Abstract
Climate change driven increases in intensity and frequency of both hot and cold extreme events contribute to coral reef decline by causing widespread coral bleaching and mortality. Here, we show that hot and cold temperature changes cause distinct physiological responses on different time scales in reef-building corals. We exposed the branching coral Acropora yongei in individual aquaria to a ± 5°C temperature change. Compared to heat-treated corals, cold-treated corals initially show greater declines in growth and increases in photosynthetic pressure. However, after 2–3 weeks, cold-treated corals acclimate and show improvements in physiological state. In contrast, heat did not initially harm photochemical efficiency, but after a delay, photosynthetic pressure increased rapidly and corals experienced severe bleaching and cessation of growth. These results suggest that short-term cold temperature is more damaging for branching corals than short-term warm temperature, whereas long-term elevated temperature is more harmful than long-term depressed temperature.
Coral reefs are one of the world's most diverse and productive ecosystems. The survival and success of reef-building corals depend on a healthy relationship between corals and their endosymbiotic dinoflagellates, which provide corals with most of their energy1,2. Similar to other photosynthetic organisms, endosymbiotic dinoflagellates rely on a delicate balance between sunlight absorbed and processed through photochemistry3, which is easily disrupted by environmental stressors. Concurrent extreme temperature and high irradiance can damage the photosynthetic system, which may generate oxidative stress and cause the collapse of the coral-algal symbiosis4,5,6,7,8,9,10,11. The breakdown of the symbiosis, also called coral bleaching, causes declines in coral health and even mortality12,13,14.
Worldwide, coral reefs are threatened because of climate change as well as other anthropogenic stressors6,15,16,17. Because climate change affects climate variability18,19,20, long-term trends in ocean warming are punctuated by episodes of extreme temperatures, which can have devastating effects on corals reefs6,16,21. Within one year alone (2010), coral reefs faced one of the coldest winters22 and one of the hottest summers23. Corals in Florida, USA experienced a 5–8°C decline in seawater temperature22, while corals in Indonesia experienced a 4°C increase in seawater temperature23; as a result, preliminary reports of the extent of coral bleaching have been of concern. Previous studies on corals have focused on the effects of heat stresse.g.7,11,24,25; however, few studies of cold stress have been carried out21,26,27,28. Systematic parallel studies of the effects of increased and decreased temperature on coral and dinoflagellate physiology would provide a realistic foundation not only for our understanding of coral biology, but also for the effective conservation and management of coral reefs.
Heat shock and to a lesser extent cold shock experiments have been able to provide better understanding of coral physiology, showing that elevated, but also depressed temperatures, can cause breakdown of the symbiosis especially when combined with high irradiance. These conditions induce severe damage to the photosynthetic apparatus, specifically, the reaction center of photosystem II (PSII), the Calvin cycle, and/or the thylakoid membranes, and can result in high levels of reactive oxygen species4,5,6,7,8,29. The ensuing oxidative stress can lead to cell apoptosis or exocytosis of the coral host10,11,26,30. As for the endosymbiotic dinoflagellates, changes in temperature elicit a number of responses including changes in population density, photosynthetic pigment concentration, photosynthetic efficiency, photosynthetic capacity, D1 reaction center protein concentration, and xanthophyll cycling7,11,21,24,25,27,28,31,32,33,34. Furthermore, PSII repair processes are also inhibited by elevated temperatures under high light intensities8. Thus host functions, such as coral calcification, growth and reproduction, can be greatly reduced by small changes in temperature12,13,14. To address the regulation and extent of these processes, we studied the effects of simultaneous cold and heat stress on physiological processes in reef-building corals. Exposures to elevated and depressed temperature were conducted in parallel in order to provide a consistent framework for the comparison of the effects of temperature on the physiological state of the coral holobiont.
