Abstract
Objective
To compare the effects of open tracheal suctioning (OS) plus intermittent mandatory ventilation (IMV) vs closed tracheal suctioning (CS) plus volume guarantee ventilation (VG) on changes in mean cerebral blood-flow velocity (CBFv) of ventilated very low birth weight (VLBW) infants.
Study Design
A total of 75 normotensive, ventilated VLBW infants (with normal cranial ultrasounds) had monitoring of mean CBFv, PCO2 and mean arterial blood pressure (MABP) before, during and after 220 tracheal suctioning sessions during the first week of life. Multiple linear regression analysis was used to determine the factor(s) influencing the magnitude of relative changes from baseline in mean CBFv after suctioning.
Result
In all, 49 VLBW infants receiving IMV had monitoring during 124 OS sessions between July 2002 and May 2005; 26 VLBW infants receiving VG had monitoring during 96 CS sessions between January 2006 and July 2007. The average magnitude of relative changes in mean CBFv was significantly less with CS + VG, and was associated with the magnitude of relative changes in PCO2 and suctioning-ventilator group.
Conclusion
The average magnitude of relative changes in mean CBFv was reduced in VLBW infants with CS + VG vs OS + IMV.
Keywords: cerebral blood-flow velocity, carbon dioxide, ventilation, suctioning, open vs closed
Introduction
Tracheal suctioning is commonly performed in intubated patients to clear secretions from the tracheobronchial airways and maintain patency of the endotracheal tube (ETT). Hypoxemia and arterial desaturation,1–9 hypercapnia,7,10 bradycardia1,3–6 and elevated mean arterial blood pressure (MABP)1–4,8,10,11 have all been observed during and briefly after open tracheal suctioning (OS) in intubated infants. These adverse events are likely related to the loss of lung volume during disconnection from the ventilator with OS.12 We previously reported that OS was associated with a 30% increase in cerebral blood-flow velocity (CBFv) in very low birth weight (VLBW, birth weight ≤1500 g) infants.10 We wondered whether closed tracheal suctioning (CS) would mitigate CBFv disturbances that we previously observed, as CS allows for at least partial ventilation and oxygenation during suctioning.
Although a majority of OS vs CS studies in premature infants observed that CS resulted in attenuated transient disturbances of systemic hemodynamics and gas exchange,5,9,13–15 a meta-analysis of tracheal suctioning without disconnection16 failed to recommend CS over OS. Two of the OS vs CS studies also examined effects on cerebral hemodynamics and found no significant benefits with CS,5,17 although there was a trend towards diminished changes in cerebral blood volume with CS.5
Coincident with the clinical practice change from OS to CS in our neonatal intensive care unit (NICU), there was also a change in primary ventilator mode from intermittent mandatory ventilation (IMV) to volume guarantee ventilation (VG). This is important because use of volume-targeted ventilation modes has been associated with reduced death or bronchopulmonary dysplasia (combined outcome), decreased rate of pneumothorax, reduced duration of ventilation, decreased hypocapnia and decreased incidence of severe cranial ultrasound abnormalities, that is, the combined outcome of periventricular leukomalacia or severe intraventricular hemorrhage (IVH), compared with IMV.18 Although cerebral hemodynamics were not measured in the Cochrane Review studies,19–23 the reduction of severe cranial ultrasound abnormalities may reflect less cerebral blood-flow disturbances due to reduced respiratory morbidities in infants managed with volume-targeted ventilation. Therefore, the main objective of this observational study was to investigate whether CS + VG attenuates acute disturbances of cerebral hemodynamics associated with OS + IMV in ventilated VLBW infants.
Methods
Subjects
Sequential ventilated normotensive VLBW infants (without cranial ultrasound abnormalities during their entire initial hospitalization) born at the University of Arkansas for Medical Sciences (UAMS) between July 2002 and July 2007 were eligible. Infants with major congenital anomalies and infants receiving highfrequency ventilation were excluded. Time and date of birth, birth weight, gestational age, race, gender, cranial ultrasound findings and Apgar scores at 1 and 5 min were abstracted from the clinical research database. Gestational age was estimated based on the best obstetrical and neonatal criteria. If there was >2 weeks’ discrepancy between the estimates, the neonatal estimate was used.
