Abstract
Passive immunization with antibodies to recombinant Plasmodium falciparum P0 riboprotein (rPfP0, 61–316 amino acids) provides protection against malaria. Carboxy-terminal 16 amino acids of the protein (PfP0C0) are conserved and show 69% identity to human and mouse P0. Antibodies to this domain are found in 10–15% of systemic lupus erythematosus patients. We probed the nature of humoral response to PfP0C0 by repeatedly immunizing mice with rPfP0. We failed to raise stable anti-PfP0C0 hybridomas from any of the 21 mice. The average serum anti-PfP0C0 titer remained low (5.1 ± 1.3 × 104). Pathological changes were observed in the mice after seven boosts. Adsorption with dinitrophenyl hapten revealed that the anti-PfP0C0 response was largely polyreactive. This polyreactivity was distributed across all isotypes. Similar polyreactive responses to PfP0 and PfP0C0 were observed in sera from malaria patients. Our data suggests that PfP0 induces a deviant humoral response, and this may contribute to immune evasion mechanisms of the parasite.
1. Introduction
Ribosomal phosphoprotein P0 is a highly conserved neutral protein found in the 60S ribosomal subunit of eukaryotes [1]. P0, along with the related acidic ribosomal phosphoproteins P1 and P2, forms a pentameric protein complex (P1)2-P0-(P2)2 that has a role in the assembly of the GTPase-binding site in the large subunit of ribosomes [2–4]. P0 is vital to cell survival as knocking it out is lethal in Saccharomyces cerevisiae and Plasmodium berghei [5, 6]. It has been postulated to have multiple other functions including apurinic-apyrimidinic endonuclease activity in Drosophila melanogaster [7], regulation of gene expression in Drosophila, and apoptosis and carcinogenesis in humans [7–10]. P0 has been shown to be present on the surface of Plasmodium spp., Toxoplasma gondii, Saccharomyces cerevisiae [11] as well as on the surface of neuronal, hepatic, and other cell lines [12, 13]. Human P proteins have been studied extensively because of their association with systemic lupus erythematosus (SLE), an autoimmune disorder. Approximately, 10 to 15% of patients suffering from SLE possess autoantibodies against the conserved 16 carboxy-terminal amino acids [14]. Clustal analysis reveals that this region of the protein is highly conserved across diverse species [15]. Human and mouse P0, for instance, differ only in six amino acids and are identical in the lupus domain (Figure 1). We have previously shown that 87% of adult residents in high-transmission malaria areas of eastern India possessed antibodies against Plasmodium falciparum P0 (PfP0) [16]. Similarly, 60% of adults residing in Kenya showed substantial T-cell responses to PfP0 protein [17]. Polyclonal and monoclonal antibodies against PfP0 have been shown to block parasite invasion of red blood cells in vitro and in vivo [15, 18, 19]. When we attempted to raise monoclonal antibodies (mAbs) against the major fragment of PfP0, recombinant PfP0 (rPfP0, 61−316 amino acids), we found that the first mouse, receiving 7 injections (4 weekly, 3 monthly), gave rise to unstable hybridomas reacting to the amino-terminus of the protein. The second mouse receiving 9 injections of the protein (4 weekly, 5 monthly), gave rise to several independent mAb clones, most of them reacting exclusively to the extreme carboxy-terminal, PfP0C0 (300−316 amino acids, Figure 1) [19]. The serum from this mouse reacted exclusively with rPfP0 and PfP0C0, but did not recognize other overlapping peptides derived from the protein [20]. PfP0C0 shows 69% identity to carboxy-terminal of human P0. This predominance of antibodies towards the lupus domain could have been a result of the age of the mouse (8 months), because of breakdown of immune tolerance following repeated immunizations, or both. Alternatively, it was possible that it was an idiosyncratic response of that mouse.
Figure 1.
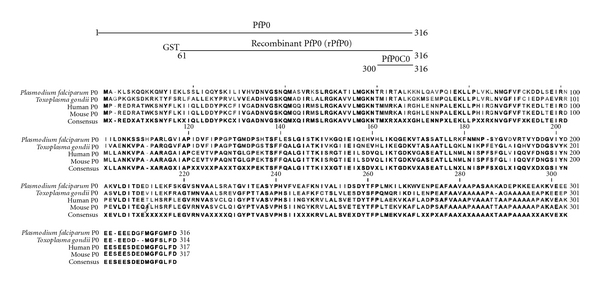
Schematic representation of PfP0, recombinant PfP0, and PfP0C0 and P0 multiple sequence alignment.
PfP0 is a potential vaccine candidate since anti-PfP0 antibodies were shown to protect against malarial infection in the murine model [18, 19]. Because of its conserved nature and the homology of the carboxy-terminal domain to the human protein, it is also likely to behave like an autoantigen. It was important to ascertain the quality and quantity of humoral response induced by the protein after repeated immunizations. We therefore undertook this systematic study wherein we attempted to raise mAbs against PfP0C0 after repeated immunizations with rPfP0. Selection processes in the central and peripheral levels govern the survival of B cells capable of responding to a particular immunogen, whereas peripheral antigen-driven selection processes determine the type and extent of humoral response. We reasoned that if splenic B cells are a reflection of the whole B cell response and that B cell specificity does not bias hybrid formation, then the frequency of hybridomas formed should reflect immunogenicity of different epitopes of PfP0. We also investigated the nature of the serum anti-PfP0C0 response.
We failed to raise a single anti-PfP0C0 hybridoma from any of the 21 mice used in these subsequent experiments, suggesting that the first success in raising hybridomas against the PfP0C0 domain was probably due to an unusual response observed in that one single mouse (of combined 23 mice used in the two studies). We observed connective tissue fibrosis of the spleen from the fourth month of the immunization schedule, and this increased progressively with further immunizations. Postmortem examination revealed pathological changes in the liver, heart, kidneys, and lungs of the mice. The average serum anti-PfP0C0 titre remained low (5.1 ± 1.3 × 104) even after 10 boosters. Dinitrophenyl (DNP) adsorption studies revealed that the humoral response was largely polyreactive. This polyreactivity was not confined to any particular immunoglobulin isotype, but was distributed across all isotypes. Sequence analysis of the seven hybridomas obtained in the initial study revealed that they were derived from a single clone. We analysed sera from uncomplicated malaria patients to determine the extent of polyreactivity to the protein. Our analysis revealed a similar polyreactive response to PfP0 and the PfP0C0 epitope in these sera. Our data suggests that PfP0 induces a deviant humoral response in mice. Induction of deviant, low-titre, polyreactive responses is likely a method of evading the host immune system.
