Abstract
The goal of the study was to determine the association between diabetes and inflammation in clinically diagnosed diabetes patients. We hypothesized that low-grade inflammation in diabetes is associated with the level of glucose control. Using a cross-sectional design we compared pro and anti-inflammatory cytokines in a community recruited cohort of 367 Mexican Americans with type 2-diabetes having a wide range blood glucose levels. Cytokines (IL-6, TNF-α, IL-1β, IL-8) and adipokines (adiponectin, resistin and leptin) were measured using multiplex ELISA. Our data indicated that diabetes as whole was strongly associated with elevated levels of IL-6, leptin, CRP and TNF-α, whereas worsening of glucose control was positively and linearly associated with high levels of IL-6, leptin. The associations remained statistically significant even after controlling for BMI and age (p = 0.01). The association between TNF-α, however, was attenuated when comparisons were performed based on glucose control. Strong interaction effects between age and BMI and diabetes were observed for IL-8, resistin, and CRP. The cytokine/adipokine profiles of Mexican Americans with diabetes suggest an association between low-grade inflammation and quality of glucose control. Unique to in our population is that the chronic inflammation is accompanied by lower levels of leptin.
Keywords: Cytokine, Inflammation, Mexican Americans, Diabetes
1. Introduction
Type 2 diabetes, particular when poorly controlled, involves disease of the innate immune system and manifest as chronic low-grade inflammation (1, 4, 7, 26). Consistent with this hypothesis, numerous studies have demonstrated that in individuals with impaired fasting blood glucose, circulating levels of inflammatory markers such as C-reactive protein (CRP), sialic acid and Interleukin-6 (IL-6) are independent predictors of the future development of diabetes (8, 29). Furthermore high levels of circulating acute phase proteins in particular IL-6 (29), tumor necrosis factor α (TNF-α) (31) and other mediators of inflammation such as serum-amyloid A (S-AA) (17, 35), and CRP (30) and their association with obesity and insulin resistance have been previously documented (8, 17). Additionally several studies have demonstrated elevated levels of IL6 and TNF-α among individuals with insulin resistance and clinically diagnosed diabetes. An important factor that could potentially contribute to inflammation is chronic hyperglycemia (1, 26). Metabolic end products resulting from poorly controlled glucose are known to up-regulate the innate immune system leading to inflammation (3, 4, 31). In a recent report published by Duncan and colleagues IL-6 was found to be strongly associated with levels of glucose and was a strong predictor of diabetes in at-risk individuals (8). Inadequate glucose control and its associated inflammation in diabetes have been implicated in the pathogenesis of atherosclerosis, impaired lung function and cardiovascular disease (5, 7, 8).
This study was therefore designed to determine the association between glucose control and levels of pro and anti-inflammatory markers. The fact that inflammation is an underlying cause of several co-morbidities associated with diabetes, particular when poorly controlled, it is essential to understanding the association between glucose control and inflammation.
We used specimens from participants from our Cameron County Hispanic Cohort (CCHC): a randomly selected and well-characterized community-recruited cohort of Mexican Americans residing on Texas-Mexico border (10). This population has several health disparities with high weighted prevalence of obesity (50.9%) and diabetes (29.7%), most of the latter poorly controlled. We first compared the pro and anti-inflammatory profile in specimens from participants with and without diabetes for baseline characterization of chronic inflammation in this population. Secondly we determine the association between glucose control and inflammation using glycated hemoglobin (A1c) as a marker of glucose control. To our knowledge this is the first study reporting these associations in a homogenous population of Mexican Americans with diabetes.
2. Material and Methods
Study participants
In this cross sectional study we measured adipokines and cytokines in 367 baseline specimens from CCHC participants, obtained between 2005 and 2008. Specimens were aliquoted and stored at −80°C until ready to be used. All participants completed a face-to-face administered questionnaire that includes personal and family medical history, socio-demographic data, history of medication, substance use, and history of chronic illness including diabetes and heart disease. Participants also completed an extensive physical exam that includes anthropometric and clinical and mental health examination as previously described (10).
Laboratory measurements
High sensitivity C-reactive protein (CRP) levels and lipid panel estimations were obtained from a CLIA-approved clinical laboratory. Fasting blood glucose (FBG) was determined at our Clinical Research Unit (CRU) using a Glucostat® analyzer (Model 27, YSA Inc. Yellow Springs, OH). Insulin levels were determined in our own laboratory using ELISA assays (Mercodia, Uppsala, Sweden), as were glycosylated hemoglobin (AIc) levels measured on frozen whole blood using GLYCO-Tek® Affinity columns (Helena Laboratories, Beaumont, Tx) (27, 34). HOMA-IR was calculated using the method of Keskin et al (19). Briefly HOMA index was calculated as fasting insulin concentration (µU/ml) X fasting glucose concentration (mmol/L)/22.5.
2.1. Diabetes definition
Participants were classified as having diabetes if they met the 2006 ADA criteria: FBG ≥126 mg/dl, a doctor’s diagnosis of diabetes and/or on medication for diabetes. No diabetes was defined as absence of a history of diabetes and FBG <100 mg/dl (2). Glycated hemoglobin (A1c) was used as a marker for glucose control where values <6.5% indicated well controlled glucose and values >6.5% indicated poor glucose control (2).