Typically, intubated VLBW infants received prophylactic surfactant ≤20 min of life. Until December 2005, time-cycled, pressure-limited, continuous flow ventilators (InfantStar, San Diego, CA, USA) in the non-synchronized, intermittent mandatory mode were used. Initial settings: peak inspiratory pressure 16 to 20 cm H2O, positive end-expiratory pressure 5 cm H2O, inspiratory time 0.25 s and respiratory rate 50 to 60 breaths per min. Since January 2006, volume-targeted, time-cycled, pressure-limited ventilators (Babylog 8000 +, Draeger, Germany) in the assist/control VG mode have been used. Initial settings: tidal volume 4.5 to 5 ml kg−1, peak inspiratory pressure limit 5 cm H20 above peak inspiratory pressure, positive end-expiratory pressure 5 cm H20, inspiratory time 0.25 s and respiratory rate 40 to 60 breaths per min. During the entire study period, oxygen saturation targets were 85 to 92% and a permissive hypercapnic ventilation strategy (PaCO2 45 to 55 mm Hg) was used.
Monitoring equipment
Measurements of right middle cerebral artery CBFv were made using a transcranial Doppler ultrasound (Nicolet Pioneer, Madison, WI, USA). A lightweight 2-MHz pulsed-wave button transducer was placed transtemporally anterior to the external ear and above the zygomatic arch and held in place by a crocheted hat (courtesy of the Arkansas Extension Homemakers Council). A depth of 16 to 22 mm was used to study the proximal portion of the middle cerebral artery. A 100-Hz low-pass filter was used to dampen ‘noise’ from the vessel wall. Transducer placement was optimized when the highest intensity acoustic signal was perceived and the highest intensity Doppler spectra were visualized. Fast Fourier analysis was performed on the CBFv signal to determine systolic, diastolic and mean CBF velocities. The ultrasound intensity (5 to 21 mW cm−2) was kept very low. CBFv tracings were consistent for >1 h, with minimal to no drift in signal intensity. Almost 93% of Doppler recordings were of excellent quality and used for the analysis.
Continuous blood-gas monitoring was carried out with a Neotrend system (Diametrics Medical, St Paul, MN, USA) or a transcutaneous monitor (MicroGas 7650 rapid, Radiometer, Westlake, OH). Briefly, the skin on either side of the chest was prepped with a small amount of Aquaphor Emollient Ointment (Beiersdorf, Norwalk, CT, USA). A double-sided adhesive ring of tape was applied to the skin, and one drop of an electrolyte solution was placed into the center of the adhesive ring. The probe was then affixed to the adhesive ring. Comparative PCO2 values from the NICU laboratory, and Neotrend and transcutaneous PCO2 values, were rarely >3 mm Hg different. There was no apparent drift in Neotrend and transcutaneous PCO2 values.
Continuous BP monitoring was performed with an umbilical arterial catheter (Diametrics Argyle/Tyco Healthcare/Kendall, Mansfield, MA, USA) attached to a BP transducer (Transpac IV, Abbott, North Chicago, IL, USA).
Experimental protocol
Informed consent was obtained from a parent before participation. CBFv, PCO2 and BP were monitored from each VLBW infant before, during and after tracheal suctioning during the first week of life. Infants were monitored up to twice daily during the first 3 days and once daily during the next 4 days, if still intubated. Baseline monitoring began ~10 to 15 min before suctioning, when infants were quiet and not undergoing any other procedures, and continued for up to ~45 min. The UAMS IRB approved the study protocol.
Standard tracheal suctioning protocols
Suctioning was performed only when clinically indicated. Indications for suctioning were determined by the bedside nurse: oxygen desaturation, hypercapnia, clinical deterioration, visible secretions in the ETT, coarse or decreased breath sounds and/or decreased or absent chest excursion. Research personnel were immediately contacted, if the nurse suspected that an infant would soon require suctioning. For OS, the oral ETT was disconnected from the ventilator circuit and an appropriately sized suction catheter was sterilely inserted into the ETT. Wall suction (80 to 100 cm H2O) was applied to the catheter, which was withdrawn with one consistent movement. This procedure took ~10 s. The ETT was then reconnected to the breathing circuit with pre-suction ventilator settings. For CS, a suction multi-access catheter (Kimberly-Clark Ballard Neonatal Trach Care MAC catheter, Draper, UT, USA) was placed in-line and connected to the ETT. The in-line suction catheter was introduced into the ETT to the desired depth, wall-suction pressure was applied, the thumb control valve was depressed and held for 3 s, and then the catheter was withdrawn with one consistent movement.
During either OS or CS, if the oxygen saturation was <80% or the heart rate was <100 beats per min, several ventilator manual breaths were given. If the infant was still desaturated or bradycardic, the inspired FiO2 was increased by 10%. If problems persisted, bag mask ventilation with similar mean airway pressure was initiated with increased FiO2, and additional suction passes were delayed until vital signs normalized. The suction process was repeated once or twice more until the airway was clear. Saline was rarely used with suctioning during the first week of life.
Statistical analysis
Data are reported as mean ± s.d., median (interquartile range), or number where appropriate.
