Abstract
The aim of this study was to investigate the independent associations among cardiorespiratory fitness, metabolic syndrome (MetS), and C-reactive protein (CRP) in children. The sample consisted of 112 children (11.4 ± 0.4 years). Data was obtained for children's anthropometry, cardiorespiratory fitness, MetS components, and CRP levels. MetS was defined using criteria analogous to the Adult Treatment Panel III definition. A MetS risk score was also computed. Prevalence of the MetS was 5.4%, without gender differences. Subjects with low fitness showed significantly higher MetS risk (P < 0.001) and CRP (P < 0.007), compared to the high-fitness pupils. However, differences in MetS risk, and CRP between fitness groups decreased when adjusted for waist circumference. These data indicate that the mechanisms linking cardiorespiratory fitness, MetS risk and inflammation in children are extensively affected by obesity. Intervention strategies aiming at reducing obesity and improving cardiorespiratory fitness in childhood might contribute to the prevention of the MetS in adulthood.
1. Introduction
The prevalence and severity of obesity is increasing dramatically among children and adolescents in many parts of the world, whereas prevalence rates are estimated to increase in the next decades [1]. In children, excess body fat appears to be strongly associated with the clustering of risk factors, such as hyperglycemia, dyslipidemia, and hypertension, which play a key role in the pathogenesis of the metabolic syndrome (MetS) [2].
Obesity and the MetS risk in children have been recently associated with systemic inflammatory markers, in particular C-reactive protein (CRP) [3, 4], implying that low-grade inflammation can already exist in childhood and may be a potential link between the obesity and the MetS. Among behavioral variables, cardiorespiratory fitness has a protective role in MetS and inflammatory factors; however, it is not entirely clear if the interrelations among cardiorespiratory fitness, MetS risk, and inflammation in children are independent or partly due to the mediating effect of obesity, since the existing data are limited and equivocal [5, 6].
Recent evidence indicates that the prevalence rates of childhood obesity in Greece remain high [1, 7] and often coexist with low cardiorespiratory fitness [8] and an unfavorable cardiometabolic risk profile [9]. For the Greek pediatric population these data suggest an increased cardiovascular morbidity in adulthood, given that high-risk children and adolescents are likely to become high-risk adults [10]. Although the relationship among obesity and dyslipidemia in Greek children has been thoroughly investigated [9, 11], there is a paucity of data regarding the clustering of metabolic risk factors, inflammation, and their relationship with cardiorespiratory fitness. The present study was undertaken in an attempt to investigate the prevalence of the MetS and examine the associations among cardiorespiratory fitness, MetS risk, and CRP in 11-year-old children.
2. Materials and Methods
2.1. Participants and Procedures
This study sample consisted of 112 pupils (54 females, 58 males, mean age 11.4 ± 0.4 years), registered in the sixth grade of 15 randomly selected primary schools in North Attica, Greece. Participants represented ~12% of the total 11-year-old population in the area. Subjects were healthy at the time of investigation and had no chronic or acute illnesses. Prior to the commencement of the study, permission from the school principal was obtained and parental consent was secured after full explanation of study objectives and data collection procedures. The children provided their verbal assent. The research ethics committee of Democritus University of Thrace approved the investigation and permission was granted by the Greek Ministry of Education. All procedures were in accordance with the Helsinki Declaration of 1975. Measures of anthropometry and fitness were conducted in the school environment (during PE classes) by the same experienced investigator (the principal author), using the same order of testing procedures and under similar conditions. Blood samples and blood pressure data were obtained at the same clinical biochemistry accredited laboratory by a medical practitioner.
3. Data Collection
3.1. Anthropometry
Age (accurate to 1 month) was recorded. Students were weighed on an electronic scale to the nearest 0.5 kg (Seca Beam Balance 710), lightly dressed and barefooted. Standing height was measured to the nearest 0.5 cm (Seca Stadiometer 208) with each subject's shoes off, feet together, and head in the Frankfort horizontal plane. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). BMI values were also converted to standard deviation scores (SDSs), whereas the IOTF's age- and gender-specific BMI cut-off points were used to calculate the prevalence rates for “overweight/obesity” [12]. Waist circumference, measured at the level of the umbilicus with a tape measure to the nearest 0.1 cm, was used as an indicator of central obesity.