2. Materials and Methods
2.1. Mice
Six-week-old female Balb/c mice were obtained from the local animal breeding facility at the Tata Institute of Fundamental Research, Mumbai, India. Mice were bred and maintained under specific pathogen-free conditions. All experimental research was conducted according to recommendations of the CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals).
2.2. Immunizations and Establishment of Hybridomas
Immunogens and peptides: the expression and purification of recombinant PfP0 (amino acids 61−316) as a GST fusion protein was done as described earlier [21]. The recombinant E. coli cells containing PfP0 gene fragment were grown for 2 h at 37°C, induced with IPTG, harvested, and lysed. The cell lysates were centrifuged, and insoluble particulate fraction containing the fusion protein was resolved on SDS-polyacrylamide preparative gel. A strip of the gel corresponding to MW of the recombinant protein was excised, electroeluted, dialysed against PBS, and concentrated. The identity of the protein was confirmed by Western blot analysis as described [22]. Ant brain homogenate, P. falciparum phosphofructokinase, P. falciparum enolase were prepared as described [23–25]. P0 protein from Toxoplasma gondii (TgP0) was cloned in pGEX4T1 and expressed as a GST fusion protein. The recombinant, full-length GST−TgP0 was extracted from induced cell pellets using sarkosyl [26], purified from the lysate using Glutathione sepharose beads (GE Healthcare), washed thrice with PBS containing 150 mM NaCl, and eluted using 100 mM glutathione. The identity of the protein was confirmed using SDS-PAGE and Western blotting (data not shown) Bovine serum albumin coupled to PfP0C0 (BSA-PfP0C0) was prepared as described earlier [19].
The synthetic peptides MoP0C0 (EESEESDEDMGFGLFD), PfP0C0 (EEEEEEDGFMGFGMFD) were obtained from Mimotopes, Canberra, Australia. Bovine insulin, ssDNA, and dsDNA were obtained from Sigma (St Louis, MO, USA).
Immunization and hybridoma establishment was done as described by Rajeshwari et al., 2004 [19]. Briefly, 50 μgs of protein emulsified in Freund's adjuvant was administered intraperitoneally in mice. The animals received four weekly injections followed by monthly injections. Except for the first immunization where Freund's complete adjuvant was used, the rest of the immunizations employed Freund's incomplete adjuvant. 4 days before fusion, the mice were boosted with 250 μg of the immunogen in saline. Mice were sacrificed after each monthly injection, their serum was collected, their spleens were harvested, and the splenocytes were fused with the mouse myeloma Sp2/0 cells. Antibody-secreting clones were selected by ELISA [27]. Immunogen-recognizing hybridomas were subcloned to monoclonality by limiting dilution. For rPfP0 immunizations, mice were given up to seven monthly immunizations; three mice were sacrificed after each monthly immunization.
2.3. Human Serum Samples
Serum samples were collected from patients of uncomplicated malaria as well as immune adults living in high endemicity area in Orissa, India. Presence or absence of P. falciparum infection was confirmed by microscopic examination of Giemsa-stained thick and thin blood smears or by using commercial kits. The samples were collected after informed consent and after obtaining the requisite clearances from the Ethics Committee, SCB Medical College and Hospital, Cuttack, India.
2.4. ELISA
Antibody response was determined by ELISA [19]. Briefly, Maxisorp plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with whole antigens (5 μg/mL) or synthetic peptides (200 ng/mL) in PBS, pH 7.2. The plates were blocked with 1% BSA before adding hybridoma supernatants or appropriate dilutions of the serum, developed using rabbit anti-mouse Ig conjugated to horseradish peroxidase (Boehringer Mannheim, Mannheim, Germany) and ABTS (Boehringer Mannheim), and the absorbance (optical density; OD) was measured at 405 nm with EL808 Ultra Microplate reader (Biotek Instruments Inc, Winooski, VT, USA).
To detect polyreactivity, DNP was conjugated to CNBr-activated sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK) as per manufacturer's instructions. Briefly, 2,4-DNP-ε-lysine (Sigma) was dissolved in 0.1 M NaHCO3 pH 8.3 containing 0.5 M NaCl, added to sepharose beads swollen in 1 mM HCl, and incubated on an end-over-end mixer for 1 h at room temperature. After washing away the excess ligand, the active sites were blocked using 0.1 M Tris-HCl buffer, pH 8.0. The beads were washed with three cycles of 0.1 M acetic acid/sodium acetate, pH 4.0 containing 0.5 M NaCl followed by a wash with 0.1 M Tris-HCl, pH 8 containing 0.5 M NaCl. Aliquots of serum dilutions were incubated or not with DNP-sepharose beads for 1.5 hour on a nutator and centrifuged. The supernatant was diluted further for use in ELISA. Percent polyreactivity was calculated as under:
| (1) |
2.5. Determination of RNA Sequence of Anti-PfP0C0 mAb Clones
Anti-PfP0C0 antibody producing hybridoma clones (1A4, 1B3, 2C11, 1E5F4, 1F6, 2G1, and 2H1) were lysed in Trizol (Invitrogen, Carlsbad, CA, USA), and RNA was prepared according to manufacturer's protocol. cDNA was prepared from the RNA using 3′ primer (heavy or light chain constant primer) and MMLV reverse transcriptase (NEB). PCR amplifications of light and heavy chains were performed using primers described in Table 1. Conditions used to amplify PCR fragments were as follows: DNA melting at 91°C for 1 min, primer annealing 52°C for 2 min, and polymerase extension at 72°C for 1.5 min for 30 cycles were carried out. The PCR products were TA cloned in pGEM-T vector system (Promega, Madison, WI, USA), and ligation mixes were used to transform XL-1 Blue E. coli bacteria. Positive clones were sequenced using M13F (5′d [GTAAAACGACGGCCAG]3′) and M13R (5′d [CAGGAAACAGCTATGAC]3′) primers.
Table 1.