2.2. Laboratory measurement of analytes
A total of eight biomarkers (adipokines/cytokines) were selected for analysis based on their documented role in inflammation (9, 15–17, 28, 30, 33). Measurement was performed using multiplex ELISA (Milliplex Map, Millipore CA) using two panels (panel A and B). Panel B was comprised of IL-6, IL-1β, TNF-α, interleukin-8 (IL-8) and the adipokine leptin, whereas panel A contained the adipokines, resistin and total adiponectin. Plasma specimens were thawed on ice and diluted 1:400 for panel A, and used undiluted for panel B. Undiluted and diluted plasma samples were then incubated with antibody coated beads read using the Luminex 200 system (Luminex corp. Austin TX).
2.3. Statistical analysis
Procedures for data collection, management and confidentiality have been previously described (10). Non-parametric methods of analysis were used since the distributions of cytokines, and adipokines were found to be non-normal and highly skewed with the exception of total cholesterol. Levels of adipokines, cytokines and anthropometric variables were compared by diabetes status test and by diabetes status after controlling for BMI and age using Wilcoxan test, p values represent differences in medians. Spearman rank test was used to determine correlations between cytokines, and adipokines. Medians and inter-quartile ranges of cytokines and adipokines were compared by diabetes status and by A1c status, using chi-square test. Quantile regression was performed to calculate association of diabetes and glucose control by quantiles (5th, 10th, 50th, 90th, and 95th) of cytokines/adipokines after controlling for age and BMI. All analyses were considered significant with p-values < 0.05 using SAS© v. 9.2 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Demographic and baseline characteristics
The analysis was performed on a total of 367 plasma specimens (67.3% female mean (M) age = 42.63 ± 15.33). The majority (80.9%), of the participants were overweight or obese and nearly one-third of the sample met the ATP III definition of metabolic syndrome (12). A total of 13.6% had diabetes (2), Descriptive, statistics compared median values (interquartile ranges IQ) for age, BMI, CRP, FBG, A1c, total cholesterol, high-density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides with adjustment for age and BMI Table 1a and after adjustment of age and BMI Table 1b. There was no significant gender differences but as expected individuals with diabetes were significantly older than those without diabetes (median age 53 vs. 38 years of age) and had higher BMI (p value = 0.001). Similar differences were observed for, FBG (161 vs. 97 mg/dl), and HbA1c (8.6 vs. 5.4%) as expected. Though there was no significant difference in total cholesterol between groups, the HDL was higher in participants without diabetes (47 vs. 43 mg/ml: p = 0.01), whereas triglycerides were higher in participants with diabetes (153 vs. 116 mg/ml) group compared to non-diabetes (116 mg/ml) (p = 0.003). Most differences between the two groups remained significant after controlling for BMI except for HDL and fasting insulin where no difference was observed
Table 1.
| a-Baseline anthropometric and metabolic characteristic by diabetes status | |||
|---|---|---|---|
| Baseline Characteristics | Non-DM (n=304) | DM (n=63) | P-value |
| Gender Male N(%) | 31.6 | 36.5 | 0.45 |
| Age (Yrs) | 38.0 [29.0–52.0] | 53.0 [44.0–62.0] | <0.0001*** |
| BMI (Kg/m2) | 29.5 [25.8–33.1] | 31.7 [27.5–38.2] | 0.008** |
| CRP mg/L | 3.1 [1.9–10.5] | 5.0 [2.2–8.7] | 0.01* |
| Mean FBG mmol/L | 97.0 [93.0–104.0] | 161.0 [134.0–224.0] | <0.0001*** |
| % HbA1c | 5.4 [5.0–6.0] | 8.6 [7.1–10.8] | <0.0001** |
| HOMA-IR mmol/La | 3.1 [2.3–4.9] | 7.0 [3.9–12.0] | <0.0001** |
| Insulin pmol/La | 13.2 [9.4–20.6] | 16.7 [10.1–26.9] | 0.01* |
| Total cholesterol mg/dL | 170.0 [148.0–196.0] | 178.0 [148.0–199.0] | 0.57 |
| HDL mg/dL | 47.0 [41.0–55.0] | 43.0 [38.0–52.0] | 0.01* |
| LDL mg/dL | 98.8 [78.6–117.8] | 93.0 [75.8–119.0] | 0.83 |
| Triglycerides mg/dL | 116.0 [79.5–178.0] | 153.5 [91.0–243.0] | 0.003** |
| b- Baseline anthropometric and metabolic characteristic by diabetes status after adjusting for age and BMI | |||
|---|---|---|---|
| Baseline Characteristics | Non-DM (n=304) | DM (n=63) | P-value |
| CRP mg/L | 5.6 (4.4, 6.8) | 7.2 (6.3, 8.2) | <0.0001*** |
| Mean FBG mmol/L | 109.8 (102.9, 117.6) | 119.4 (113.8, 125.6) | <0.0001*** |
| % HbA1c | 6.0 (5.7, 6.3) | 6.4 (6.2, 6.6) | <0.0001*** |
| HOMA-IR mmol/La | 4.7 (3.9, 5.7) | 5.2 (4.3, 7.0) | 0.038* |
| Insulin pmol/La | 16.8 (14.4, 20.2) | 17.4 (14.8, 23.5) | 0.49 |
| Total cholesterol mg/dL | 170.4 (163.7, 180.0) | 182.1 (175.7, 188.2) | <0.0001*** |
| HDL mg/dL | 48.1 (46.8, 49.0) | 48.0 (45.4, 49.0) | 0.68 |
| LDL mg/dL | 98.8 (95.7, 104.4) | 105.0 (101.9, 108.4) | <0.0001*** |
| Triglycerides mg/dL | 143.5 (117.6, 170.3) | 168.1 (146.3, 192.8) | <0.0001*** |
N = 62 for DM due a missing observation
Median baseline anthropometric, and metabolic characteristics of participants were compared by diabetes status. Values are presented as Medians (inter-quartile ranges).
