestingly, we found that CNS2 was bound by histone remodeling enzymes p300 and JMJD3 in Th17 cells, which are shown to induce permissive acetylation of histone H3 or to remove the repressive histone marker H3K27me3, respectively (Agger et al., 2007; De Santa et al., 2007; Wang et al., 2008; Xiang et al., 2007), suggesting a histone remodeling enzymatic complex associates with CNS2 in Th17 cells, possibly by the interaction between CNS2 and RORγt. The looping of CNS2 with the promoters thus is able to bring CNS2-associated histone remodeling enzymes to the promoters for chromatin activation at distal regions.
In summary, this study provides a molecular basis of how cis- and trans-acting factors may function together in Th17 cells to drive selective cytokine gene expression. The commitment to Th17 lineage may begin by inducing one or more crucial early transcription factors, such as ROR factors, which bind to CNS2 and the Il17 and Il17f promoters to induce looping of the cytokine locus by interacting with each other or the basic transcriptional machinery assembled on the promoter regions. During this process, chromatin remodeling enzymes are recruited to CNS2 and possibly more other cis-elements, and cause permissive and lineage-specific chromatin remodeling of the Th17 cytokine locus. The opening of the Il17-Il17f locus then leads to binding of more cell type-specific trans-acting factors, which interact with the basic transcriptional machinery due to close spatial proximity and then activate gene transcription in a robust and lineage-specific manner. Considering the importance of CNS2 in regulating IL-17 and IL-17F, characterization of the transcription factors and other cis-elements that associate with CNS2 may reveal additional mechanisms underlying the differentiation of Th17 cells, a pro-inflammatory effector Th subset important in many inflammatory diseases.
MATERIALS AND METHODS
Primers
All the primers used for ChIP-PCR, mutagenesis, luciferase reporter, CNS2-targeting plasmid construction, and genotyping were listed in Supplementary Table 1. The real-time RT-PCR primers used to quantify Il17, Il17f, Il21, RORα, RORγt and β-actin were described previously (Yang et al., 2008b).
Mice
The CNS2-targeting plasmid was generated by cloning the sequences flanking the CNS2 site into pEASY-Flox vector which uses NeoR as positive and thymidine kinase as negative selection markers (Figure S2a). CNS2-deficient mice were generated by replacing the CNS2 fragment with a NeoR gene cassette flanked by Floxp sites using the 129/TC1 embryonic stem cell line (Figure S2a). Targeted embryonic stem cell clones were selected and injected into C57BL/6 blastocysts to generate chimeras. High percentage chimeras were bred with C57BL/6 mice for germline transmission. The CNS2+/− mice were obtained by crossing with the CMV-Cre strain to remove the NeoR cassette. The deletion of CNS2 alleles was detected by PCR (Figure S2b). Homozygous WT and CNS2-deficient animals on the same 129×C57BL/6 F1 mixed background were bred and used in experiments. All the mice were housed in the SPF animal facility at the M. D. Anderson Cancer Center and the animal experiments were performed at the age of 6–8 weeks with the use of protocols approved by the Institutional Animal Care and Use Committee.
Rorc−/− (RORγ-deficient) mice were generated before (Kurebayashi et al., 2000) and their splenocytes were used for isolation of naïve CD4+ T cells in this study.
Luciferase reporter assays
The Il17, Il17f and Ifng promoters, without or together with CNS2, were cloned into PGL3 luciferase reporter plasmid. The ROR binding sites in CNS2 were mutated by site-directed mutagenesis. The PGL3-Il4p reporter construct was made before (Lee et al., 2001). For all the reporter constructs, CNS2 was cloned into the MluI and XhoI sites and the promoters were cloned into XhoI and Hind III sites in PGL3 plasmid. All the primers used for cloning and mutagenesis were listed in Supplementary Table 1. Naïve CD4+ T cells were isolated from C57BL/6 mice and differentiated under Th1 and Th17 conditions. On day 3, the cells were transfected with different luciferase reporter constructs together with a control Renilla luciferase reporter plasmid by using a Mouse T Cell Nucleofector® Kit (LONZA, cat# VPA-1006) and Amaxa Nucleofector™ I (Amaxa Biosystem) according to manufacturer’s instructions. The dual-luciferase reporter system (Promega) was used to examine firefly and Renilla luciferase activity. Renilla luciferase was used to normalize transfection efficiency and luciferase activity.
