Abstract
Objective
REM sleep behavior disorder (RBD) is associated with neurodegenerative disease and particularly with the synucleinopathies. Convenience samples involving subjects with idiopathic RBD have suggested an increased risk of incident mild cognitive impairment (MCI), dementia (usually dementia with Lewy bodies) or Parkinson’s disease (PD). There is no data on such risk in a population-based sample.
Methods
Cognitively normal subjects aged 70–89 in a population-based study of aging who screened positive for probable RBD using the Mayo Sleep Questionnaire were followed at 15 month intervals. In a Cox Proportional Hazards Model, we measured the risk of developing MCI, dementia, PD among the exposed (pRBD+) and unexposed (pRBD−) cohorts.
Results
Forty-four subjects with pRBD+ at enrollment (median duration of pRBD features was 7.5 years), and 607 pRBD− subjects, were followed prospectively for a median of 3.8 years. Fourteen of the pRBD+ subjects developed MCI and one developed PD (15/44=34% developed MCI / PD); none developed dementia. After adjustment for age, sex, education, and medical comorbidity, pRBD+ subjects were at increased risk of MCI / PD [Hazard Ratio (HR) 2.2, 95% Confidence Interval (95%CI) 1.3 – 3.9; p=0.005]. Inclusion of subjects who withdrew from the study produced similar results, as did exclusion of subjects with medication-associated RBD. Duration of pRBD symptoms did not predict the development of MCI / PD (HR 1.05 per 10 years, 95%CI 0.84 – 1.3; p=0.68).
Interpretation
In this population-based cohort study, we observed that pRBD confers a 2.2-fold increased risk of developing MCI / PD over four years.
Keywords: sleep disorders, parasomnias, dementia, Alzheimer’s disease, dementia with Lewy bodies, parkinsonism, synuclein
INTRODUCTION
REM sleep behavior disorder (RBD) is a parasomnia in which subjects appear to act out their dreams. It is associated with the synucleinopathies of Parkinson’s disease (PD), PD with Dementia (PDD), Dementia with Lewy Bodies (DLB), Multiple System Atrophy (MSA) and Pure Autonomic Failure.1 Autopsy studies support a reasonably specific association of RBD with synuclein disorders.2, 3 The onset of RBD typically precedes the development of cognitive impairment and/or parkinsonism by years or even decades.4 Finally, persons with “idiopathic” RBD appear to have an increased risk of cognitive impairment and/or parkinsonism.5–8 These findings, suggesting that RBD represents an evolving synucleinopathy, raise the possibility that selected subjects with RBD might be enrolled in therapeutic trials to prevent progression of the pathology.9, 10
Estimates of the risk of parkinsonism or dementia associated with RBD vary from 18%5 to over 65%.6, 11 When mild cognitive impairment (MCI) is included in these calculations, the five-year risk of developing a neurodegenerative syndrome is 45%.7 However, all risk estimates to date have been determined from clinical samples from sleep or movement disorder clinics. These high estimates could therefore be due to referral bias. Compared to persons in the general population, subjects recruited from the clinical setting may have a more severe form of the disorder in regard to the frequency, severity, or type of RBD behaviors. More severe REM atonia is associated with a greater risk of developing a neurodegenerative syndrome.12 A population based estimate would give a more valid estimate across the range of RBD severity. To our knowledge, such an estimate has not been previously reported. We sought to address these issues by identifying and following subjects with probable RBD in a population-based sample.
DESIGN/METHODS
Subjects
Subjects were 70–89 year-old residents of Olmsted County, Minnesota, USA at enrollment in October, 2004. Using the medical records linkage system of the Rochester Epidemiology Project, we randomly selected subjects from the population to participate in the Mayo Clinic Study of Aging, a longitudinal population-based study of aging and MCI.13 Written consent for participation was provided by the subjects and their informants, which was typically the spouses or adult children of the subject. All study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.13
Diagnostic evaluation
Subjects are evaluated using a standardized clinical protocol and followed prospectively at 15-month intervals using the same protocol as at baseline. A clinician obtains a medical history from the subject and performs a comprehensive neurological examination. The clinician routinely asks about pre-existing dream enactment behavior, and always asks about new-onset dream enactment as part of the structured interview. Particular attention is paid to detecting the cardinal features of PD, and the modified part III of the Unified Parkinson Disease Rating Scale (UPDRS) is completed. The Charlson Comorbidity Index, a measure of illness burden, is computed using information from the participant’s medical record.13 The clinician, study coordinator and neuropsychologist evaluate and/or diagnose the subject independently prior to a weekly consensus meeting. A consensus diagnosis of normal cognition, or one of the neurodegenerative syndromes (e.g., MCI, dementia, PD) as defined by published criteria, is determined for each subject.13 Ninety-one percent of eligible subjects had at least one follow-up visit. Additional details of the MCSA are published elsewhere.13
Only cognitively normal subjects without PD at enrollment and with at least one follow-up evaluation were included in the present study. Patients unable to attend the clinic and unwilling to have a home visit were followed with in-depth telephone interviews. Almost all MCSA subjects seek their medical care from one of two provider systems: both use a medical records-linkage system that enables tracking of all patient information. We systematically reviewed the clinical records of participants who withdrew from the study for evidence of neurodegenerative syndrome.13 If no clinical diagnosis is made, the subjects were assumed to be cognitively normal. These data are included in a sensitivity analysis.
