Abstract
While childhood trauma appears to be a risk factor for the onset of depression and subclinical depressive symptomatology in Mexican Americans, the specific physiological mechanisms contributing to this relationship remain to be clarified. Stress-induced dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis is associated with depressive symptomatology in non-Hispanics. The current study assessed the extent to which the cortisol awakening response (CAR) predicts subclinical depressive symptomatology beyond the influence of childhood trauma in a sample of 55 Mexican American males and females ages 18–38 years, without a diagnosis of clinical depression. Participants were assessed for exposure to early trauma and current depressive symptomatology. Salivary cortisol samples were collected on two consecutive days at awakening, 30, 45, and 60 minutes thereafter, and again at 3pm, 6pm and 9pm. Data were analyzed using general linear models with repeated measures at four morning time points, and again, at three afternoon and evening time points. Results indicated a significant Symptoms × Time interaction for the CAR (p <.05). The Symptom × Time interaction was not significant for afternoon and evening cortisol concentrations. Moreover, subclinical symptomatology was associated with attenuation of the initial rise in CAR, after controlling for the total frequency of exposure to childhood traumas. Hierarchical analyses show attenuation of the initial rise in the CAR was the best predictor of greater subclinical depressive symptomatology beyond the influence of trauma, and independent of a current diagnosis of major depression in a sample of adult Mexican Americans.
Keywords: Childhood Trauma, HPA, Cortisol, Depression, Mexican Americans, Hispanics
Introduction
Hispanic children comprise a significant percentage of children exposed to childhood trauma in the U.S. (NCANDS, 2002; NCTSN, 2005). The stress associated with childhood trauma increases the risk for depression in adulthood (Gillespie et al., 2009; Heim et al., 2008, 2009; Kendler et al., 2001), and Mexican American adults with a history of childhood trauma report higher rates of clinical depression and more subclinical depressive symptomatology compared to their trauma-free counterparts (Aranda et al., 2001; Martinez-Taboas & Bernal, 2000; Mennen, 2000; Zayas et al., 2002). In addition, clinical depression is more likely to go undetected, untreated, and lead to more persistent depressive symptomatology in Mexican Americans compared to their non-Hispanic counterparts (Marin et al., 2006). Thus, the identification of specific biological risk factors associated with subclinical depressive symptomatology in Mexican Americans with a history of childhood trauma is of particular importance. There is interest in the extent to which exposure to traumatic stress is associated with injury to crucial biological, stress response systems. Stress-induced dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis is one neurobiological pathway that may link childhood trauma to greater depressive symptomatology (Goodwin & Stein, 2004; Heim et al., 2006). Indeed, exposure to traumatic stress is associated with alterations of HPA axis functioning and greater risk for depression in non-Hispanics (Goodwin & Stein, 2004; Heim et al., 2006). The Cortisol Awakening Response (CAR) is utilized as a means to investigate stress-induced injury to the HPA Axis (Pruessner et al., 1999). Basal HPA axis activity is characterized by a distinct diurnal rhythm, with increasing cortisol secretion in the second half of the night followed by peak cortisol levels in the early morning hours. Cortisol levels steadily decline throughout the day to nadir during the first half of the night (Dallman et al.,2000; Tsigos and Chrousos, 2002). The CAR is a discrete phenomenon superimposed on the circadian rhythm characterized by a sharp increase in cortisol concentration between twenty and thirty minutes following awakening in the morning (Wilhelm et al., 2007). Changes in the CAR can yield important information regarding the relationship between altered stress responsivity and injury to the awakening portion of the cortisol circadian rhythm. The CAR is a reliable biological marker of HPA activity, dependent on a moderate genetic influence (Bartels et al., 2003; Schmidt-Reinwald et al., 1999). The sensitivity/capacity of the adrenal cortex is proposed to play a crucial role in the magnitude of the CAR (Kudielka and Kirschbaum, 2003; Pruessner et al., 1997, 1999).
Studies in non-Hispanic adults suggest that childhood trauma is associated with attenuation of the CAR (Meinlschmidt & Heim, 2005; Shea et al., 2007). Although studies in Hispanics are practically non-existent, a single study conducted in our laboratory demonstrated attenuation of the CAR in Mexican American adults with a history of childhood trauma (Mangold et al., 2010). Moreover, prospective evidence suggests that following childhood trauma there is a transition from a higher CAR in childhood to an attenuated CAR in adulthood (Trickett et al., 2010). Indeed, some studies show less decline in afternoon and evening cortisol levels coupled with attenuation of the CAR reflecting an overall flattened diurnal cortisol rhythm with a higher daily output in non-Hispanic adults with a history of trauma (Miller et al., 2007; Weissbecker et al., 2005).
The CAR is also attenuated in non-Hispanic adults with clinical (Huber et al., 2006; Oquendo et al., 2003; Peeters et al., 2004;Strickland et al., 2002; Taylor et al., 2009) and subclinical depressive symptoms independent of a formal diagnosis of major depression (Dedovic et al., 2010). This is of particular interest because attenuation of the CAR may be a biological risk factor that is a precursor to the development of clinical depression in some high risk groups (Adam et al., 2010; Dedovic et al., 2010). However, studies examining the CAR and depressive symptomatology in Mexican Americans are scarce. The single available study in distressed, adult, Hispanic caregivers suggests an association between subclinical depressive symptomatology and a flatter slope to the daytime salivary cortisol curve (Gallagher-Thompson et al., 2006).
Equally lacking, are studies designed to differentiate the extent to which the CAR compared with waking day cortisol concentrations (e.g., 12 hour AUC) predicts subclinical depressive symptomatology in Hispanics and non-Hispanics. Cross-sectional studies of clinically depressed, non-Hispanics show elevated evening cortisol concentrations (Forbes et al., 2006; Shirtcliff & Essex, 2008; Van den Bergh & Van Calster, 2009). However, recent prospective evidence suggests an enhanced initial rise in the CAR, but not evening cortisol concentrations predicts greater risk for future episodes of major depressive disorder (Adam et al., 2010). Thus the differential effects of the CAR compared with afternoon and evening cortisol concentrations on subclinical depressive symptomatology remains to be clarified.