p <0.05,
p <0.01.
p <0.001
N = 62 due to one missing observation in DM group
Median values for baseline anthropometric characteristics were compared between DM and non-DM after adjusting for age and BMI.
3.2. Correlation between anthropometric markers and inflammatory markers
A wide range of moderately positive correlations were observed between anthropometric markers, and pro and anti-inflammatory markers in all subjects (Table 2), most significant among which were between age and FBPG (0.36), age and TNF-α (0.34), and age and adiponectin (0.29) (p = < 0.01). A moderate positive correlation was also observed between BMI, and CRP (0.46) and BMI and leptin (0.59) (p = <0.01). CRP was also moderately and positively correlated with IL-6 (0.31), and leptin (0.36) (P < 0.01). Lastly, IL-6 was moderately and positively correlated with IL-8 (0.32) and TNF-α (0.33) (p = 0.01)
Table 2.
Spearman correlation coefficients between age, BMI, CRP, mean FBG and cytokines and adipokines among all subjects.
| Age | BMI | CRP | MFBG | HbA1c | IL-1B | IL-6 | IL-8 | TNF-α | Leptin | Adiponectin | Resistin | HOMA -IR |
Insulin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| BMI | 0.10 | 1.00 | – | – | – | – | – | – | – | – | – | – | – | – |
| CRP | 0.06 | 0.46* | 1.00 | – | – | – | – | – | – | – | – | – | – | – |
| MFBG | 0.36* | 0.24* | 0.16* | 1.00 | – | – | – | – | – | – | – | – | – | – |
| HbA1c | 0.24* | 0.13* | 0.17* | 0.43* | 1.00 | – | – | – | – | – | – | – | – | – |
| IL-1B | −0.10* | −0.03 | 0.01 | −0.04 | −0.07 | 1.00 | – | – | – | – | – | – | – | – |
| IL-6 | 0.20* | 0.16* | 0.31* | 0.12* | 0.17* | 0.04 | 1.00 | – | – | – | – | – | – | – |
| IL-8 | 0.23* | 0.02 | −0.03 | 0.09 | 0.00 | 0.08 | 0.32* | 1.00 | – | – | – | – | – | – |
| TNF-α | 0.34* | 0.11* | 0.09 | 0.15* | 0.07 | −0.04 | 0.33* | 0.43* | 1.00 | – | – | – | – | – |
| Leptin | 0.21* | 0.59* | 0.36* | 0.07 | −0.01 | 0.05 | 0.23* | 0.03 | 0.06 | 1.00 | – | – | – | – |
| Adiponectin | 0.29* | −0.20* | −0.18* | −0.06 | 0.08 | −0.16* | 0.10* | 0.13* | 0.03 | 0.11* | 1.00 | – | – | – |
| Resistin | 0.03 | 0.10 | 0.17* | 0.00 | 0.05 | 0.12* | 0.19* | 0.23* | 0.12* | 0.21* | −0.02 | 1.00 | – | – |
| HOMA-IR | 0.09 | 0.49* | 0.28* | 0.49* | 0.27* | 0.06 | 0.18* | 0.08 | 0.16* | 0.32* | −0.38* | 0.16* | 1.00 | – |
| Insulin | −0.02 | 0.48* | 0.25* | 0.25* | 0.12* | 0.06 | 0.15* | 0.04 | 0.12* | 0.38* | −0.41* | 0.17* | 0.94* | 1.00 |
Values represent Spearman correlation coefficents. Values in bold indicate corelation coefficients ≥ 0.25 or ≤ −0.25.
3.2. Comparison of cytokines and adipokines by diabetes status and A1c status
Levels of pro-inflammatory cytokines and adipokines were compared by diabetes status. Values presented are medians (IQ ranges) (Table 3a). To study the role of glucose control in inflammation we also performed comparison by A1c values. As mentioned in materials and methods, a value of A1c 6.5% or less was used as an indicator of well controlled diabetes whereas a value of 6.5% represented poorly controlled diabetes. Significantly high levels of IL-6, IL-8 and TNF-α were observed in individuals with diabetes, compared to non-diabetes (p = < 0.01). Interestingly, participants with diabetes tended to have higher levels of, adiponectin compared to individuals without diabetes but this was not statistically significant. Levels of resistin did not show any difference between the two groups. These difference remained significant even when data was compared by A1c levels, suggesting an important role of these cytokine in both diabetes and poorly controlled diabetes.