The experiment we conducted induced cold (−5°C) and heat (+5°C) treatments on the common reef-building coral Acropora yongei, endemic the Indo-West Pacific. Acropora is generally regarded as susceptible to bleaching6, which makes it a model organism to investigate temperature stress. The 20 d laboratory experiment included intermediary analyses at days 5, 9, and 12 to elucidate shorter and longer-term time scales of the effects of temperature changes on coral physiology. Coral branches in individual aquaria were subjected to either cold (21°C), control (26°C), or heat (31°C) treatments under constant controlled lighting conditions (see Methods). As expected, both cold and heat stress negatively affected corals, yet to our surprise the experiment identified two divergent and critical time scales of physiological responses: an initial phase from 0–5 d, and a final phase extending until the end of the experiment (6–20 d). In the initial phase, the decrease in temperature caused more stress to the coral holobiont; whereas in the final phase, the increase in temperature was more stressful.
Results
Coral growth can be considered an integrated assessment for the overall health of the coral holobiont. Remarkable declines in coral growth were observed in both temperature treatments, yet divergent responses between cold and heat treatments emerged over the course of the experiment suggesting different temperature-driven physiological modes of action (Fig. 1a, Supplementary Table S1 online). During the initial phase, cold-treated coral growth rates were <0.5x of heat-treated corals. Growth rates of cold-treated corals reached lowest values during days 5–9 (15x less than controls); rates stabilized and actually increased 2.5x from days 9–12 to 12–20, but remained about 5x lower than controls. In contrast, the growth rates of heat-treated corals decreased throughout the experiment until there was no detectable growth during days 12–20, beyond which death would have likely occurred.
Figure 1. Effect of temperature change on coral growth and endosymbiotic dinoflagellate populations of Acropora yongei.
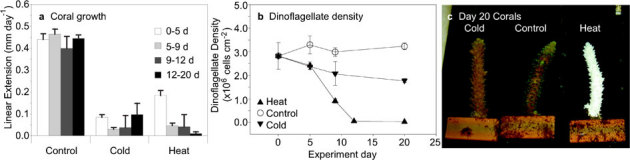
(a) Coral linear extension (mm day−1; mean ± s.e.m.) during days 0–5 (N = 15−19), days 5–9 (N = 10−14), days 9–12 (N = 5−9), and days 12–20 (N = 4−5). (b) Coral dinoflagellate density (x106 cells cm−2; mean ± s.e.m.; N = 4−5) over time during the experiment. (c) Single image of 3 representative corals after 20 d thermal stress. The heat treatment coral has bleached, a discoloration from the reduction of endosymbiotic dinoflagellates resulting in transparent coral tissue and visible white skeleton. Two-way ANOVAs of growth and dinoflagellate density revealed significant effects of treatment (P<0.0001), time (P<0.01), and treatment x time (P<0.01) (Supplementary Table S1 online).
Dinoflagellate populations were reduced by both cold and warm temperature treatments, following similar trends at first, and then diverging in the final phase. In the initial phase, dinoflagellate density declined by ∼20% in both cold and heat-treated corals as compared to controls (Fig. 1b, Supplementary Table S1 online). During the final phase, dinoflagellate density in cold-treated corals appeared to level off to ∼40% of controls by the end of the experiment. In contrast, in heat-treated corals, dinoflagellate density dropped precipitously to below ∼1% of controls (<1x106 cells cm−2) by 9 d, and remained at this low concentration until the end of the experiment. The decline in dinoflagellate density was coincident with visible coral bleaching in the heat treatment (Fig. 1c). Heat-treated corals bleached by 9 d and remained so until the end of the experiment; thin transparent tissue present over the entire skeleton indicated that the coral was still alive. Within dinoflagellates, cellular concentrations of chlorophyll a (Chl a) remained relatively constant throughout the experiment regardless of treatment (Fig. 2a, Supplementary Tables S1 and S2 online), suggesting that bleaching resulted from the reduction in dinoflagellate density rather than a reduction of Chl a per dinoflagellate cell.
Figure 2. Effect of temperature change on photosynthetic pigments from endosymbiotic dinoflagellates of the coral Acropora yongei.