Probable RBD diagnosis
Assessment for RBD was based on information from the Mayo Sleep Questionnaire (MSQ) provided by someone who sleeps in the same room as the participant. The diagnosis of pRBD is less reliable if informants do not sleep in the same room as the subject; therefore we excluded those subjects. The presence of pRBD is based on an affirmative response to the question: “Have you ever seen the patient appear to ‘act out his/her dreams’ while sleeping? (punched or flailed arms in the air, shouted or screamed).” Informants answer yes only if dream enactment behavior has occurred at least three times. The MSQ is a validated screening measure14 which has 100% sensitivity and 95% specificity for polysomnogram (PSG)-confirmed RBD in this population;15 it is freely available from http://www.mayoclinic.org/sleep-disorders/research.html.
Statistical analysis
We used the Cox proportional hazards model to compute Hazards Ratios, and Kaplan-Meier curves to display the data. The analyses were made to: (a) compare rates of MCI, dementia and PD in subjects with and without pRBD, (b) estimate risk at four years, (c) determine if duration of pRBD predicted diagnosis, and (d) determine if measures of mild Parkinsonism (UPDRS) at enrollment, or changes in these scores over time, differed between the pRBD+ and pRBD− groups. We conducted a stratified analysis to compare rates of MCI, dementia and PD within the groups of patients taking, or not taking, antidepressant medications.
Sensitivity Analyses
Obstructive sleep apnea
Some patients with obstructive sleep apnea (OSA) exhibit dream enactment behavior, and upon detailed questioning of patients and their bed-partners, many of the other historical features of RBD may be present.2, 16 If electromyographic atonia is present during REM sleep, then such patients have OSA only, and are often said to have “pseudo-RBD.” Thus a false-positive diagnosis of pRBD can be made in subjects with OSA whose RBD has not been confirmed by polysomnography. We reviewed the data from all available polysomnograms from pRBD+ subjects. We also compared the frequency of OSA features queried on the MSQ between pRBD+ and pRBD− subjects, and added them to our statistical model in a sensitivity analysis. OSA features are captured on the MSQ by the questions, “Has the patient ever snorted or choked him/herself awake?” and “Does the patient ever seem to stop breathing during sleep?”
Antidepressants
Selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tri- and tetra-cyclic antidepressants can precipitate or aggravate RBD.2, 17, 18 Therefore, we compared the use of these medications (collectively referred to as “antidepressants” henceforth) between the pRBD+ and pRBD− groups. Subjects whose pRBD features began within six months of drug commencement were defined as having medication-associated pRBD. We performed a sensitivity analysis to determine if the exclusion of medication-associated pRBD subjects affected results.
Inclusion of subjects with prevalent MCI
We also determined if pRBD is associated with subsequent diagnosis of dementia or PD when patients with prevalent MCI at enrollment are included. We also adjusted for baseline cognitive status in this model.
Role of the funding sources
The funding sources had no role in the design, data collection, analysis, interpretation or reporting. The corresponding author had full access to all data and was responsible for the decision to publish the report.
RESULTS
Sample characteristics
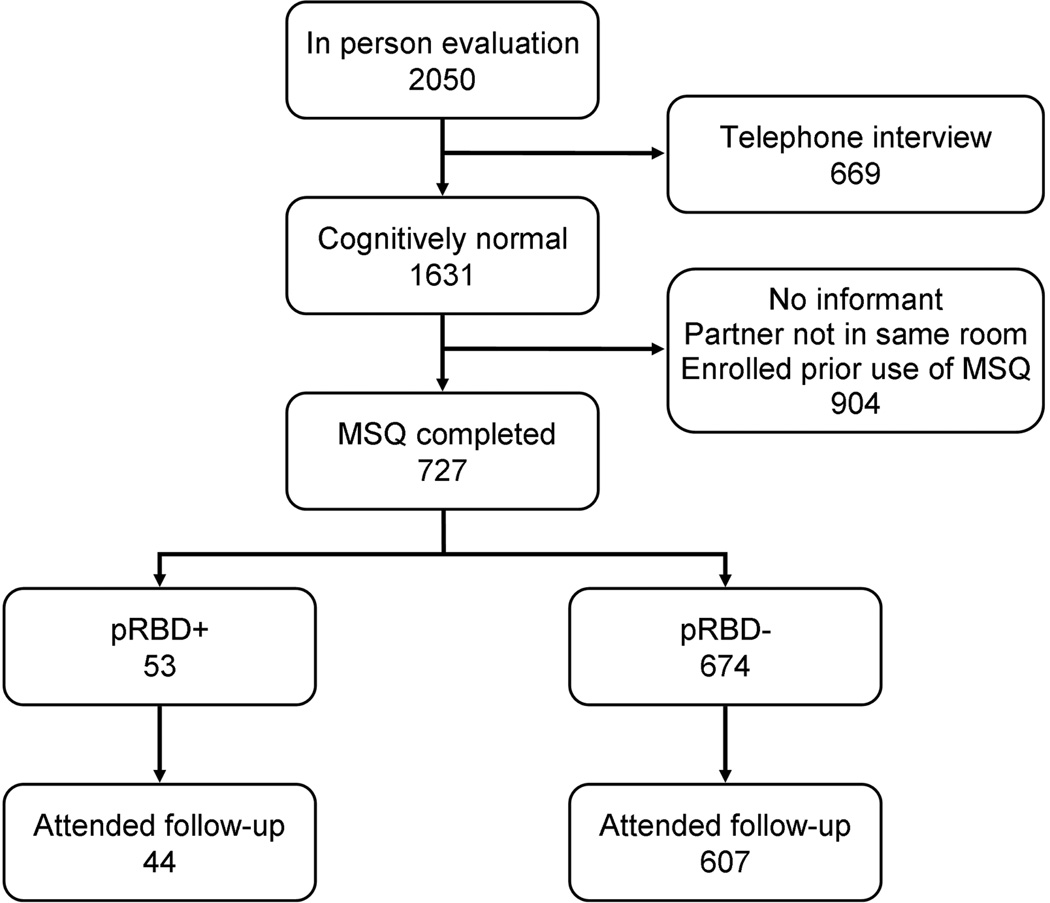
Of the 1631 subjects who were cognitively normal at baseline, 727 completed the MSQ. Of these, 651 had at least one follow-up evaluation and are included in the analyses (Figure 1, Table 1). None of the incident MCI subjects in the pRBD+ group were taking cholinesterase inhibitors at enrollment when the MSQ was originally completed; one started a cholinesterase inhibitor after development of MCI.