Table 3.
Comparison of median cytokine/adipokine values by diabetes status
| Cytokines and Adipokines | HbA1c ≤ 6.5 (n=304) |
HbA1c >6.5 (n=63) |
Pr>χ2 |
|---|---|---|---|
| IL-1B pg/mL | 0.2 [0.2–0.3] | 0.2 [0.2–0.3] | 0.68 |
| IL-6 pg/mL | 2.8 [1.5–4.2] | 4.1 [2.3–5.6] | <0.0001*** |
| IL-8 pg/mL | 4.4 [3.2–5.9] | 5.2 [3.7–6.6] | 0.01* |
| TNF-α pg/mL | 3.2 [2.3–4.3] | 3.8 [3.0–5.0] | 0.003** |
| Leptin ng/mL | 17.3 [8.9–29.5] | 16.1 [7.9–32.5] | 0.97 |
| Adiponectin ng/mL | 36.6 [26.1–48.8] | 42.3 [28.4–57.7] | 0.07 |
| Resistin pg/mL | 45.5 [35.9–61.2] | 45.7 [31.5–60.4] | 0.65 |
N= 62 due to one missing observation in DM
Medians and (interquartile ranges) of cytokines/adipokines were compared diabetes and non-diabetes diabetes and non-diabetes groups by. The p value indicates that the medians in two groups are significantly different
Additionally, when pro-inflammatory cytokines and adipokines were compared by levels of A1c, we observed that IL-6, IL-8 and TNF-α were significantly elevated in group with high A1c vlaues (Table 3b) (p = 0.001). These findings were consistent with those observed when cytokines were comapred by diabetes status (Table 3a).
3.4. Association of diabetes with inflammation after controlling for age and BMI
To determine what porportion of our observed diabetes-inflammation association could be attributed to obesity, we performed quantile regression where all models were adjusted for age and BMI. The advantage of quantile regression is that it provides more useful predictive relationships and is more roubust in response to large outliers. We measured effects of diabetes on 5th, 10th, 50th 75th, 90th and, 95th quantiles of cytokines (Table 4a) after adjusting for both age and BMI. A significant affect of diabetes was observed on the 10th and 50th quantiles of IL-6 where levels of IL-6 were significantly and positively affected by diabetes, (P = <0.01). A moderate effect of diabetes was also observed on the 5th [0.800 (95% CI, 0.073, 1.527)] and 10th [0.640 (95% CI 0.113, 1.167)] quantile of TNF-α (P = 0.05). Interestingly we observed a significant negative effect of diabetes on third quantile of leptin [−6.6441 (95% CI −10.776, −2.105] P 0.01. To confirm these trends we performed logistic regression (Table 6). We found that individuals with diabetes were more likely to have elevated IL-6 values. In contrast, individuals with diabetes were more likely to have lower leptin values (data not shown). To determine the association of glucose control in diabetes with inflammation we performed another quantile regression where we performed the analysis by A1c status (Table 4b) where A1c values <6.5% served as a reference. Our findings suggested a positive association between of IL-6 with poorly controlled diabetes whereas the leptin remained negatively associated even with poorly controlled diabetes.
Table 4.
| a – Association of diabetes on cytokine after adjusting for age and BMI | |||||
|---|---|---|---|---|---|
| Cytokine | Quantile | ||||
| 5 | 10 | 50 | 90 | 95 | |
| IL1B | 0.000 | 0.000 | 0.000 | 0.070 (−0.17, 0.31) | 0.080 (−0.49, 0.65) |
| IL6 | 0.520 (−0.07, 1.11) | 1.110 (0.35, 1.86)** | 1.150 (0.30, 1.99)** | 4.160 (−2.37, 0.69) | 8.540 (−4.63, 21.71) |
| IL8 | 0.58 (−0.35, 1.52) | 0.34 (−0.38, 1.06) | 0.56 (−0.20, 1.32) | 1.38 (−0.14, 2.90) | 1.74 (−2.43, 5.91) |
| Leptin | −0.32 (−3.64, 2.99) | −0.64 (−2.87, 1.57) | −6.4 (−10.77, −2.10)** | −14.16 (−39.37, 11.04) | −8.95 (−48.34, 30.42) |