Primers used to amplify antibody variable regions.
| Heavy chain 3′ primer | |
|---|---|
| IgG1 | 5′ AGG CTT ACT AGT ACA ATC CCT GGG CAC AAT 3′ |
| Heavy chain 5′ primers | |
| HC1 | 5′ AGG TCC AGC TGC TCG AGT CTG 3′ |
| HC2 | 5′ AGG TCC AGC TGC TCG AGT CAG 3′ |
| HC3 | 5′ AGG TCC AGC TTC TCG AGT CTG 3′ |
| HC4 | 5′ AGG TCC AGC TTC TCG AGT CAG 3′ |
| HC5 | 5′ AGG TCC AAC TGC TCG AGT CTG 3′ |
| HC6 | 5′ AGG TCC AAC TGC TCG AGT CAG 3′ |
| HC7 | 5′ AGG TCC AAC TTC TCG AGT CTG 3′ |
| HC8 | 5′ AGG TCC AAC TTC TCG AGT CAG 3′ |
|
| |
| Kappa light chain 3′ primer | |
| MKC | 5′ GCG CCG TCT AGA ATT AAC ACT CAT TCC TGT TGA A 3′ |
|
| |
| Kappa light chain 5′ primers | |
| LC1 | 5′ CCA GTT CCG AGC TCG TTG TGA CTC AGG AAT CT 3′ |
| LC2 | 5′ CCA GTT CCG AGC TCG TGT TGA CGC AGC CGC CC 3′ |
| LC3 | 5′ CCA GTT CCG AGC TCG TGC TCA CCC AGT CTC CA 3′ |
| LC4 | 5′ CCA GTT CCG AGC TCC AGA TGA CCC AGT CTC CA 3′ |
| LC5 | 5′ CCA GAT GTG AGC TCG TGA TGA CCC AGA CTC CA 3′ |
| LC6 | 5′ CCA GAT GTG AGC TCG TCA TGA CCC AGT CTC CA 3′ |
| LC7 | 5′ CCA GTT CCG AGC TCG TGA TGA CAC AGT CTC CA 3′ |
2.6. Statistical Analysis
Paired Wilcoxan signed-rank test was used to compare and evaluate the statistical significance of the unadsorbed and adsorbed OD of mouse sera. Nonparametric Mann-Whitney test was used to evaluate the statistical significance of the human serum data. GraphPad Instat software was used for analysis.
3. Results and Discussion
3.1. Recombinant PfP0 Immunizations Failed to Yield Stable Anti-PfP0C0 mAb Producing Hybridomas
We were interested in raising mAbs against the recombinant P. falciparum ribosomal protein P0 (PfP0) as polyclonal antibodies to this protein had inhibited parasite invasion of red blood cells [15, 19]. In a previous study, we were successful in raising stable mAbs against PfP0 after multiple immunizations only in one mouse, and most of these were against PfP0C0 [19]. Since the extreme C-terminal domain of human P0 protein is homologous to PfP0 carboxy-terminus (PfP0C0; 300−316 amino acids) and is the major P-protein autoantigen involved in lupus [14], we were interested in probing the nature of the humoral response to PfP0C0 and wanted to determine the frequency with which we would obtain mAbs against this domain of PfP0 protein. The full-length protein shows extremely poor expression, hence we used the major fragment of the protein (rPfP0, 61−316 amino acids) for immunizations.
We immunized 6-week-old Balb/c mice with four weekly injections of rPfP0 in Freund's complete adjuvant, followed by monthly boosters. Spleens from 3 mice each were harvested after each monthly injection (5th through 11th immunization) for hybridoma preparation. Table 2, shows the results for 5th, 7th, 9th, and 11th immunization. Similar results were obtained for the remaining time points. As is seen from Table 2, we failed to obtain any stable clones against PfP0C0, despite repeated immunizations or the age of the mice. Screening of about 1800 clones yielded only 22 stable clones. Most clones were either polyreactive or stopped antibody production within a few days and failed to recognize the carboxy-terminal epitope. Thus, neither age of the animal nor repeated immunizations with a potential autoantigen could be the reason for the success of raising hybridomas in the initial study.
Table 2.
Serum titre and hybridomas obtained following repeated immunizations with various proteins.
| Immunization number | Number of mice screened | Number of clones screened | Stable clones (specificity) | Serum titre (×104) | Splenic fibrosis |
|---|---|---|---|---|---|
| Recombinant PfP0 | |||||
| 4 | 16 | ND | − | 2.5 ± 0.6 | − |
| 5 | 2 | 120 | 0 | ND | − |
| 7 | 3 | 282 | 0 | 2.4 ± 0.1 | + |
| 9 | 3 | 478 | 9 (Mouse P0, polyreactive) | 5.3 ± 1.3 | ++ |
| 11 | 2 | 571 | 13 (non-PfP0C0, GST) | 4.0 ± 1.0 | +++ |
|
| |||||
| Ant-brain homogenate | |||||
| 4 | 1 | 700 | 33 | 5 | − |
|
| |||||
| Drosophila Translin | |||||
| 3 | 1 | 290 | 22 | >10 | − |
|
| |||||
| Plasmodium falciparum Phosphofructokinase | |||||
| 3 | 1 | 190 | 7 | >50 | − |
|
| |||||
| Plasmodium falciparum enolase | |||||
| 5 | 1 | 290 | 10 | >50 | − |
|
| |||||
| Toxoplasma gondii P0 | |||||
| 5 | 1 | 300 | >70 | >17 | − |
ND: not done; −: negative, +: mild, ++: moderate, +++: severe.
Our failure to raise stable hybridomas against PfP0C0 cannot be attributed to faulty technique, since we succeeded in obtaining multiple stable clones against a variety of proteins (Table 2). These include ant brain homogenate, Drosophila translin which bears 52% homology with the mouse protein [28], parasite proteins such as P. falciparum fructokinase-β, and potential autoantigenic proteins such as P. falciparum enolase [29, 30] and TgP0. The identity of PfP0 and mouse P0 C-terminus over the extreme 16 amino acids is 69% (Figure 1). This homology of PfP0 to mouse P0 alone cannot be the only reason for lack of hybridomas, since both P. falciparum enolase and TgP0 show considerable homologies with mouse enolase and mouse P0 (75% and 62.5%, resp.), and these were amenable to generation of a large number of stable hybridomas. Secondary antigenic challenges result in the generation of long-lived plasma cells capable of secreting high-affinity antibodies to the immunizing antigen [31]. It is tempting to speculate that PfP0 is an unusual antigen in that it failed to induce a robust B cell response, since repeated immunizations yielded very few stable hybridomas, and none of them were against the immunogenic carboxy-terminus of the antigen.