3.2. Cardiorespiratory Fitness
Cardiorespiratory fitness was assessed by using the 20 m multistage shuttle run test [13], a standard field test included in the European fitness test battery [14]. In brief, 5–10 subjects performed a series of runs across a 20 m track, changing direction at the end of each run to coincide with an audio signal that was getting progressively faster. Subjects started running at a speed of 8.5 km/hr, and speed increased at various stages (0.5 km/hr every minute). Each stage was made up of several shuttle runs, and pupils were instructed to keep pace with the signals as long as possible. The number of laps fully completed were recorded for each pupil and subsequently used to estimate cardiorespiratory fitness as predicted maximal oxygen uptake (VO2max) in mL/kg/min. Based on gender- and age-specific cut-off values of VO2max [15] participants were classified into two fitness groups.
3.3. Clinical and Biochemical Screening
Blood pressure (BP) was measured in the right arm, using a mercury sphygmomanometer with appropriate size cuffs and cuff bladders, after the subject had been sitting quietly for at least 5 min. The mean of two measurements at the first and the fifth Korotkoff phase was recorded for systolic and diastolic BP, respectively [16].
Blood samples were obtained from each subject early in the morning, following an overnight fast. Blood was centrifuged for plasma separation, and 1.5 mL aliquots were pipetted into plastic Eppendorf tubes and stored at −70°C until further analyses. Plasma glucose, triglycerides, and HDL cholesterol (HDL-C) were determined using chromatographic enzymatic method on an automated MB II analyzer (Dade Behring, Marburg, Germany). CRP was measured by high-sensitive latex-enhanced immunoassay on a BN II nephelometer from the same company. The lower limit of detection of this assay was 0.15 mg/L.
3.4. Metabolic Syndrome
The MetS was defined as meeting ≥3 of the following age- and gender-specific criteria: waist circumference ≥90th percentile, glucose ≥100 mg/dL, triglycerides ≥100 mg/dL, HDL-C < 50 mg/dL, and systolic or diastolic BP ≥ 90th percentile [17, 18]. We further computed a MetS risk score [19] as follows: for each of the MetS components, an absolute Z score was computed. For HDL-C the Z score was multiplied by −1 to indicate higher metabolic risk with increasing value. The Z scores of systolic and diastolic BP were averaged and then added to the rest of the Z scores. This sum was then divided by 5 to compile the average MetS risk score. This risk score is statistically more sensitive and less error-prone by comparison to dichotomous approaches [20]. We also computed a nonobesity MetS risk score, omitting the Z score from waist circumference.
3.5. Statistical Analysis
The Kolmogorov-Smirnov test of normality was adopted to assess data distribution. Because of a nonnormal distribution, CRP values were logarithmically transformed prior to the analyses. Descriptive statistics and simple contingency table analyses were performed. Continuous variables are presented as mean values ± standard error of means, while qualitative variables are presented as absolute and relative frequencies. Gender differences in the examined variables were explored using unpaired t-test. Relationships among cardiorespiratory fitness, MetS risk score, and CRP levels were summarized with Pearson's correlations. The independent effect of low fitness level on anthropometric indices, metabolic characteristics, MetS risk score, and CRP was assessed using one-way ANCOVA. The level of significance was set at P < 0.05. Statistical analyses were performed with SPSS 13.0 software.
4. Results
Descriptive characteristics of the subjects by gender and for the entire sample are presented in Table 1. Anthropometric parameters are in line with recently published paediatric data [1, 7, 8], suggesting that the sample is representative of periadolescent children in Greece. Mean BMI values, mean plasma concentrations of the MetS components, and mean BP recordings were within acceptable ranges and did not differ between genders. SDS-BMI was significantly higher in males compared to females (t (110) = 2.52, P = 0.013).