| TNF-α | 0.80 (0.07, 1.52)* | 0.64 (0.11, 1.16)* | 0.22 (−0.25, 0.69) | 1.43 (−0.40, 3.27) | 0.33 (−1.76, 2.42) |
| Adiponectin | −2.09 (−9.72, 5.54) | 0.92 (−5.34, 7.19) | −1.97 (−10.16, 6.20) | 3.63 (−21.50, 28.77) | 18.86 (−12.92, 50.64) |
| Resistin | −0.39 (−8.50, 7.72) | 1.67 (−3.26, 6.60) | −1.39 (−8.68, 5.90) | −2.71 (−22.94, 17.52) | −5.53 (−36.39, 25.32) |
| CRP | 0.30 (−0.21, 0.81) | 0.50 (−0.04,1.04) | 1.700 (0.55, 2.85)** | 10.00 (0.11, 19.88)* | 15.20 (−2.89, 33.29) |
| b – Association of blood glucose control in diabetes (based on definition of HbA1c < = 6.5% and >6.5%) on cytokines after adjusting for age and BMI | |||||
|---|---|---|---|---|---|
| Cytokine | Quantile | ||||
| 5 | 10 | 50 | 90 | 95 | |
| IL1B | 0.000 | 0.000 | 0.000 | 0.06 (−0.17, 0.29) | 0.22 (−0.35, 0.79) |
| IL6 | 0.520 (0.07, 0.96)* | 0.70 (0.01, 1.38)* | 0.88 (0.08, 1.67)* | 3.86 (−0.29, 8.01) | 3.09 (−9.28, 15.46) |
| IL8 | 0.60 (−0.25, 1.45) | 0.22 (−0.24, 0.68) | 0.36 (−0.33, 1.05) | 0.46 (−1.02, 1.94) | 0.57 (−1.67, 2.81) |
| Leptin | 0.14 (−3.52, 3.81) | −2.04 (−4.10, 0.02) | −5.37 (−10.10, −0.63)* | 6.50 (−30.09, 43.10) | 5.404 (−67.96, 78.77) |
| TNF-α | 0.52 (−0.38, 1.42) | 0.43 (−0.12, 0.98) | 0.11 (−0.32, 0.54) | 0.05 (−1.64, 1.74) | −0.63 (−2.91, 1.64) |
| Adiponectin | 0.91 (−8.71, 10.54) | 2.43 (−2.90, 7.77) | 4.14 (−2.96, 11.25) | 9.73 (−11.53, 31.00) | 18.86 (−12.92, 50.64) |
| Resistin | −1.93 (−9.90, 6.04) | −0.19 (−4.92, 4.54) | −2.03 (−8.36, 4.29) | −7.51 (−27.99, 12.97) | −11.47 (−42,03, 19.09) |
| CRP | 0.30 (−0.20, 0.80) | 0.40 (−0.16,0.96) | 0.90 (−0.12, 1.92) | 3.10 (−7.44, 13.64) | 15.00 (0.94, 29.05)* |
Data represents regression coefficient (95% confidence intervals). Inflammatory markers were distributed into quantiles and a regression model was used to estimate assocation of diabetes with increasing quantiles of biomakers. Model was adjusted for age and BMI. Non-diabetes was used as reference
Data represents regression coefficient (95% confidence intervals) of diabetes demonstrating association of glucose control with different quantiles of cytokines after adjusting for age. HbA1c <6.5% was used as a reference
3.5. Interaction effects of age and BMI on pro-inflammatory cytokines in individuals with diabetes
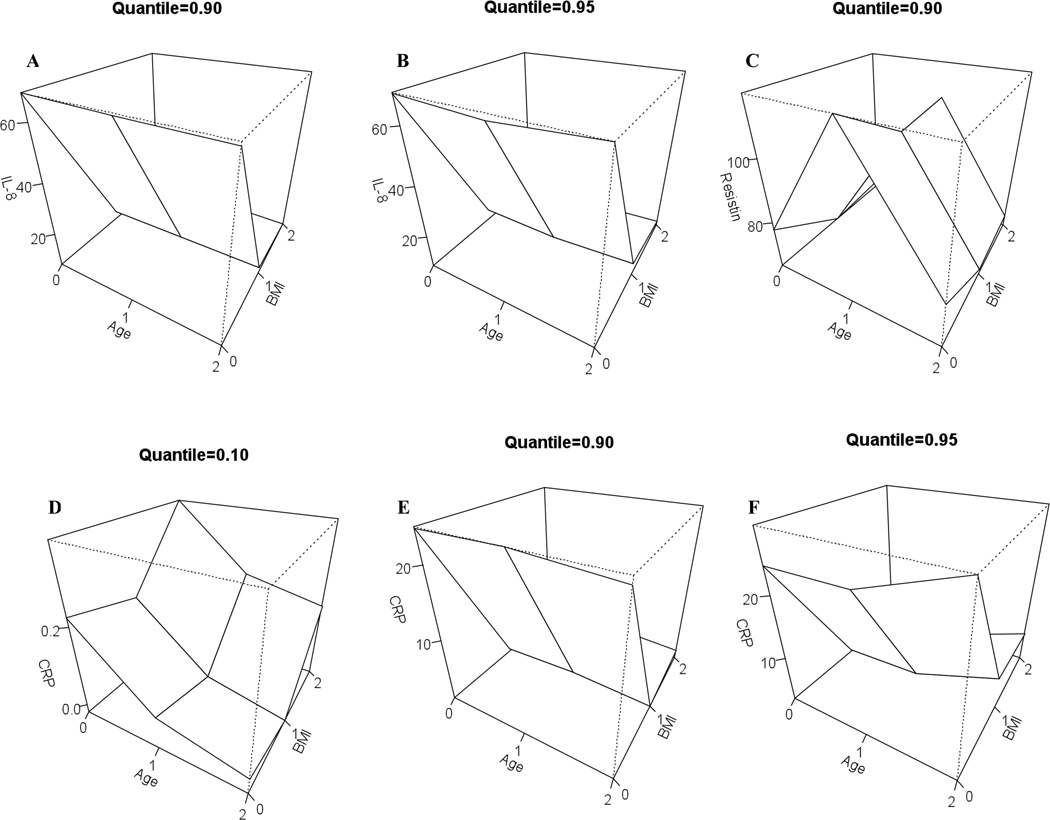
To adjust for the interaction effects for diabetes status and BMI and diabetes status and age we fitted quantile regression models for the following quantiles of cytokines: 5th, 10th, 50th, 90th and 95th. Results for significant (p value = <0.05) interactions are presented as three-dimensional plots in Fig 2. We observed that the 90th (Fig 1A) and 95th (Fig 1B) quantiles of IL-8 were associated with diabetes (p < 0.01) in older age groups (50–64 years old) in comparison to younger age group (18 – 49). IL-8 was also significantly lower (p < 0.01) in higher BMI groups (BMI 25 –30) in comparison to lower BMI group (BMI ≤ 25). The 90th quantile of value of resistin (Fig 1C) was higher in older individuals with diabetes (50 – 65 years of age) in comparison to younger individuals with diabetes (18 –29 years of age). We also observed that older individuals with diabetes had lower levels of CRP (10th quantile) whereas younger individuals with diabetes were associated with higher quantiles (90th) of CRP (Fig 1D). A similar relationship was also observed with BMI where CRP was higher in individuals who were both obese (BMI 25 – 30) and had diabetes, whereas lower quantiles of CRP were associated with diabetes without obesity (BMI ≤ 25) (Fig 1E and F) P = <0.01.
