Abstract
Background
Parkinson’s disease [PD] is a degenerative process affecting the striato nigral system [SN]. Its etiology, although obscure may involve oxidative damage. Selenium, an anti-oxidant, was shown to protect the SN in animal models. In the current study we investigate the association between plasma selenium concentrations and the presence of “soft” neurological signs related to the SN.
Methods
Plasma selenium concentration was assessed in participants age ≥65y in the InCHIANTI study, a population-based cohort study in Tuscany, Italy. PD was defined based on standard criteria. “Soft” neurological signs were ascertained by physical examination.
Results
A total of 1012 participants were included. No association was found between the presence of PD and plasma selenium. There was, however, a strong association between plasma selenium and timed performance-based assessments.
Conclusions
Lower levels of selenium were significantly associated with decreased performance in neurological tests of coordination among older adults. Prospective studies are needed to further investigate the effects of selenium on SN dysfunction.
Keywords: selenium, trace elements, Parkinson’s disease, antioxidants, elderly
INTRODUCTION
Dopamine [DA] secreting neurons have been found to be sensitive to oxidative stress (1–4) as well as other neurodegenerative disorders [NDG] (5). DA itself readily oxidizes to react with proteins, lipids and nucleic acids. It forms neurotoxic derivatives, and interacts with intracellular iron or products of monoamine oxidase to form toxic oxygen radicals (4). DA neurons have evolved multiple mechanisms to protect themselves from cytosolic DA stress (6). Nonetheless, these defense mechanisms can be overwhelmed by cellular events, subjecting the cell to substantial oxidative damage, which may have a part in selective DA neuronal loss (7).
Selenium is the third element in the chalcogen group - or oxygen family. In the body, selenium is incorporated into specific proteins called selenoproteins (8, 9) and can participate in redox type reactions. As such selenium protects cellular elements from oxidative damage. The best known mechanism utilizes glutathione peroxidase [GPX], in which selenium occupies the enzymatic center. Nevertheless, other mechanisms do exist (10). Selenium is preferentially taken-up by brain tissue and seems to have additional roles in the brain beside the glutathione mechanism (8, 11). Animal experiments have documented the ability of selenium to prevent or even reverse brain changes, including Striato-nigral [SN] changes induced by oxidation (11, 12). Selenium reduces both the pathologic changes in the SN and the decline in DA that is normally induced by methamphetamine toxicity (11). Over the recent years, attention has also focused on cerebellar oxidative damage; considerable part of this damage is mediated by glutamate. A recent in-vitro study reveals that Se compounds were able to counteract oxidative damage mediated by glutamate (13–16) Still, the evidence relating to cerebellar damage is merely in vitro evidence and lags behind considerably, compared to the SN system.
Given the sensitivity to oxidative stress of DA secreting neurons and the neuroprotective properties of selenium (16–19), we tested the hypothesis that higher plasma selenium levels may be related to better SN system function in older persons.
METHODS
Study sample
The design and data collection methods of the InCHIANTI study have been described previously (20). The study conformed to the ethical principles contained in the Declaration of Helsinki, and the InCHIANTI Study protocol was approved by the Ethical Committee of the Italian National Institute of Research and Care of Aging. It is a longitudinal study among residents of two towns in Tuscany, Italy. The study commenced in 1998, when 1270 people aged 65 years or older were randomly selected from the two towns. Excluding 17 men and 22 women who had died or moved away from the area, a total of 1155 persons (91.1%) agreed to participate in the study and signed an informed consent. In cases of physical or cognitive impairment interfering with the ability to consent, proxy consent was obtained from a close relative. The next component was a structured home interview. Among those interviewed, 1026 (89%) were seen in the clinic and had a complete medical and functional evaluation performed by a geriatrician and an experienced physical therapist. A total of 1012 participants had both a complete physical exam and plasma selenium concentration measured.
Major neurological conditions were ascertained based on standard criteria. Parkinson’s disease or Parkinsonism was defined as either a self-reported or documented previous physician diagnosis or as newly diagnosed based on the neurologic examination. De-novo diagnosis required typical clinical features, including slowness of voluntary movement plus one of the following signs: resting tremor, axial instability, rigidity, stooped posture, or festinating gait (21).