While studies in non-Hispanics indicate injury to the CAR may be a biological risk factor associated with both subclinical and clinical depressive symptomatology, there are few studies examining this potential risk factor in Mexican Americans. There are several likely pathways to the onset of clinical depression in which specific patterns of injury to the HPA axis are linked to the development of particular forms of depressive symptomatology in certain high-risk groups (Adam et al., 2010; Dedovic et al., 2010; Ehlert et al., 2001). In view of the fact that Mexican Americans with a history of childhood trauma are at greater risk for undetected and untreated depression, the identification of early biological risk factors independent of a formal diagnosis are of particular importance (Marin et al., 2006). Recent findings from our laboratory have shown that childhood trauma is associated with attenuation of the CAR in highly acculturated Mexican Americans without a lifetime diagnosis of major depressive disorder (Mangold et al., 2010). However, the extent to which the CAR compared with afternoon and evening cortisol concentrations predicts subclinical depressive symptomatology beyond the influence of childhood trauma remains to be clarified. Therefore, the objectives of the current investigation were to: 1) examine the association between the CAR and subclinical depressive symptomatology in Mexican Americans utilizing a carefully constructed sample monitoring system with respect to time of awakening and while carefully controlling for the known effects of childhood trauma and demographic factors previously shown to influence the CAR and; 2) distinguish to what extent the CAR compared with 12hr waking day AUC predicts subclinical symptomatology.
Materials and Methods
Participants
The current study was reviewed and approved by the University of Texas Institutional Review Board. Participants, primarily college students of Mexican descent (n=55), aged 18 to 38 years, were recruited from the San Antonio metropolitan area, through advertisements in the local community and college campuses. Complete details of the recruitment and screening procedures for the current study are reported elsewhere (Mangold et al., 2010). Participants were fully informed of study procedures and consent was obtained prior to enrollment in the study. During an initial visit to the laboratory, participants underwent a screening interview and a battery of self-report assessments designed to identify and exclude factors known to potentially affect the HPA axis, including current depression (Huber et al., 2006; Oquendo et al., 2003), use of oral contraceptives in the past 60 days (Meulenberg & Hofman, 1990; Pruessner et al., 1997,1999), current pregnancy (Meulenberg & Hofman, 1990), menstrual cycle abnormalities in the past 60 days (Bao et al., 2003, 2004; Suh et al., 1988), strenuous aerobic exercise (more than 2 hours per day for 4 or more days per week in the past 60 days (Hansen et al., 2008; Kelly et al., 2008), certain reported medical conditions, history of head trauma, use of medications, severe obesity (defined as a body mass index of > 30.0 kg/m2) and alcohol or other drug use disorders (Hansen et al., 2008;Wand & Dobs, 1991). Additional exclusion criteria included abnormal sleeping patterns, (Lasikiewicz et al., 2008) and shift and overtime work (Clow et al., 2004; Lundberg & Hellstrom, 2002; Hanrahan et al., 2006).
Psychometric Assessments
Diagnosis of Major Depression
Participants were screened for depression using the Hamilton Depression Inventory Short Form (HDI-SF; Reynolds & Kobak, 1995) and excluded from participation based on a score of 10 or greater indicating a strong likelihood of depressive disorder (Reynolds & Kobak, 1995). Reliability for the HDI-SF for the sample was acceptable (α = .78).
Substance Abuse/Dependence
Participants were screened and excluded for current or lifetime alcohol and other drug use disorders using the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (WHO ASSIST; Humeniuk et al., 2008). Reliability for the sample was acceptable (α = .81).
Assessment of Ethnicity/Nativity
Participants were determined to be of Mexican descent if both biological parents and both maternal and paternal grandparents were of Mexican descent. Generational status was characterized as first generation (subject immigrated to U.S. as child or as an adult, with both parents born in Mexico; Second Generation (subject was born in the U.S. with at least one parent born in Mexico), Third Generation or higher (subject was born in the U.S. and at least one parent and one grandparent born in Mexico).
General Health
Subjective general health was assessed with the RAND 36-Item Health Survey (RAND-36; Stewart et al., 1988). Reliability for the RAND-36 is demonstrated for Mexican American samples (Ayuso-Mateos et al., 1999) with good reliability for the current sample (α = .85).
Childhood Trauma
Exposure to childhood trauma was assessed using the Early Trauma Inventory–Short Form (ETISR-SF; Bremner et al., 2007). The ETI-SF assesses General Trauma, Physical Punishment, Emotional Abuse and Sexual Events, and has strong psychometric properties (Bremner et al., 2007; Hyman et al., 2005) with good reliability for the current sample (α = .80).
Psychiatric Symptoms
Severity of depressive symptomatology was assessed with the Revised Symptom Checklist (SCL-90-R; Derogatis,1976, 1977; Derogatis & Cleary 1977a, 1977b). The SCL-90-R is a self-report, clinical rating scale providing global indices and scores on symptom dimensions psychological distress. The instrument is comprised of 90 items across nine symptom categories: Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobia, Paranoid Ideation, and Psychoticism. Subjects’ responses to each of these items provide information on the intensity of experienced symptoms (5-point scale: not at all; a little bit; moderately; quite a bit; extremely) in the past seven days before completing the questionnaire. Each of the scales has exhibited good internal consistency (coefficient alpha from 0 .77 to 0.90) and high concurrent validity with MMPI clinical profiles (Derrogatis et al., 1976). Good psychometric properties are reported for Hispanic samples (Martinez et al., 2005) with good reliability for the depression subscale used for the current sample (α = .97).
Acculturation
The revised Acculturation Rating Scale for Mexican Americans (ARSMA-II; Cuéllar et al., 1995) was used to characterize the sample on level of acculturation by measuring subjects’ orientation towards Anglo culture and Mexican culture. The ARSMA-II has strong psychometric properties with good reliability for the current sample (α = .78).