Fig 1.
Three dimensional plots for the model predicting interaction effects between diabetes status, BMI, age and cytokines
Three dimensional plots of model predicting cytokine values for specific quantile values. The cytokines and the quantile are reported for which the interactions effects between diabetes status and BMI and diabetes status and age are statistically significant at least 5% level.
DISCUSSION
In this study we demonstrated the unique cytokine/adipokine profile associated with diabetes in a randomly selected cohort of Mexican Americans. Dissection of levels of cytokine and adipokine expression indicated elevated levels of IL-6, TNF-α and paradoxically, adiponectin to be associated with diabetes. The elevation of IL-6 and TNF-α remained statistically significant after controlling for age and BMI. In contrast, IL-8 was elevated only in older participants with diabetes, and leptin levels were low in diabetes at all ages. Consistent with our observations, Ho et al (14) also reported elevated levels of TNF-α in non-diabetic Mexican Americans. Given the fact that Mexican Americans are more susceptible to metabolic syndrome and its associated co-morbidities, the pre-existing levels might suggest a predisposition of this population to certain chronic conditions.
Elevated levels of IL-6 in both poorly controlled diabetes and well-controlled diabetes are well studied in other ethnic groups (11, 13, 20). When stratified on the basis of AIc values we found that IL-6, TNF-α and IL-8 were most significantly elevated in the group with A1c values >6.5%. In a correlation analysis we were then able to confirm that the quality of glucose control does correlate with levels of pro-inflammatory cytokines in a linear fashion. These findings were similar to what we have observed with in our comparison by diabetes status. One likely explanation for this could be that most of our diabetes is poorly controlled and therefore the two analysis showed similar results
Elevated levels of IL-8 were significantly associated with TNF-α, and both of these with diabetes. Elevation of IL-8 may be in response to high levels of TNF-α as TNF-α regulates epithelial tissue-derived IL-8 (1, 25). The associations between IL-8 and TNF-α have not been previously reported in any other ethnic/racial groups and therefore warrants further investigation. Interestingly we found IL-8 to be down regulated in obese participants. This strengthens our hypothesis that the IL-8 in diabetes is mainly derived from the epithelium rather than adipose tissue. Additionally possibility of existence of other pathways of IL-8 synthesis cannot be excluded. It has been shown recently that B cell-derived IL-8 is elevated in individuals with diabetes through activation of Toll like receptor 2 (TLR2) on B cells (18), where metabolic end products of hyperglycemia have been shown to activate TLR2 in poorly controlled diabetes (6).
We also found CRP to be significantly elevated in individuals with diabetes and to be moderately associated with elevated levels of IL-6. A sensitive marker of low-grade inflammation, CRP is the most commonly measured marker of inflammation. We also observed negative association of leptin in diabetes participants again suggesting chronic inflammation. Leptin is known to be regulated by immune responses (1), inasmuch that the acute immune response and release of TNF-α and IL-1β results in a prompt short term release and increase in plasma levels of leptin. However, chronic inflammation and its resultant constitutive up-regulation of pro-inflammatory cytokines will cause suppression of leptin, and this is presumably what we have observed (24). In contrast to our findings leptin levels have been reported to be higher in Hispanic women (21). This could be partially explained by the fact that the observation made by Kings et al was in Hispanics as whole whereas we are reporting our observations in a homogenous group of Mexican Americans. Additionally our measurements were made mainly in diabetics whereas King et al studied these relationships in terms of adiposity, which could result in observed differences in findings between two studies. Moreover, our findings are further strengthen by the observations made by King et al suggesting ethnic diversity in levels of adiponectin and its association with several chronic diseases.
Inversely it also possible that obese individuals who have high levels of leptin may be in the acute phase of inflammation and therefore still have high levels of leptin. Given this relationship of leptin with acute and chronic inflammation, leptin could serve as a valuable marker for predicting acute inflammation in individuals with both obesity and diabetes.
