Abstract
Despite the advent of antiretroviral therapy, complications of HIV-1 infection with concurrent drug abuse are an emerging problem. Opiates are well-known to modulate immune responses by preventing the development of cell-mediated immune responses. Their effect on the pathogenesis of HIV-1 infection however, remains controversial. Using the Simian Immunodeficiency Virus/macaque model of HIV pathogenesis, we sought to explore the impact of morphine on disease progression and pathogenesis. Sixteen Rhesus macaques were divided into 2 groups; 4 were administered saline and twelve others morphine routinely. Both groups of animals were then inoculated with SIVmacR71/17E and followed longitudinally for disease pathogenesis. The morphine group (M+V) exhibited a trend towards higher mortality rates and retardation in weight gain compared to the virus alone group. Interestingly, a subset of M+V animals succumbed to disease within weeks post-infection. These rapid progressors also exhibited a higher incidence of other end-organ pathologies. Despite the higher numbers of circulating CD4+ and CD8+T cells in the M+V group, CD4:CD8 ratios between the groups remained unchanged. Plasma and CSF viral load in the M+V group was at least a log higher than the control group. Similarly there was a trend toward increased virus build-up in the brains of M+V animals compared with controls. A novel finding of this study was the increased influx of infected monocyte/macrophages in the brains of M+V animals.
Keywords: Morphine, SIV, Neuropathogenesis
Introduction
Currently it is estimated that over 33 million people are living with HIV-1 worldwide (CDC 2009). Though the overwhelming majority has acquired infection through sexual contact, a growing number acquire the virus via intravenous drug use. The most commonly injected illicit drug is heroin. Combined HIV-1 infection and opiate dependence is becoming an emerging problem in the post-HAART era as infected individuals continue to live longer. It is estimated that drug abuse may be the leading cause of HIV transmission in USA. The interplay of these two thus raises concerns regarding their effects on disease severity and rate of progression (Donahoe, 2004; Kapadia et al., 2005). Morphine is known to function as an immunosuppressant; however, information on its effects on pathogenesis remains scant. In vitro and in vivo studies examining the effects of opiates on AIDS progression and severity have yielded conflicting results (Chuang et al., 1993; Suzuki et al., 2002; Donahoe, 2004; Kumar et al., 2004; Kumar et al., 2006; Marcario et al., 2008). Several epidemiological studies have also led to contrasting conclusions with some showing protective effects of opiates on HIV pathogenesis (Spijkerman et al., 1996) while others demonstrating deleterious effects (Bouwman et al., 1998) or even no effect at all (Thorpe et al., 2004). These studies are problematic due to high variability in drug usage patterns, length of use, and multidrug use as well as differences in nutritional status and access to medical care (Kapadia et al., 2005). Also, most studies rely on self-reported data, which may not be accurate. In addition, many studies base their conclusions on comparisons between homosexual males and injection drug users, which can be confounding due to possible differences in socioeconomic status, and behavior between these two groups. Animal models therefore provide a valuable approach to study the effects of opiates on lentivirus pathogenesis in a more controlled environment.
Several studies have utilized the simian immunodeficiency virus (SIV)/AIDS macaque model to better understand the influence of opiate dependency on HIV progression to AIDS. SIV infection in rhesus macaques is one of the best models of HIV-1 infection in humans, since the virus has a CCR5 (R5) phenotype similar to that of HIV (Chen et al., 1997; Kirchhoff et al., 1997; Marx and Chen, 1998).
The rationale for using R71/17E (Raghavan et al., 1999) a SIVmac239 derivative, in the present study is that analogous to the human situation, this virus causes a highly productive infection in memory CD4+ T cells that are primarily located in the secondary and gut-associated lymphoid tissues (Veazey et al., 1998; Veazey et al., 2000a; Veazey et al., 2000b), leading to increased viremia. Various studies have explored the effect of opiate dependence on progression of SIV infection, but similar to epidemiological studies, the results have led to contrasting conclusions. One study concluded that opiate dependency was beneficial in slowing the progression to AIDS (Donahoe et al., 2009), while another reported an accelerated progression (Chuang et al., 2005). Still another study demonstrated both accelerated progression in some monkeys and slowed progression in others (Kapadia et al., 2005). These discrepancies may be attributed to different types and virulence of virus used, differing amounts and scheduling of opiate administration, small sample sizes, and/or possible differences in genetic background between test subjects. Though the results were inconsistent in these published studies, the SIV/AIDS macaque model still represents the best system to examine the effects of addictive drugs on the pathogenesis of infection by allowing controlled administration of the drug into animals infected with a biologically characterized virus with the ability to follow the resultant effects over time.