Procedure
Measurement of Waking Cortisol Concentrations
Complete details of the salivary collection procedure have been previously reported (Mangold et al., 2010). Participants were free to wake up as usual (using an alarm or spontaneously) and instructed to start sampling immediately at awakening, 30, 45 and 60 minutes thereafter, and again at 3pm, 6pm and 9pm. Participants were instructed to collected samples on two consecutive weekdays and log wake-up times along with sampling times for comparison with electronic monitoring data. Additional instructions included refraining from strenuous exercise, brushing teeth, eating or drinking, and use of alcohol, caffeine and nicotine during sampling to avoid contamination of cortisol samples (Badrick et al., 2007; Clow et al., 2004). Immediately after awakening, participants collected saliva in a salivette test tube (Sarstedt; Rommelsdorft, Germany), and remained sitting upright in bed until the second sample was obtained. They were then free to follow their normal weekday routine during the remaining sampling period. Cortisol was assessed in females during the early follicular phase of their menstrual cycle (Bao et al., 2003, 2004). We utilized electronic monitoring devices together with self-reported time of sampling to determine concordance between monitored times and self-reported times, detect deviations from the protocol, and maximize accuracy in the documentation of sample times (Jacobs et al., 2005; MEMS; Aardex; Zug, Switzerland).
Hormonal Assays
Participants stored the samples in their home freezers until they were returned to the laboratory the next day and stored at −80 C. On the day of analysis for cortisol, saliva samples were thawed at room temperature and centrifuged at 2500g for fifteen minutes at −4 C. Saliva samples were assayed for cortisol level in duplicate using a high sensitivity enzyme immunoassay kit (Diagnostic Systems Laboratories; Webster, Texas) with a mean lower sensitivity limit of 0.11 ug/dL, standard curve range from 0.1 to 10.0 ug/dL. The intra and inter-assay coefficients of variation were less than 5% at all levels of the calibrator curve. The concentration of cortisol in saliva was expressed as ug/dL. To minimize the potential effects of exposure to stressful events during the sampling period, participants who were currently students were not sampled the week prior to scheduled class examinations. In addition, participants indicating daily hassles, or exposure to stressful daily events during the sampling period (Van Eck et al., 1996), sleep disturbances (Lasikiewicz et al., 2008), and other unusual events or protocol noncompliance (teeth brushing, eating, etc), during sampling periods were excluded from the final analyses.
Statistical Analyses
The current sample includes data from 55 of 59 original participants in a previous study examining the effects of childhood trauma and acculturation on the CAR in Mexican Americans (Mangold et al., 2010). As an extension of the previous study, 55 participants consented to complete additional study phases (including completion of an assessment of depressive symptom severity and three additional cortisol sampling sessions in the afternoon and evening) designed to test a priori hypotheses to determine the extent to which the CAR compared with 12 hr waking day AUC predicts subclinical symptomatology while controlling for the effects of childhood trauma.
The primary statistical design included two General Linear Models with repeated measures at four morning time points (awakening, 30, 45, and 60minutes thereafter) and three afternoon and evening time points (3pm, 6pm and 9pm). Fixed design effects were Subclinical Depressive Symptomatology, used as a continuous, dimensional variable (SCL-90-R scores ranged from 0 to 3.38) and Time (a fixed classification factor with four levels and again with three levels). The Subclinical Symptomatology × Time interactions were the primary focus of the current study, testing the association between symptoms and initial changes in morning, afternoon and evening salivary cortisol concentrations over time. Summary cortisol statistics are presented separately for days one and two for comparison with previous data and to demonstrate minimal between-day variability in cortisol concentrations. Inferences are based on the results of the analyses using continuous measures, but analyses were also performed by grouping depressive symptom severity scores into classes (high, moderate and low scores) to aid in the interpretation of the graphics presented.
In addition, two cortisol summary measures were calculated using salivary cortisol concentrations sampled across the morning, afternoon and evening to measure: (1) 12hr area under the curve (AUC) calculated across seven time points (Altman, 1991) and; (2) Delta calculated as the change in cortisol concentration in the period between awakening and thirty minutes thereafter. A series of four hierarchical regressions assessed the extent to which the set of demographic and health variables (general health, sex, BMI, age and household income) each of the four childhood trauma variables of interest (emotional abuse, sexual abuse, physical abuse and general traumas), and the set of two cortisol summary variables (Delta and AUC) predicted subclinical depressive symptomatology. Statistical analyses were performed using SPSS (version 11.0), and all statistical tests were two tailed. The accepted level of significance was set at an alpha level of 0.05.
Results
Demographic Characteristics
Demographic characteristics for the participant sample are presented in Table 1. Participants were first (31.0%), second (34.5%), and third generation or higher (34.5%), Mexican Americans, ranging in age from 18 to 38 (M = 21.80, SD = 5.2), and primarily undergraduate college students (26 males and 29 females). Results of chi-square tests (χ2) showed no significant differences in the composition of the sample between males and females. Participants were healthy with mean scores on the General Health scale of the RAND-36 for the current sample (M = 80.05, SD = 16.68) in line with those previously reported for healthy, young adults (M = 77.60, SD = 20.10, Vander Zee et al., 1996). The mean score on the HDI for the sample was lower than normative means reported for college-aged participants (M = 5.16, SD = 4.48; Reynolds & Kobak, 1995; Vander Zee et al., 1996) due to exclusion criteria including a diagnosis of current and lifetime depression and HDI scores higher than 10. Participants endorsed a range of depressive symptoms with HDI scores ranging from 0 to 9.50 (M = 2.68, SD = 2.26) for the sample. Participants reported no use of psychotropic medication.
Table 1.