Plasma selenium, total caloric intake, and cognitive function
Selenium was measured in the plasma. Blood was drawn in the morning after an overnight fast. Aliquots of plasma were shipped on dry ice to the laboratory for the measurement of plasma selenium. Selenium was measured using graphite furnace atomic absorption spectrometry with an Analyst 600 with Zeeman background correction (Perkin Elmer, Norwalk, CT). Samples were diluted 1:4 with Triton-X(Sigma Chemical, St Louis, MO) and nitric acid solution (Fisher Scientific, Pittsburgh, PA), and the matrix modifier was a palladium and magnesium nitrate solution (both from Perkin Elmer). The instrument was calibrated daily by using known plasma selenium standards (UTAK Laboratories Inc, Valencia, CA). Within-run and between-run CVs were 3.1% and 7.1%, respectively (11, 12). In addition, total caloric intake was calculated by using the European Prospective Investigation into Cancer (EPIC) food-frequency questionnaire (FFQ), which has been validated in the Italian population (22). The Mini-Mental State Examination (MMSE) was used to measure cognitive function (23)
Neurological examination
The neurological examination followed a pre-specified protocol designed to minimize the subjective component of the assessment (24). Test-retest reliability of the examination was evaluated by repeated examinations, separated by 1 week, in 16 geriatric outpatients. The intraclass correlation coefficients for test-retest reliability for each item were all above 0.8.
Muscle tone was examined by passive rotation of the participant’s neck and flexion/extension motion of the elbows and knees. Rigidity was defined as steady resistance throughout the entire range of movement as perceived by the examiner. Coordination was evaluated measuring the rate of rapid alternating movements. The pronation/supination test [pro/sup], was done in the sitting position with palms on the thighs. The participant was asked to rapidly lift the hands, turn them upside down, and return to the initial position. Pro/sup was repeated sequentially for 20 seconds as quickly as possible, and the number of complete cycles was recorded. In the finger-tapping test, the participant was asked to tap on the table with the middle finger as fast and as regularly as possible for 20 seconds. In the heel-to-shin coordination test, the participant, while lying supine, was instructed to place the right heel on the left shin just below the knee and then slide it down to the foot, repeating this movement sequentially for 10 times. The time needed to complete 10 cycles was recorded. Posture and gait were assessed during the examination by a trained geriatrician who diagnosed and charted any of the following abnormalities: neck bent forward, back curved forward, cautious attitude while walking, taking short steps, and walking with a widened base of support. Each abnormality was assigned a score of 1 and the total number of posture and gait abnormalities was calculated for each participant with scores ranging 0 to 5. Several activities of daily living were also observed by the above mentioned team. Any difficulty or limitation noted was recorded by the team on a graded scale. These tasks were grouped as activity of daily living (ADL) items. For our analysis we used rising from a chair, dressing and undressing for the examination, and getting in and out of bed. These ADL’s are related to speed of motion and to maintaining the center of gravity, both functions that are related to the EPS. If a participant was unable to independently carry out any one of these 3 ADL tasks (without the assistance of another person), then that participant was classified as having an ADL limitation.
Statistical analysis
Selenium concentration was categorized into quartiles because preliminary analyses suggested non-linear associations with the outcomes. The study sample characteristics were described with means (±SD) for continuous variables (including the neuro-motor performance outcomes that were recorded as integers, but approximate continuous variables) and proportions for categorical ones across quartiles of plasma selenium (Table 1). To determine whether individual participant characteristics in Table 1 were associated with levels of selenium, the F statistic from analysis of variance and the chi square statistic were used for continuous and categorical variables, respectively. Tests of trend were also performed by entering the quartiles of selenium as an ordinal variable in linear and logistic regression models. In addition, Pearson correlation coefficients between selenium concentration and the neuro-motor performance outcomes that approximate continuous variables are reported in the text of the Results section. We used multivariable analysis to assess the strength of association between selenium level and the outcomes in Table 2, adjusting for the potential confounding effects of age, sex, study site, total energy intake, cognitive function (measured by the MMSE) and neuroleptic medications. Linear regression was used for the continuous outcome variables (e.g., finger tapping tests) and logistic regression was used for the dichotomous outcomes (e.g., presence of rigidity). Participants with Parkinson’s disease were excluded from the analysis of soft neurological signs. In Figure 2, linear models were used to regress selected neuro-motor performance outcomes on selenium concentration as a continuous variable adjusting for the covariates used in Table 2. To graphically characterize these associations, Kernel-weighted local polynomial regression smoothing was used to generate the graphs in Figures 1 and 2. All analyses were performed using Stata version 10.0 (College Station, TX).