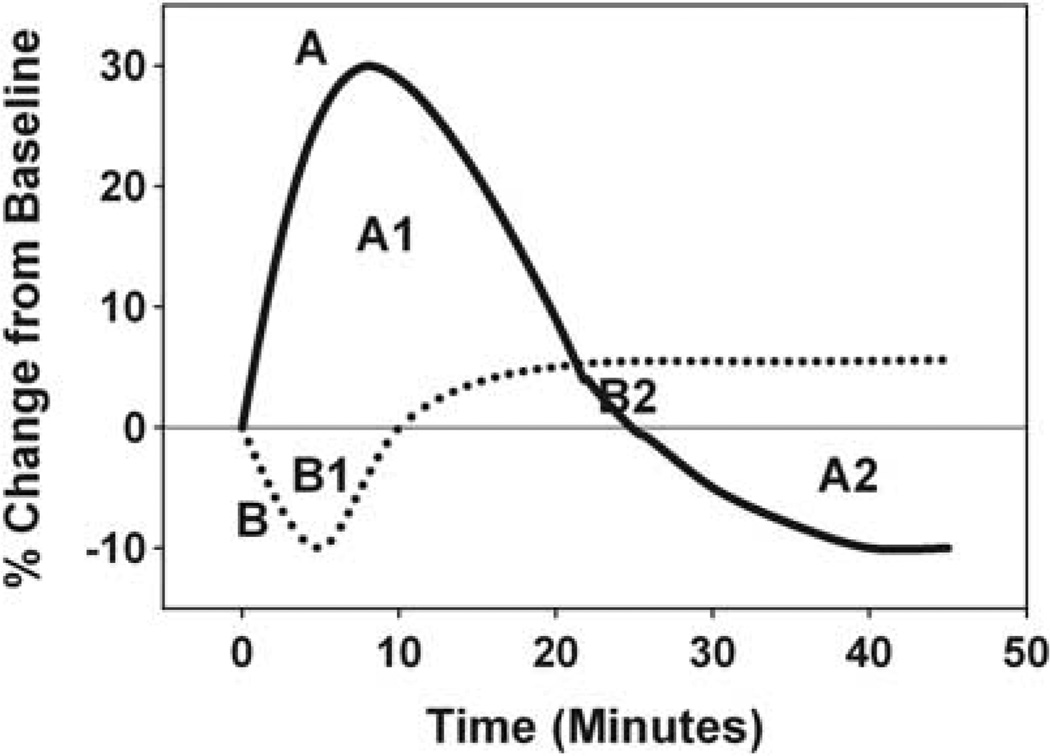
For each suctioning session for each infant, a locally weighted regression (LOESS)24 technique was used to reveal the pattern of the relative changes (percent changes from baseline) for mean CBFv, PCO2 and MABP for up to 45 min after suctioning. For example, the patterns of the relative changes in mean CBFv for 45 min after suctioning for two hypothetical infants are shown in Figure 1 for the purpose of illustrating our statistical methods. For Infant A, the relative change in mean CBFv peaked at 30% above baseline, remained above baseline for 25 min, and then fell to 10% below baseline for the duration of monitoring. The area under the curve (AUC) for the first 25 min is designated ‘A1’ and AUC for the last 20 min is ‘A2’. For Infant B, mean CBFv fell to 10% below baseline after suctioning, was decreased for 10 min, and then remained just above baseline for the last 35 min. The AUC for the first 10 min is ‘B1’ and the AUC for the last 35 min is ‘B2’. This same concept was also applied for PCO2 and MABP for each suctioning session for each infant.
Figure 1.
Relative changes in mean cerebral blood-flow velocity (CBFv) for 45 min after suctioning for two hypothetical Infants (A (solid line) and B (dotted line)). A1 and A2 represent areas under the curve for Infant A, and B1 and B2 represent areas under the curve for Infant B. For example, Total area under the curve (AUC) (mean CBFv) for Infant A is the sum of the absolute values of A1 and A2.
As stability of physiological variables for premature newborns undergoing intensive care is desired, in order to quantify the magnitude of the percent changes from baseline after suctioning for mean CBFv, PCO2 and MABP separately, the sum of the absolute values of the individual AUCs, calculated using the trapezoidal rule,25 for each of these three variables was computed separately for each suctioning session for each infant, yielding a Total AUC for each variable for each suctioning session. To illustrate this calculation for a suctioning session for Hypothetical Infant A for mean CBFv, the absolute value of A1 is added to the absolute value of A2 to obtain the desired sum. We shall hereafter refer to such sums as Total AUC (mean CBFv), Total AUC (PCO2), and Total AUC (MABP) for mean CBFv, PCO2 and MABP, respectively. We decided to use both increases and decreases from baseline when calculating Total AUC because any acute physiological changes may be problematic for VLBW infants. A low Total AUC value implies minimal changes after suctioning, and is most desirable, and a high Total AUC value implies large changes.