Sample Demographics for Mexican American Adult, Males and Females
| Variable | Overall Sample | Males | Females |
|---|---|---|---|
| Gender | 55(100.0%) | 26(47.3%) | 29(52.7%) |
| Age | |||
| (Mean and SD) | 21.80(5.2) | 24.20(5.8) | 21.00(4.7) |
| Body Mass Index | |||
| (Mean and SD) | 24.28(4.21) | 24.20(3.8) | 24.35(4.6) |
| Participants’ Education Level | |||
| Freshman | 27(49.1%) | 14(53.8%) | 13(44.8%) |
| Sophomore | 11(20.0%) | 2(7.7%) | 9(31.0%) |
| Junior | 6(10.9%) | 4(15.4%) | 2(6.9%) |
| Senior | 11(20.0%) | 6(23.1%) | 5(17.3%) |
| Participants’ Generational Status | |||
| First Generation* | 17(31.0%) | 9(34.6%) | 8(27.6%) |
| Second Generation | 19(34.5%) | 9(34.6%) | 10(34.5%) |
| Third Generation (or above) | 19(34.5%) | 8(30.8%) | 11(37.9%) |
| Acculturation Level** | |||
| High Mexican Orientation | 16(29.1%) | 8(30.8%) | 8(27.6) |
| Moderate Mexican Orientation | 17(30.9%) | 7(26.9%) | 10(34.5%) |
| Low Mexican Orientation | 22(40.0%) | 11(42.3%) | 11(37.9%) |
| Household Income | |||
| Below 40K | 31(56.3%) | 11(42.3%) | 20(69.0%) |
| 40K–80K | 15(27.3%) | 9(34.6%) | 6(20.7%) |
| 80K and above | 9(16.4%) | 6(23.1%) | 3(10.3%) |
| Parental Occupational Status | |||
| Executive | 19(34.5%) | 10(38.4%) | 9(31.0%) |
| Administrative/Management | 12(21.8%) | 6(23.1%) | 6(20.7%) |
| Clerical/Skilled Manual Labor | 20(36.4%) | 8(30.8%) | 12(41.4%) |
| Semi-skilled or Unskilled Labor | 4(7.3%) | 2(7.7%) | 2(6.9%) |
Subjects who emigrated from Mexico to the United States either as adults or as children are grouped together as ‘First Generation’.
The revised Acculturation Rating Scale for Mexican Americans (ARSMA-II; Cuéllar et al., 1995) was used to assess acculturation by measuring subjects’ orientation towards Anglo culture and Mexican culture. Scores on the ARSMA-II reflect the extent to which participants endorsed Anglo relative to Mexican orientations.
The majority of respondents reported speaking English very often or almost always (91%). However, approximately 67% reported enjoying speaking Spanish very often or almost always and nearly 38% reported enjoying Spanish language movies very often or almost always, suggesting many of the participants were bilingual. There was little intra-individual variability in the timing of cortisol sampling. Approximately ninety percent of the samples were obtained within less than 10 minutes of protocol. Calculations of the change in mean cortisol concentration from awakening to 30 min post-awakening for trial one (12.70 ± 13.8 nmol/l) and trial two (9.38 ± 15.18 nmol/l) comparable to the mean change previously reported for normals, although with greater dispersion (9.3 ± 3.1 nmol/l) (Clow et al., 2004). The correlation coefficients between days one and two for Delta were r = .42 (df = 51, p = .002; Spearman rho = .29, p = .04) and, for AUC were r = .70 (df = 51, p = .001; Spearman rho = .66, p = .001) suggesting minimal variability in cortisol responses between days one and two for the current sample. Results from analyses of day one data are presented, however, analyses using summary variables aggregated from Days 1 and 2 produced similar results.
Mean number of exposures for each subtype of trauma and composite exposures are reported in Table 2. Total trauma exposures were lower than those previously reported in a healthy non-Hispanic sample of trauma victims, without psychiatric diagnoses (Klassens et al., 2009). Males in the current sample reported more exposure to total traumas (p< .01), physical punishment (p< .01), and general traumas (p < .05) compared with females. There were no differences in trauma exposures among first, second and third generation groups or low, moderate and high acculturation groups.
Table 2.
Childhood Trauma Exposures amd Psychiatric Symptomatology for Mexican-American Males and Females (Means and SD).
| Overall Sample (N=55) | Males (N=26) | Females (N=29) | |
|---|---|---|---|
| Childhood Trauma Exposures† | |||
| Total Traumas | 5.33 (4.22) | 7.01 (4.91) | 3.83 (2.80)** |
| Emotional Abuse | 1.15 (1.53) | 1.42 (1.75) | 0.90 (1.29) |
| Physical Punishment | 1.38 (1.46) | 1.96 (1.59) | 0.86 (1.13)* |
| General Traumas | 2.37 (1.76) | 2.97 (1.89) | 1.83 (1.47)* |
| Sexual Events | 0.44 (0.88) | 0.65 (1.02) | 0.24 (0.69) |
| Psychiatric Symptomatology†† | |||
| Anxiety | 0.54 (.52) | 0.61 (.55) | 0.47 (.48) |
| Depression | 0.78 (.77) | 0.89 (.83) | 0.68 (.68) |
| Interpersonal Sensitivity | 0.88 (.81) | 1.04 (.85) | 0.74 (.76) |
| Obsessive-Compulsive | 1.06 (.81) | 1.27 (.89) | 0.88 (.70) |
| Paranoid Ideation | 0.78 (.82) | 0.86 (.81) | 0.71 (.83) |
| Phobic Anxiety | 0.23 (.38) | 0.24 (.29) | 0.22 (.44) |
| Psychoticism | 0.48 (.55) | 0.60 (.56) | 0.37 (.54) |
| Somatization | 0.47 (.49) | 0.56 (.53) | 0.39 (.44) |
| Hostility | 0.46 (.48) | 0.58 (.55) | 0.36 (.38) |
p < .05,
p < .01,
p< .001
Early Trauma Inventory (ETISR-SF; Bremner et al., 2007); Although there were no gender differences in trauma exposure overall, males reported significantly more exposures to General Traumas [F(1,16.9) =5.83, p <.05], Physical Punishment [F(113.12) = 6.50, p< .05], and Total Traumas [F(1, 125.13) =7.32, p < .01] compared with females. Mean ETI-SF scores are lower than those previously reported in healthy non-Hispanic participants without psychiatric diagnoses (Klaassens et al., 2009).