Figure One.
Study participants.
Table 1.
Demographic Characteristics At Enrollment of Participants with MCSA Study Follow-up
| pRBD+ | pRBD− | Total | p value | |
|---|---|---|---|---|
| Subjects | 44 | 607 | 651 | |
| Age (years) | 78 (75, 82) | 77 (74, 82) | 77 (74, 82) | 0.36 |
| Male gender | 34 (77.3) | 421 (69.4) | 455 (69.9) | 0.27 |
| Education (years) | 12 (12, 16) | 14 (12, 16) | 14 (12, 16) | 0.38 |
| Charlson Index of Comorbid Disease | 2 (1, 4) | 2 (0, 3) | 2 (0, 4) | 0.13 |
| Parkinsonism | ||||
| UPDRS Motor Subtest – Total | 0 (0, 5) | 0 (0, 2) | 0 (0, 2) | 0.63 |
| Total ≥ 4 | 12 (27.3) | 111 (18.3) | 123 (18.9) | 0.16 |
| Total ≥ 10 | 2 (4.5) | 33 (5.4) | 35 (5.4) | 1.00 |
| Kokmen Short Test of Mental Status | 34 (33, 36) | 35 (33, 36) | 35 (33, 36) | 0.49 |
| Study Follow-up (months) | 46 (31, 47) | 46 (33, 48) | 46 (33, 48) | |
| Neurodegenerative Syndrome Evolved | 15 (34.1) | 94 (15.5) | 109 (16.7) | |
| MCI† | 14 (31.8) | 90 (14.8) | 104 (16.0) | |
| Dementia† | 0 (0.0) | 8 (1.3) | 8 (1.2) | |
| PD† | 1 (2.3) | 4 (0.7) | 5 (0.8) | |
| Age at onset of pRBD (years) | 69 (35, 76) | |||
|
Duration of pRBD [median; mean (25th, 75th percentile)] |
7.5; 18.7 (2.0, 41.5) | |||
| Use of antidepressant# | 10 (22.7) | 40 (6.6) | 50 (7.7) | 0.001 |
| pRBD temporally related to use | 2 (4.5) | |||
| OSA features | ||||
| Snorted/choked themselves awake | 20 (45.5) | 139 (22.9) | 159 (24.4) | 0.002 |
| Stopped breathing when asleep | 12 (27.3) | 92 (15.2) | 104 (16.0) | 0.052 |
Numbers in table are N (%) or Median (25th percentile, 75th percentile). Data at enrollment are presented, unless otherwise indicated. MCI: Mild Cognitive Impairment, MCSA: Mayo Clinic Study of Ageing, OSA: Obstructive Sleep Apnoea, PD: Parkinson’s Disease, pRBD: probable REM Sleep Behavior Disorder, UPDRS: Unified Parkinson’s Disease Rating Scale (Part III).
Subjects can be included in multiple categories: 2 pRBD- subjects developed PD then MCI, 6 pRBD- subjects developed MCI then dementia. 2 pRBD- subjects did not have MCI detected before dementia and are only counted as dementia.
Includes selective serotonin reuptake inhibitors (SSRI), serotonin-norepinephrine reuptake inhibitors (SNRI), and tri- and tetra-cyclic antidepressants. Data includes incident use.
Cognitive and parkinsonism diagnoses
Forty-four subjects with pRBD at enrollment, and 607 without pRBD, were followed for a median of 46 months. Fourteen subjects in the pRBD+ group developed MCI and one developed PD; none developed dementia. Therefore, 15/44=34% of the pRBD+ group developed MCI / PD compared to 94/607=15% in the pRBD− group. Because of the large size of the pRBD− group, most of the subjects that developed a neurodegenerative syndrome were pRBD− at baseline: MCI (90 vs. 14), dementia (8 vs. 0) and PD (4 vs. 1). UPDRS scores did not differ between pRBD+ and pRBD− subjects at enrollment (see Table 1), nor was the change over time in UPDRS scores different between the groups (0.13 per year decrease in both groups, p=0.76).