It is well documented that opiates can modulate the immune system either directly via opioid receptors located on immune cells or indirectly via opioid receptors in the CNS. Because heroin acts largely via its conversion to morphine in the brain, morphine is commonly used as a drug of choice to study opiate effects both in vivo and in vitro. Chronic morphine exposure affects both arms of the immune system. Activation of mu opioid receptors (MOR) on macrophages leads to inhibition of phagocytosis (Szabo et al., 1993; Tomei and Renaud, 1997) and nitric oxide (NO) production(Fecho et al., 1994); while on CD4+ T cells, activation inhibits Th1 cytokines IL-2 and IFN-γ while potentiating Th2 cytokines IL-4 and IL-5, ultimately biasing them towards a Th2 pathway (Roy et al., 2004; Roy et al., 2005). Morphine can also induce increased CCR5 receptor expression on neutrophils and monocytes/macrophages (Miyagi et al., 2000; Suzuki et al., 2002).
These effects, among many others, on the immune system have important implications for the pathogenesis of HIV-1. For example, CCR5 is a critical co-receptor in the pathogenesis of HIV. In the early stages of the infection, activated and resting memory CD4+T cells, both expressing CCR5, are the primary substrates for viral replication. Naïve CD4+T cells generally lack CCR5 and therefore do not support viral replication. Since morphine up-regulates CCR5 expression, it could be a major factor contributing to increased viral replication. Viremia is established early and leads to invasion of the CNS where perivascular macrophages in the brain are the primary cell type that becomes infected.
In the present study we used the macaque model of HIV -1 infection with the SIVmac239-derived virus, R71/17E; along with chronic morphine administration to determine the role opiates play in HIV-1 neuropathogenesis. Our findings suggest that morphine has a trend of potentiating virus replication and end-organ pathogenesis leading to increased mortality in a subset of macaques compared with the virus alone animals
Material and Methods
Animals and viruses
Macaques
Sixteen, 2- to 3-year-old, Indian rhesus macaques (Macaca mulatta) were purchased from the Caribbean Research Primate Center and individually housed in steel holding cages, in two dedicated rooms within the AAALAC-approved animal facility at the University of Kansas Medical Center. The monkeys were exposed, daily, to 12-hour light-dark cycles and given laboratory chow and water ad libitum along with daily snacks. All cages were equipped with environmental enrichments. The animals were tested for tuberculosis, herpes B virus and simian retrovirus and found negative in all these tests. All animal protocols were approved by the local animal care committee (IACUC) at the University of Kansas in accordance with the Guide for the Care and Use of Laboratory Animals. The animals were randomly divided into two groups: SIV only (V, n=4) and morphine + SIV (M+V, n=12) (Table 1). Morphine was administered intramuscularly (IM) to M+V macaques 4 times daily, at 6 hour intervals, at a dose of 3 mg/kg with a 1 ml syringe (27.5G needle). The macaques were gradually acclimated to morphine by starting with 1mg/kg for one week and escalating to a final dose of 3mg/kg in 1mg/kg increments per week. The V group animals were injected with saline IM at the same time intervals. Morphine administration was maintained throughout the study to avoid withdrawal effects. For all animal studies, clinical grade morphine was purchased from the University of Kansas Pharmacy.
Table 1. Layout of the study. Sixteen macaques were divided into 2 groups; V and M+V.