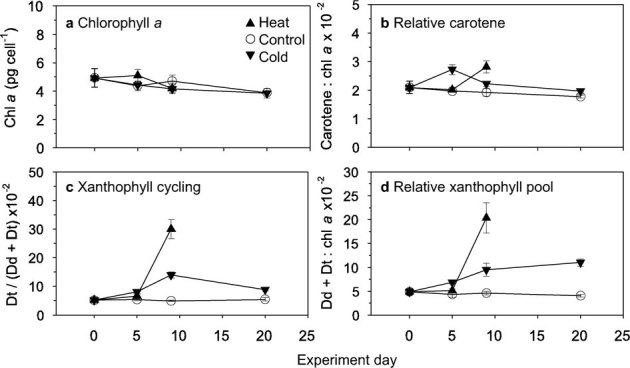
(a) Chlorophyll a, (b) relative carotene, (c) xanthophyll cycling, and (d) relative total xanthophyll pool over time during the experiment (mean ± s.e.m.; N = 5). The xanthophyll cycle protects photosystem II from excess excitation by dissipating energy through the de-epoxidation of diadinoxanthin (Dd) to diatoxathin (Dt). Heat treatment 20 d pigment concentrations were below detection limit of the instrument. Two-way ANOVA and t-tests of chlorophyll a concentration reveals no significant effects of treatment, time, nor treatment x time (Supplementary Tables S1 and S2 online). Two-way ANOVA of relative carotene reveals significant effects of treatment (P<0.01), time (P<0.05), and treatment x time (P<0.01) (Supplementary Table S1 online), while 20 d t-test reveals no significant difference between cold and control treatments (Supplementary Table S2 online). Two-way ANOVA and t-tests of xanthophyll cycling and relative xanthophyll pool reveal significant effects of treatment (P<0.01), time (P<0.0001), and treatment x time (P<0.0001) (Supplementary Tables S1 and S2 online).
When the balance of energy absorbed and processed through photochemistry is disrupted, photoprotective pigments such as carotene and xanthophylls safely dissipate excess light energy to prevent oxidative stress3. Different responses in photoprotective pigment concentrations were observed between cold and heat treatments (Fig. 2, Supplementary Tables S1 and S2 online). In the initial phase, carotene, total xanthophylls, and xanthophyll cycling increased in the cold treatment suggesting that the cold treatment initiated a photoprotective response. In contrast, concentrations of photoprotective pigments initially remained unchanged in the heat treatment. During the final phase of the cold treatment, carotene, total xanthophylls, and xanthophyll cycling decreased or remained constant indicating a decrease in the need for photoprotection, a sign of acclimation. In contrast, photoprotective pigments dramatically increased during the final phase of the heat treatment, clearly indicating a harsh environment (measurements at 20 d were not possible due to the large reduction in dinoflagellate populations; see Methods).
Declining photochemical efficiency of PSII revealed the stress on the photosynthetic system of the endosymbiotic dinoflagellates (Fig. 3, Supplementary Tables S1 and S2 online). Values of maximum excitation pressure over PSII (Qm), the effective quantum yield at midday relative to maximum quantum yield (see Methods), reflected the differences between cold and heat-treated corals. During the initial phase of the experiment, the cold treatment caused an increase in Qm as a result of a decrease in effective quantum yield and limited change in maximum quantum yield. During the final phase, effective quantum yield and Qm stabilized in the cold treatment. In contrast, heat-treated corals initially did not experience a loss in effective quantum yield or an increase in Qm. In the final phase, Qm increased rapidly while both effective and maximum quantum yield sharply declined in heat-treated corals.
Figure 3. Effect of temperature change on photophysiology of the coral Acropora yongei.