Even though the association was attenuated after controlling for BMI we observed high levels of adiponectin and resistin in diabetes. Previous reports have suggested low levels of adiponectin to be markers of metabolic syndrome, type-2 diabetes and cardiovascular disease in both non-Hispanic whites and African Americans (22, 23, 36). Therefore higher levels of adiponectin observed in diabetes in our population suggests, that this might be a compensatory mechanism to overcome the downstream detrimental effects of inflammation such as cardiovascular disease. Although not statistically significant, the trend for higher adiponectin in participants with higher HbA1c may have a protective role. It is of note that this cannot be the result of medications such as rosiglitazone, which are known to increase adiponectin and TNF-α as none of the participants in this study were on rosiglitazone.
There are limitations to our study. The sample size is small, and this may be have led to weaker associations among some of the cytokines. Most of the adiponectin we measured was low molecular weight adiponectin; nevertheless our findings provide a foundation for understanding how adiponectin may be affected by diabetes in this population of mainly Mexican Americans. Our population was homogenously Mexican American and therefore it would be difficult to generalize our findings to all Hispanics, which are genetically disparate. Additionally the cross-sectional nature of the study also allow for measurement of associations between pro-inflammatory markers and diabetes. In order to fully understand the association and the biological role they might play in development of co-morbidities in diabetes we need a prospective study with a significantly larger sample size.
In conclusion we show that the cytokine/adipokine profile of Mexican Americans with diabetes is one of low-grade inflammation. Since the levels of pro-inflammatory cytokines strongly correlated with AIc values indicates that quality of glucose control plays a role in initiation of the acute phase response. The increase in circulating levels of IL-6, IL-8 and TNF-α is similar to that previously reported for African Americans and non-Hispanic whites with diabetes (5, 32). What is unique in our population is that, the increase in circulating levels of pro-inflammatory markers is accompanied by lower levels of leptin.
Table 5.
Odds ratios and adjusted odds ratios indicating associations of cytokines and adipokines with diabetes.
| Variable | OR [95% CI] | Adjusted OR [95% CI] | |
|---|---|---|---|
| IL-1B pg/mL | |||
| ≤ 0.16 | Ref. | Ref. | |
| 0.17–0.23 | 1.6 [0.7–3.4] | 1.2 [0.5–2.6] | |
| 0.24–0.31 | 1.3 [0.7–2.6] | 1.5 [0.7–3.1] | |
| > 0.31 | 1.0 [0.4–2.5] | 1.5 [0.6–3.7] | |
| IL-6 pg/mL | |||
| ≤ 1.52 | Ref. | Ref. | |
| 1.53–2.82 | 4.0 [1.3–12.6]* | 3.6 [1.1–11.8]* | |
| 2.83–4.85 | 7.5 [2.3–22.8]* | 6.2 [2.0–19.6]* | |
| > 4.85 | 7.3 [2.4–22.3]* | 5.0 [1.5–15.9]* | |
| IL-8 pg/mL | |||
| ≤ 3.23 | Ref. | Ref. | |
| 3.24–4.53 | 0.9 [0.3–2.4] | 0.9 [0.3–2.5] | |
| 4.54–5.97 | 2.8 [1.2–6.4]* | 2.5 [1.0–6.0] | |
| > 5.97 | 3.5 [1.5–8.0]* | 2.4 [1.0–5.8] | |
| TNF-α pg/mL | |||
| ≤ 2.41 | Ref. | Ref. | |
| 2.42–3.31 | 1.2 [0.5–3.2] | 0.8 [0.3–2.2] | |
| 3.32–4.33 | 3.4 [1.4–8.2]* | 2.6 [1.0–6.4] | |
| > 4.33 | 3.7 [1.5–8.7]* | 1.8 [0.7–4.5] | |
| CRP mg/L | |||
| ≤ 2.00 | Ref. | Ref. | |
| 2.01–3.33 | 0.8 [0.3–1.8] | 0.8 [0.3–2.0] | |
| 3.34–6.40 | 1.6 [0.8–3.5] | 1.5 [0.7–3.5] | |
| > 6.40 | 2.1 [1.0–4.3] | 1.7 [0.7–3.9] | |
| Leptin ng/mL | |||
| ≤ 8.69 | Ref. | Ref. | |
| 8.70–17.00 | 1.0 [0.5–2.0] | 0.7 [0.3–1.6] | |
| 17.01–29.80 | 0.6 [0.3–1.3] | 0.2 [0.1–0.5]* | |
| > 29.80 | 0.8 [0.4–1.7] | 0.1 [0.03–0.3]* | |
| Adiponectin ng/mL | |||
| ≤ 26.51 | Ref | Ref. | |
| 26.52–37.02 | 0.8 [0.3–1.7] | 0.6 [0.2–1.4] | |
| 37.03–51.14 | 0.8 [0.3–1.7] | 0.5 [0.2–1.2] | |
| > 51.14 | 1.4 [0.8–2.9] | 0.9 [0.4–2.0] | |
| Resistin pg/mL | |||
| ≤ 35.92 | Ref. | Ref. | |
| 35.93–45.51 | 0.5 [0.2–1.2] | 0.5 [0.2–1.2] | |
| 45.52–61.22 | 0.8 [0.4–1.7] | 0.7 [0.3–1.5] | |
| > 61.22 | 1.1 [0.5–2.2] | 0.8 [0.4–1.8] | |
Odds ratios (Ors) and adjusted odds ratios (AORs) were calculated for cytokines and adipokines and comparisons were made between diabetes and non-diabetes groups. Cytokines and adipokines values were categorized by quartiles with the lowest quartile serving as the reverence group. For adjusted Ors, variables were adjusted for covariates age and BMI.