Next, a multiple linear regression analysis was performed where the response variable was Total AUC (mean CBFv), and the predictor variables were Total AUC (PCO2), Total AUC (MABP), suctioning-ventilator group (OS + IMV vs CS + VG) and birth weight. In this analysis, White’s empirical covariance structure estimation method was used, which accounted for the heteroskedasticity in the data and any correlation resulting from suctioning sessions that were performed on the same infant.26
The last objective was to develop curves illustrating the relative changes from baseline after suctioning for 45 min for mean CBFv, PCO2 and MABP for each of the suctioning-ventilator groups. This was accomplished by calculating the mean percent change from baseline at every second of monitoring for infants in each suctioning-ventilator group and plotting these as continuous curves.
Results
Subject characteristics
A convenience sample of 75 sequential normotensive, ventilated VLBW infants, without cranial ultrasound abnormalities, had monitoring of mean CBFv, PCO2 and MABP before, during and after 220 tracheal suctioning sessions during the first week of life (during the PI’s K-award). A total of 49 VLBW infants born between July 2002 and May 2005 received OS + IMV. After clinical care changes in the NICU from OS to CS, and IMV to VG, 26 VLBW infants had monitoring between January 2006 and July 2007 (Table 1).
Table 1.
Characteristics of the subjects by suctioning and ventilator mode
| Characteristics | OS + IMV | CS + VG | P |
|---|---|---|---|
| n | 49 | 26 | |
| Suctioning sessions | 124 | 96 | |
| Date | 7/02–5/05 | 1/06–7/07 | |
| Birth weight (g) | 1016 ± 233 | 752 ± 194 | <0.0001 |
| Gestational age (weeks) | 27.5 ± 1.9 | 26.1 ± 1.4 | 0.001 |
| Gender | 28 female | 17 female | 0.488 |
| 21 male | 9 male | ||
| Race | 16 Black | 12 Black | 0.373 |
| 6 Hispanic | 1 Hispanic | ||
| 27 White | 13 White | ||
| Delivery | 34 Cesarean | 17 Cesarean | 0.724 |
| 15 vaginal | 9 vaginal | ||
| Apgar 1 min | 4 (3.0–6.0) | 4 (3.0–5.0) | 0.499 |
| Apgar 5 min | 7 (6.0–8.0) | 6 (5.0–7.0) | 0.046 |
Abbreviations: OS + IMV, open tracheal suctioning + intermittent mandatory ventilation; CS + VG, closed tracheal suctioning + volume guarantee ventilation.
Mean ± s.d., median and interquartile range, and number where appropriate.