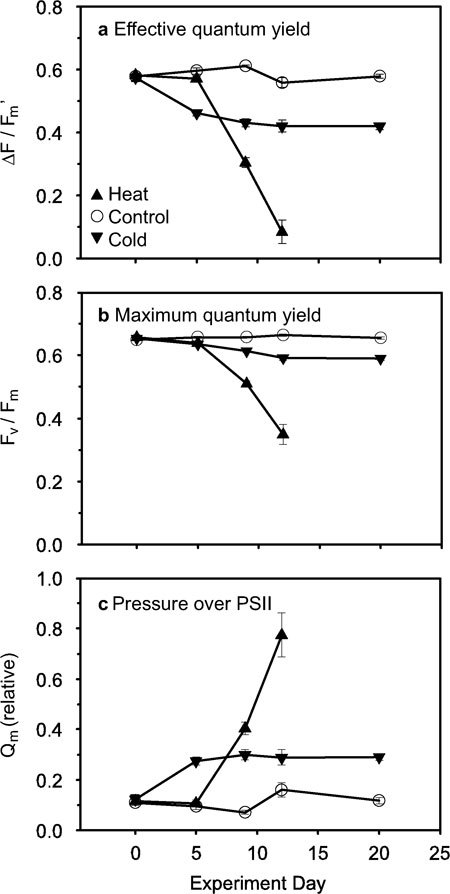
(a) Effective quantum yield (ΔF/Fm'), (b) maximum quantum yield (Fv/Fm), and (c) maximum excitation pressure over photosystem II (Qm) over time during the experiment (mean ± s.e.m.; N = 10−19 for 0–9 d, N = 5−8 for 12–20 d). Heat treatment 20 d measurements were below detection limit of the methodology. Two-way ANOVAs and t-tests of effective quantum yield and maximum quantum yield reveal significant effects of treatment (P<0.0001), time (P<0.0001), and treatment x time (P<0.0001) (Supplementary Tables S1 and S2 online).
Discussion
This study investigated the effects of cooling and warming seawater on coral physiology, both of which are likely to become more frequent due to global climate change. The present study used temperature changes similar to the extreme changes experienced during the summer and winter of 201035,36, but likely on somewhat faster time scales than occurring naturally on reefs. In contrast to most previous studies, which have focused on the effects of either cold temperaturee.g.21,27,28 or warm temperaturee.g.11,24,25,32,33, the present study on a simultaneous cold and heat stress experiment combined with data collected during multiple time points helps elucidate the effects of temperature change on the physiology of corals and their endosymbionts. Both cold and heat stress negatively affected corals. Our experiment identified two critical time scales of physiological responses: during the initial phase (0–5 d), the decrease in temperature caused more stress to the coral holobiont, whereas during the final phase (6–20 d), the increase in temperature caused more stress to the coral holobiont.
The acute effects of the cold treatment included declines in coral growth and endosymbiotic dinoflagellate density, and increases in photoprotective pigments. The decrease in effective quantum yield during the initial phase suggests that the treatment caused an immediate imbalance between the amount of light energy absorbed and processed through PSII. This imbalance is likely due to temperature dependant reduction of enzyme activities37, which decreased rates of photosynthetic reactions, caused a build up of excess light energy, and resulted in an increased need for photoprotection. The down-regulation of PSII photochemistry may have been photoprotective. The maximum quantum yield data provide evidence that the photosynthetic system recovered during the nighttime. Declines in photochemical efficiency and dinoflagellate density, combined with the general metabolic decrease at cold temperature, may have ultimately contributed to the large reductions in coral growth. Extreme rapid cold shock (4 hrs) has been reported to cause a reduction in dinoflagellate density in corals, and thus is consistent with our study26,27. The loss of dinoflagellates seems to occur from the release of intact dinoflagellate containing coral endoderm cells into the surrounding water, which was documented to occur under heat stress as well26. Short-term cold stress (≤18 h) in Montipora digitata affects maximum quantum yield, dinoflagellate density and chlorophyll a concentration, which could reflect the organism's ability for rapid photoacclimation since the responses were dependent on light intensity and magnitude of temperature change28. During the final phase of our experiment, the stabilization and improvement in coral and dinoflagellate physiological states suggest that cold-treated corals are able to acclimate to cooler temperatures and initiate recovery. During the final phase, the effective quantum yield and pressure over PSII stabilized, suggesting that the photosynthetic machinery was able to compensate by changing the concentration of proteins, pigments, and/or enzymes involved in photosynthesis, which could explain the observed increase in xanthophyll pool. The continued cold treatment did not cause sustained stress: in the final phase, carotene and xanthophyll cycling decreased, dinoflagellate density stabilized, and coral growth increased.