Isolation of different immune cells and T cell differentiation
For Th differentiation, naïve T cells were isolated by sorting CD4+CD25−CD62LhiCD44lo cells from spleens and lymph nodes, differentiated under several T-helper conditions, and analyzed as described (Nurieva et al., 2007; Yang et al., 2008b). Th0 (for retroviral infection): 0.5 μg ml−1 anti-CD3 (plate-bound), 0.5 μg ml−1 anti-CD28 (soluble), 10 μg ml−1 anti-IL4 (11B11), 10 μg ml−1 anti-IFNγ (XMG), and 30 U ml−1 IL-2. Th1: 1 μg ml−1 anti-CD3 (plate-bound), 1 μg ml−1 anti-CD28 (plate-bound), 10 μg ml−1 anti-IL4 (11B11), 10 ng ml−1 IL-12, and 30 U ml−1 IL-2. Th2: 0.5 μg ml−1 anti-CD3 (plate-bound), 0.5 μg ml−1 anti-CD28 (soluble), 10 μg ml−1 anti-IFNγ, 10 ng ml−1 IL-4, and 30 U ml−1 IL-2. iTreg: 1 μg ml−1 anti-CD3 (plate-bound), 1 μg ml−1 anti-CD28 (plate-bound), 10 μg ml−1 anti-IL4 (11B11), 10 μg ml−1 anti-IFNγ, and 2.5 ng ml−1 TGFβ. Th17: 1 μg ml−1 anti-CD3 (plate-bound), 1 μg ml−1 anti-CD28 (plate-bound), 15 ng ml−1 IL-6, and 2.5 ng ml−1 TGFβ. When indicated, 50 ng ml−1 IL-23, 10 ng ml−1 tumour necrosis factor (TNF)-α, and 10 ng ml−1 IL-1β were used for optimal Th17 differentiation.
Memory CD4+, γδ T and LTi-like cells were isolated by sorting or gating on CD4+CD62LloCD44hiCD25−, γδ+ TCR or CD4+CD3− cells from spleens and lymph nodes, respectively, and analyzed by intracellular staining after phorbol 12-myristate 13-acetate (PMA) and ionomycin restimulation in the presence of Golgi-stop or by real-time RT-PCR after ex vivo restimulation. IELs and LP cells were isolated from the small intestine as previously described (Goodman and Lefrancois, 1988; Laky et al., 1997) and the cells was analyzed by intracellular staining after PMA and ionomycin restimulation in the presence of Golgi stop.
Chromosome conformation capture (3C) assays
The 3C assays were performed as described with minor modifications (Tolhuis et al., 2002). PstI and NsiI were used to generate restriction fragments of the Il17-17f locus in Th1 and Th17 cells. The ligation of CNS2 to the Il17 and Il17f promoters was detected by PCR amplification using primers shown in the Supplementary Table 1. Undigested C57BL/6 genomic DNA was used as a negative control for the PCR reactions.
Retrovrial transduction
RORα (Genbank Acc. XM_903197) and RORγt (Genbank Acc.AJ132394) were cloned into bicistronic retroviral vector pMIG-hCD2 containing IRES-regulated human CD2 as described (Nurieva et al., 2007; Yang et al., 2008b). Naïve CD4+ T cells were activated using 0.5 μg ml−1 anti-CD3 (plate-bound), 0.5 μg ml−1 anti-CD28 (soluble), and differentiated under Th0 and Th17 conditions. On day 1, the cells were infected by retroviruses RORα-hCD2, RORγt-hCD2 or control empty vector pMIG-hCD2. On day 4, the cells were restimulated with PMA and ionomycin in the presence of Golgi-stop for 5 hr, stained for surface marker hCD2 first, and then stained for IL-17 and IL-17F intracellularly.
ChIP-PCR assays
Cells were first cross-linked using 2% paraformadehyde for 10 min at 37°C, sonicated, and then the DNA-protein complexes were isolated using a ChIP assay kit (Millipore, Cat# 17-295) according to manufacturer’s instructions with antibodies against p300 (SantaCruz), acetylated histone H3 (H3Ac) (Millipore), trimethylated histone H3-K27 (H3K27me3) (Millipore), RNA Polymerase II (SantaCruz), or JMJD3 (Abcam). The precipitated DNA was quantified by real-time PCR using primers list in Supplementary Table 1. The results were normalized relative to the input control.