Table 1.
Subject characteristics and prevalence rates of metabolic risk factors and the MetS.
| Females (n = 54) | Males (n = 58) | P value | Overall (n = 112) | |
|---|---|---|---|---|
| Anthropometric and fitness characteristics | ||||
| Age (years) | 11.28 ± 0.04 | 11.41 ± 0.05 | 0.06 | 11.35 ± 0.04 |
| Height (cm) | 150.86 ± 0.85 | 150.78 ± 0.87 | 0.94 | 150.82 ± 0.61 |
| Weight (kg) | 44.98 ± 1.43 | 47.05 ± 1. 45 | 0.31 | 46.05 ± 1.02 |
| BMI (kg/m2) | 19.65 ± 0.53 | 20.54 ± 0.51 | 0.23 | 20.11 ± 0.37 |
| SDS-BMI | 0.49 ± 0.18 | 1.08 ± 0.15 | 0.01 | 0.79 ± 0.12 |
| Normal, n (%) | 34 (63) | 33 (56.9) | 67 (59.8) | |
| Overweight/obese, n (%) | 20 (37) | 25 (43.1) | 0.513 | 45 (40.2) |
| VO2max (mL/kg/min) | 29.86 ± 0.72 | 31.26 ± 0.79 | 0.15 | 30.58 ± 0.54 |
|
| ||||
| MetS components and CRP concentration | ||||
| Waist circumference (cm) | 68.83 ± 1.29 | 71.97 ± 1.47 | 0.12 | 70.47 ± 0.99 |
| ≥90th percentile, n (%) | 6 (11.1) | 8 (13.8) | 0.67 | 14 (12.5) |
| Fasting glucose (mg/dL) | 93.58 ± 1.33 | 95.36 ± 1.26 | 0.30 | 94.51 ± 0.92 |
| ≥100 mg/dL, n (%) | 13 (24.1) | 18 (31.0) | 0.41 | 31 (27.7) |
| HDL-C (mg/dL) | 63.54 ± 1.73 | 63.68 ± 1.66 | 0.97 | 63.62 ± 1.19 |
| <50 mg/dL, n (%) | 9 (16.7) | 6 (10.3) | 0.33 | 15 (13.4) |
| Triglycerides (mg/dL) | 73.74 ± 6.16 | 70.15 ± 5.05 | 0.60 | 71.88 ± 3.93 |
| ≥110 mg/dL, n (%) | 10 (18.5) | 10 (17.2) | 0.86 | 20 (17.9) |
| Systolic BP (mmHg) | 100.67 ± 1.08 | 102.68 ± 1.04 | 0.19 | 101.71 ± 0.75 |
| Diastolic BP (mmHg) | 75.96 ± 0.78 | 76.16 ± 0.70 | 0.85 | 76.06 ± 0.52 |
| ≥90th percentile, n (%) | 7 (13) | 2 (3.5) | 0.06 | 9 (8) |
| MetS prevalence (≥3 risk factors), n (%) | 3 (5.6) | 3 (5.2) | 0.93 | 6 (5.4) |
| MetS Z score including adiposity | −0.09 ± 0.09 | 0.04 ± 0.07 | 0.45 | −0.02 ± 0.06 |
| MetS Z score excluding adiposity | −0.08 ± 0.09 | 0.00 ± 0.06 | 0.78 | −0.04 ± 0.05 |
| CRP (mg/dL) | 1.07 ± 0.22 | 1.47 ± 0.32 | 0.28 | 1.27 ± 0.19 |
Values are presented as means ± SEM or n (%). BMI: body mass index, BP: blood pressure, CRP: C-reactive protein, HDL-C: high-density lipoprotein cholesterol, MetS: metabolic syndrome, SDS-BMI: standard deviation scores for BMI, and VO2max: predicted maximal oxygen uptake.