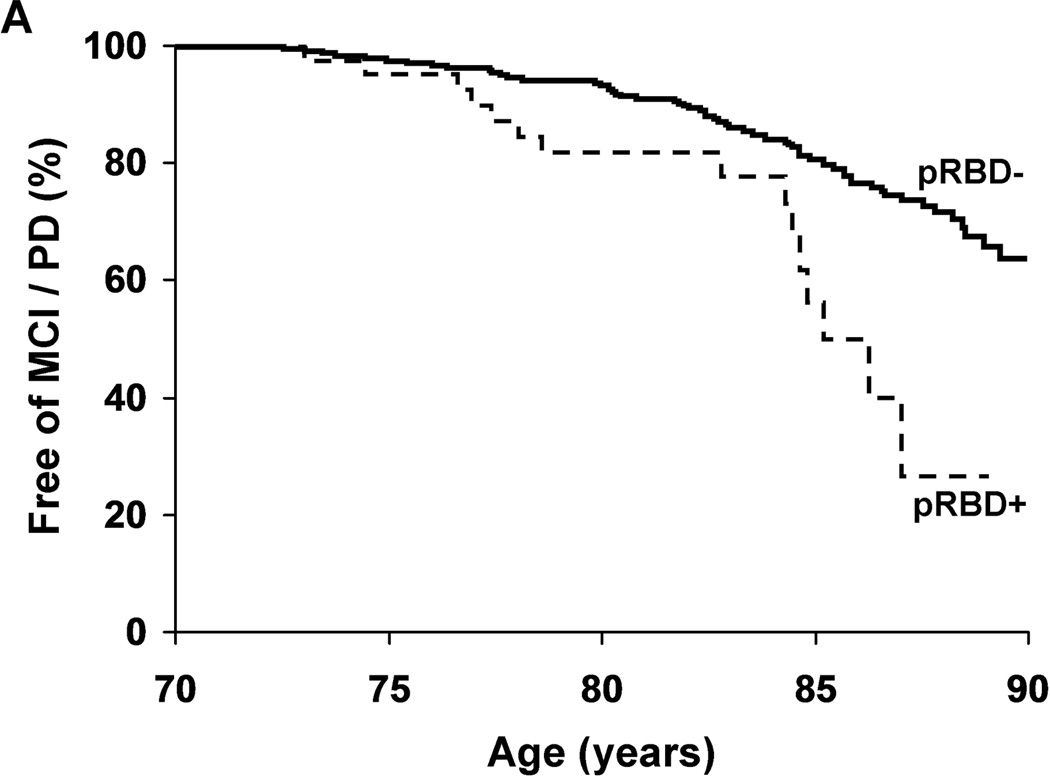
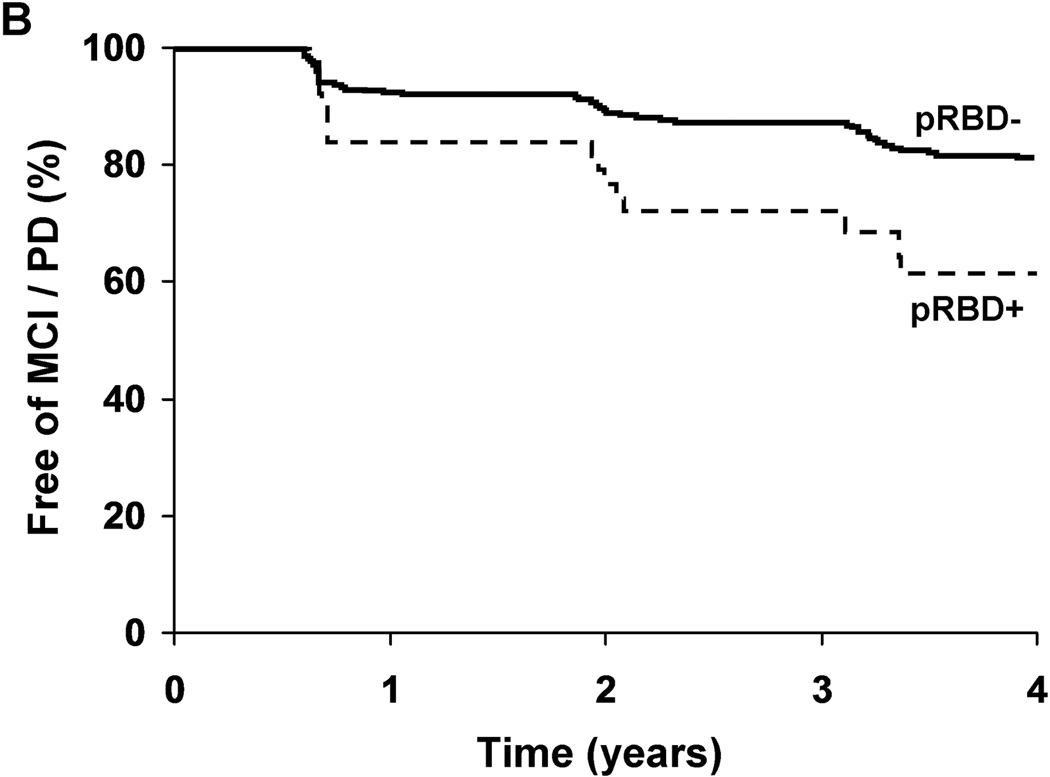
After adjustment for age, sex, education, and medical comorbidity, pRBD+ subjects were at increased risk of MCI / PD [Hazard Ratio (HR) 2.2, 95%CI 1.3 – 3.9; p=0.005, Figure 2). Results were similar after additionally adjusting for OSA symptoms [HR 2.0, 95%CI 1.1 – 3.7; p=0.016]. Inclusion of subjects who withdrew from the study led to similar results [HR 2.0, 95%CI 1.2 –3.5; p=0.01], as did exclusion of the two pRBD+ subjects with medication-associated RBD [HR 2.4, 95%CI 1.3 – 4.1; p=0.003]. A post-hoc within groups analysis revealed a significantly increased risk of MCI / PD associated with pRBD+ among those not taking an antidepressant at enrollment (HR 2.7, 95%CI 1.5 – 5.1, p=0.002), but no significant effect among those taking antidepressants (HR 0.74, 95%CI 0.20 – 2.8, p=0.66).