Quantitative real-time RT-PCR
Total RNA was isolated from T cells with the use of Trizol reagent (Invitrogen) according to manufacturer’s instructions. cDNAs were synthesized using Superscript reverse transcriptase and oligo(dT) primers (Invitrogen) and analyzed using an iCycler Optical System and an iQ SYBR green real-time PCR kit (Bio-Rad). The data were normalized to a β-actin control.
Allergic lung inflammation
Age-matched CNS2-deficient and their WT control mice were challenged intranasally twice with an allergenic fungal proteinase (FAP) derived from Aspergillus oryzae on day 1 at a dosage of 7 μg FAP and 43 μg OVA in 50 μl PBS per mice, and then analyzed IL-17 and IL-17F expression in the lung tissues or lung γδ T cells on Day 2 as described (Kiss et al., 2007; Yang et al., 2008a).
MOG immunization and induction of EAE
Both MOG immunization and EAE induction were performed by immunizing mice with 300 μg MOG35–55 peptide (amino acids 35–55; MEVGWYRSPFSROVHLYRNGK) emulsified in CFA and analyzed as previously described (Nurieva et al., 2007; Yang et al., 2008b). The disease scores were assigned on a scale of 0–5 as follows: 0, none; 1, limp tail or waddling gait with tail tonicity; 2, wobbly gait; 3, hindlimb paralysis; 4, hindlimb and forelimb paralysis; 5, death.
Calculations and Statistic Analysis
All our in vitro data were repeated at least 2–5 times with consistent results. When indicated, the statistical significance was determined by Student’s t test. (* represents p < 0.05; ** represents p < 0.03; *** represents p < 0.01).
Supplementary Material
Highlights.
CNS2 is sufficient to drive expression of both IL-17 and IL-17F in Th17 cells
CNS2 is indispensible for lineage-specific expression of IL-17
CNS2 interacts with the Il17 and Il17f promoters in a cell-type-specific manner
CNS2 modulates the chromatin accessibility of the Th17 cytokine gene locus
Acknowledgments
We thank the Dong laboratory members for their help. The work is supported by research grants from NIH (to C.D. and R.I.N.), American Heart Association (to X.O.Y.) and 973 National Basic Research Program of China (2010CB126301). X.W. receives a postdoctoral fellowship from the National Multiple Sclerosis Society, Y.Z. was supported by a fellowship from Chinese Academy of Sciences, and C.D. receives a Research Trust Fellowship from MD Anderson Cancer Center and is a Leukemia and Lymphoma Society Scholar.
Footnotes
Author contributions: C.D. and X. W. designed the research and analyzed the data. X.W., Y.Z., X.Y., R.I.N., S.H.C., S.S.O. and H.S.K. performed the experiments, and X.W., J.G., K.S.S., A.M.J. and C.D. prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O’Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, Lallone RL, Murphy KM, Weaver CT. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, et al. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–342. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Laky K, Lefrancois L, Puddington L. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J Immunol. 1997;158:1417–1427. [PubMed] [Google Scholar]
- Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U SA. 1990;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Fields PE, Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447–459. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat Immunol. 2005;6:42–48. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- Loots GG, Locksley RM, Blankespoor CM, Wang ZE, Miller W, Rubin EM, Frazer KA. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. 2000;288:136–140. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- Meireles-Filho AC, Stark A. Comparative genomics of gene regulation-conservation and divergence of cis-regulatory information. Curr Opin Genet Dev. 2009;19:565–570. doi: 10.1016/j.gde.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464:1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymar DC, Agarwal S, Bassing CH, Alt FW, Rao A. A 3′ enhancer in the IL-4 gene regulates cytokine production by Th2 cells and mast cells. Immunity. 2002;17:41–50. doi: 10.1016/s1074-7613(02)00334-5. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5:1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Motomura Y, Suzuki Y, Yagi R, Inoue H, Miyatake S, Kubo M. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat Immunol. 12:77–85. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–858. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tang Y, Cole PA, Marmorstein R. Structure and chemistry of the p300/CBP and Rtt109 histone acetyltransferases: implications for histone acetyltransferase evolution and function. Curr Opin Struct Biol. 2008;18:741–747. doi: 10.1016/j.sbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res. 2007;17:850–857. doi: 10.1038/cr.2007.83. [DOI] [PubMed] [Google Scholar]
- Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008a;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008b;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.