Symptom Checklist-90-R (SCL-90-R; Derrogatis, 1977); There were no significant gender differences in symptom severity. Mean SCL-90-R scores for each symptom dimension are in line with those previously reported for Hispanic college students (Martinez et al., 2005). Published normed SCL90-R scores for Hispanics are not available.
Raw scores on the SCL-90-R for the current sample of Mexican Americans are in line with raw scores reported by Martinez et al. (2005) for a similar Hispanic college sample (Table 2). However, raw scores are much higher than those reported by Derogatis et al. (1977) for normal, non-patient samples of non-Hispanic males and females with depressive symptomatology.
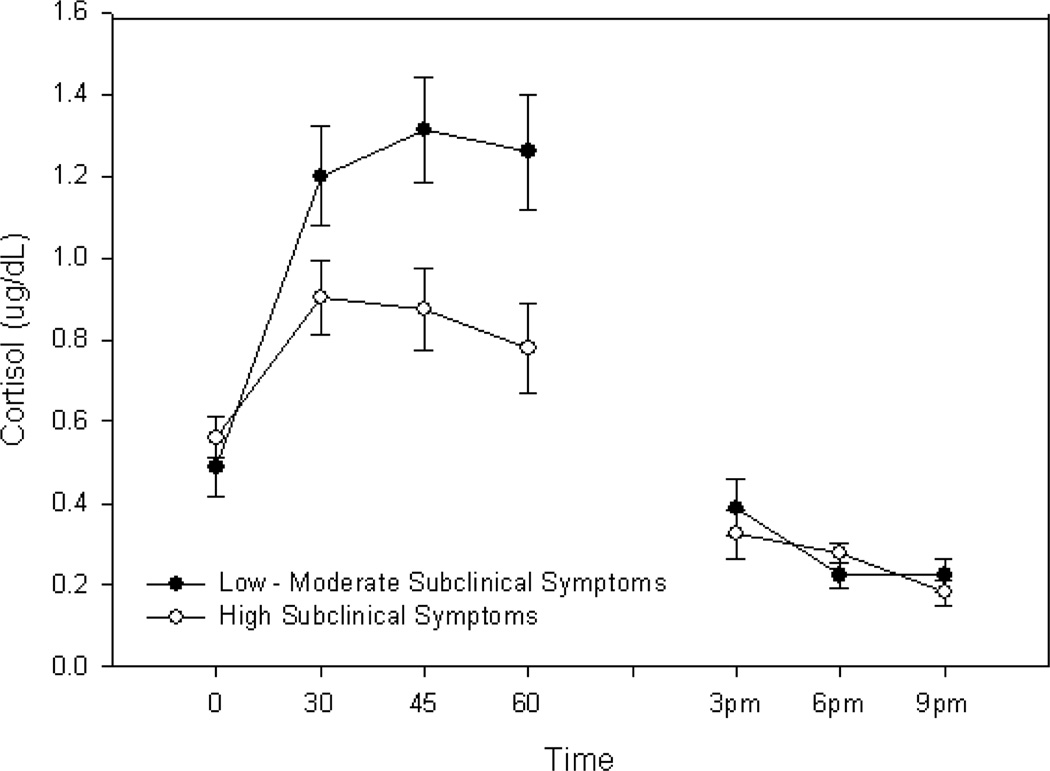
The first model examining the two- factor Symptomatology × Time interaction (morning cortisol concentrations) was significant (p = .009) when the subclinical symptomatology scale was included as a dimensional covariate and after applying the Bonferroni adjustment. To clarify this interaction, the sample was divided into approximate thirds based on symptom severity scores. The two-factor interaction using the three symptom groups in place of the dimensional covariate was again, significant (F(6,96) = 2.23, p<.05). Contrast tests indicated the CAR significantly differed in the group with the highest symptomatology when compared with the low and moderate groups (p = .014) although the low and moderate symptom groups did not differ from one another (p = .16). The low and moderate symptom groups were therefore combined and compared to the higher symptom group. A test of the change in cortisol during the first 30 minutes in the high symptom group showed that it was significantly attenuated compared to the average change in the same time interval in the low and moderate groups combined (F(1,52) = 5.99, p = .02). Figure 1 presents cortisol concentrations for the three subclinical symptom groups at awakening, 30, 45 and 60 minutes thereafter. The second model examining the two-factor Symptomatology × Time interaction (afternoon and evening) was not significant when symptomatology was included as a dimensional covariate and again when symptom scores were grouped into three classes. Figure 1 presents cortisol concentrations for the three subclinical symptom groups at 3pm, 6pm and 9pm.
Figure 1.
Morning, Afternoon and Evening Salivary Cortisol in Mexican Americans with Subclinical Depressive Symptoms
A series of hierarchical regression analyses were conducted to determine the extent to which health variables (general health, body mass index, age, sex, and household income) exposure to each of four subtypes of childhood trauma (emotional abuse, sexual abuse, general traumas, and physical punishment) and two cortisol summary variables (Delta and AUC) predict subclinical depressive symptomatology. Table 3 presents zero order correlations among the variables in the models, and shows attenuation of the CAR is associated with childhood exposure to emotional abuse and general traumas similar to previously reported findings from our laboratory (Mangold et al., 2010). In each analysis, the block of demographic/health variables were entered first, followed by the trauma variable of interest and finally, the block of two cortisol summary variables. Demographic and health variables did not predict symptomatology. However, the first three models including the block of health/demographic variables, trauma variable of interest and block of cortisol variables (Delta and AUC) significantly predicted depressive symptomatology, even after adjustments to control for experiment-wise error due to multiple tests (i.e. 4 regressions): The Holm-Bonferroni and the Benjamini-Hochberg False Discovery Rate (FDR) criteria for significance at p<0.05 (Benjamini and Hochberg, 1995; Holm 1979). The first three models were significant at p< 0.05 by unadjusted F-tests and remain significant at p< 0.05 using either criteria according to the step down Holm-Bonferroni or FDR methods: Emotional Abuse (R2adj. = 0.39, F(8, 46) = 5.23, p< .001); Sexual Abuse (R2adj. = 0.32, F(8, 46) = 4.24, p< .001) and; General Traumas (R2adj. = 0.18, F(8, 46) = 2.49, p<.05). The final model was not significant at unadjusted p< 0.05, although was very close to significance: Physical Punishment (R2adj. = 0.14, F(8, 46) = 2.13, p = .05181). Computation of standardized beta coefficients in the final models showed that increased exposure to some, but not all types of childhood trauma were significant predictors of greater subclinical depressive symptomatology: Emotional Abuse (β = 0.51; p< .001), Sexual Abuse (β = 0.44, p< .001), General Traumas (β = 0.25, p = .08), and Physical Punishment (β = 0.14, p = .32).