Among all participants, 34.8% had one component, 12.5% had two components, 2.7% had three components, 1.8% had four components, and 0.9% had five components of the MetS. Hyperglycemia was the most common metabolic abnormality in both genders (27.7%), followed by hypertriglyceridemia (17.9%). Elevated BP was more frequent in females, but differences did not achieve statistical significance (χ 2 = 3.43, P = 0.064). The prevalence of the MetS was 5.4%, without statistically significant gender differences (χ 2 = 0.008, P = 0.928).
Pearson's correlation analyses revealed that cardiorespiratory fitness was inversely associated with the MetS risk score (r = −0.386, P < 0.001), and CRP (r = −0.394, P < 0.001). MetS risk score and CRP were positively interrelated (r = 0.544, P < 0.0005). A high percentage of the study participants demonstrated cardiorespiratory fitness values considerably lower than the recommended fitness levels for metabolic health. In particular, ~72% of both genders did not achieve the acceptable fitness values for children of comparable age [15].
Children with lower fitness levels, compared to their peers with increased fitness levels, demonstrated significantly increased BMI (21.01 versus 17.91 kg/m2, F (1,111) = 17.55, P < 0.001), SDS-BMI (1.07 versus 0.14, F (1,111) = 13.43, P < 0.001) and waist circumference (72.89 versus 64.23 cm, F (1,111) = 19.82, P < 0.001). In addition, pupils in the lower fitness group showed significantly higher MetS risk score (F (1,93) = 13.03, P < 0.001) and CRP values (F (1,93) = 7.67, P < 0.007), compared to the high-fitness group (Figure 1). Adjustment for central obesity attenuated the observed differences in the MetS risk score, which were no longer significant (Figure 1(A)). Differences in CRP values between fitness groups decreased also when adjusted for waist circumference (Figure 1(B)); however, they remained borderline significant (F (1,93) = 3.49, P < 0.067). Analyses including BMI or SDS-BMI instead of waist circumference yielded similar results (data not shown).
Figure 1.
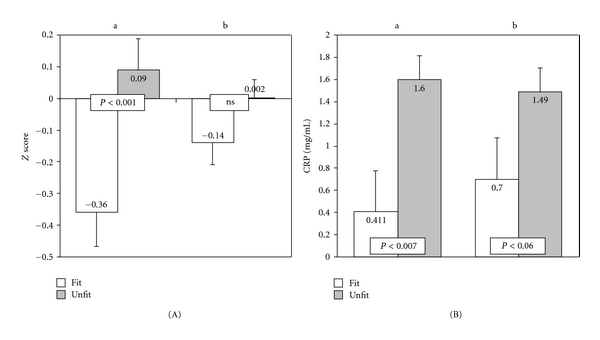
Differences in MetS risk score (A) and CRP values (B) between high- and low-fitness groups. (a) ANCOVA results after adjustment for age and gender. (b) ANCOVA results after further adjustment for waist circumference.
5. Discussion
The MetS has a multifactorial aetiology, and the mechanisms underlying its onset are not completely understood. In the present study we investigated the prevalence of the MetS and examined the independent associations among cardiorespiratory fitness, MetS risk, and CRP in Greek children. Using a paediatric definition based closely on ATP III [17, 18], we found that the prevalence of MetS in our sample was 5.4%. This prevalence rate is similar to those reported in US and French adolescents (4–5.8%) [21–23] but lower than the prevalence reported in Iranian paediatric populations (14.1%) [24]. These data indicate that the MetS is a common health problem among different paediatric populations, underlining the need for effective policies and appropriate intervention strategies at global, national, and regional level.
We further found that the protective effect of cardiorespiratory fitness on the MetS risk score was explained by the confounding effect from central obesity. Our results are in agreement with previous findings [6], showing that cardiorespiratory fitness is not independently associated with markers of the MetS. However, recent data from the European Youth Heart Study (EYHS) [19, 25, 26] and the National Health and Nutrition Examination Survey (NHANES) 1999–2002 [27] revealed that cardiorespiratory fitness is a significant and independent predictor of the MetS in children, whereas the benefits of higher cardiorespiratory fitness seem to extend also to children at lower metabolic risk [28]. Differences in body composition and fitness levels are known to affect the metabolic profile in children and could explain at least part of these discrepancies among studies.