The V group was placed on saline whereas the M+V group was administered morphine in an escalating dose from 1mg/kg up to 3mg/kg, which was then continued throughout the duration of the study. Following nine weeks of morphine or saline treatment, all the animals were inoculated intravenously with SIV R17/71E.
| Time | |||||
|---|---|---|---|---|---|
| Group | Number of Macaques | -9 weeks | -4 weeks | 0 weeks | 12 months |
| V | 4 | Saline | Saline | SIV (i.v.) R17/17E | Necropsy |
|
| |||||
| M+V | 12 | Morphine (1 mg/kg) | Morphine (3 mg/kg) | SIV (i.v.) R17/17E | Necropsy |
Viruses
SIVmacR71/17E(Raghavan et al., 1999) was originally prepared from pooled brain homogenates from macaques infected with R17 and R71E both of which cause encephalitis. Virus stock was prepared in CD8+T cell depleted, ConA-activated macaque peripheral blood mononuclear cells (PBMC) and assayed for infectivity in CEMx174 cells and by plaque assay in GHOST Hi5 cells. Animals were inoculated intravenously with approximately 104 plaque forming units (PFU) of virus nine weeks following initiation of morphine administration.
Routine Collection of Samples
Blood and cerebrospinal fluid (CSF) samples were collected at weekly intervals for the first month and monthly thereafter. Animals were anesthetized with ketamine and peripheral blood was collected in EDTA. Approximately 1 mL of CSF was collected from the cistern magna. Levels of circulating CD4+ and CD8+T cells as well as viral loads in plasma and CSF were monitored for over eight months post-virus inoculation (PI).
Necropsy
All monkeys were monitored daily for evidence of disease (diarrhea, respiratory difficulties, progressive neurological signs, etc) and alterations in attitude, appetite, or behaviors suggestive of illness (lack of movement, severely withdrawn behavior, lack of eating and/or drinking, etc). Once these symptoms become unresponsive to treatment, the monkeys were euthanized. In preparation for necropsy, the animals were deeply anesthetized with ketamine (3mg/kg) and medetomidine (0.15mg/kg) given IM. Laparotomies were then performed, and the animals exsanguinated from the descending aorta and perfused transcardially with normal saline. Samples of all the tissues including brain were harvested. Brains were dissected and portions of the parietal cortex, basal ganglia, frontal cortex, occipital cortex and brainstem were collected. Adjacent portions of each tissue were frozen in liquid nitrogen-cooled isopentane and stored frozen at -80°C for later determination of viral RNA content. Other portions were placed into cassettes and fixed in 4% paraformaldehyde or 10% formalin. These cassettes were then processed for routine histopathological analysis.
Flow Cytometry
CD4+ and CD8+T cell numbers in peripheral blood were determined as described previously (Mackay et al., 2004). One hundred microliters of whole blood was immunostained with an antibody mix against cell surface markers CD3, CD4 and CD8 (BD Biosciences, SanJose, CA). Antibodies were conjugated to 3 different fluorophores and incubated in the dark at 4°C for 30 minutes. Red blood cells were lysed by incubating the blood/antibody mix with lysing solution (BD Biosciences) for 10 minutes. Cells were fixed with 1% formalin and analyzed in a flow cytometer (BD Biosciences FACS Calibur).
Viral burdens in plasma and CSF
Viral burdens in the plasma and CSF were assessed by Real-time RT-PCR as previously described (Marcario et al., 2008). Briefly, plasma and CSF were centrifuged at 20,000g for 1 hour at 4°C and pellets were resuspended in PBS. RNA was isolated from the pellets using the QIAgen RNA mini-kit (Invitrogen, Carlsbad, CA). Real-time PCR was performed using SIV gag-specific primers and a Taqman probe (Applied Biosystems, Austin, TX) and analyzed on the 7500 Fast Real Time PCR system. Thermal cycling conditions were as follows: 50°C (2 min), 60°C (30 min), 95°C (10 min), 95°C (15 sec; 44 cycles) and 60°C (30 sec; 44 cycles). Viral RNA copies were calculated per milliliter of plasma and CSF. The minimum level of detection was 18 copies.
Tissue RNA isolation and Real-time PCR
Brain tissue collected from monkeys at the time of necropsy and snap frozen in liquid nitrogen was used for the extraction of RNA using Trizol reagent (Invitrogen, Carlsbad, CA). RNA extracted from these tissues was subjected to Real-time RT-PCR for SIV gag.