Table 3.
Zero-Order Correlation Coefficients for the Total Sample
| (N=55) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Depressive Symptomatology | - | |||||||||||
| 2. General Health | −.19 | - | ||||||||||
| 3. Body Mass Index | .17 | .29* | - | |||||||||
| 4. Gender | −.14 | −.10 | .02 | - | ||||||||
| 5. Age | .13 | −.21 | .29* | −.16 | - | |||||||
| 6. Household Income | .06 | .29* | −.28* | −.25 | −.18 | - | ||||||
| 7. Emotional Abuse | .57** | −.07 | .07 | −.17 | .13 | .18 | - | |||||
| 8. Sexual Abuse | .48** | −.00 | .02 | −.24 | .23 | .15 | .37** | - | ||||
| 9. General Traumas | .38** | .05 | .03 | −.33* | .17 | .15 | .35** | .43** | - | |||
| 10. Physical Abuse | .28* | .09 | −.02 | −.38** | .04 | .22 | .51** | .36** | .39** | - | ||
| 11. CAR (Delta) | −.35** | .14 | −.08 | .30* | −.38** | .15 | −.28* | −.23 | −.34* | −.25 | - | |
| 12. Cortisol (AUC) | −.12 | .19 | −.17 | .20 | −.23 | .13 | −.17 | −.09 | −.12 | −.07 | .68** | - |
p<.05,
p<.01
Of particular interest, attenuation of the initial morning rise in cortisol expressed as Delta, significantly contributed to the prediction of subclinical depressive symptomatology beyond the contribution of each type of trauma: Emotional Abuse (Delta: β = −0.40, p< .05); Sexual Abuse (β = −0.45, p< .01); General Traumas (β = 0.45, p<.05); and Physical Punishment (β = −0.50, p<.01). In two of the final models Delta, but not Physical Punishment and General Traumas, exclusively predicted subclinical depressive symptomatology. Moreover, Delta cortisol was a better predictor of subclinical depressive symptomatology compared to 12 hr AUC, and in each case when Delta was removed from the final analyses, AUC alone did not significantly predict subclinical symptomatology.
Discussion
The current study assessed the extent to which exposure to childhood trauma and injury to the CAR predict subclinical symptomatology in Mexican Americans. This is the first study to examine this relationship in Mexican Americans, and thus the first to demonstrate that attenuation of the initial rise in CAR is associated with greater subclinical depressive symptomatology in this minority group. Notably, this is also the first study to distinguish the effects of alterations in cortisol concentrations from the effects of childhood trauma on subclinical depressive symptomatology in adults, and thus the first to demonstrate that attenuation of the initial rise in CAR (delta) predicts greater subclinical depressive symptomatology beyond the contribution of trauma, even after controlling for sex, health, age, body mass index and household income. An additional novel finding demonstrates that after controlling for trauma, attenuation of the initial rise in the CAR is a better predictor of subclinical symptoms than 12 hr AUC.
Several strengths of the study increase our confidence in our findings. Our findings are not likely attributable to the known effects of antidepressant use or substance abuse on the CAR given that subjects were assessed and excluded for use of psychotropic medications, and current alcohol, nicotine and other drug use disorders which helped to minimize the potential influence of these confounds on the HPA axis (Aihara et al., 2007; Wand & Dobs, 1991). Female participants were screened and excluded for use of oral contraceptives prior to enrollment in the current study and sampled during the early follicular phase to minimize the effects of estrogen and progesterone on the HPA axis. Subjects were allowed to wake naturally and measurements were performed with strict reference to time of awakening (Federenko et al., 2004; Williams et al., 2005; Wust et al., 2000) to minimize the influence of time on measurement of the CAR (Edwards et al., 2001; Federenko et al, 2004). Further, we minimized sampling error through the use of electronic monitoring devices (Broderick et al., 2004; Kudielka et al., 2003; Mangold et al., 2010; review in Hansen et al., 2008).
Our finding that attenuation of the initial rise in CAR is associated with greater subclinical depressive symptomatology in Mexican American adults is consistent with previous findings in non-Hispanics (Ahlberg et al., 2002; Dedovic et al., 2010; Tops et al., 2008). Findings are also consistent with the majority of cross-sectional studies showing attenuation of the CAR in non-Hispanics with a formal diagnosis of major depression (Huber et al., 2006; Stetler & Miller, 2005) with a few exceptions (Bhagwagar et al., 2003; Vreeburg et al., 2009). However, our findings are in contrast with studies showing an enhanced CAR in asymptomatic, high risk adults with a family history of major depression, and in adolescents who develop future major depressive episodes (Adam et al., 2010). The discrepancies in findings may be due to widespread variations in sampling methodologies across studies, and temporal differences in the development of the disorder. Indeed, it is proposed that subclinical depressive symptomatology is a less severe condition that may be a precursor to the onset of a formal diagnosis of major depression (Cuijpers & Smit, 2004; Shankman et al., 2009). In line with this contention, studies have shown a negative association between the CAR, length of time following the onset of trauma or chronic stress and the severity of depressive symptomatology (Chida & Steptoe, 2009; Miller et al., 2007).