Compared to their North European and North American age-related peers, Greek children exhibit higher rates of overweight/obesity and worse cardiorespiratory fitness performance [8, 19, 27, 29–31]. These findings suggest that children in the present study did not achieve the required threshold in cardiorespiratory fitness, in order to profit from its beneficial effects with respect to the risk of MetS in the face of obesity. Indeed, Nassis et al. [32] have shown that exercise-related cardiorespiratory fitness enhancement in overweight girls can lead to marked improvements in insulin sensitivity, without changes in body composition. In addition, even if central obesity is an intermediate link between low fitness and MetS risk, fitness will still be the principal cause, even if its association with MetS risk disappears after adjustment for an intermediate variable in the causal chain (i.e., central obesity).
In line with a previous report in children and young adults [5], we found that the association between cardiorespiratory fitness and CRP was largely explained by their interrelation to obesity. Thomas et al. [33] reported no relationship between CRP and cardiorespiratory fitness in 12-13-years-old schoolchildren. In contrast, results from the Ten Towns Children's Study [34] and more recent data from South African children [35] reported strong correlations between fitness and CRP. An explanation for this discrepancy may be the fact that in the Ten Towns Children's Study a crude estimate of physical fitness (i.e., resting heart rate) was used, whilst in the latter study [35] the investigators did not take into account the confounding effect of obesity. More studies are needed to improve our understanding of the interrelations among cardiorespiratory fitness, CRP, and obesity in children.
Our findings indicate that the mechanisms linking cardiorespiratory fitness, MetS risk, and CRP are extensively affected by obesity. Further, the favourable effect of cardiorespiratory fitness on MetS risk may not become evident when fitness levels underlie a critical threshold. Given that high levels of cardiorespiratory fitness improve the metabolic state of obese children [32], attenuate the proinflammatory milieu associated with obesity [36], and offer protection against the development of the insulin resistance syndrome from childhood into adolescence and young adulthood [37–39], children should be encouraged to achieve the recently recommended physical activity levels (90 min/day of at least moderate intensity), in order to prevent clustering of cardiovascular disease risk factors and improve their metabolic and overall health profile [28].
The interpretation of the present findings may be affected by several potential limitations. A root cause in the development of the MetS is insulin resistance, which was not assessed in the present study. However, epidemiologic studies indicate that a substantial proportion of subjects with the MetS do not have evidence of insulin resistance and the correlation between insulin resistance and individual components of the MetS is weak to moderate [40]. In addition, cardiorespiratory fitness was indirectly assessed. However, it is unlikely that our findings are invalidated by the use of the 20 m multistage shuttle run test since it has been extensively used in the literature and is recommended for paediatric studies as reliable, valid, and cost-effective [8, 13, 14, 30, 31]. Further, a strict random sampling of all eligible pupils was virtually impossible, given that the study was dependent on the principals' disposal and the willingness of individual children and their parents to participate. The prevalence of overweight/obesity, however, was similar to that reported for school-aged children from other Greek regions [7–9, 11]. Finally, the sample size and the age range of our subjects limit the generalization of the findings.
Within the above-mentioned limitations, it is concluded that the metabolic consequences of excessive body fat and low fitness are detectable early in life. Taking into consideration the growing epidemic of childhood obesity, the public health authorities should seriously consider the design and early implementation of effective prevention strategies. Health education programs aiming at the prevention of weight gain and the improvement of cardiorespiratory fitness in childhood could anticipate the metabolic abnormalities predisposed to or associated with MetS risk and inflammation and thus contribute to the prevention of the MetS and its complications later in life.