Immunohistochemical Staining
Immunohistochemical staining for macrophages and the SIV antigen was conducted using a mouse monoclonal anti-CD68 antibody (KP1 clone, Dako, Glostrup Denmark) and a mouse monoclonal anti-SIV gp120 antibody (VM-18S clone, NIH AIDS Reference Reagent Program, Bethseda, MD), respectively. Paraffin embedded monkey brain tissue sections were deparaffinized in xylene for 30 minutes and rehydrated in a graded series of alcohol. Following washes in PBS and blocking in donkey serum for 30 minutes, sections were incubated in anti-CD68 (1:100) or anti-SIV gp120 (1:100) antibody at 4°C overnight. Sections were then washed three times in PBS and incubated in biotin-conjugated donkey anti-mouse antibody (1:200). Vectastain ABC kit and diaminobenzidine (DAB) were used to visualize the antigen bound antibody.
In situ hybridization
SIV RNAs were detected using in situ hybridization (ISH) as previously described (Li et al., 1997). In brief, 6- to 8-μm sections on silanized slides were deparaffinized in xylene, rehydrated in phosphate-buffered saline, and permeabilized by treatment of the sections with HCl, digitonin, and proteinase K. After acetylation, the sections were hybridized to 35S-labeled SIV-specific riboprobes. Following washing and digestion with ribonucleases, the sections were coated with nuclear track emulsion, exposed, developed, and counterstained with hematoxylin and eosin.
Statistical methods
The longitudinal data of CD4+ and CD8+T cell counts, as well as, plasma and CSF RNA replicates were analyzed by the generalized linear mixed-effects (GLIMMIX) models. The CD4 and CD8 counts were assumed to follow Poisson distributions, while the distributions of the RNA replicates were skewed to the right and were compared on their logarithms assuming the log-normal distributions. Considering the small sample size, we intended not to control type I errors for multiplicity (multiple outcome comparisons). The analysis was performed on SAS® version 9.1.3, PROC GLIMMIX (Production June 2006).
Results
Clinical symptoms of SIV infection
Sixteen macaques were used in this study and randomly divided into two groups as described in the methods section. Depending on the time animals succumbed to disease; they were classified either as rapid progressors (RPs) or long-term progressors (LPs) (Table 2). Three of the RP in the M+V group (B4, B5 and B6) developed severe disease symptoms that necessitated euthanasia at the early time point of 5 weeks PI. These animals exhibited symptoms of ataxia, diarrhea and loss of appetite. In addition, B4 and B5 displayed symptoms of severe neurologic disease including ticks and tremors. Two of the other RP animals (A1 from the M+V group and C3 from the V group) developed symptoms of ataxia, diarrhea and loss of appetite at week 12 that was unresponsive to treatment and therefore they were euthanized at week 13. At week 20, animal B2 from the M+V group died unexpectedly. Post-mortem examination of the spleen revealed possible follicular lymphoma. Other M+V animals (A2, A4, B3) developed symptoms of diarrhea and/or loss of appetite, however, they responded well to treatment with pedialyte and fresh fruit treats and, hence, were allowed to recover.
Table 2. Timeline of mortality and the underlying pathology in the infected macaques.
Mortality in the M+V group was as early as 5 weeks PI (3/8 animals) compared to 13 weeks PI in the V group (1/4 animals). Three out of five of the M+V rapid progressors died of meningitis/encephalitis and pneumonia whereas one died of lymphoma. The rapid progressor in the V group had mild encephalitis.
| Animal ID | Treatment | Death (p.i) Weeks | Encephalitis | Other Diagnosis | |
|---|---|---|---|---|---|
| Rapid Progressors | A1 | M+V | 13 | Mild | Pneumonia |
| B4 | M+V | 5 | Moderate | Meningitis, Pneumonia | |
| B5 | M+V | 5 | Moderate | Meningitis, Pneumonia | |
| B6 | M+V | 5 | None | Meningitis, Pneumonia | |
| B2 | M+V | 16 | None | Follicular lymphoma of spleen, moderate lung congestion, GALT & IN hyperplasia | |
| C3 | V | 13 | Mild | Pneumonia | |
| Long-term Progressors | A2 | M+V | 51 | Moderate | Pneumonia |
| A3 | M+V | 51 | Mild | Meningoencephalitis | |
| A4 | M+V | 51 | Mild | Pneumonia | |
| B3 | M+V | 51 | Mild | Pneumonia | |
| C1 | V | 52 | Mild | Meningitis | |
| C2 | V | 52 | Moderate | Pneumonia | |
| C4 | V | 52 | Mild | Meningitis, Pneumonia |
All of the M+V animals showed significant retardation in weight gain compared with the V group. For example, at four weeks prior to virus inoculation, the V group showed an average weight gain of 15.5% in contrast to an average of only 2.1% in M+V animals relative to their initial weight at week -36. At week 32 PI, the V group showed an average weight gain of 44.8%, whereas, the M+V group demonstrated a modest 20.1% weight gain (Figure 1A). Of the 12 M+V animals, 5 (42%) succumbed to infection or related illness in less than 3 months post-virus inoculation, whereas, only 1 out of 4 (25%) V animals developed symptoms prompting euthanasia (Figure 1B). This difference, however, was not statistically significant.