The infants in the CS + VG group were significantly smaller, less mature and had lower 5-min Apgar scores than the OS + IMV group (Table 1).
Tracheal suctioning
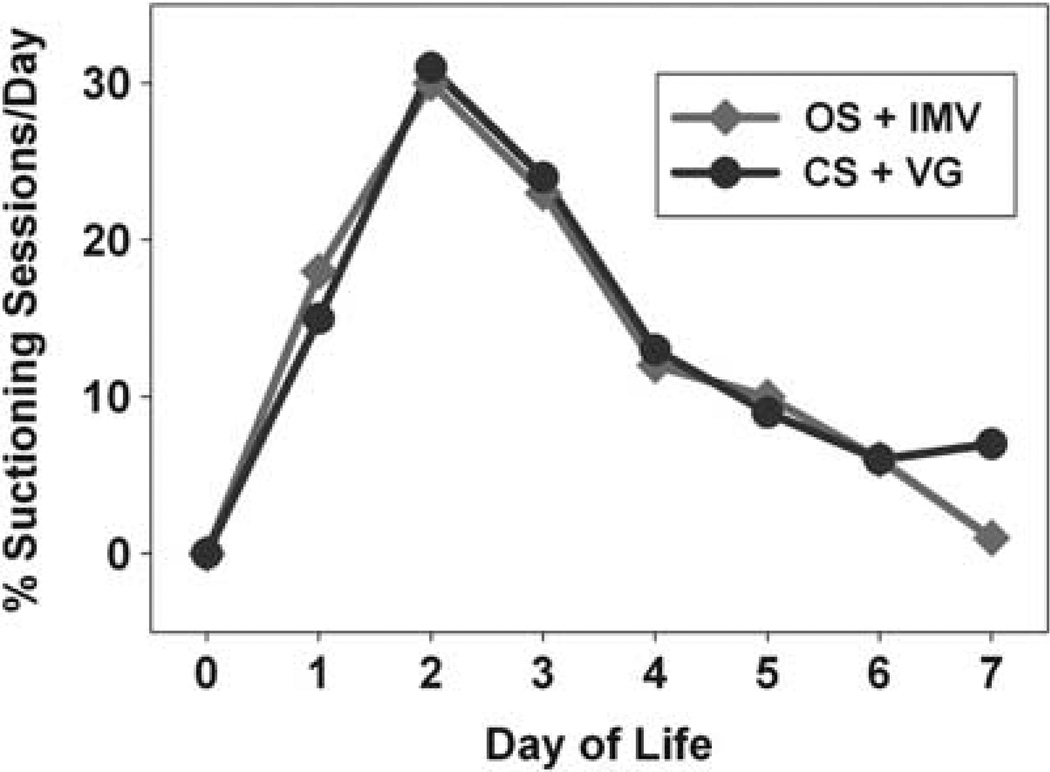
For the OS + IMV group, there was a total of 124 suctioning sessions during the first week of life: 22 on day of life (DOL) 1, 37 on DOL 2, 29 on DOL 3, 15 on DOL 4, 12 on DOL 5, 8 on DOL 6 and 1 on DOL 7. For the CS + VG group, there were a total of 96 suctioning sessions during the first week of life: 14 on DOL 1, 30 on DOL 2, 23 on DOL 3, 12 on DOL 4, 9 on DOL 5, 6 on DOL 6 and 2 on DOL 7. There was no difference in the distribution of suctioning sessions per day during the first week of life for infants with OS + IMV vs CS + VG (Figure 2).
Figure 2.
Distribution of suctioning sessions per day during the first week of life for each suctioning-ventilator group (open tracheal suctioning (OS) + intermittent mandatory ventilation (IMV) (red) vs closed tracheal suctioning (CS) + volume guarantee ventilation (VG) (blue)). See online version for color information.
Total AUC and multiple regression
The average Total AUC (mean CBFv) was significantly less for CS + VG vs OS + IMV (P = 0.0003). The average Total AUC (PCO2) was also significantly less for CS + VG vs OS + IMV (P = 0.0004). Total AUC (MABP) was not different for CS + VG vs OS + IMV (P = 0.4963). All three analyses were performed using a one-sided t-test.
As the P-value for birth weight was 0.584, birth weight was removed from the regression model. In the final model, Total AUC (PCO2) (P < 0.0001) and suctioning-ventilator group (P = 0.015) were statistically significant predictors of Total AUC (mean CBFv), whereas Total AUC (MABP) was not (P = 0.123).
Relative changes of physiological variables for both suctioning-ventilator groups
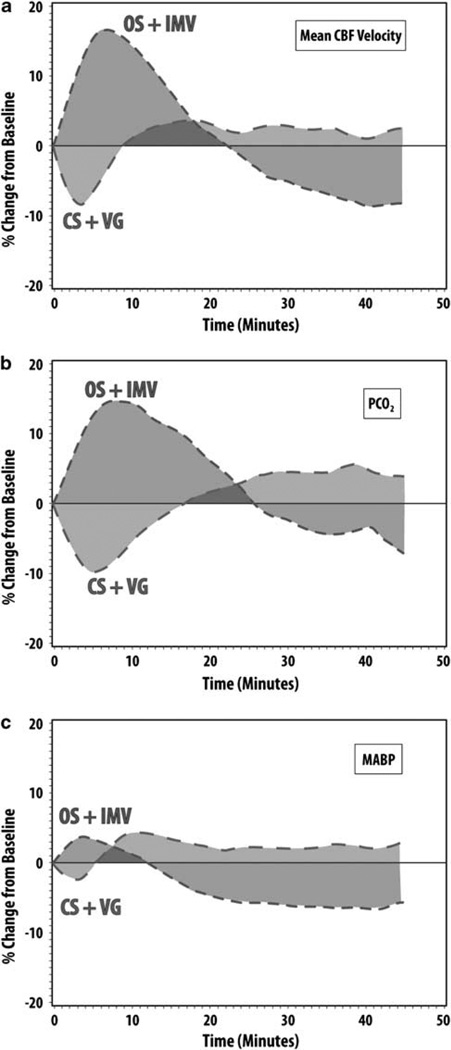
Descriptive curves for the mean relative changes from baseline for 45 min after suctioning for mean CBFv (A), PCO2 (B) and MABP (C) for each suctioning-ventilator group are shown in Figure 3. By ~6 min after OS + IMV (red), mean CBFv reached its maximum mean relative increase of ~17%, gradually returned to baseline by ~23 min, and then remained below baseline for the remainder of the monitoring. For CS + VG (blue), mean CBFv initially reached a minimum mean relative decrease of −8% at ~3 min, returned to baseline by ~8 min, and then remained just above baseline for the remainder of the period (Figure 3a).