3.2. Earlier mAbs Raised against PfP0C0 Arose from a Single B Cell Clone
Since in our previous studies we obtained several mAbs exclusively against PfP0C0 from one mouse, we analyzed the isotype and sequences of the seven mabs (1A4, 1B3, 2C11, 1E5F4, 1F6, 2G1, and 2H1) from this earlier study to establish their relatedness, if any. Isotype analysis showed that all the mAbs belonged to IgG1 isotype. We performed RT-PCRs and amplified products of the heavy and the light chains of these seven mAb clones and sequenced them (Figure 2). It was observed that the CDR2 and CDR3 regions were identical for the heavy chain in all seven clones. Two single base changes in CDR1 region of 1A4 and 1E5F4 and 3 base changes in 2C11 gave rise to some amino acid changes in the CDR1 region of 3 out of 7 clones. Regarding the light chain, 1A4 and 2C11 showed certain base changes, while all the other five clones were identical. Although the CDR3 region of 1A4 was decidedly different, the CDR1 and 2 regions were near identical. The remaining regions of the IgG1 chains of these clones were identical (data not shown). It is therefore apparent that the clones obtained in the earlier study originated from a single B cell clone. This could be due to an unusual expansion of the clone, possibly due to breakdown of tolerance, and was definitely an aberrant incidence since subsequent analysis failed to reproduce our earlier result of obtaining mAb clones against PfP0C0 (Table 2).
Figure 2.
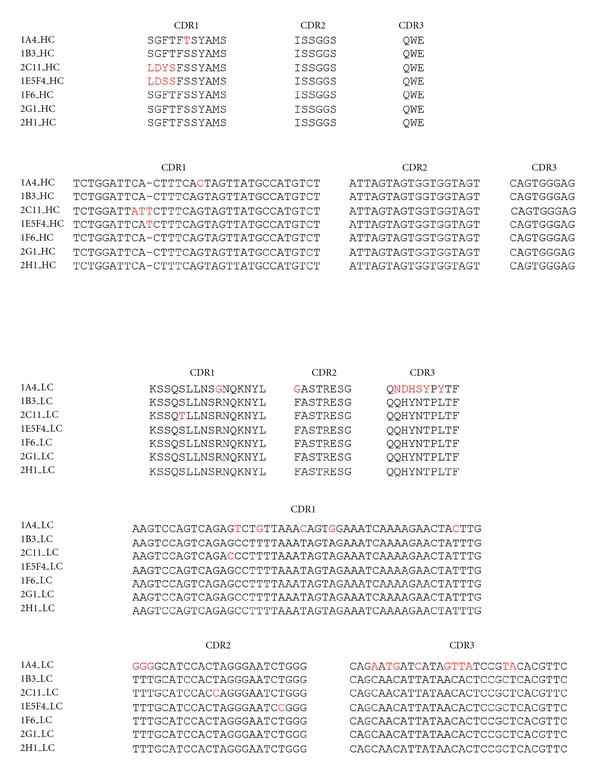
Sequence determination of anti-PfP0C0 mAbs obtained in the first study. Seven mAbs obtained in our earlier study [19], namely, 1A4, 1B3, 2C11, 1E5F4, 1F6, 2G1 and 2H1 were sequenced to determine their relatedness. Except for some minor variations (in red), all seven mAbs were nearly identical, suggesting that they had arisen from a single B cell clone.
3.3. Recombinant PfP0 Immunizations Resulted in Pathological Changes in the Mice
During the course of spleen harvesting for hybridoma generation, we noticed mild connective tissue fibrosis of the spleen from the 7th immunization (Table 2). The animals started to become sick following the booster immunization. This fibrosis worsened progressively, and by 11th immunization the spleen became difficult to harvest (Figure 3). Marked splenic fibrosis had not been observed in the earlier study. Since the aim of that study was to obtain mAbs to PfP0C0, postmortem pathological examination had also not been undertaken. Postmortem analysis of the mice revealed pathological changes in all the major organs such as the heart, lungs, liver, and kidneys (Table 3). Interestingly, no abnormality was detected in the brain. These findings suggested that repeated immunizations with rPfP0 resulted in a humoral autoimmune response, possibly because of the homology between the parasite P0 and mouse P0. The brain is well separated from blood by the blood-brain barrier. Antibodies do not normally cross this barrier [32]. The pathological changes observed in all the major organs excluding the brain suggested autoantibodies as a possible cause of the damage. We therefore investigated the serum Ig response to PfP0C0.
Figure 3.
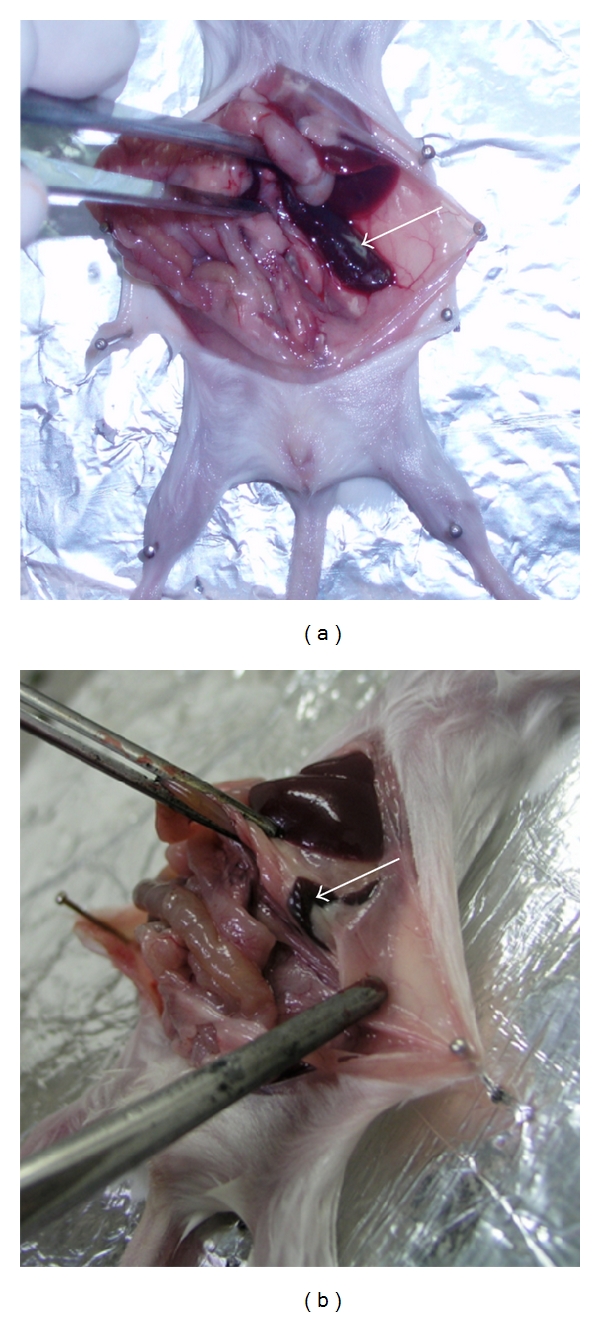
Splenic fibrosis observed after repeated immunizations with recombinant PfP0. (a) Spleen of control mice. (b) Spleen of a mouse following repeated immunization with recombinant PfP0 (four weekly and seven monthly). Arrows point to the spleen. Note the reduced size of the spleen and fibrosis around it. Histopathological analysis revealed a loss of lymphoid cells and extensive replacement of splenic tissue with connective tissue.