Acute effects induced by the heat treatment were less severe than those induced by the cold treatment, however, chronic effects were more deleterious. Initially, the growth rate of heat-treated corals did decrease, although not as substantially as those of cold-treated corals; however the pressure over PSII remained unchanged until after 5 d. It is reported that rates of photosynthesis increase in short-term heat (<2 h) stress experiments on corals and on symbiotic dinoflagellates in culture, until temperatures of 31°C or 30°C, respectively38,39. The temperature ∼30°C seems to be a critical threshold from the photobiological standpoint, perhaps representing an inherent limit of PSII. Symbiotic dinoflagellates in culture have impaired photosynthesis above 30°C and photosynthesis ceased by 34–36°C39. Different clades of symbionts have varying degrees of susceptibility to thermal stress and discrete responses in culture40. Here, we propose that photodamage accumulated in the corals during our heat experiment, and that after 5 d, the photosynthetic system could no longer process the excess light energy. The decreased shading caused by the reduction in dinoflagellate density creates a higher local light field within the coral cells41. The combination of the locally increased light and inhibited repair processes of PSII8 may have further stressed the remaining dinoflagellate population. Corresponding increases in photoprotective pigments after the initial phase were observed in heat-treated corals suggesting that the photosynthetic system was struggling to compensate for the rapidly accumulating stress. In the final phase of the heat treatment, dinoflagellate populations experienced a fast decline, and corals bleached and growth ceased. These results suggest that the heat-treated symbionts, which often become a source of oxidative stress with increased temperature5,10,42, were under considerable photostress causing rapid disruption of the coral-algal symbiosis. Obviously, bleached heat-treated corals were beyond typical acclimation responses43 and clearly under severe irreversible stress. Similar to many other coral heat stress experiments7,24,25, decreases in photosynthetic yield and dinoflagellate density were also observed in our study; however, the direct comparison with the cold response analyzed in parallel at multiple time points allowed us to elucidate the dynamics of differential responses from the corals and their endosymbionts between treatments, which clearly provided evidence that the heat treatment was ultimately more harmful than the cold treatment for the corals.
There has been one previous study on the effects of temperature on growth and mortality in Hawaiian corals12. Similar to the present study, reduced growth was observed in both heat and cold treatments. In contrast to the present study, those experiments found that heat causes faster stress and mortality (<2 d), but lower long-term (30 d) mortality than cold. Such discrepancy with our data suggests that species and locations might be important factors to understand corals' response to temperature change, and that some corals might live closer to their upper thermal limits while others closer to their lower one. Nonetheless, both studies conclude that both heat and cold conditions can be deleterious to corals, inducing stress through divergent physiological mechanisms over time.
Although both cold and heat treatments had large negative effects on coral and dinoflagellate physiology, the treatments had distinct responses at different time scales. The present study indicates that transient decreases in seawater temperature can be very deleterious to corals, but that prolonged increases in seawater temperature will eventually be much more harmful. This result has serious implications for the future of coral reefs and their management, and suggests that in areas with cooler temperatures, corals and their symbionts may be able to acclimate to new environments and survive. However, reduced growth may also make the reefs more susceptible to sea level rise, another aspect of climate change44. Endosymbiotic dinoflagellates appear very sensitive to temperature changes and this research supports monitoring their photophysiology as an indicator of coral health45. Because of climate change, corals will experience more temperature anomalies that will not only cause physiological stress, but also have long-term repercussions on growth and fitness, ultimately affecting stability of coral reefs. Although temperature history can also influence coral physiological responses to current stressors, repeated and synergistic stressors may reduce coral resiliency15. Additional effects of abnormal temperatures such as coral diseases44 and further stressors such as ocean acidification15 increase the pressure on corals and multiple stresses provide less time for recovery. Nevertheless, extreme temperature events have been and will continue to be a major contributor to coral reef decline at a global and long-term scale.