Figure 1. Morphine-treated animals exhibit rapid disease progression, retardation in weight gain and higher mortality rates.
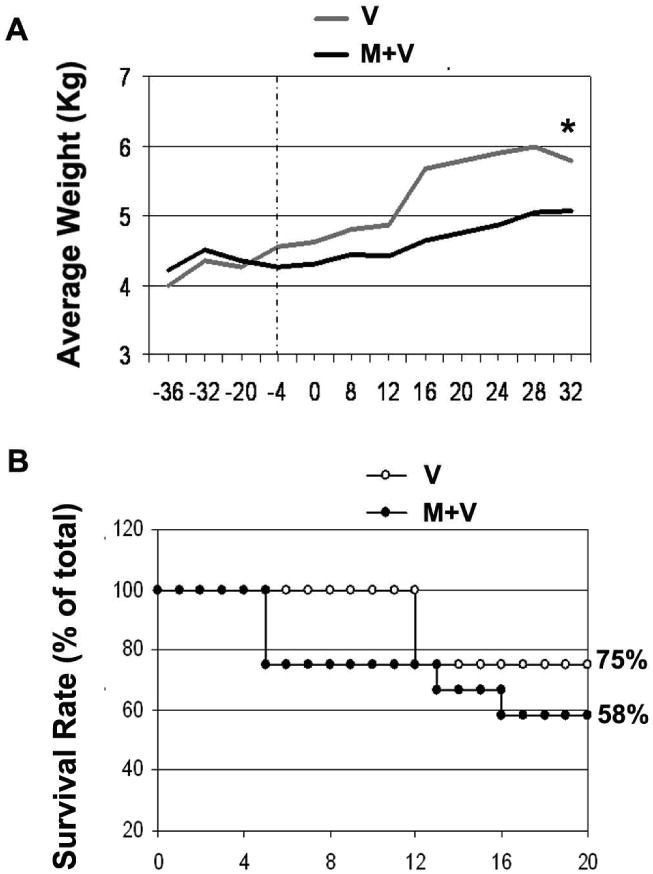
(A) Average weight gain over time, in the M and M+V groups, expressed as percent of body weight at week 36 prior to viral inoculation. *p<0.05 vs V group using the Wilcox signed rank test. (B) Survival (expressed as percentage of total animals) of morphine-treated and untreated animals infected with SIV.
Histopathological changes
Pathological findings of the brain and lung sections from the RP animals (B4 and B5) in the M+V group, exhibited mild meningioencephalitis and mild pneumonitis, with lesions that were consistent with inflammation typical of R71/17E. The M+V RP animal B6 had meningitis that resembled cytomegalovirus though there were no herpes virus inclusions. This animal also had mild pneumonitis that was not typical of a classical R71/17E infection. RP animal A1 in the M+V group had both mild encephalitis with perivascular glial nodules and pneumonia. In contrast, LP macaque B2 in the M+V group was diagnosed post-mortem with follicular lymphoma with death possibly due to gamma herpesvirus, a virus associated with lymphoma in immunocompromised macaques. The V group macaque C3 demonstrated mild encephalitis as summarized in Table 2. Pathological examination of the brain regions from C3 revealed fewer small glial nodules in the basal ganglia, frontal cortex and the brainstem. Other regions such as the cerebellum, occipital and parietal cortices showed either minor mononuclear or histiocytic infiltrates around blood vessels.