Figure Two.
Neurodegenerative syndrome-free survival adjusted for age (a), or over time in the study (b), in subjects with (+) and without (−) probable REM sleep behavior disorder (pRBD).
RBD characteristics
The median duration of pRBD symptoms was 7.5 years at enrollment (mean 18.7; range: 2 months – 60 years; inter-quartile range (years): 2.0 – 41.5). The median time between onset of dream enactment behavior and diagnosis of MCI / PD was 20.7 years. Despite this apparent difference in duration of pRBD between those who developed MCI / PD and those that did not, duration of pRBD did not predict the outcome in parametric (HR 1.05 per 10 years, 95%CI 0.84 – 1.3; p=0.68) or non-parametric analyses (HR 1.07 per 2-fold increase, 95%CI 0.86 –1.3; p=0.55).
Potential confounds in RBD determination
Medication-associated pRBD
pRBD+ subjects were significantly more likely to be taking an antidepressant (23% vs. 7%, p=0.001). Ten of the 44 pRBD+ subjects were taking an antidepressant. Two had medication-associated pRBD;2, 17, 18 the eight remaining subjects had pRBD symptom onset 2.5– 51 years before antidepressant treatment.
OSA features
Significant differences in the features of OSA were found between the pRBD+ and pRBD− groups (Table 1). Six of the 44 pRBD+ subjects had previously undergone a PSG: all six had periodic limb movements during sleep, and five had OSA (apnea-hypopnea index ≥ 5). Three had undergone PSG within two years of enrollment into the MCSA and so their PSG’s were available for blinded, randomized review as part of another study protocol. All three demonstrated the electrophysiologic substrate for RBD: REM sleep without atonia.
Inclusion of subjects with prevalent MCI
When subjects with prevalent MCI at enrollment are added to the original analysis and the outcome measure is changed to dementia or PD, the risk associated with pRBD is no longer significant (HR 0.69, p=0.48). Amongst the subjects with MCI at enrollment, 31 of 104 pRBD− subjects developed dementia or PD, whereas 3 of 16 pRBD+ subjects developed dementia or PD.
DISCUSSION
In this population-based cohort of cognitively-normal 70–89 year-olds, pRBD confers a 2.2-fold increased risk of developing MCI / PD within four years. MCI predicts subsequent progression to dementia,19 and in this population the risk is increased 2.1-fold (Petersen RC et al., unpublished data). These results support and extend the previous clinic-based findings of risk associated with RBD.5–7 Despite not coming to clinical attention, the risk of MCI / PD associated with pRBD in the older, cognitively intact population is still quite high. pRBD is worth identifying for clinical and research purposes, and may become far more important for experiment trials of α-synuclein disease-modifying therapies, particularly considering that therapy may be most effective if started early in the disease.
Many patients with apparently idiopathic RBD subsequently develop PD (Table 2). In this study, however, subjects with pRBD developed a cognitive disorder far more frequently than a movement disorder (i.e. 14 patients with MCI vs. 1 patient with PD). Since the development of both cognitive and movement disorders were rigorously assessed by evaluators blinded to the MSQ data and previous evaluations, ascertainment bias is unlikely. Nor do the relatively stringent diagnostic criteria for PD (e.g. requiring akinesia plus one other cardinal feature) account for the findings: there was no between-group difference in the degree or progression of mild parkinsonism (as reflected by the UPDRS scores). Instead the different pattern may reflect clinical sample bias in earlier studies5–7 in which subjects had presented to a sleep disorder centre, and not all subjects were rigorously assessed for possible mild cognitive impairment. Conversely, by including only subjects with bed-partners, we may have excluded many subjects with severe RBD. The pattern could also relate to the advanced age of our cohort at enrollment. We suspect that many patients with RBD had developed PD prior to age 70 and thus were excluded from our analysis. This suspicion is heightened further as the median age of onset of PD in Olmsted County, Minnesota, is 71 years.20, 21 Enrolling and following a younger cohort of subjects using a similar methodology is warranted.