Table 3.
Results of histopathological investigations of mice following repeated recombinant PfP0 administrations.
| Organ | Severity | Changes observed |
|---|---|---|
| Liver | +++ | Severe swelling and derangement of cords, infiltration of mononuclear cells around the portal triad |
| Kidneys | +++ | Tubular necrosis and diffuse necrotic changes along with infiltration of inflammatory cells around the necrotic area |
| Lungs | +++ | Edema, congestion, MNC infiltration, diffuse interstitial and patchy pneumonia |
| Heart | + | Mild congestion and swelling of fibres |
| Spleen | +++ | Loss of lymphoid cells, replaced by connective tissue |
| Brain | − | No abnormality detected |
−: negative, +: mild, +++: severe.
3.4. Recombinant PfP0 Induces a Low-Titre, Largely Polyreactive Serum Anti-PfP0C0 Response
Sera collected from the mice after each monthly immunization with rPfP0 were tested for their anti-PfP0C0 response. The serum titre remained low even after the 11th administration of the protein (Table 2). By contrast, high-titre responses were obtained after just 4 or 5 immunizations against a variety of other proteins, including those that displayed a high degree of homology to mouse proteins such as P. falciparum enolase, Drosophila translin, and TgP0. The low titre of the sera, coupled with the unstable polyreactive hybridomas obtained, suggested the possibility that the induced anti-PfP0C0 response was polyreactive. Polyreactive antibodies are low affinity antibodies that can bind a range of biological molecules of both self- and non-self-origin [33, 34]. They are normal serum constituents and are a significant part of the initial immune response. DNP treatment is commonly used to remove polyreactive antibodies in animals not exposed to the hapten [35, 36]. We treated the sera with DNP-sepharose beads and determined the anti-PfP0C0 titre of adsorbed and unadsorbed sera in ELISA. Treatment with DNP led to a statistically significant reduction in the anti-PfP0C0 reactivity of the sera, indicating that PfP0 immunization induced a largely polyreactive anti-PfP0C0 response. Figure 4(a) shows a typical response of DNP adsorbed and unadsorbed serum sample. The extent of polyreactivity remained high (median value 32%) even after repeated boosters (Figure 4(b)). This polyreactive response was not confined to a particular isotype, but was found across all isotypes, since DNP treatment resulted in statistically significant reduction in anti-PfP0C0 response in all isotypes (Figure 4(c)). We also tested the sera for reactivity against dsDNA, ssDNA, and insulin. Figure 4(d) shows the result of a single serum sample. Similar results were obtained for other samples (data not shown).
Figure 4.
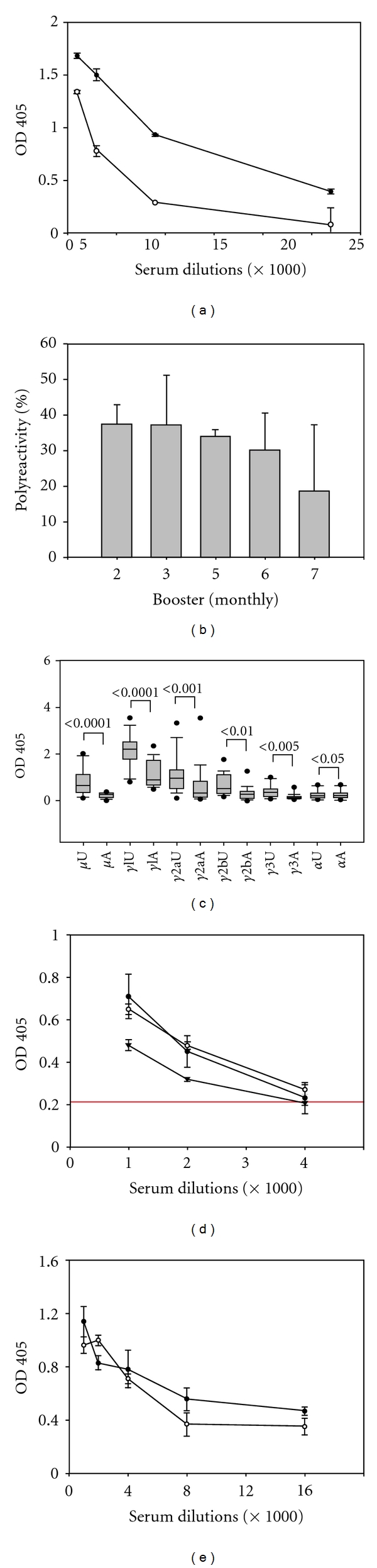
Polyreactive serum anti-PfP0C0 response induced following repeated immunizations with rPfP0 but not BSA-PfP0C0. (a) Sera of mice administered recombinant PfP0 were treated or not with DNP-sepharose beads. Anti-PfP0C0 titre before and after treatment was determined in an ELISA. A representative example of anti-PfP0C0 response of DNP adsorbed (empty circles) and unadsorbed (filled circles) sera. Error bars represent the SEM of three readings. (b) Mice were immunized by four weekly and up to seven monthly injections of rPfP0. Aliquots of the sera were treated with DNP-sepharose beads to adsorb out polyreactive antibodies. The bar graphs depict the percent polyreactivity in sera of mice after multiple immunizations as indicated. Error bars represent the SEM of 3 sera. (c) Box plots showing the 25th and 75th percentiles, together with the median, with whiskers showing the minimum and maximum difference and filled circles representing the outliers, in the ELISA OD responses of DNP unadsorbed (U) and adsorbed (A) sera of all 21 mice immunized with recombinant PfP0; μ (IgM), γ1 (IgG1) γ2a (IgG2a), γ2b (IgG2b), γ3 (IgG3), and α (IgA). Statistically significant values obtained by Paired Wilcoxan signed-rank test are indicated. (d) Autoreactivity of a representative serum sample against bovine insulin (closed circle), dsDNA (open circle), and ssDNA (closed triangle) of mice immunized with rPfP0. Red line represents the response of control unimmunized sera at the lowest dilution (1 : 1000). Error bars represent the SEM of three readings. Similar results were obtained with other sera. (e) Mice were administered BSA-PfP0C0, and the sera were treated or not with DNP-sepharose beads. Anti-PfP0C0 titre before and after treatment was determined in ELISA. A representative example of anti-PfP0C0 response of DNP adsorbed (empty circles) and unadsorbed (filled circles) sera. Error bars represent the SEM of three readings.