References
- 1.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa heart study. Diabetes. 2002;51(1):204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 3.Hiura M, Kikuchi T, Nagasaki K, Uchiyama M. Elevation of serum C-reactive protein levels is associated with obesity in boys. Hypertension Research. 2003;26(7):541–546. doi: 10.1291/hypres.26.541. [DOI] [PubMed] [Google Scholar]
- 4.Lambert M, Delvin EE, Paradis G, O’Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clinical Chemistry. 2004;50(10):1762–1768. doi: 10.1373/clinchem.2004.036418. [DOI] [PubMed] [Google Scholar]
- 5.Isasi CR, Deckelbaum RJ, Tracy RP, Starc TJ, Berglund L, Shea S. Physical fitness and C-reactive protein level in children and young adults: the Columbia University BioMarkers study. Pediatrics. 2003;111(2):332–338. doi: 10.1542/peds.111.2.332. [DOI] [PubMed] [Google Scholar]
- 6.Ball GDC, Shaibi GQ, Cruz ML, Watkins MP, Weigensberg MJ, Goran MI. Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight hispanic youth. Obesity Research. 2004;12(1):77–85. doi: 10.1038/oby.2004.11. [DOI] [PubMed] [Google Scholar]
- 7.Tambalis KD, Panagiotakos DB, Kavouras SA, et al. Eleven-year prevalence trends of obesity in greek children: first evidence that prevalence of obesity is leveling off. Obesity. 2010;18(1):161–166. doi: 10.1038/oby.2009.188. [DOI] [PubMed] [Google Scholar]
- 8.Tokmakidis SP, Kasambalis A, Christodoulos AD. Fitness levels of Greek primary schoolchildren in relationship to overweight and obesity. European Journal of Pediatrics. 2006;165(12):867–874. doi: 10.1007/s00431-006-0176-2. [DOI] [PubMed] [Google Scholar]
- 9.Magkos F, Manios Y, Christakis G, Kafatos AG. Secular trends in cardiovascular risk factors among school-aged boys from Crete, Greece, 1982–2002. European Journal of Clinical Nutrition. 2005;59(1):1–7. doi: 10.1038/sj.ejcn.1602023. [DOI] [PubMed] [Google Scholar]
- 10.Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Clustering of cardiovascular disease risk factors among obese schoolchildren: the Taipei children heart study. American Journal of Clinical Nutrition. 1998;67(6):1141–1146. doi: 10.1093/ajcn/67.6.1141. [DOI] [PubMed] [Google Scholar]
- 11.Manios Y, Magkos F, Christakis G, Kafatos AG. Changing relationships of obesity and dyslipidemia in Greek children: 1982–2002. Preventive Medicine. 2005;41(5-6):846–851. doi: 10.1016/j.ypmed.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20-m shuttle run test for aerobic fitness. Journal of Sports Sciences. 1988;6(2):93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- 14.EUROFIT. European Test of Physical Fitness. Rome, Italy: Council of Europe,Committee for the Development of Sport; 1988. [Google Scholar]
- 15.Bell RD, Máček M, Rutenfranz J, Saris WHM. Health indicators and risk factors of cardiovascular diseases during childhood and adolescence. In: Rutenfranz J, Mocellin R, Klimt F, editors. Children and Exercise 7. Champaign, Ill, USA: Human Kinetics; 1986. pp. 19–27. [Google Scholar]
- 16.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Update on the 1987 task force report on high blood pressure in children and adolescents: a working group report from the national high blood pressure education program. Pediatrics. 1996;98(4):649–658. [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 18.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the third national health and nutrition examination survey. Circulation. 2004;110(16):2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 19.Brage S, Wedderkopp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European youth heart study (EYHS) Diabetes Care. 2004;27(9):2141–2148. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 20.Ragland DR. Dichotomizing continuous outcome variables: dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology. 1992;3(5):434–440. doi: 10.1097/00001648-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third national health and nutrition examination survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28(4):878–881. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 23.