Methods
Experimental design
Prior to temperature treatments, Acropora yongei fragments (∼5 cm) were glued onto terracotta tiles and placed in individual 1 L glass aquaria (seawater flow rate ∼0.7 L min−1) and maintained in steady-state at 26°C for 16 d under 12∶12 h light:dark photoperiod (300 μmol photons m−2 s−1 intensity) as previously described43. During this acclimation period, there was no coral mortality and tissue grew completely over the cut region within days. The ±5°C temperature changes were introduced incrementally over a 5 h period starting at sunrise on 1 d. There was no coral mortality or tissue sloughing on any coral in any treatment throughout the 20 d experiment. Coral measurements were conducted at 0, 5, 9, 12, and 20 d, and when appropriate, a sub-set of corals was collected 1 ± 0.5 hr prior to sunset and stored at −80°C for destructive sampling/analyses.
Coral growth rates
Growth rates of the corals during the experiment were determined by linear extension of the tip of the coral calculated by digital imagery as previously described43. Briefly, stereoscope (Nikon SMZ1500) digital images were captured perpendicular to the growth axis on corals fixed to square tiles to maintain orientation. Linear extension was measured from a landmark with image analyses software (ImageJ). Average daily growth was calculated during each time period.
Dinoflagellate densities
A 1.5 cm long section of the coral (starting 2.4 cm from the tip) was used for dinoflagellate density analyses. Dinoflagellates were isolated by removing coral tissue with an artist's airbrush and purifying dinoflagellates through centrifugation43. The dinoflagellate density was determined in triplicate using a Neubauer ruled hemocytometer and normalized to the surface area of the coral, which was calculated using simple cylinder geometry43.
Photosynthetic pigments
Photosynthetic pigments were extracted in acetone from dinoflagellates isolated from the same coral region used to determine dinoflagellate density. Pigment concentrations were determined using an Agilent 1100 series high-pressure liquid chromatography (HPLC) system (Agilent Technologies)43. The xanthophyll cycling was calculated as the relative proportion of diatoxathin to the total xanthophyll pool (Dt/(Dd+Dt)). Concentrations of photosynthetic pigments were below levels of detection for the heat treatment at 20 d.
Chlorophyll fluorescence
Chlorophyll fluorescence measurements were obtained using the pulse amplitude-modulated fluorometer (Diving-PAM, Walz Inc.). Dark-acclimated maximum quantum yield of PSII (Fv/Fm; Fv, variable fluorescence; Fm, maximum fluorescence) was measured pre-dawn as previously described43. Light-acclimated effective quantum yield of PSII (ΔF/Fm') was measured at experimental solar noon. The pressure over PSII was determined as: Qm = 1 – [(ΔF/Fm' at noon)/(Fv/Fm at pre-dawn)]45,46. Because of the large decline in the dinoflagellate density, chlorophyll fluorescence measurements could not be obtained for the heat treatment at 20 d.
Statistical analyses
Data were tested for assumptions of normality and homoscedasticity, and data were arcsine or log transformed accordingly prior to analyses. Two-way ANOVA tests were used to test the effects of temperature treatment and time (Supplementary Table S1 online). For all significant factors in the ANOVA tests, post-hoc Tukey-Kramer HSD pairwise comparisons were used to test which groups were significantly different. Because chlorophyll fluorescence and photosynthetic pigment concentrations for heat treatment 20 d were not obtained, t-tests were used instead of ANOVA tests (Supplementary Table S2 online). Equal variance was tested and appropriate t-tests were used. Data are represented as mean ± s.e.m. Statistical differences were significant at the α = 0.05 level.
Author Contributions
MSR and DDD designed the research; MSR performed the research and analyzed the data; RG conducted HPLC; and MSR and DDD wrote the manuscript. All authors reviewed the manuscript.