Table 1.
Baseline characteristics by quartiles of plasma selenium (N=1,012)*
| Baseline Characteristics | Plasma Selenium by Quartiles | Overall P value | P value for trend | |||
|---|---|---|---|---|---|---|
| 4th quartile (>82.3 μg/L) | 3rd quartile (74.3–82.3 μg/L) | 2nd quartile (66.8–74.2 μg/L) | 1st quartile (<66.7 μgl/L) | |||
| Age in years, M (SD) | 72.8 (5.9) | 73.6 (6.4) | 75.7 (7.5) | 78.1 (8.4) | <0.001 | <0.001 |
| Women, % | 57.3 | 57.3 | 56.1 | 53.4 | 0.784 | 0.350 |
| Diagnosed Parkinson’s disease, % | 0.8 | 2.0 | 2.4 | 3.6 | 0.200 | 0.039 |
| Mini-Mental State Examination Score, M (SD) | 25.4 (3.7) | 25.3 (3.9) | 24.4 (4.4) | 23.1 (6.1) | <0.001 | <0.001 |
| Total energy intake in kcal/day, M (SD) | 1998 (563) | 1967 (535) | 1909 (567) | 1792 (570) | <0.001 | <0.001 |
| Any medication causing rigidity, % | 5.9 | 9.9 | 7.5 | 10.3 | 0.246 | 0.172 |
| Rigidity present at any site, % | 33.2 | 31.2 | 30.4 | 37.9 | 0.274 | 0.310 |
| Rest-tremor present at any site, % | 2.8 | 3.2 | 4.7 | 4.7 | 0.529 | 0.168 |
| Cycles of pronation / supination per 20 seconds, M (SD) | 25.8 (7.1) | 24.0 (6.3) | 23.7 (6.2) | 22.1 (6.5) | <0.001 | <0.001 |
| Fingertaps per 20 seconds, M (SD) | 39.8 (10.8) | 37.5 (10.6) | 36.5 (11.5) | 34.6 (11.8) | <0.001 | <0.001 |
| Time to complete 10 heel-tibia cycles in seconds, M (SD) | 12.1 (3.7) | 13.0 (4.1) | 13.1 (4.5) | 14.1 (4.4) | <0.001 | <0.001 |
| Total number of postural abnormalities, M (SD) | 2.3 (1.5) | 2.5 (1.5) | 2.8 (1.5) | 2.8 (1.7) | <0.001 | <0.001 |
| Any limitation in activities of daily living, % | 7.5 | 9.5 | 16.6 | 29.6 | <0.001 | <0.001 |
Note: For the overall P value, the F statistic from analysis of variance and the chi square statistic were used for continuous and categorical variables, respectively. The tests for trend were performed by entering the quartiles of selenium as an ordinal variable in unadjusted linear and logistic regression models.
Table 2.