3.5. Malarial Infection Also Results in a Low-Titre, Largely Polyreactive Serum Anti-PfP0 and Anti-PfP0C0 Response
We wished to determine if serum from patients of uncomplicated malaria also showed a similar polyreactive response to PfP0. The protein is highly conserved across P. falciparum. Amongst the 13 strains reported in PlasmoDB [37], the DNA sequence is identical except for a single synonymous mutation in one strain (Figure 5). PfP0 is also abundantly expressed on the surface of merozoites [18]. Although we normally see elevated antibody responses to PfP0 in malaria immune adults as compared to patients [18], we had not assessed the polyreactive component earlier. We tested sera from immune adults as also patients of uncomplicated malaria for anti-PfP0 and anti-PfP0C0 antibodies. In agreement with our earlier results, immune adults, but not patients, showed an elevated response to PfP0 (Figure 6(a), P < 0.01). Analysis of the response for polyreactivity to PfP0 revealed that the patient's sera were highly polyreactive (Figure 6(b); median value 44.8%). By contrast, sera from immune adults exhibited minimal polyreactivity to the protein (P = 0.001; median value 13.9%). Similar results were obtained for PfP0C0 (data not shown).
Figure 5.

DNA sequence alignment of P0 across P. falciparum strains showing one synonymous mutation (marked in yellow) in the Santa Lucia strain. The rest of the sequences were identical across strains.
Figure 6.
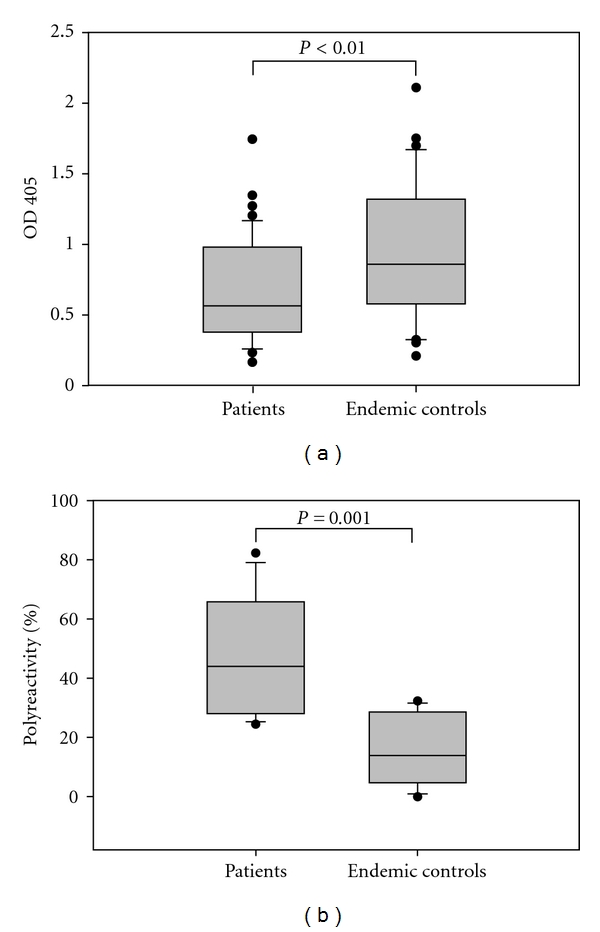
Polyreactive serum anti-PfP0C0 response observed in uncomplicated malaria patients, but not in immune adults living in high endemicity areas. (a) Box plots showing the 25th and 75th percentiles, together with the median, with whiskers showing the minimum and maximum difference and filled circles representing the outliers in anti-PfP0 responses of sera from patients of uncomplicated malaria (n = 49) and immune adults (n = 30) as determined in ELISA. (b) Box plots showing the 25th and 75th percentiles, together with the median, with whiskers showing the minimum and maximum difference and filled circles representing the outliers of % polyreactivity in anti-PfP0 responses of sera from patients and immune adults. The sera were treated or not with DNP-sepharose beads, and anti-PfP0 titre before and after treatment was determined in ELISA to determine the % polyreactivity.
Polyreactive autoantibodies have been reported in Plasmodium-chabaudi-infected mice [38]. The autoantibodies found in clinically protected persons resident in malaria hyperendemic areas are similar to those present in disorders such as SLE, rheumatoid arthritis, Sjogren's syndrome, polymyositis, scleroderma, and Hashimoto's thyroiditis. These can bind to double- and single-stranded DNA, erythrocytes, immunoglobulins, ribonucleoproteins, and enolase. However, anti-thyroglobulin antibodies, autoreactive to B cells and found in normal persons, are not enhanced in such a population, indicating that it is not a matter of random nonspecific polyclonal B cell activation against conserved antigens [18, 25, 39, 40]. It is unlikely that homology of PfP0 to the host protein is responsible for the observed polyreactive response since repeated immunizations with Pf enolase or bovine serum albumin coupled to PfP0C0 (BSA-PfP0C0) failed to induce a polyreactive response. Interestingly, although specific, BSA-PfP0C0 administration did not induce a high titre anti-PfP0C0 response (titre ~2 ×104, Figure 4(e)). However, this response was autoreactive, since the sera were reactive to mouse P0C0, albeit at a tenfold lower concentration (titre ~2 ×103, data not shown). Thus, the potentially autoreactive epitope coupled to a carrier protein failed to induce a polyreactive response, but the recombinant protein containing 61–316 amino acids induced a low-titre, polyreactive response. This suggests that the capacity to induce polyreactivity probably lies in the 61−300 region of the protein. It is also possible that the conformation of the protein is an important factor in the induction of polyreactivity or that rPfP0 acts like a B cell mitogen. Further investigations are needed to determine if a particular region and/or the conformational structure of the protein is responsible for induction of a polyreactive response.
Normal human serum contains natural autoantibodies that recognize self-antigens [41]. Analysis of human monoclonal autoantibodies derived from normal peripheral blood B cells has shown that natural autoantibodies are polyreactive and express germline immunoglobulin variable region genes with little or no somatic mutation [42, 43], although polyreactivity has also been observed in affinity-matured antibodies [44]. The capacity to adopt multiple conformational states in equilibrium allows polyreactive antibodies to bind multiple, structurally unrelated antigens [45]. Polyreactive antibodies have been reported to be induced in murine model of malaria [38]. We show that immunization with a parasite-derived protein can also induce a polyreactive response. Interestingly, we find people suffering from malaria also exhibit a polyreactive response to PfP0. This polyreactive response was confined to patients, since sera from immune adults showed a comparatively nonpolyreactive response to the protein. We have earlier shown that PfP0 is expressed on the surface of merozoites [18]. Thus, it could be that exposure to low levels of the protein (as would happen in the case of immune endemic adults) results in specific response to the protein, and this specific response protects against malarial disease. Exposure to high doses of the protein (e.g., during active malarial disease or during immunization protocols), on the other hand, possibly results in a polyreactive response. Further investigations are needed to see if low doses of PfP0 with or without a strong adjuvant result in a specific anti-PfP0 response.