Table 2.
Studies of the Risk of Developing a Neurodegenerative Syndrome Associated with Probable or Definite RBDa
| Study | Setting | Cohort N |
Percent Male |
Mean Age at RBD / pRBD Diagnosisc |
Developed Degenerative Syndrome N |
Mean Years of RBD Symptoms Prior to Degenerative Syndrome |
Diagnoses (%) | Cohort Neurodegenerative Syndrome Risk |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCI | AD | DLB | MSA | PD | ||||||||
| Schenck et al, 19965 | Patients referred to sleep disorder center | 29 | 100b | 52 | 12 | 12.7e | n.a. | 8 | 0 | 0 | 92 | 39% at 3.7 yearsf |
| Iranzo et al, 20066 | Patients referred to sleep disorder center | 44 | 89 | 69 | 20 | 10.7 | 20 | 0 | 30 | 5 | 45 | 45% at 5 years |
| Postuma et al, 20094 | Patients referred to sleep disorder center | 93 | 80 | 65 | 26 | 11.5 | n.a. | 15 | 27 | 4 | 54 | 18% at 5 yearsf 41% at 10 yearsf |
| This study | Cognitively normal community dwellers | 44 | 70 | 78d | 15 | 23.9 | 93 | 0 | 0 | 0 | 7 | 34% at 4 years |
AD: Alzheimer’s disease, DLB: dementia with Lewy bodies, MCI: mild cognitive impairment, MSA: multiple system atrophy, N: number, n.a.: not assessed, PD: Parkinson’s disease, pRBD: probable REM sleep behavior disorder, RBD: REM sleep behavior disorder.
Important methodological differences exist between these studies which limit direct comparisons, see text.
88% of original cohort of 96 subjects were male.
All subjects.
Enrolled 70 – 89 year old subjects only.
PD subjects only.
Does not include MCI.
False-positive pRBD cases suggest true risk of RBD is underestimated
While some of the pRBD+ subjects developed cognitive impairment and/or parkinsonism, many more did not. Some of these subjects may not have RBD (i.e. represent false-positives), and hence their risk of developing cognitive impairment and/or parkinsonism is similar to the normal population. Polysomnography in those who screen positive will allow identification of those with definite RBD. Using the sensitivity and specificity of the Mayo Sleep Questionnaire for true RBD, and the prevalence of RBD, one can determine the positive predictive value of the questionnaire. The sensitivity and specificity of the Mayo Sleep Questionnaire for RBD are 100% and 95% respectively,15 but the prevalence of RBD is not known. However, we have determined that the prevalence of pRBD in this population is 8.9%.22 Keeping in mind the risk of circularity related to the use of pRBD rather than RBD prevalence data, and noting that subjects are excluded from the Mayo Clinic Study of Aging if they already carry a diagnosis of dementia, we can estimate the positive predictive value of the Mayo Sleep Questionnaire to be 0.66. Therefore our reported hazard ratios likely underestimate the true risk of MCI / PD associated with RBD by this fraction.
Inclusion of subjects with MCI
We did not find an increased risk of dementia or PD when we added subjects with prevalent MCI to our analysis. The strength of the association between MCI and dementia may overshadow the association between RBD and dementia/PD, or may reflect the differing rate at which RBD-associated and non-RBD-associated dementias progress.
Comparison with previous studies
Important differences exist between our study and the others which have characterized RBD-associated risk (Table 2). Subjects in those studies were patients referred to a sleep disorder centre for clinical investigation who had PSG-confirmed RBD and were prospectively followed, or whose data was analyzed retrospectively. In contrast, we prospectively studied cognitively normal, community-dwelling subjects whose bed-partners witnessed recurrent dream enactment behavior. After nearly four years of follow-up, only one subject (2.3%) in the pRBD+ group was diagnosed with dementia or PD. This rate is significantly lower than the lowest 5-year risk estimate (18% of subjects) observed in sleep disorder centre samples.5 When MCI is included in the calculations, the five-year risk was 45%,7 compared to 39% (after four years) in our sample. Despite these differences, the five-year risk of a neurodegenerative syndrome in our cohort may yet approach those of the clinic-based samples.5, 23 The frequency of MCI is higher in the pRBD+ group (31.8% vs.14.7%), and those with MCI convert to dementia at twice the rate of those without (8.4% vs. 4.0% per year; Petersen et al, unpublished data). Therefore we can expect greater rates of dementia in the pRBD+ vs. pRBD− groups over a longer duration of follow-up. Recent clinicopathologic analyses suggest that those with RBD and MCI – regardless of the MCI subtype – very likely reflect evolving Lewy body disease.1–4, 7, 23 All four subtypes of MCI (as represented by amnestic vs. non-amnestic, and single vs. multi-domain dichotomies) were observed in the 14 pRBD+ subjects who developed MCI. This was also the case among several subjects with RBD who evolved from MCI to DLB before Lewy body disease was diagnosed at autopsy in this population.24 Whether those in this cohort with pRBD plus MCI will evolve into DLB will be determined in future assessments.