Association of selenium with Parkinson’s disease and soft neurological signs adjusted for age, sex, total energy intake, MMSE score, and neuroleptic medication
| Plasma Selenium by Quartiles | |||||
|---|---|---|---|---|---|
| Outcome | 4th quartile (>82.3 μg/L) | 3rd quartile (74.3–82.3 μg/L) | 2nd quartile (66.8–74.2 μg/L) | 1st quartile (<66.7 μgl/L) | P value Test for trend* |
| Parkinson’s disease † | 1.00 | 2.26 (0.40, 12.57) | 2.69 (0.47, 15.41) | 3.96 (0.73, 21.43) | 0.103 |
| Presence of rigidity† | 1.00 | 1.11 (0.70, 1.77) | 0.71 (0.44, 1.14) | 1.34 (0.80, 2.24) | 0.745 |
| Presence of rest-tremor† | 1.00 | 1.10 (0.33, 3.74) | 1.32 (0.41, 4.27) | 0.93 (0.26, 3.37) | 0.981 |
| Number of postural abnormalities‡ | Reference | 0.07 (−0.16, 0.30) | 0.18 (−0.05, 0.41) | −0.12 (−0.36, 0.12) | 0.584 |
| Cycles of pronation / supination per 20 secs.‡ | Reference | −1.14 (−2.17, −0.12) | −0.90 (−1.93, 0.14) | −1.47 (−2.55, −0.39) | 0.017 |
| Number of Fingertaps per 20 seconds‡ | Reference | −1.87 (−3.62, −0.13) | −1.95 (−3.74, −0.18) | −2.45 (−4.29, −0.60) | 0.012 |
| Time to complete 10 heel-tibia cycles‡ | Reference | 0.54 (−0.15, 1.24) | 0.43 (−0.28, 1.13) | 1.02 (0.27, 1.76) | 0.016 |
The tests for trend were performed by entering the quartiles of selenium as an ordinal variable in the adjusted linear and logistic regression models
Odds ratios and (95% CI) from logistic regression models are reported for dichotomous outcomes with the highest quartile as the reference group
b coefficients and (95% CI) from linear regression models are reported for continuous outcomes with the highest quartile as the reference group
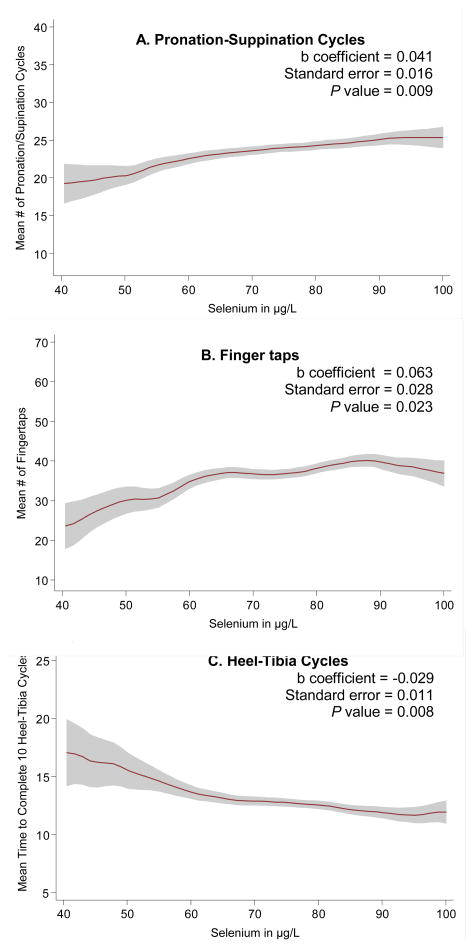
Figure 2.
Figure 2a–c. Selected neuro-motor performance outcomes by plasma selenium concentration*
*Note: Kernel-weighted local polynomial regression smoothing was used to generate the graphs (red line = mean and shaded areas = 95% confidence intervals. B coefficients were obtained from linear models regressing the neuro-motor performance outcome on selenium as a continuous variable adjusting for age, sex, total energy intake, MMSE score, and neuroleptic medication.
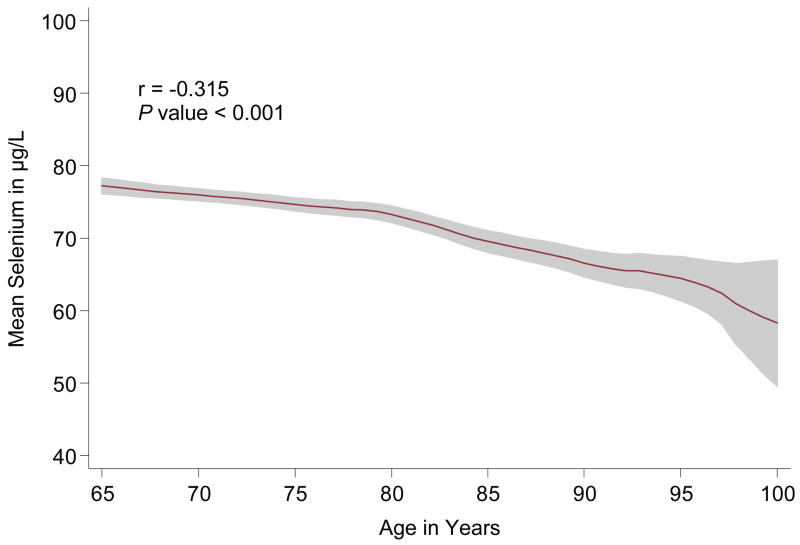
Figure 1. Plasma selenium concentration by age*.