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006;49(9):2078–2085. doi: 10.1007/s00125-006-0320-6. [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi R, Razaghi EM, Gouya MM, et al. Association of physical activity and the metabolic syndrome in children and adolescents: CASPIAN study. Hormone Research. 2007;67(1):46–52. doi: 10.1159/000096121. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz JR, Rizzo NS, Ortega FB, Loit HM, Veidebaum T, Sjöstrom M. Markers of insulin resistance are associated with fatness and fitness in school-aged children: the European Youth Heart Study. Diabetologia. 2007;50(7):1401–1408. doi: 10.1007/s00125-007-0678-0. [DOI] [PubMed] [Google Scholar]
- 26.Ekelund U, Anderssen S, Andersen LB, et al. Prevalence and correlates of the metabolic syndrome in a population-based sample of European youth. American Journal of Clinical Nutrition. 2009;89(1):90–96. doi: 10.3945/ajcn.2008.26649. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DM, Dubose KD, Imai S, Collier DN. Fitness versus fatness and insulin resistance in U.S. adolescents. Journal of Obesity. 2010;2010:7 pages. doi: 10.1155/2010/195729. Article ID 195729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European youth heart study) The Lancet. 2006;368(9532):299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 29.Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K. Biological cardiovascular risk factors cluster in Danish children and adolescents: the European youth heart study. Preventive Medicine. 2003;37(4):363–367. doi: 10.1016/s0091-7435(03)00145-2. [DOI] [PubMed] [Google Scholar]
- 30.Flouris AD, Canham CH, Faught BE, Klentrou P. Prevalence of cardiovascular disease risk in Ontario adolescents. Archives of Disease in Childhood. 2007;92(6):521–523. doi: 10.1136/adc.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olds T, Tomkinson G, Léger L, Cazorla G. Worldwide variation in the performance of children and adolescents: an analysis of 109 studies of the 20-m shuttle run test in 37 countries. Journal of Sports Sciences. 2006;24(10):1025–1038. doi: 10.1080/02640410500432193. [DOI] [PubMed] [Google Scholar]
- 32.Nassis GP, Papantakou K, Skenderi K, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54(11):1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Thomas NE, Baker JS, Graham MR, Cooper SM, Davies B. C-reactive protein in schoolchildren and its relation to adiposity, physical activity, aerobic fitness and habitual diet. British Journal of Sports Medicine. 2008;42(5):357–360. doi: 10.1136/bjsm.2007.043604. [DOI] [PubMed] [Google Scholar]
- 34.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149(1):139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 35.Harmse B, Kruger HS. Significant differences between serum CRP levels in children in different categories of physical activity: the PLAY study. Cardiovascular Journal of Africa. 2010;21(6):316–322. doi: 10.5830/CVJA-2010-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. Journal of the American College of Cardiology. 2006;48(9):1865–1870. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Dwyer T, Magnussen CG, Schmidt MD, et al. Decline in physical fitness from childhood to adulthood associated with increased obesity and insulin resistance in adults. Diabetes Care. 2009;32(4):683–687. doi: 10.2337/dc08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira I, Twisk JWR, van Mechelen W, Kemper HCG, Stehouwer CDA. Development of fatness, fitness, and lifestyle from adolescence to the age of 36 years: determinants of the metabolic syndrome in young adults: the Amsterdam Growth and Health Longitudinal Study. Archives of Internal Medicine. 2005;165(1):42–48. doi: 10.1001/archinte.165.1.42. [DOI] [PubMed] [Google Scholar]
- 39.Flouris AD, Bouziotas C, Christodoulos AD, Koutedakis Y. Longitudinal preventive-screening cutoffs for metabolic syndrome in adolescents. International Journal of Obesity. 2008;32(10):1506–1512. doi: 10.1038/ijo.2008.142. [DOI] [PubMed] [Google Scholar]
- 40.Mikhail N. The metabolic syndrome: insulin resistance. Current Hypertension Reports. 2009;11(2):156–158. doi: 10.1007/s11906-009-0027-4. [DOI] [PubMed] [Google Scholar]