Confounds and limitations
OSA
A major limitation of the current study is that we rely on bed-partner reports of dream enactment behavior to define pRBD, rather than establishing the diagnosis with a PSG. The bed-partners of pRBD+ subjects were significantly more likely to report features of OSA. Patients with OSA can mimic the symptoms of RBD,16 and hence some of our pRBD+ subjects may not in fact have RBD. Because OSA has not been shown to be associated with an increased risk of neurodegenerative syndromes, the hazard ratios reported here may represent under-estimates of the true population RBD risk. However, adding OSA symptoms to our statistical model did not substantially change our results. We plan to investigate our pRBD+ subjects with PSG in order to better assess this risk.
Medication-associated RBD
Subjects with pRBD were significantly more likely to be taking antidepressant medications known to be associated with RBD (22.7 vs. 5.4%, p<0.001). In eight of the ten RBD+ subjects taking antidepressants, the onset of dream enactment behavior substantially preceded antidepressant use. We conducted a separate analysis which excluded the remaining two subjects and this did not significantly affect the MCI / PD risk estimates. It is unclear if previous clinic-based studies of RBD associated cognitive impairment or parkinsonism sufficiently accounted for medication-associated RBD. Iranzo et al7 included only “idiopathic RBD” subjects, Schenck et al6 withdrew “psychotropic medication” for one month prior to PSG, and Postuma et al5 withdrew all medications “known to affect sleep” at least two weeks prior to the PSG. While a two- to four-week medication washout is certainly reasonable, there are no data on the natural history of medication-associated RBD after cessation of such drugs. Given the very slow offset of action of these medications,25, 26 patients with medication-associated RBD may have been included in previous risk estimates. Whether their inclusion would falsely decrease RBD associated neurodegenerative risk estimates is unknown.
Separate from the possible direct effect of antidepressant medications on RBD is the association of depression and neurodegenerative disease. It is likely that depression is both a prodromal feature of neurodegenerative disease and a reaction to the sense of loss associated with incipient cognitive decline. This may account for the high proportion (23%) of subjects in the pRBD+ group taking antidepressant medication. Given that depression is an independent risk factor for neurodegenerative disease27, 28 and that antidepressant medications can precipitate RBD, inclusion of subjects with RBD, depression and antidepressant medication may confound RBD-associated neurodegenerative disease risk assessment. This was reflected in our data: pRBD was associated with a significantly increased risk of MCI / PD among subjects not taking antidepressants (HR 2.7, p=0.002), whereas there was no significant association among subjects on an antidepressant (HR 0.74, p=0.66).
Clinician not blind to possible RBD
Although clinicians in this study are blind to the MSQ data, they always ask subjects about a history of dream enactment behavior as part of the structured interview. This knowledge could potentially increase their willingness to diagnose MCI, PD or dementia and thus produce an artificially high estimate of the risk associated with pRBD. This is unlikely for several reasons. The diagnosis of normal cognition, MCI, or dementia used in this analysis is based on formal criteria and is made by consensus: the clinician’s diagnosis cannot “trump” the others’ diagnoses. Furthermore, we reviewed the independent diagnoses at each wave of the study: agreement between the three groups (clinician, study coordinator and neuropsychologist) was 89–92%. The clinicians did not diagnose disease at a rate greater than the other study members (p≥0.5).
These findings underscore the value of using a sensitive and specific measure for probable RBD in epidemiologic research, particularly to assess the association of RBD with neurodegenerative syndromes and to assess risk that pRBD portends to future course. Screening for RBD will become increasingly important as investigators identify subjects with pRBD, confirm or refute the diagnosis by PSG, and prepare for experimental trials of disease-modifying therapies in neurodegenerative diseases.
ACKNOWLEDGEMENTS
We thank the staff of MCSA and especially the subjects and family members involved in the study. This research was funded by the following grants: U01 AG06786, P50 AG016574, RO1 AG15866, K01 AG028573, K01 MH068351, R01 AR030582, R01 AG034676, Harold Amos Medical Faculty Development Program (RWJ foundation), and Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
ABBREVIATIONS
- AD
clinically probable Alzheimer’s disease
- DLB
dementia with Lewy bodies (as defined by the clinical syndrome)
- FTD
behavioral variant frontotemporal dementia
- HR
hazard ratio
- LBD
Lewy body disease (as defined by pathology)
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Ageing
- MSA
multiple system atrophy
- MSQ
Mayo Sleep Questionnaire
- OSA
obstructive sleep apnea
- PD
Parkinson’s disease
- pRBD
probable REM sleep behavior disorder
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
Footnotes
DISCLOSURES
Brendon P. Boot, MBBS – nothing to disclose.
Bradley F. Boeve, MD – has served as an investigator for clinical trials sponsored by Cephalon, Inc., and Allon Pharmaceuticals. He receives royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2009). He has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging [P50 AG16574 (Co-Investigator), U01 AG06786 (Co-Investigator), RO1 AG32306 (Co-Investigator)] and the Center for Inherited Disease Research (CIDR) [U24 AG026395 (Co-Investigator)].
Rosebud O. Roberts, PhD – receives research support from the National Institutes of Aging [P50 AG016574; U01 AG006786 (Co-Investigator)] and previously received support through K01 AG028573 (Principal Investigator).