*Note: Kernel-weighted local polynomial regression smoothing was used to generate this graph (red line = mean and shaded areas = 95% confidence intervals.
RESULTS
Among the 1,012 participants included in the study, the average age was 75 years, 56% were women, and the average concentration of plasma selenium was 74.5 μg/L (SD = 12.5). Figure 1 shows that selenium concentration decreased with advancing age (r = −0.32; p<0.001). Table 1 presents the baseline characteristics of study participants by quartiles of plasma selenium. Lower levels of selenium were significantly associated with decreased total energy intake, decreased MMSE score, and more limitation in ADLs. Additionally, performance-based assessments of coordination were significantly worse in those with lower levels of selenium, and there was a significant trend of increased Parkinson’s disease prevalence in the lower selenium quartiles. The correlation coefficients between selenium concentration and the number of postural problems, cycles of pronation/suppination in 20 seconds, finger taps in 20 seconds, and time to complete 10 heel-tibia cycles were −0.13, 0.21, 0.18, and −0.18, respectively (p < 0.001 for each correlation), The proportion of women and of participants with signs of rest-tremor or rigidity at any site did not significantly vary across selenium levels.
The multivariable regression analyses showed that selenium concentration was not associated with Parkinson’s disease, rigidity, rest-tremor, or the number of postural abnormalities (Table 2). However, selenium was significantly associated with the number of pronation-suppination cycles per 20 seconds, the number of fingertaps per 20 seconds, and the time needed to complete 10 heel-tibia cycles. Participants in the lowest quartile of selenium concentration had significantly worse neuro-motor performance on these objective tests compared with those in the highest quartile of selenium. These results are consistent with Figures 2a–c, which show the effect of selenium modeled as continuous variable on selected neuro-motor outcomes.
DISCUSSION
Our results show that low plasma selenium concentrations are associated with subtle neurological impairments reflected in soft neurological signs. Such impairments were seen in coordination tests of both upper and lower extremities. Our observation is consistent with findings of other research showing higher prevalence of disability in the lower plasma selenium group (16, 25). A somewhat similar finding was also found in participants with low vitamin E by another researcher (26). Given that the InChianti study was conducted in community-dwelling elderly we chose to focus on minor neurological findings, bearing potential relation to the SN system.
The average selenium concentration in our study sample was relatively low, 74.5μg/L (0.94 μmol/L). These values are consistent with other studies conducted in Italy. For example, in the Veneto study (27) the mean plasma selenium concentrations were 65.2 μg/L (0.82 μmol/L) in adults and 89.1 μg/L (1.12 μmol/L ) and 68.4 μg/L (0.86 μmol/L ) in adults aged 65–89 and ≥ 90 respectively from Bologna (28). In other countries, and particularly in the US, selenium concentrations are higher, depending on the area where the assessment was conducted (29). In the third National Health and Nutrition Examination Survey, Phase 2 (1991–1994) (NHANES III) the mean serum selenium among non-anemic and anemic adults was 127.3 μg/L (1.60 μmol/L) and 120.1 μg/L (1.51 μmol/L) respectively (30). These differences may reflect different assay methods as well as differences in bioavailability of selenium from foods that may stem from the amount of selenium in the soil (30).
From a biological point of view, the findings in this study may reflect the putative protective effect of selenium against oxidative damage in neuronal cells. A wealth of scientific evidence relates selenium to the anti-oxidative mechanisms. Much of the confusion related to the popular term “anti-oxidants” stems from the generality of this term. In fact, anti-oxidants should be grouped according to several categories by their functions and chemical structures, which are highly divergent. Selenium, as previously mentioned, is an element, with close resemblance to sulfur, which serves as a component of the active site of several enzymes. The common feature of these enzymes is their ability to “disarm” super-oxide molecules (8, 11). From a theoretical standpoint, even though aging is not fully explained by any single mechanism, it is widely believed that oxidative damage plays a role in the aging process.