Figure 3.
Relative changes of mean cerebral blood-flow velocity (CBFv) (a) PCO2 (b) and mean arterial blood pressure (MABP) (c) following tracheal suctioning for each suctioning-ventilator group (open tracheal suctioning (OS) + intermittent mandatory ventilation (IMV) (red) vs closed tracheal suctioning (CS) + volume guarantee ventilation (VG) (blue)). See online version for color information.
The mean percent increase in PCO2 during OS + IMV reached its peak of ~7% at ~8 min, gradually returned to baseline by ~26 min, and then remained below baseline for the remainder of the monitoring, with a pattern similar to relative changes in mean CBFv. For CS + VG, relative changes in PCO2 decreased to a minimum of −4 to −5% for ~5 min, returned to baseline by ~16 min, and then remained just above the baseline for the remaining period; again this was similar to the pattern of relative changes in mean CBFv with CS + VG (Figure 3b). Relative changes in MABP (Figure 3c) had similar temporal patterns to relative changes in PCO2 for each suctioning-ventilator group.
Discussion
Although necessary in ventilated VLBW infants, tracheal suctioning and other routine intensive-care procedures produce numerous undesirable side effects on systemic hemodynamics and gas exchange, which may adversely affect the developing brain.2,27,28 As CS vs OS tended to decrease disturbances of cerebral hemodynamics,5 and volume-targeted ventilation has been associated with reduced severe cranial ultrasound abnormalities,18 we wondered whether CS + VG would mitigate disturbances of CBFv that we previously observed with OS + IMV in ventilated VLBW infants.10
We have shown in this observational study for the first time that CS + VG is associated with less disturbances of cerebral hemodynamics than OS + IMV in normotensive, conventionally ventilated VLBW infants without cranial ultrasound abnormalities. This is fortunate for VLBW infants in our NICU as CS + VG has been our first-line suctioning and ventilation method since January 2006. We also observed that Total AUC (PCO2) was associated with Total AUC (mean CBFv), which is consistent with our previous observations.10, 27 Thus, intensive-care procedures that promote less PCO2 fluctuations should be considered for use.
Although CS in ventilated neonates has been shown to reduce physiological instability14,15 and may be better tolerated by small premature infants,29 theoretical benefits including reduced nosocomial infections and ventilator-associated pneumonias have not been realized.29 Moreover, concerns that CS is not as effective as OS in removing secretions has not been shown in ventilated infants. The benefits of CS over OS are believed to be because of the maintenance of functional residual capacity because CS obviates the need for ETT disconnection from the ventilator; again, this has not been demonstrated in human infants. Lastly, although there is a trend towards less-disturbed cerebral hemodynamics with CS,5 this has not been definitively shown. On the other hand, in the pooled analysis of volume-targeted ventilation vs IMV studies, volume-targeted ventilation was associated with a significant-risk reduction for severe cranial ultrasound abnormalities.18 Thus, integrating results from our study, together with observations from the two Cochrane Reviews,16,18 we speculate that CS (over OS) plus VG (over IMV) may have additive benefits for reducing CBFv disturbances.
Although we observed significantly decreased disturbances of CBFv with CS + VG vs OS + IMV, are these results clinically significant? We could not directly answer this question from this observational trial of normotensive, ventilated VLBW infants, without cranial ultrasound abnormalities, who likely had intact cerebral autoregulation (no relationship between CBFv and MABP). When possible, cerebral hemodynamic fluctuations in ventilated premature infants are best avoided. It is unclear from this study, however, whether avoiding CBFv fluctuations makes a difference in preventing brain injury.
There are some limitations to this study. First, we used transcranial Doppler ultrasound instead of more direct measures of CBF. Good correlations, however, have been observed between relative changes of CBFv and near-infrared spectroscopy measures of cerebral hemodynamics.30–32 Another limitation of this trial is that we examined physiological variables only after clinically indicated procedures rather than using objective criteria. Although our results could have been influenced by the subjective judgment of the neonatal nurses, we felt that this was a strength of our study, as we left the determination of when to suction to the ‘real-world’ decision makers. As we did not measure oxygen saturation during the study, we cannot comment on how oxygen desaturation and changes in PCO2 during suctioning may have produced additive effects on cerebral hemodynamics. Also, we did not prospectively examine suctioning methods and ventilator modes in a randomized manner, but instead compared physiological measures after a NICU clinical practice change. Thus, we did not have monitoring sessions with OS + VG, or CS + IMV, could not determine how ventilator synchronization influenced cerebral hemodynamics, and could not differentiate whether CS or VG was more important in mitigating changes in CBFv. Although the study period spanned 5 years, and intensive care procedures and policies may have changed that could have affected our results, our policies for treating hypotension, using permissive hypercapnia, administering surfactant, antibiotics, newborn resuscitation and the group of attending neonatologists did not change during this time. And finally, infants in the second cohort were smaller and less mature. One would assume, however, that the most immature infants would have the largest hemodynamic disturbances following suctioning compared with the more mature infants from the first cohort. This was not observed, and in fact, birth weight was not a significant predictor of changes in cerebral hemodynamics and was removed from the regression model.