Antibodies have a crucial role in the control of malarial infection. Opsonization by antibodies to parasite proteins expressed on the surface of merozoites or infected erythrocytes facilitates their removal and eventual destruction by macrophages [46]. The malarial parasite has been shown to subvert the host immune system in multiple ways including antigenic variation and antigenic polymorphisms, interference with dendritic cell maturation, and induction of apoptosis in memory B and T cells [47–50]. Here, we show that a conserved, parasite-derived protein induces a deviant humoral response in the host. A propensity to express or secrete conserved antigens that induce a weak polyreactive humoral response could be one more mechanism of enhancing parasite survival.
Acknowledgments
The authors gratefully acknowledge Dr. Pierre-André Cazenave for critical reading of the manuscript. They also thank Dr. S. Das and N. Jindal for their inputs. This study was conducted using funds received from the Department of Atomic Energy, Government of India. S. Pathak and K. Rajeshwari contributed equally to the paper.
Abbreviations
- PfP0:
Plasmodium falciparum P0
- rPfP0:
Recombinant PfP0
- PfP0C0:
Carboxy-terminal 16 amino acids of PfP0
- SLE:
Systemic lupus erythematosus
- mAbs:
Monoclonal antibodies
- DNP:
Dinitrophenyl
- TgP0:
Toxoplasma gondii P0
- BSA-PfP0C0:
Bovine serum albumin coupled to PfP0C0.
References
- 1.Rich BE, Steitz JA. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Molecular and Cellular Biology. 1987;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saenz-Robles MT, Remacha M, Vilella MD, Zinker S, Ballesta JPG. The acidic ribosomal proteins as regulators of the eukaryotic ribosomal activity. Biochimica et Biophysica Acta. 1990;1050(1–3):51–55. doi: 10.1016/0167-4781(90)90140-w. [DOI] [PubMed] [Google Scholar]
- 3.Uchiumi T, Wahha AJ, Traut RR. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by cross linking. Proceedings of the National Academy of Sciences of United States of America. 1987;84(16):5580–5584. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchiumi T, Kominami R. Direct evidence for interaction of the conserved GTPase domain within 28 S RNA with mammalian ribosomal acidic phosphoproteins and L12. Journal of Biological Chemistry. 1992;267(27):19179–19185. [PubMed] [Google Scholar]
- 5.Santos C, Ballesta JPG. Ribosomal protein P0, contrary to phosphoproteins P1 and P2, is required for ribosome activity and Saccharomyces cerevisiae viability. Journal of Biological Chemistry. 1994;269(22):15689–15696. [PubMed] [Google Scholar]
- 6.Das S, Basu H, Korde R, et al. Arrest of nuclear division in Plasmodium through blockage of erythrocyte surface exposed ribosomal protein P2. In press. [DOI] [PMC free article] [PubMed]
- 7.Yacoub A, Kelley MR, Deutsch WA. Drosophila ribosomal protein PO contains apurinic/apyrimidinic endonuclease activity. Nucleic Acids Research. 1996;24(21):4298–4303. doi: 10.1093/nar/24.21.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frolov MV, Birchler JA. Mutation in P0, a dual function ribosomal protein/apurinic/apyrimidinic endonuclease, modifies gene expression and position effect variegation in Drosophila. Genetics. 1998;150(4):1487–1495. doi: 10.1093/genetics/150.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockstedt E, Rickers A, Kostka S, et al. Identification of apoptosis-associated proteins in a human Burkitt lymphoma cell line: cleavage of heterogeneous nuclear ribonucleoprotein A1 by caspase 3. Journal of Biological Chemistry. 1998;273(43):28057–28064. doi: 10.1074/jbc.273.43.28057. [DOI] [PubMed] [Google Scholar]
- 10.Kondoh N, Wakatsuki T, Akihide R, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Research. 1999;59(19):4990–4996. [PubMed] [Google Scholar]
- 11.Singh S, Sehgal A, Waghmare S, Chakraborty T, Goswami A, Sharma S. Surface expression of the conserved ribosomal protein P0 on parasite and other cells. Molecular and Biochemical Parasitology. 2002;119(1):121–124. doi: 10.1016/s0166-6851(01)00394-2. [DOI] [PubMed] [Google Scholar]
- 12.Hirohata S, Nakanishi K. Antiribosomal P protein antibody in human systemic lupus erythematosus reacts specifically with activated T cells. Lupus. 2001;10(9):612–621. doi: 10.1191/096120301682430195. [DOI] [PubMed] [Google Scholar]
- 13.Koren E, Reichlin MW, Koscec M, Fugate RD, Reichlin M. Autoantibodies to the ribosomal P proteins react with a plasma membrane- related target on human cells. Journal of Clinical Investigation. 1992;89(4):1236–1241. doi: 10.1172/JCI115707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frampton G, Moriya S, Pearson JD, et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein PO as an endothelial cell autoantigen. Rheumatology. 2000;39(10):1114–1120. doi: 10.1093/rheumatology/39.10.1114. [DOI] [PubMed] [Google Scholar]
- 15.Goswami A, Singh S, Redkar VD, Sharma S. Characterization of P0, a ribosomal phosphoprotein of Plasmodium falciparum. Antibody against amino-terminal domain inhibits parasite growth. Journal of Biological Chemistry. 1997;272(18):12138–12143. doi: 10.1074/jbc.272.18.12138. [DOI] [PubMed] [Google Scholar]
- 16.Lobo CA, Kar SK, Ravindran B, Kabilan L, Sharma S. Novel proteins of Plasmodium falciparum identified by differential immunoscreening using immune and patient sera. Infection and Immunity. 1994;62(2):651–656. doi: 10.1128/iai.62.2.651-656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra I, Mungai P, Muchiri E, et al. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infection and Immunity. 2005;73(6):3462–3470. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee S, Singh S, Sohoni R, et al. Antibodies against ribosomal phosphoprotein P0 of Plasmodium falciparum protect mice against challenge with Plasmodium yoelii. Infection and Immunity. 2000;68(7):4312–4318. doi: 10.1128/iai.68.7.4312-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajeshwari K, Patel K, Nambeesan S, et al. The P domain of the P0 protein of Plasmodium falciparum protects against challenge with malaria parasites. Infection and Immunity. 2004;72(9):5515–5521. doi: 10.1128/IAI.72.9.5515-5521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajeshwari. Unpublished observation.