Afternoon and evening cortisol concentrations were not associated with subclinical depressive symptomatology in the current sample of Mexican Americans. Our findings are similar to those of a few previous studies in clinically depressed non-Hispanic samples demonstrating minimal differences in afternoon and evening cortisol levels (Burke et al., 2005) and in a symptomatic subjects with a family history of mental illness (Hsiao et al., 2010). However, our findings are in contrast to the majority of cross-sectional studies demonstrating increased evening cortisol levels in clinically depressed non-Hispanic adults (Dahl et al., 1991). Previous studies examining the relationship between afternoon and evening cortisol concentrations and subclinical depressive symptomatology are scarce hence; our current findings are difficult to interpret. It is possible that attenuation of the CAR coupled with minimal difference in afternoon and evening cortisol concentrations is a unique neuroendocrine profile in subjects with a history of childhood trauma who develop subclinical depressive symptomatology.
Final results from hierarchical models including health, childhood trauma and summary cortisol variables accounted for up to thirty-nine percent of the variance in subclinical depressive symptomatology. The heterogeneity of depression suggests numerous possible mechanisms influence the onset of the disorder and specific alterations in HPA axis dynamics may be linked to the development of particular symptom profiles in certain high risk groups (Adam et al., 2010; Dedovic et al., 2010; Ehlert et al., 2001; Heim et al., 2000). It is plausible that specific patterns of HPA axis injury are linked to the development of a specific profile of subclinical depressive symptomatology in Mexican Americans with a history of childhood trauma.
When compared with overall waking day cortisol concentrations (12 hr AUC), attenuation of the CAR is a better predictor of greater subclinical depressive symptomatology. This is consistent with recent investigations showing that the CAR is distinct from diurnal variations in HPA axis activity, possibly reflecting a unique role related to the process of awakening (Wilhelm et al., 2007), and with prospective evidence suggesting the CAR is a better predictor of future depressive episodes when compared with other predictors (Adam et al., 2010).
There are some limitations to the current study. The study was not prospective, but retrospective in design. Therefore, we are unable to determine causal pathways between CAR and subclinical depressive symptomatology. While it is conceivable that an attenuated CAR is present prior to the development of a formal diagnosis and is a biological risk factor that may play a role in the pathophysiology of depression (Dedovic et al., 2010; Van Praag, 2004), it is also possible that injury to the CAR is merely an epiphenomenon of subclinical depressive symptomatology. Additional prospective studies utilizing larger sample sizes are necessary to address this debate.
Determination of the precise developmental mechanisms underlying the relationship between attenuation of the CAR and greater subclinical depressive symptomatology in individuals with a history of childhood trauma is beyond the scope of the current study. However, given that the HPA axis is particularly sensitive to adverse psychosocial experiences in early life during critical periods of neuronal plasticity (Halligan et al., 2004) it is possible that the CAR is a potential biological risk factor that confers risk for subclinical depressive symptomatology in individuals with a history of childhood trauma. According to the “attenuation hypothesis” exposure to childhood trauma may alter the set point of the hypothalamic CRF system leading to enhanced stress reactivity followed by down regulation of central glucocorticoid receptors over time. The hypothalamic CRF system may undergo desensitization leading to attenuated cortisol responses to stress and an attenuated CAR (Gunnar & Vazquez, 2001; Heim et al., 2008; Susman, 2006; Trickett et al., 2010). Alternatively, it is also plausible that early childhood stress or persistent chronic stress produces a blunted CAR with no antecedent HPA axis hyperactivity. This is unlikely given that adolescents with elevated morning cortisol are at greater risk for future depressive episodes (Adam et al., 2010; Halligan et al., 2007) and studies demonstrating a transition from initial childhood hypercortisolism to adult hypocortisolism following exposure to childhood trauma (Trickett et al., 2010).
Taken together, these findings show that greater subclinical depressive symptomatology is associated with attenuation of the CAR, but not 12 hr waking day AUC in Mexican American adults with a history of childhood trauma. It will be important to determine if injury to the HPA axis confers greater risk for additional hypocortisolemic syndromes in this minority group.
Acknowledgements
We thank Dr. Ray Garza, Director, South Texas Initiative for Mental Health Research, for his support and assistance during the project. We also thank Gary Wand, M.D., who kindly assisted with the editing and proof reading of the manuscript.