In ventilated VLBW infants during the first week of life, OS + IMV was associated with larger disturbances in cerebral hemodynamics than CS + VG. Thus, we propose that the combined suctioning-ventilator protocol of CS + VG should be considered for use in ventilated VLBW infants during the first week of life.
Acknowledgments
We gratefully acknowledge the technical assistance of Natalie C Sikes and Melanie J Mason, and the support of the University of Arkansas for Medical Sciences neonatologists, NICU nurses, respiratory therapists and ultrasound technicians.
Dr Kaiser was supported by the National Institutes of Health (1 K23 NS43185, RR20146 and 1 R01 NS060674), the University of Arkansas for Medical Sciences Center for Clinical & Translational Research (1UL1RR029884), and the University of Arkansas for Medical Sciences Children’s University Medical Group.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
This research was presented in part at the 2nd Congress of the European Academy of Paediatrics in Nice, France, October 2008 and at the Pediatric Academic Society, Society for Pediatric Research meeting in Baltimore, Maryland, May 2009.
References
- 1.Simbruner G, Coradello H, Fodor M, Havelec L, Lubec G, Pollak A. Effect of tracheal suction on oxygenation, circulation, and lung mechanics in newborn infants. Arch Dis Child. 1981;56:326–330. doi: 10.1136/adc.56.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlman JM, Volpe JJ. Suctioning in the preterm infant: Effects on cerebral blood flow velocity, intracranial pressure, and arterial blood pressure. Pediatrics. 1983;72:329–334. [PubMed] [Google Scholar]
- 3.Fanconi S, Duc G. Intratracheal suctioning in sick preterm infants: Prevention of intracranial hypertension and cerebral hypoperfusion by muscle paralysis. Pediatrics. 1987;79:538–543. [PubMed] [Google Scholar]
- 4.Shah AR, Kurth CD, Gwiazdowski SG, Chance B, Delivoria-Papadopoulos M. Fluctuations in cerebral oxygenation and blood volume during endotracheal suctioning in premature infants. J Pediatr. 1992;120:769–774. doi: 10.1016/s0022-3476(05)80246-x. [DOI] [PubMed] [Google Scholar]
- 5.Mosca FA, Colnaghi M, Lattanzio M, Bray M, Pugliese S, Fumagalli M. Closed versus open endotracheal suctioning in preterm infants: effects on cerebral oxygenation and blood volume. Biol Neonate. 1997;72:9–14. doi: 10.1159/000244460. [DOI] [PubMed] [Google Scholar]
- 6.Kohlhauser C, Bernert G, Hermon M, Popow C, Seidl R, Pollak A. Effects of endotracheal suctioning in high-frequency oscillatory and conventionally ventilated low birth weight neonates on cerebral hemodynamics observed by near infrared spectroscopy (NIRS) Pediatr Pulmonol. 2000;29:270–275. doi: 10.1002/(sici)1099-0496(200004)29:4<270::aid-ppul6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Skov L, Ryding J, Pryds O, Greisen G. Changes in cerebral oxygenation and cerebral blood volume during endotracheal suctioning in ventilated neonates. Acta Paediatr. 1992;81:389–393. doi: 10.1111/j.1651-2227.1992.tb12255.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgess GH, Oh W, Brann BS, Brubakk AM, Stonestreet BS. Effects of phenobarbital on cerebral blood flow velocity after endotracheal suctioning in premature neonates. Arch Pediatr Adolesc Med. 2001;155:723–727. doi: 10.1001/archpedi.155.6.723. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson LP, McPhee AJ, Donovan EF. Partially ventilated endotracheal suction. Use in newborns with respiratory distress syndrome. AJDC. 1986;140:462–465. doi: 10.1001/archpedi.1986.02140190072029. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser JR, Gauss CH, Williams DK. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol. 2008;28:34–41. doi: 10.1038/sj.jp.7211848. [DOI] [PubMed] [Google Scholar]
- 11.Ninan A, O’Donnell M, Hamilton K, Tan L, Sankaran K. Physiologic changes induced by endotracheal instillation and suctioning in critically ill preterm infants with and without sedation. Am J Perinatol. 1986;3:94–97. doi: 10.1055/s-2007-999841. [DOI] [PubMed] [Google Scholar]