Yonas E Geda MD – Dr. Geda receives research support from the NIH (K01 MH68351; AG06786), Mayo CTSA (RR024150 [Career Transition Award]), the RWJ Foundation (Harold Amos Scholar), and from the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Eric G. Tangalos, MD – Serves as a consultant to Novartis and Janssen. He has served on a data safety monitoring board for a clinical trial sponsored by Lilly. He is an investigator in a clinical trial sponsored by Baxter.
V. Shane Pankratz, PhD – nothing to disclose.
Robert J. Ivnik, PhD – nothing to disclose.
Tanis J. Ferman, PhD – nothing to disclose.
Glenn E. Smith, PhD – nothing to disclose.
Eric McDade DO – serves as a consultant to UpToDate, Inc. and receives research support from the National Institute on Aging [P50 AG05133].
Teresa J.H. Christianson, BSc – nothing to disclose.
David S. Knopman, MD - serves on a Data Safety Monitoring Board for Lilly Pharmaceuticals, and is an investigator for clinical trials sponsored by Élan Pharmaceuticals, Forest Pharmaceuticals and Baxter Healthcare. He is deputy editor of Neurology, and receives compensation for editorial activities. He receives research support from the National Institute on Aging [U01 AG006786; P50 AG016574].
Michael H. Silber, MBChB – has received honoraria from the American Academy of neurology and the American Academy of Sleep Medicine. He receives royalties from two books (Sleep Medicine in Clinical practice, 2nd edition, 2010, and Atlas of Sleep Medicine, 2010, Informa Medical).
Ronald C. Petersen, PhD, MD - Chairs Data Monitoring Committees for Pfizer, Inc. and Janssen Alzheimer Immunotherapy and consults to Elan Pharmaceuticals and GE Healthcare. He receives research support from the National Institute on Aging [U01 AG006786; P50 AG016574].
REFERENCES
- 1.Boeve B, Silber M, Saper C, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 2.Boeve B. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann NY Acad Sci. 2010;1184:17–56. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferman T, Boeve B, Smith G, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011 Aug 17; doi: 10.1212/WNL.0b013e31822c9148. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claassen D, Josephs K, Ahlskog J, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postuma R, Gagnon J, Vendette M, Fantini M, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46(2):388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 7.Iranzo A, Molinuevo J, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon J, Vendette M, Postuma R, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, Lomeña F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2010;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 10.Boeve B. Predicting the future in idiopathic rapid-eye movement sleep behaviour disorder. Lancet Neurol. 2010;9:1040–1042. doi: 10.1016/S1474-4422(10)70221-0. [DOI] [PubMed] [Google Scholar]
- 11.Schenck C, Bundlie S, Mahowald M. REM behavior disorder (RBD): Delayed emergence of parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic RBD, and an analysis of the minimum & maximum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep. 2003;26:A316. [Google Scholar]
- 12.Postuma R, Gagnon J, Rompré S, Montplaisir J. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74:239–244. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts R, Geda Y, Knopman D, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeve B, Molano J, Ferman T, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12:445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeve B, Molano J, Ferman T, et al. Screening for REM sleep behavior disorder in the community-dwelling elderly: Validation of the Mayo Sleep Questionnaire in the Mayo Clinic Study of Aging. Neurology. 2010;74 suppl 2:A432. [Google Scholar]
- 16.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 17.Winkelman J, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;15:317–321. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 18.Onofrj M, Luciano AL, Thomas A, Iacono D, D'Andreamatteo G. Mirtazapine induces REM sleep behavior disorder (RBD) in parkinsonism. Neurology. 2003;60(1):113–115. doi: 10.1212/01.wnl.0000042084.03066.c0. [DOI] [PubMed] [Google Scholar]
- 19.Petersen R. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Bower J, Maraganore D, McDonnell S, Rocca W. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 21.Elbaz A, Peterson B, Yang P, et al. Nonfatal cancer preceding Parkinson's disease: a case-control study. Epidemiology. 2002;13:157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Molano J, Boeve B, Roberts R, et al. Frequency of sleep disorders in the community-dwelling elderly: The Mayo Clinic Study of Aging. Neurology. 2009;72 Suppl 3:A107. [Google Scholar]
- 23.Terzaghi M, Sinforiani E, Zucchella C, et al. Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease? Sleep Med. 2008;9:343–351. doi: 10.1016/j.sleep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: A clinicopathological study. Brain. 2009;133:540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid I, Stewart C. How antidepressants work: new perspectives on the pathophysiology of depressive disorder. Br J Psychiatry. 2001;178:299–303. doi: 10.1192/bjp.178.4.299. [DOI] [PubMed] [Google Scholar]
- 26.Baldessarini R, Tondo L, Ghiani C, Lepri B. Illness risk following rapid versus gradual discontinuation of antidepressants. Am J Psychiatry. 2010;167:934–941. doi: 10.1176/appi.ajp.2010.09060880. [DOI] [PubMed] [Google Scholar]
- 27.Geda Y, Knopman D, Mrazek D, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 28.Savica R, Rocca W, Ahlskog J. When does Parkinson disease start? Arch Neurol. 2010;67:798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]