Supplementary Material
Supplementary Info
Acknowledgments
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship (MSR), National Science Foundation under Grant No. 0333444, and the Air Force Office of Scientific Research Biomimetics, Biomaterials, and Biointerfacial Sciences program under award FA9550-07-1-0027 (DDD). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Air Force Office of Scientific Research. The authors would like to thank M. Latz for helpful discussions and providing lab space, J. Smith for providing the diving-PAM, M. Roadman for assistance with HPLC, E. Kisfaludy and F. Nosratpour for aquarium support, the Birch Aquarium at the Scripps Institution of Oceanography for providing corals, and C. Clefton for general assistance.
References
- Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals., in Ecosystems of the world, Vol. 25. (ed. Dubinsky Z., ed. ) 75–87 (Elsevier, Amsterdam; 1990). [Google Scholar]
- Goreau T. F. The physiology of skeleton formation in corals. 1. A method for measuring the rate of calcium deposition by corals under different conditions. Biol. Bull. 116, 59–75 (1959). [Google Scholar]
- Niyogi K. K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Molec. Biol. 50, 333–359 (1999). [DOI] [PubMed] [Google Scholar]
- Jones R. J., Hoegh-Guldberg O., Larkum A. W. D. & Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 21, 1219–1230 (1998). [Google Scholar]
- Lesser M. P. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 41, 271–283 (1996). [Google Scholar]
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 50, 839–866 (1999). [Google Scholar]
- Warner M. E., Fitt W. K. & Schmidt G. W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 96, 8007–8012 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nakamura T., Sakamizu M., van Woesik R. & Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 45, 251–255 (2004). [DOI] [PubMed] [Google Scholar]
- Weis V. M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 211, 3059–3066 (2008). [DOI] [PubMed] [Google Scholar]
- Lesser M. P. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187–192 (1997). [Google Scholar]
- Lesser M. P. & Farrell J. H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23, 367–377 (2004). [Google Scholar]
- Jokiel P. L. & Coles S. L. Effects of temperature on mortality and growth of Hawaiian reef corals. Mar. Biol. 43, 201–208 (1977). [Google Scholar]
- Jokiel P. L. & Guinther E. B. Effects of temperature on reproduction in the hermatypic coral Pocillopora damicornis. Bull. Mar. Sci. 28, 786–789 (1978). [Google Scholar]
- Goreau T. J. & Macfarlane A. H. Reduced growth rate of Montastrea annularis following the 1987–1988 coral-bleaching event. Coral Reefs 8, 211–215 (1990). [Google Scholar]
- Hoegh-Guldberg O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- Baker A. C., Glynn P. W. & Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008). [Google Scholar]
- Pandolfi J. M. et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 (2003). [DOI] [PubMed] [Google Scholar]
- Urban F. E., Cole J. E. & Overpeck J. T. Influence of mean climate change on climate variability from a 155-year tropical Pacific coral record. Nature 407, 989–993 (2000). [DOI] [PubMed] [Google Scholar]
- Abram N. J., Gagan M. K., Cole J. E., Hantoro W. S. & Mudelsee M. Recent intensification of tropical climate variability in the Indian Ocean. Nat. Geosci. 1, 849–853 (2008). [Google Scholar]
- Gergis J. L. & Fowler A. M. A history of ENSO events since AD 1525: Implications for future climate change. Clim. Change 92, 343–387 (2009). [Google Scholar]
- Hoegh-Guldberg O. et al. Coral bleaching following wintry weather. Limnol. Oceanogr. 50, 265–271 (2005). [Google Scholar]
- Chilly February caps coldest winter in three decades over south Florida. (NOAA, National Weather Service, February 2010); www.srh.noaa.gov/images/mfl/news/Feb2010WinterSummary.pdf.
- Kintisch E. Record hot summer wreaks havoc. Science NOW (15 September 2010); http://news.sciencemag.org/sciencenow/2010/09/record-hot-summer-wreaks-havoc.html.