The SN system seems an important indicator of oxidative damage in the brain. First, it contains a population of dopaminergic neurons, which are unique, highly specialized cells, with a specific neurological function. Thus, an injury to dopaminergic neurons in the substantia-nigra is far more likely to be clinically relevant than a similar size injury elsewhere in the brain. Secondly, dopaminergic neurons are highly susceptible to oxidative damage given the nature of the biochemical reactions in these cells (25, 31). Specifically, the dopamine molecule itself has the potential to induce oxidative damage, unless rapidly sequestered in the cell (5, 25, 31). On the other hand, other parts of the brain are also susceptible to oxidative damage. The cerebellum, which is highly important in movement modulation, is also a target for such hits. Ataxia-telangiectasia (A-T), a hereditary illness is a suitable example. A-T is an autosomal recessive disorder caused by mutations in the ATM gene. The hallmark of A-T is fulminant degeneration of cerebellar Purkinje cells accompanied by a progressive ataxia with features of both cerebellar and basal ganglia dysfunction. Recently, it has been suggested that abnormalities in redox status contribute to the A-T phenotype. In a recent study (13), microscopic examination revealed elevated superoxide levels in cerebellar Purkinje cells and nigral dopaminergic neurons but not cortical neurons. These findings map increased superoxide levels onto the vulnerable neuronal populations selectively affected in A-T.
Our findings are also consistent with findings from animal studies using the methamphetamine nigrostriatal toxicity model. A study by Kim (11) showed prevention and even reversal of functional and anatomic nigrostriatal destructive changes using dietary enrichment with Se. This study is the most extensive one done in animals and its results are very convincing. Nonetheless, it is worth remembering that the methamphetamine toxicity model may not be fully analogous to degenerative Parkinson’s disease (3, 32).
The cross-sectional design of the current study limits causal inference that can be drawn from these findings. Indeed, reverse causation is possible as SN dysfunction might cause malnutrition leading to lower Se levels. This would be particularly of great concern in various patient populations (e.g., those diagnosed with Parkinsonism); however, our study sampled community-dwelling older adults and we excluded participants with Parkinson’s diagnosis from the analyses of neuro-motor performance, reducing concern for reverse causality. Nonetheless, this study is observational and constitutes the first level of scientific investigation into the role of selenium in protecting the EPS. Thus the findings should be taken cautiously. The next phase should be a longitudinal cohort study, which would add a dimension of temporal sequence to this association. Finally, a neurological clinical trial could evaluate the potential benefit of increasing selenium concentrations.
Acknowledgments
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002);supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland
Footnotes
Disclosure Statement
- No actual or potential conflict of interest was reported by any of the authors. No competing financial interests exist by any of the authors.
- The study conformed to the ethical principles contained in the Declaration of Helsinki, and the InCHIANTI Study protocol was approved by the Ethical Committee of the Italian National Institute of Research and Care of Aging.
Financial Disclosures
None for any of the authors. In addition:
- No conflict of interest was reported by any of the authors
- No contracts relating to this research through which it or any other organization may stand to gain financially now or in the future were signed by any of the authors.
- No other agreements of any of the authors or their institutions that could be seen as involving a financial interest in this work was signed
Author Roles:
Shahar A and Patel KV-main writers and the developers of the idea for the current manuscript.