- 21.Chatterjee S, Singh S, Sohoni R, et al. Characterization of domains of the phosphoriboprotein P0 of Plasmodium falciparum. Molecular and Biochemical Parasitology. 2000;107(2):143–154. doi: 10.1016/s0166-6851(99)00226-1. [DOI] [PubMed] [Google Scholar]
- 22.Aruna K, Chakraborty T, Nambeesan S, et al. Identification of a hypothetical membrane protein interactor of ribosomal phosphoprotein P0. Journal of Biosciences. 2004;29(1):33–43. doi: 10.1007/BF02702559. [DOI] [PubMed] [Google Scholar]
- 23.Hofbauer A. Eine Bibliothek monoklonaler Antikorper gegen das Gehirn von Drosophila melanogaster. Wurzburg, Germany: University of Wurzburg; 1991. Habilitation thesis. [Google Scholar]
- 24.Mony BM, Mehta M, Jarori GK, Sharma S. Plant-like phosphofructokinase from Plasmodium falciparum belongs to a novel class of ATP-dependent enzymes. International Journal for Parasitology. 2009;39(13):1441–1453. doi: 10.1016/j.ijpara.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Pal-Bhowmick I, Vora HK, Roy J, Sharma S, Jarori GK. Generation and characterisation of monoclonal antibodies specific to Plasmodium falciparum enolase. Journal of Vector Borne Diseases. 2006;43(2):43–52. [PubMed] [Google Scholar]
- 26.Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Analytical Biochemistry. 1993;210(1):179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 27.Engvall E. Enzyme immunoassay ELISA and EMIT. Methods in Enzymology. 1980;70:419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- 28.Claussen M, Koch R, Jin ZY, Suter B. Functional characterization of Drosophila Translin and Trax. Genetics. 2006;174(3):1337–1347. doi: 10.1534/genetics.106.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii A, Yoneda M, Ito T, et al. Autoantibodies against the amino terminal of α-enolase are a useful diagnostic marker of Hashimoto’s encephalopathy. Journal of Neuroimmunology. 2005;162(1-2):130–136. doi: 10.1016/j.jneuroim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-α-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. Journal of Clinical Immunology. 2007;27(2):181–192. doi: 10.1007/s10875-006-9065-8. [DOI] [PubMed] [Google Scholar]
- 31.Calame KL. Plasma cells: finding new light at the end of B cell development. Nature Immunology. 2001;2(12):1103–1108. doi: 10.1038/ni1201-1103. [DOI] [PubMed] [Google Scholar]
- 32.Bouras C, Riederer BM, Kövari E, Hof PR, Giannakopoulos P. Humoral immunity in brain aging and Alzheimer’s disease. Brain Research Reviews. 2005;48(3):477–487. doi: 10.1016/j.brainresrev.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Tchernychev B, Cabilly S, Wilchek M. The epitopes for natural polyreactive antibodies are rich in proline. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6335–6339. doi: 10.1073/pnas.94.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. Journal of Autoimmunity. 2007;29(4):219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berneman A, Guilbert B, Eschrich S, Avrameas S. IgG auto- and polyreactivities of normal human sera. Molecular Immunology. 1993;30(16):1499–1510. doi: 10.1016/0161-5890(93)90458-n. [DOI] [PubMed] [Google Scholar]
- 36.Bruley-Rosset M, Mouthon L, Chanseaud Y, Dhainaut F, Lirochon J, Bourel D. Polyreactive autoantibodies purified from human intravenous immunoglobulins prevent the development of experimental autoimmune diseases. Laboratory Investigation. 2003;83(7):1013–1023. doi: 10.1097/01.lab.0000077982.70800.02. [DOI] [PubMed] [Google Scholar]
- 37. http://PlasmoDB.org, 2011.
- 38.Ternynck T, Falanga PB, Unterkirscher C, Gregoire J, Pereira da Silva L, Avrameas S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. International Immunology. 1991;3(1):29–37. doi: 10.1093/intimm/3.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Daniel-Ribeiro CT, Zanini G. Autoimmunity and malaria: what are they doing together? Acta Tropica. 2000;76(3):205–221. doi: 10.1016/s0001-706x(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro CD, Alfred C, Monjour L, Gentilini M. Normal frequency of anti-thyroglobulin antibodies in hyperendemic areas of malaria: relevance to the understanding of autoantibody formation in malaria. Tropical and Geographical Medicine. 1984;36(4):323–328. [PubMed] [Google Scholar]
- 41.Hurez V, Dietrich G, Kaveri SV, Kazatchkine MD. Polyreactivity is a property of natural and disease-associated human autoantibodies. Scandinavian Journal of Immunology. 1993;38(2):190–196. doi: 10.1111/j.1365-3083.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 42.Siminovitch KA, Misener V, Kwong PC, Song QL, Chen PP. A natural autoantibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. Journal of Clinical Investigation. 1989;84(5):1675–1678. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanz I, Casali P, Thomas JW, Notkins AL, Capra JD. Nucleotide sequences of eight human natural autoantibody v(H) regions reveals apparent restricted use of V(H) families. Journal of Immunology. 1989;142(11):4054–4061. [PubMed] [Google Scholar]
- 44.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299(5611):1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 45.Sethi DK, Agarwal A, Manivel V, Rao KVS, Salunke DM. Differential epitope positioning within the germline antibody paratope enhances promiscuity in the primary immune response. Immunity. 2006;24(4):429–438. doi: 10.1016/j.immuni.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunology. 2006;28(1-2):51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 47.Urban BC, Ferguson DJP, Pain A, et al. Plasmodium falciparum infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400(6739):73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 48.Pierce SK, Miller LH. World Malaria Day 2009: what malaria knows about the immune system that immunologists still do not. Journal of Immunology. 2009;182(9):5171–5177. doi: 10.4049/jimmunol.0804153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wykes MN, Zhou YH, Liu XQ, Good MF. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. Journal of Immunology. 2005;175(4):2510–2516. doi: 10.4049/jimmunol.175.4.2510. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Wipasa J, Yan H, et al. The mechanism and significance of deletion of parasite-specific CD4+ T cells in malaria infection. Journal of Experimental Medicine. 2002;195(7):881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]