- Fitt W. K. et al. Response of two species of Indo-Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: The host does matter in determining the tolerance of corals to bleaching. J. Exp. Mar. Biol. Ecol. 373, 102–110 (2009). [Google Scholar]
- Hoegh-Guldberg O. & Smith G. J. The effect of sudden changes in temperature, light, and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 129, 279–303 (1989). [Google Scholar]
- Gates R. D., Baghdasarian G. & Muscatine L. Temperature stress causes host-cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol. Bull. 182, 324–332 (1992). [DOI] [PubMed] [Google Scholar]
- Muscatine L., Grossman D. & Doino J. Release of symbiotic algae by tropical sea anemones and corals after cold shock. Mar. Ecol. Prog. Ser. 77, 233–243 (1991). [Google Scholar]
- Saxby T., Dennison W. C. & Hoegh-Guldberg O. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 248, 85–97 (2003). [Google Scholar]
- Tchernov D. et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101, 13531–13535 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin D. J., Hoegh-Guldberg P., Jones R. J. & Berges J. A. Cell death and degeneration in the symbiotic dinoflagellates of the coral Stylophora pistillata during bleaching. Mar Ecol Prog Ser 272, 117–130 (2004). [Google Scholar]
- Dove S. et al. Response of holosymbiont pigments from the scleractinian coral Montipora monasteriata to short-term heat stress. Limnol Oceanogr 51, 1149–1158 (2006). [Google Scholar]
- Rowan R. Coral bleaching - Thermal adaptation in reef coral symbionts. Nature 430, 742–742 (2004). [DOI] [PubMed] [Google Scholar]
- Warner M. E., Fitt W. K. & Schmidt G. W. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ 19, 291–299 (1996). [Google Scholar]
- Venn A. A., Wilson M. A., Trapido-Rosenthal H. G., Keely B. J. & Douglas A. E. The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Plant Cell Environ 29, 2133–2142 (2006). [DOI] [PubMed] [Google Scholar]
- First Florida cold-water bleaching event in 30 years. (NOAA, National Ocean Service, March 2010); http://oceanservice.noaa.gov/news/weeklynews/mar10/cwcoral.html.
- Troubled waters: Massive coral bleaching in Indonesia. (Wildlife Conservation Society, 17 August 2010); http://www.wcs.org/new-and-noteworthy/aceh-coral-bleaching.aspx.
- Somero G. N. Proteins and temperature. Annu. Rev. Physiol. 57, 43–68 (1995). [DOI] [PubMed] [Google Scholar]
- Coles S. L. & Jokiel P. L. Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar. Biol. 43, 209–216 (1977). [Google Scholar]
- Iglesias-Prieto R., Matta J. L., Robins W. A. & Trench R. K. Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. USA 89, 10302–10305 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison J. D. & Warner M. E. Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta). J. Phycol. 42, 568–579 (2006). [Google Scholar]
- Terán E., Mendez E. R., Enriquez S. & Iglesias-Prieto R. Multiple light scattering and absorption in reef-building corals. Appl. Optics 49, 5032–5042 (2010). [DOI] [PubMed] [Google Scholar]
- Yakovleva I. M. et al. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar. Ecol. Prog. Ser. 378, 105–112 (2009). [Google Scholar]
- Roth M. S., Latz M. I., Goericke R. & Deheyn D. D. Green fluorescent protein regulation in the coral Acropora yongei during photoacclimation. J. Exp. Biol. 213, 3644–3655 (2010). [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. & Bruno J. F. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528 (2010). [DOI] [PubMed] [Google Scholar]
- Warner M. E., Lesser M. P. & Ralph P. J. Chlorophyll fluorescence in reef building corals., in Chlorophyll fluorescence in aquatic sciences: Methods and applications. (eds. D.J. Suggett, O. Prasil & M. A. Borowitzka) 209–222 (Springer, Berlin; 2010 [Google Scholar]
- Iglesias-Prieto R., Beltrán V. H., LaJeunesse T. C., Reyes-Bonilla H. & Thomé P. E. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. B 271, 1757–1763 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info