Role of Funding Source
Funding for this study was provided by NIMH (R24-MH070636-01A1; The South Texas Initiative for Mental Health Research). The NIMH had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlberg AC, Ljung T, Rosmond R, McEwen B, Holm G, Akesson HO, Bjorntorp P. Depression and anxiety symptoms in relation to anthropometry and metabolism in men. Psychiatry Research. 2002;112:101–110. doi: 10.1016/s0165-1781(02)00192-0. [DOI] [PubMed] [Google Scholar]
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Research. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Aranda MP, Lee PJ, Wilson S. Correlates of depression in older Latinos. Home Health Care Services Quarterly. 2001;20:1–20. doi: 10.1300/J027v20n01_01. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Lasa L, Vazquez-Barquero JL, Oviedo A, Diez-Manrique JF. Measuring health status in psychiatric community surveys: internal and external validity of the Spanish version of the SF-36. Acta Psychiatrica Scandinavica. 1999;99:26–32. doi: 10.1111/j.1600-0447.1999.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. Journal of Clinical Endocrinology and Metabolism. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bao AM, Ji YF, Van Someren EJW, Hofman MA, Liu RY, Zhou JN. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Hormones and Behavior. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bao AM, Liu RY, van Someren EJW, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. European Journal of Endocrinology. 2003;148:227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC. Heritability of cortisol levels: Review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increase in concentration of waking salivary cortisol in recovered patients with depression. American Journal of Psychiatry. 2003;160:1890–1891. doi: 10.1176/appi.ajp.160.10.1890. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. Journal of Nervous and Mental Disease. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta423 analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cuellar I, Arnold B, Maldonado R. Acculturation rating-scale for Mexican-Americans II - A revision of the original ARSMA scale. Hispanic Journal of Behavioral Sciences. 1995;17:275–304. [Google Scholar]
- Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatrica Scandinavica. 2004;109:325–331. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Bhatnagar S, Viau V. Hypothalamo–pituitary–adrenal axis. In: Fink G, editor. Encyclopedia of Stress. San Diego: Academic Press; 2000. [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biological Psychiatry. 2010;68:847–853. doi: 10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R Administration, Scoring and Procedures Manual. Minneapolis: National Computer Systems; 1977. [Google Scholar]
- Derogatis LR, Cleary PA. Confirmation of dimensional structure of SCL-90 - Study in construct-validation. Journal of Clinical Psychology. 1977a;33:981–989. [Google Scholar]
- Derogatis LR, Cleary PA. Factorial invariance across gender for primary symptom dimensions of SCL-90. British Journal of Social and Clinical Psychology. 1977b;16:347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Rickels K, Rock AF. SCL-90 and MMPI - Step in validation of a new self-report scale. British Journal of Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- Ebrecht M, Buske-Kirschbaum A, Hellhammer D, Kern S, Rohleder N, Walker B, Kirschbaum C. Tissue specificity of glucocorticoid sensitivity in healthy adults. Journal of Clinical Endocrinology and Metabolism. 2000;85:3733–3739. doi: 10.1210/jcem.85.10.6891. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sciences. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, Sephton SE, Thompson LW. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. American Journal of Geriatric Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychological Medicine. 2004;34:509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, Unger ER, Reeves WC. Early adverse experience and risk for chronic fatigue syndrome - Results from a population-based study. Archives of General Psychiatry. 2006;63:1258–1266. doi: 10.1001/archpsyc.63.11.1258. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6. 1979;2:65–70. [Google Scholar]
- Hsiao F-H, Yang T-T, Hoe RTH, Jow G-M, Ng S-M, Chan CLW, Laif Y-M, Chen Y-T, Wang K-C. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology. 2010;35:503–515. doi: 10.1016/j.psyneuen.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Kemp K, Mazure CM, Sinha R. A gender specific psychometric analysis of the early trauma inventory short form in cocaine dependent adults. Addictive Behaviors. 2005;30:847–852. doi: 10.1016/j.addbeh.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N, Nicolson NA, Derom C, Delespaul P, van Os J, Myin-Germeys I. Electronic monitoring of salivary cortisol sampling compliance in daily life. Life Sciences. 2005;76:2431–2443. doi: 10.1016/j.lfs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33:1257–1268. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. American Journal of Psychiatry. 2001;158:582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, Zitman FG. Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:889–894. doi: 10.1016/j.pnpbp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Hellstrom B. Workload and morning salivary cortisol in women. Work & Stress. 2002;16:356–363. [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Hormones and Behavior. 2010 doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin H, Escobar JI, Vega WA. Mental illness in Hispanics: a review of the literature. Focus. 2006;4:23–37. [Google Scholar]
- Martinez-Taboas A, Bernal G. Dissociation, psychopathology, and abusive experiences in a nonclinical Latino university student group. Cultur Divers Ethnic Minor Psychol. 2000;6:32–41. doi: 10.1037/1099-9809.6.1.32. [DOI] [PubMed] [Google Scholar]
- Martinez S, Stillerman L, Waldo M. Reliability and validity of the SCL-90-R with Hispanic college students. Hispanic Journal of Behavioral Sciences. 2005;27:254–264. [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30:568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mennen FE. Psychological symptoms in a sample of Latino abused children. Journal of Multicultural Social Work. 2000;8:193–213. [Google Scholar]
- Meulenberg PMM, Hofman JA. The effect of oral-contraceptive use and pregnancy on the daily rhythm of cortisol and cortisone. Clinica Chimica Acta. 1990;190:211–221. doi: 10.1016/0009-8981(90)90175-r. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- National Child Abuse and Neglect Data System (NCANDS) Ithaca, NY: Cornell University Family Life Development Center; 2002. Detailed case data component, 1995–1999. [Google Scholar]
- National Child Traumatic Stress Network (NCTSN) Washington, DC: 2005. Culture and trauma brief: Promoting culturally competent trauma-informed practices (Report) [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, Cooper TB, Malone KM, Mann JJ. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Research. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27:477–489. [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychological Assessment. 1995;7:472–483. [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurneyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64(18):1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, Altman SE. Subthreshold conditions as precursors for full syndrome disorders: a 15-year longitudinal study of multiple diagnostic classes. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50:1485–1494. doi: 10.1111/j.1469-7610.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey: Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community - Interactions between social adversity, cortisol and serotonin neurotransmission. British Journal of Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. Journal of Clinical Endocrinology and Metabolism. 1988;66:733–739. doi: 10.1210/jcem-66-4-733. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology. 2009;34:1184–1188. doi: 10.1016/j.psyneuen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Tops M, Riese H, Oldehinkel AJ, Rijsdijk FV, Ormel J. Rejection sensitivity relates to hypocortisolism and depressed mood state in young women. Psychoneuroendocrinology. 2008;33:551–559. doi: 10.1016/j.psyneuen.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic. Research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Van Praag HM. Can stress cause depression? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Vander Zee K, Sanderman R, Heyink J, de Haes H. Psychometric qualities of the RAND 36-item health survey 1.0: A multidimensional measure of general health status. International Journal of Behavioral Medicine. 1996;3:104–122. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of General Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the Hypothalamic-Pituitary-Adrenal axis in actively drinking alcoholics. Journal of Clinical Endocrinology and Metabolism. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Williams E, Magid K, Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005;30:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise and Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Zayas LH, Cunningham M, McKee MD, Jankowski KRB. Depression and negative life events among pregnant African-American and Hispanic women. Womens Health Issues. 2002;12:16–22. doi: 10.1016/s1049-3867(01)00138-4. [DOI] [PubMed] [Google Scholar]