- 12.Choong K, Chatrkaw P, Frndova H, Cox PN. Comparison of loss in lung volume with open versus in-line catheter endotracheal suctioning. Pediatr Crit Care Med. 2003;4:69–73. doi: 10.1097/00130478-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Cabal LA, Devaskar S, Siassi B, Plajstek C, Waffarn F, Blanco C, et al. New endotracheal tube adaptor reducing cardiopulmonary effects of suctioning. Crit Care Med. 1979;7:552–555. doi: 10.1097/00003246-197912000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Tan AM, Gomez JM, Mathews J, Williams M, Paratz J, Rajadurai VS. Closed versus partially ventilated endotracheal suction in extremely preterm neonates: physiologic consequences. Intensive Crit Care Nurs. 2005;21:234–242. doi: 10.1016/j.iccn.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kalyn A, Blatz S, Feuerstake S, Paes B, Bautista C. Closed suctioning of intubated neonates maintains better physiologic stability: a randomized trial. J Perinatol. 2003;23:218–222. doi: 10.1038/sj.jp.7210883. [DOI] [PubMed] [Google Scholar]
- 16.Woodgate PG, Flenady V. Tracheal suctioning without disconnection in intubated ventilated neonates. Cochrane Database of Systematic Reviews. 2001;2 doi: 10.1002/14651858.CD003065. CD003065. [DOI] [PubMed] [Google Scholar]
- 17.Rieger H, Kuhle S, Ipsiroglu OS, Popow CN. Effects of open vs closed system endotracheal suctioning on cerebral blood flow velocities in mechanically ventilated extremely low birth weight infants. J Perinat Med. 2005;33:435–441. doi: 10.1515/JPM.2005.077. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database of Systematic Reviews. 2010;11 doi: 10.1002/14651858.CD003666.pub3. CD003666. [DOI] [PubMed] [Google Scholar]
- 19.Keszler M, Abubakar K. Volume guarantee: stability of tidal volume and incidence of hypocarbia. Pediatr Pulmonol. 2004;38:240–245. doi: 10.1002/ppul.20063. [DOI] [PubMed] [Google Scholar]
- 20.Lista G, Colnaghi M, Castoldi F, Condò V, Reali R, Compagnoni G, et al. Impact of targeted-volume ventilation on lung inflammatory response in preterm infants with respiratory distress syndrome (RDS) Pediatr Pulmonol. 2004;37:510–514. doi: 10.1002/ppul.10458. [DOI] [PubMed] [Google Scholar]
- 21.Singh J, Sinha SK, Clarke P, Byrne S, Donn SM. Mechanical ventilation of very low birth weight infants: Is volume or pressure a better target variable? J Pediatr. 2006;149:308–313. doi: 10.1016/j.jpeds.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 22.D’Angio CT, Chess PR, Kovacs SJ, Sinkin RA, Phelps DL, Kendig JW, et al. Pressure-regulated volume control ventilation vs synchronized intermittent mandatory ventilation for very low-birth-weight infants: a randomized controlled trial. Arch Pediatr Adolesc Med. 2005;159:868–875. doi: 10.1001/archpedi.159.9.868. [DOI] [PubMed] [Google Scholar]
- 23.Sinha SK, Donn SM, Gavey J, McCarty M. Randomised trial of volume controlled versus time cycled, pressure limited ventilation in preterm infants with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1997;77:F202–F205. doi: 10.1136/fn.77.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neter J, Kutner M, Nachtsheim C, Wasserman W. Applied Linear Statistical Models. 4th ed. Boston: WCB/McGraw-Hill; 1996. [Google Scholar]
- 25.Yeh S, editor. Using trapezoidal rule for the area under a curve calculation. SAS Users Group International 27 Proceedings.2002. [Google Scholar]
- 26.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 27.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very low birth weight infants. J Pediatr. 2004;144:809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Gressens P, Rogido M, Paindaveine B, Sola A. The impact of neonatal intensive care practices on the developing brain. J Pediatr. 2002;140:646–653. doi: 10.1067/mpd.2002.123214. [DOI] [PubMed] [Google Scholar]
- 29.Cordero L, Sananes M, Ayers LW. Comparison of a closed (Trach Care MAC) with an open endotracheal suction system in small premature infants. J Perinatol. 2000;3:151–156. doi: 10.1038/sj.jp.7200330. [DOI] [PubMed] [Google Scholar]
- 30.Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 31.Pellicer A, Valverde E, Gayá F, Quero J, Cabañas F. Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol. 2001;24:103–109. doi: 10.1016/s0887-8994(00)00239-3. [DOI] [PubMed] [Google Scholar]
- 32.Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr. 1997;131:549–554. doi: 10.1016/s0022-3476(97)70060-x. [DOI] [PubMed] [Google Scholar]