Semba RD – Writing and advisor in the area of selenium
Bandinelli S and Ferrucci L- PI of the InChianti study, reviewers of the current manuscript and helping in refining the idea of the paper
Shahar DR-Paper drafting, statistical analyses and nutrition expert
Guralnik JM-Writing and advising in all the steps of developing the idea and analysis strategy
References
- 1.Visser M, Marinus J, Stiggelbout A, van Hilten J. Responsiveness of impairments and disabilities in parkinson’s disease. Parkinsonism and Related Disorders. 2006;12(5):314–8. doi: 10.1016/j.parkreldis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in parkinson’s disease. Trends Neurosci. 2007;30(5):244–50. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. Description of parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 4.Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotoxicity Research. 1999;1(3):181–95. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence M, Paula I, Mark A. Metal ions and oxidative protein modification in neurological disease. Ann Ist Super Sanita. 2005;41(2):143–64. [PubMed] [Google Scholar]
- 6.Nakamura K, Bindokas VP, Kowlessur D, Elas M, Milstien S, Marks JD, et al. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J Biol Chem. 2001;276(37):34402. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- 7.Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in china. Am J Clin Nutr. 1993 Feb;57(2 Suppl):259S–63S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- 8.Schweizer U, Schomburg L, Savaskan NE. The neurobiology of selenium: Lessons from transgenic mice. J Nutr. 2004;134(4):707. doi: 10.1093/jn/134.4.707. [DOI] [PubMed] [Google Scholar]
- 9.Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology. 2006;21(5):307. doi: 10.1152/physiol.00021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The unified parkinson disease rating scale (UPDRS) National Parkinson foundation; 2007. [Google Scholar]
- 11.Kim HC, Jhoo WK, Choi DY, Im DH, Shin EJ, Suh JH, et al. Protection of methamphetamine nigrostriatal toxicity by dietary selenium. Brain Res. 1999;851(1–2):76–86. doi: 10.1016/s0006-8993(99)02122-8. [DOI] [PubMed] [Google Scholar]
- 12.Rederstorff M, Krol A, Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cellular and molecular life sciences. 2006;63(1):52–9. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quick KL, Dugan LL. Superoxide stress identifies neurons at risk in a model of ataxia-telangiectasia. Ann Neurol. 2001;49(5):627–35. [PubMed] [Google Scholar]
- 14.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497(1):1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 15.Ghisleni G, Porciúncula LO, Mioranzza S, Boeck CR, Rocha JBT, Souza DO. Selenium compounds counteract the stimulation of ecto-nucleotidase activities in rat cultured cerebellar granule cells: Putative correlation with neuroprotective effects. Brain Res. 2008;1221:134–40. doi: 10.1016/j.brainres.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Beck J, Ferrucci L, Sun K, Walston J, Fried LP, Varadhan R, et al. Low serum selenium concentrations are associated with poor grip strength among older women living in the community. Biofactors. 2007;29(1):37–44. doi: 10.1002/biof.5520290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartali B, Semba RD, Frongillo EA, Varadhan R, Ricks MO, Blaum CS, et al. Low micronutrient levels as a predictor of incident disability in older women. Arch Intern Med. 2006;166(21):2335. doi: 10.1001/archinte.166.21.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walston J, Xue Q, Semba R, Ferrucci L, Cappola A, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163(1):18. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 19.Lauretani F, Semba RD, Bandinelli S, Ray AL, Guralnik JM, Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: The InCHIANTI study. Am J Clin Nutr. 2007;86(2):347. doi: 10.1093/ajcn/86.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci L, Bandinelli S, Benvenutie E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 21.Gibb W. Neuropathology in movement disorders. J Neurol Neurosurg Psychiatr. 1989;52(Suppl):55. doi: 10.1136/jnnp.52.suppl.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasanisi P, Berrino F, Bellati C, Sieri S, Krogh V. Validity of the italian EPIC questionnaire to assess past diet. IARC Sci Publ. 2002;156:41–4. [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, et al. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004;116(12):807–15. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97(22):11869. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ble A, Cherubini A, Volpato S, Bartali B, Walston JD, Windham BG, et al. Lower plasma vitamin E levels are associated with the frailty syndrome: The InCHIANTI study. Journals of Gerontology Series A: Biological and Medical Sciences. 2006;61(3):278. doi: 10.1093/gerona/61.3.278. [DOI] [PubMed] [Google Scholar]
- 27.Bellisola G, Perona G, Galassini S, Moschini G, Guidi G. Plasma selenium and glutathione peroxidase activities in individuals living in the veneto region of italy. J Trace Elem Electrolytes Health Dis. 1993;7(4):242–4. [PubMed] [Google Scholar]
- 28.Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Savarino L, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. Journal of Clinical Endocrinology & Metabolism. 2000;85(6):2260. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 29.Finley JW. Bioavailability of selenium from foods. Nutr Rev. 2006;64(3):146–51. doi: 10.1111/j.1753-4887.2006.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 30.Semba R, Ricks M, Ferrucci L, Xue Q, Guralnik J, Fried L. Low serum selenium is associated with anemia among older adults in the united states. Eur J Clin Nutr. 2007;63(1):93–9. doi: 10.1038/sj.ejcn.1602889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: A neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26(11):578–80. doi: 10.1016/j.tins.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in parkinson disease. NeuroRx. 2004;1(1):139–54. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]