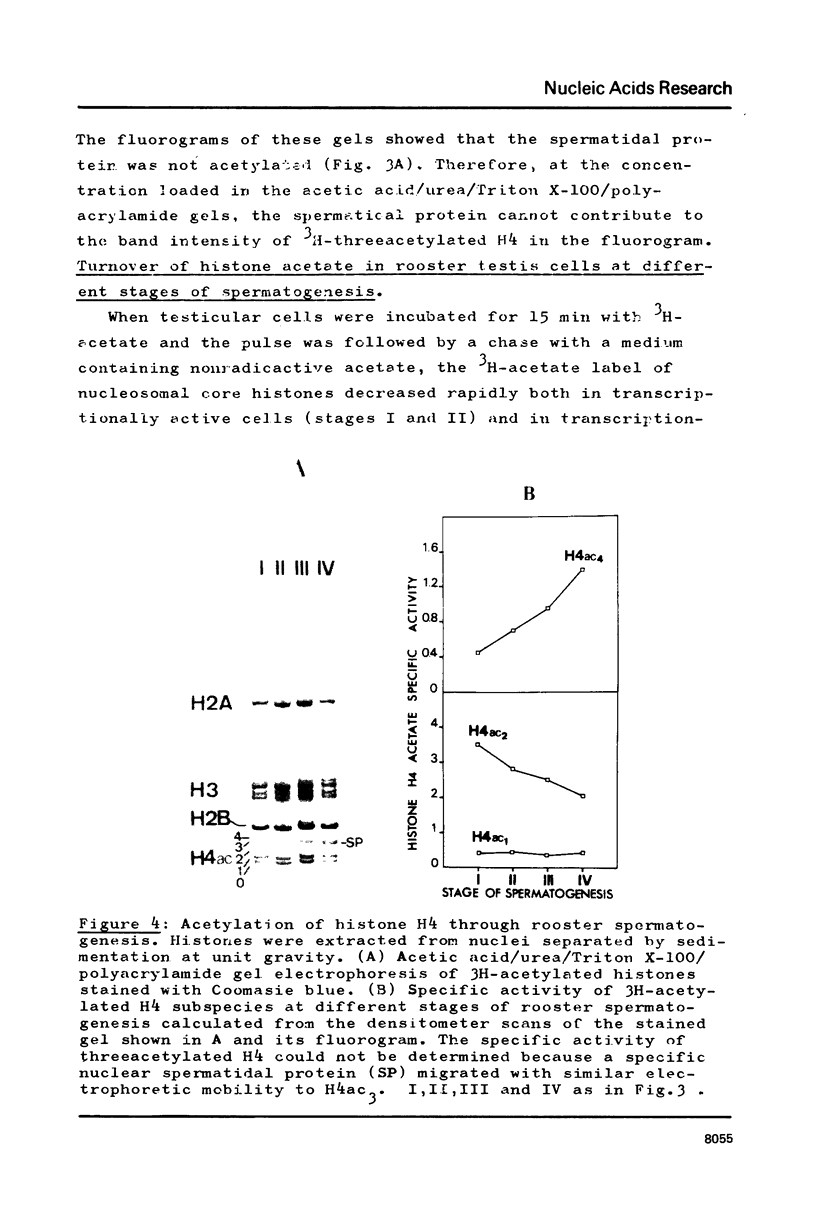
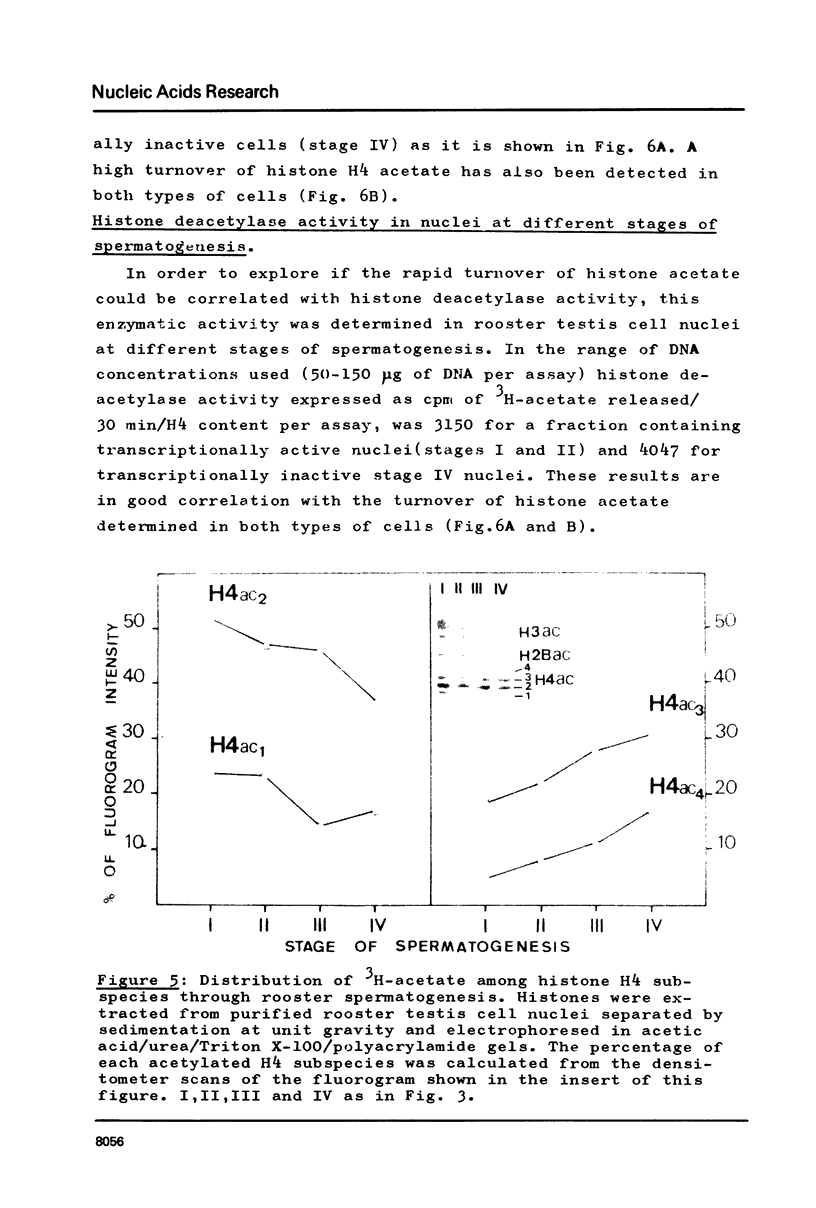
Abstract
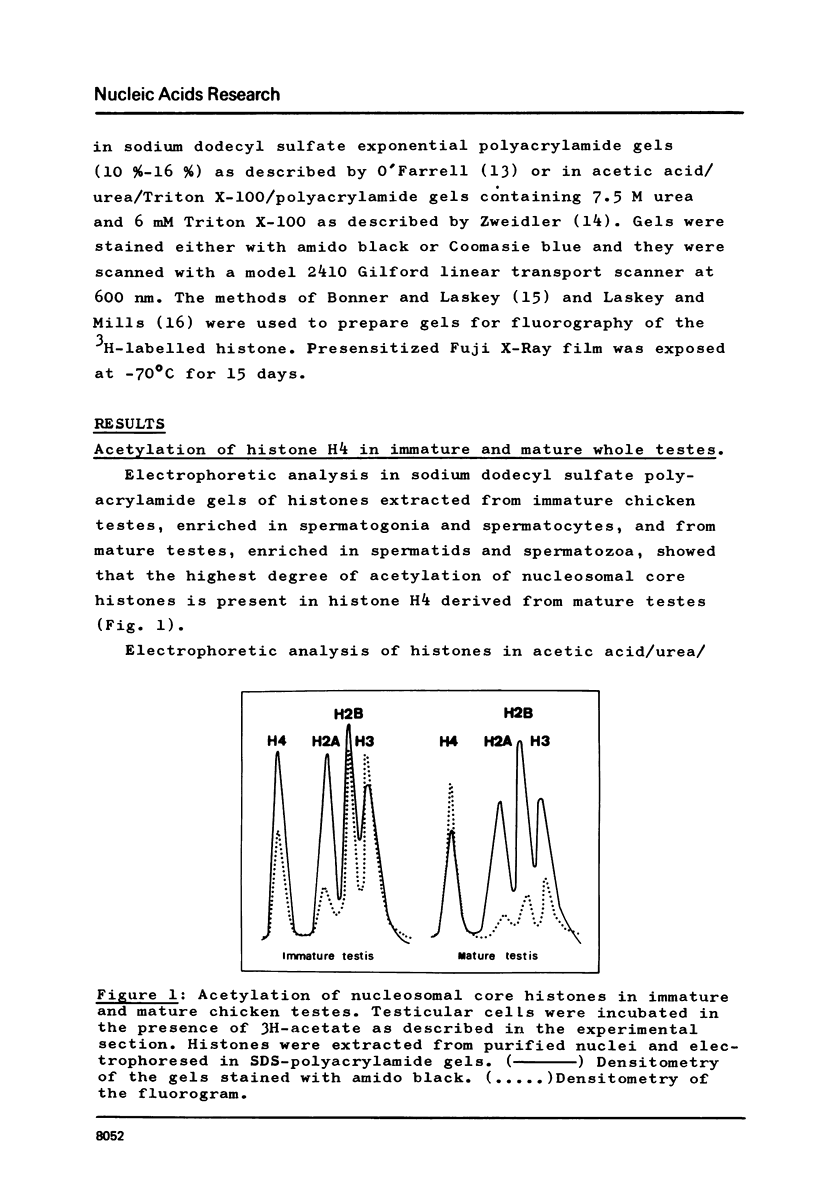
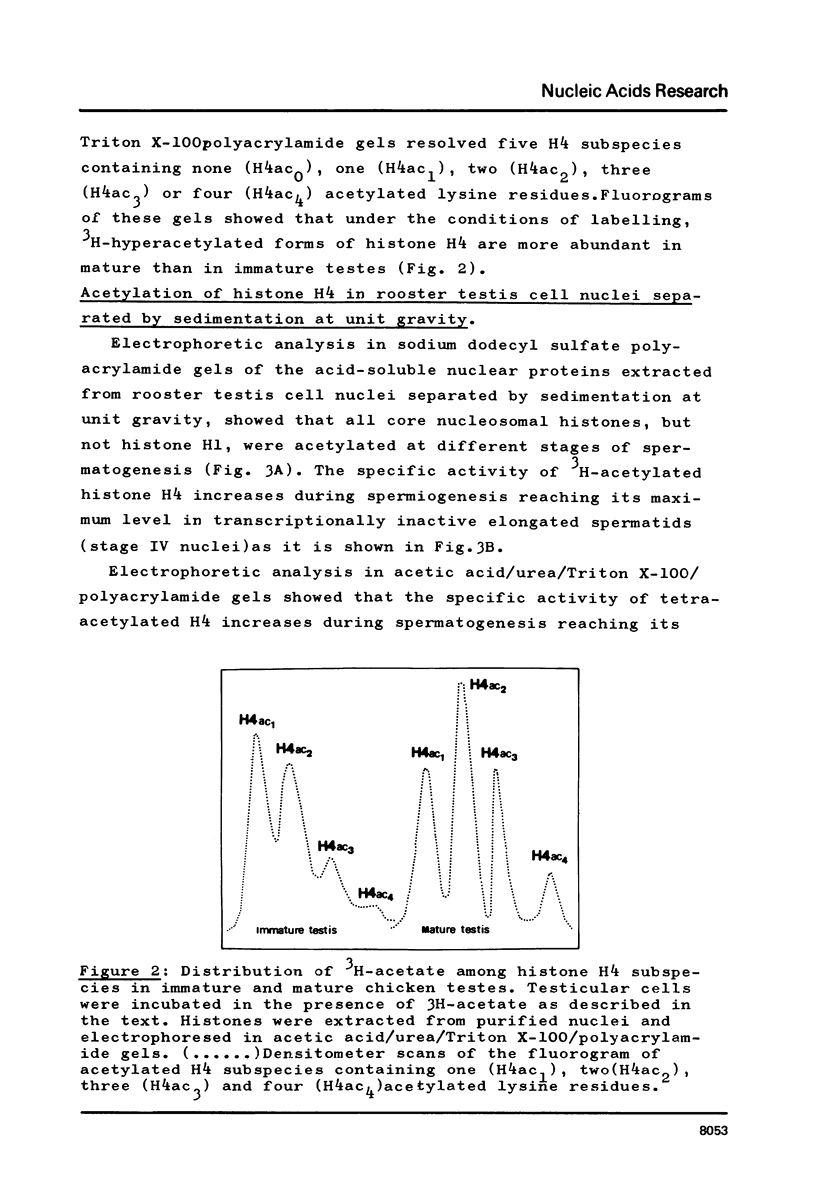
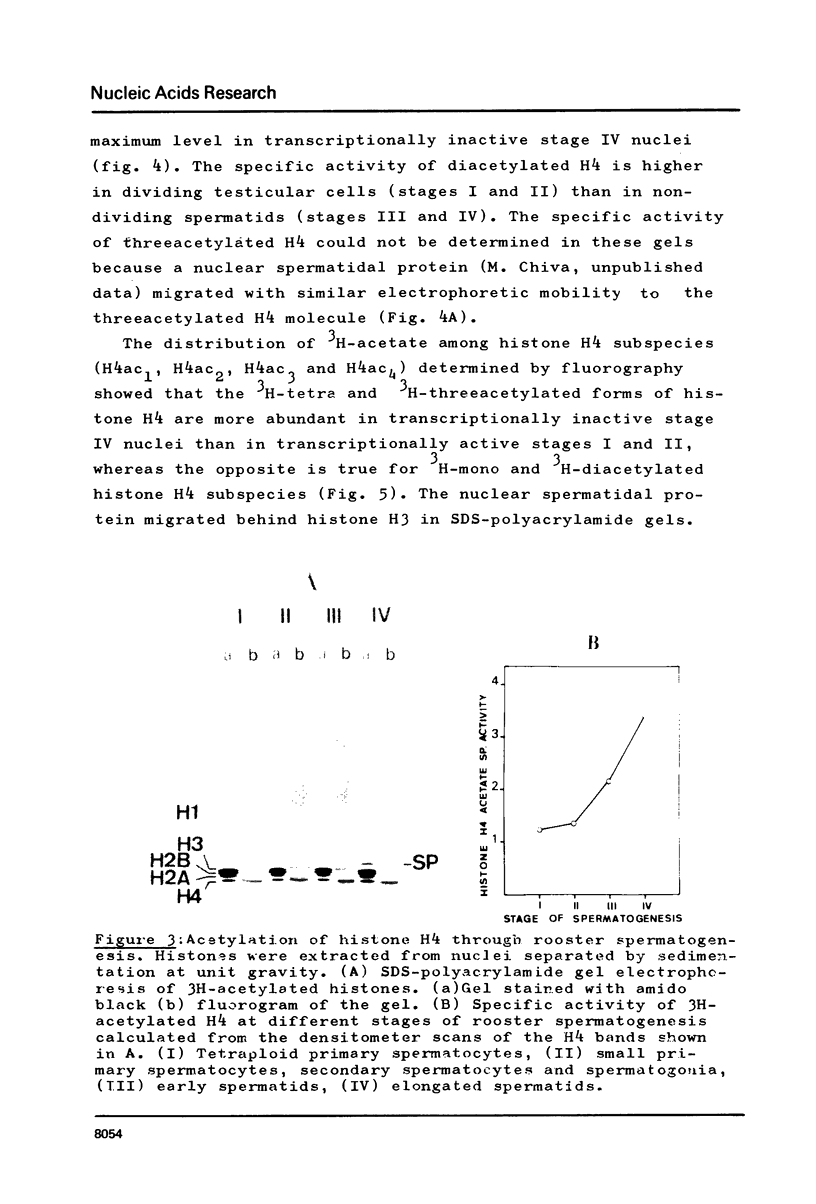
In order to study the relationship between acetylation of histones, chromatin structure and gene activity, the distribution and turnover of acetyl groups among nucleosomal core histones and the extent of histone H4 acetylation were examined in rooster testis cell nuclei at different stages of spermatogenesis. Histone H4 was the predominant acetylated histone in mature testes. Hyperacetylation of H4 and rapid turnover of its acetyl groups are not univocally correlated with transcriptional activity since they were detected in both genetically active testicular cells and genetically