Acknowledgments
We thank our cohort team, particularly Rocio Uribe, Elizabeth Braunstein, and Julie Ramirez at the Hispanic Health Research Center in the Lower Rio Grande Valley. We also thank Christina Villarreal at the University of Texas School of Public Health for administrative support. We would like to acknowledge the participant of the Cameron County Hispanic cohort who should tremendous enthusiasm, and support for the study. We are also grateful to the Valley Baptist Medical Center, Brownsville, for hosting our Clinical Research Unit. The Study was supported by grant number MD000170 P20 funded from the National Center on Minority Health and Health disparities (NCMHD), and Centers for Translational Science Award U54RR023417-01 from the National Center for Research Resources (NCRR).
Footnotes
Conflict of Interest: None declared
References
- 1.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 2.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29 Suppl 1:S43–S48. [PubMed] [Google Scholar]
- 3.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2422–2429. doi: 10.1210/jc.2003-030178. [DOI] [PubMed] [Google Scholar]
- 6.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis RJ, Maldonado D, Rojas MX, Aschner P, Rondon M, Charry L, Casas A. Inadequate glucose control in type 2 diabetes is associated with impaired lung function and systemic inflammation: a cross-sectional study. BMC Pulm Med. 10:38. doi: 10.1186/1471-2466-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 9.Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, Hoogeveen RC, Heiss G. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473–2478. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 10.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, Restrepo BI, Wilson JG, Hossain MM, Rahbar MH, Hanis CM, McCormick JB. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev Chronic Dis. 7:A53. [PMC free article] [PubMed] [Google Scholar]
- 11.Goran MI. Ethnic-specific pathways to obesity-related disease: the Hispanic vs. African-American paradox. Obesity (Silver Spring) 2008;16:2561–2565. doi: 10.1038/oby.2008.423. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 13.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21 Suppl 3:C11–C14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 14.Ho RC, Davy KP, Hickey MS, Melby CL. Circulating tumor necrosis factor alpha is higher in non-obese, non-diabetic Mexican Americans compared to non-Hispanic white adults. Cytokine. 2005;30:14–21. doi: 10.1016/j.cyto.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 18.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–e503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 20.Kim CX, Bailey KR, Klee GG, Ellington AA, Liu G, Mosley TH, Jr., Rehman H, Kullo IJ. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One. 5:e9065. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King GA, Deemer SE, Thompson DL. Relationship between leptin, adiponectin, bone mineral density, and measures of adiposity among pre-menopausal Hispanic and Caucasian women. Endocr Res. 35:106–117. doi: 10.3109/07435800.2010.496090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 23.Lu JY, Huang KC, Chang LC, Huang YS, Chi YC, Su TC, Chen CL, Yang WS. Adiponectin: a biomarker of obesity-induced insulin resistance in adipose tissue and beyond. J Biomed Sci. 2008;15:565–576. doi: 10.1007/s11373-008-9261-z. [DOI] [PubMed] [Google Scholar]
- 24.Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82:3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 25.Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, Vonk R, Rezaee F. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller S, Martin S, Koenig W, Hanifi-Moghaddam P, Rathmann W, Haastert B, Giani G, Illig T, Thorand B, Kolb H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia. 2002;45:805–812. doi: 10.1007/s00125-002-0829-2. [DOI] [PubMed] [Google Scholar]
- 27.Peterson CM, Jovanovic L, Raskin P, Goldstein DE. A comparative evaluation of glycosylated haemoglobin assays: feasibility of references and standards. Diabetologia. 1984;26:214–217. doi: 10.1007/BF00252410. [DOI] [PubMed] [Google Scholar]
- 28.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 29.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 30.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 31.Rytter E, Vessby B, Asgard R, Johansson C, Sjodin A, Abramsson-Zetterberg L, Moller L, Basu S. Glycaemic status in relation to oxidative stress and inflammation in well-controlled type 2 diabetes subjects. Br J Nutr. 2009;101:1423–1426. doi: 10.1017/s0007114508076204. [DOI] [PubMed] [Google Scholar]
- 32.Saltevo J, Laakso M, Jokelainen J, Keinanen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Levels of adiponectin, C-reactive protein and interleukin-1 receptor antagonist are associated with insulin sensitivity: a population-based study. Diabetes Metab Res Rev. 2008;24:378–383. doi: 10.1002/dmrr.831. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 34.Selvin E, Coresh J, Jordahl J, Boland L, Steffes MW. Stability of haemoglobin A1c (HbA1c) measurements from frozen whole blood samples stored for over a decade. Diabet Med. 2005;22:1726–1730. doi: 10.1111/j.1464-5491.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, Duncan BB, Schmidt MI. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab. 95:5097–5104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]