inactive elongated spermatids. During the transition from nucleohistone to nucleoprotamine in elongated spermatids the chromatin undergoes dramatic structural changes with exposition of binding sites on DNA (1). Hyperacetylation of H4 and rapid turnover of its acetyl groups could be correlated with the particular conformation of chromatin in elongated spermatids and might represent a necessary condition for binding of chromosomal proteins to DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chahal S. S., Matthews H. R., Bradbury E. M. Acetylation of histone H4 and its role in chromatin structure and function. Nature. 1980 Sep 4;287(5777):76–79. doi: 10.1038/287076a0. [DOI] [PubMed] [Google Scholar]
- Doenecke D., Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem. 1982 Apr 30;44(2):113–128. doi: 10.1007/BF00226895. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Chae C-B, Irvin J. L. Acetylation of histones of rat testis. Arch Biochem Biophys. 1975 Jun;168(2):425–435. doi: 10.1016/0003-9861(75)90271-4. [DOI] [PubMed] [Google Scholar]
- Inoue A., Fujimoto D. Histone deacetylase from calf thymus. Biochim Biophys Acta. 1970 Nov 11;220(2):307–316. doi: 10.1016/0005-2744(70)90015-x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in chromatin structure during spermatogenesis in maturing rooster testis as demonstrated by the initiation pattern of ribonucleic acid synthesis in vitro. Biochem J. 1978 Feb 15;170(2):203–210. doi: 10.1042/bj1700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in nuclear and chromatin composition and genomic activity during spermatogenesis in the maturing rooster testis. Biochem J. 1977 Apr 15;164(1):99–111. doi: 10.1042/bj1640099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter E., Candido M., Dixon G. H. Acetylation of trout testis histones in vivo. Site of the modification in histone IIb 1 . J Biol Chem. 1972 Jun 25;247(12):3868–3873. [PubMed] [Google Scholar]
- Sealy L., Chalkley R. Modification of histones immediately following synthesis. Arch Biochem Biophys. 1979 Oct 1;197(1):78–82. doi: 10.1016/0003-9861(79)90221-2. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Martin-Ponthieu A., Sautiere P., Biserte G. Acetylation of histone H4 in chicken erythrocyte and cuttle-fish testis chromatin. FEBS Lett. 1981 Jun 15;128(2):195–200. doi: 10.1016/0014-5793(81)80079-8. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]