Representative brain sections from RPs in the M+V group (macaques A1, B4 and B5) demonstrated focal histiocytic nodules as shown in Figure 2 (panels A-macaque A1 and panel B- macaque B4). Formation of nodules was observed in all the brain regions and this was accompanied by the presence of golden pigment, known to be associated with oxidative stress (arrows in panel B). Additionally, frontal cortex of macaque A1 also demonstrated histiocytic infiltrations around the blood vessel (panels D and E) accompanied with syncytial cell formation (black arrow in panel D) and presence of 2 single mononuclear cells containing golden pigment (white arrows in panel D). However, there were no histiocytic nodules (panel C) or histiocytic infiltrates (panel F) in the basal ganglia of macaque C1 in the V group (Figure 2).
Figure 2. Pathological findings in the brains of the M+V and V rapid progressors were comparable.
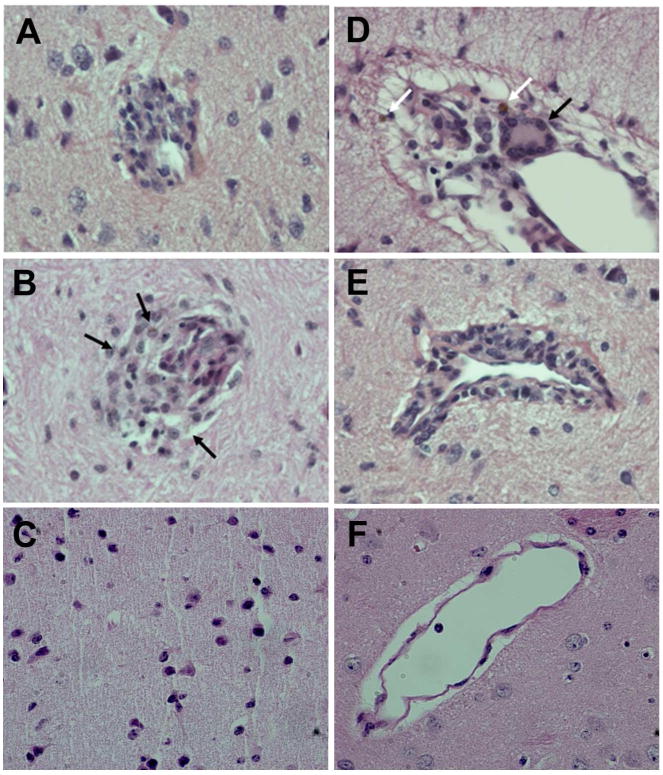
Focal histiocytic nodules in the basal ganglia of M+V group [Macaques A1 (A) & B4 (B)]; note a hint of golden pigment (associated with oxidative stress) in several cells (arrows). Histiocytic infiltrates around a blood vessel in the frontal cortex of A1 (D); note the syncytial cell formation (black arrow) and 2 single mononuclear cells containing golden pigment (white arrows). Perivascular cuff in the Parietal cortex of A1 (E). No Histiocytic (C) nodule and histiocytic infiltrates (F) in the basal ganglia from V group (macaque C3). Magnification: 40×.
Characterization of the infection
1) Viral RNA in Plasma
All the animals in the V and M+V groups demonstrated plasma viremia (105-108 viral RNA copies/mL) as early as one week PI, indicating a highly productive systemic infection. Virus load continued to rise until week 4 PI in both groups of animals. Plasma viral titers in the V animals were maintained at levels equal to or below 106 for the duration of the study. Macaque C3 in the V group however, failed to control virus replication with levels peaking up to 108 copies/ml of plasma at week 12 PI. At this point the animal was euthanized having developed ataxia, loss of appetite, and untreatable diarrhea. At week 8 PI in the M+V group, 6 of the 9 animals had plasma viral loads equal to or greater than 106; whereas, only 1 of 4 animals from the V group had greater than 105 viral copies indicating less effective control of the infection in the presence of morphine (Figure 3A and B). Comparison of plasma viral loads among RPs in the M+V versus the V group (p=0.21, p=0.17 respectively) or LPs in the M+V versus the V group (p=0.79, p=0.78 respectively) indicated no significant differences in the group-by-time interactions (Figure 3C).
Figure 3. Viral load in plasma in SIV-infected monkeys treated with morphine compared to those without morphine.
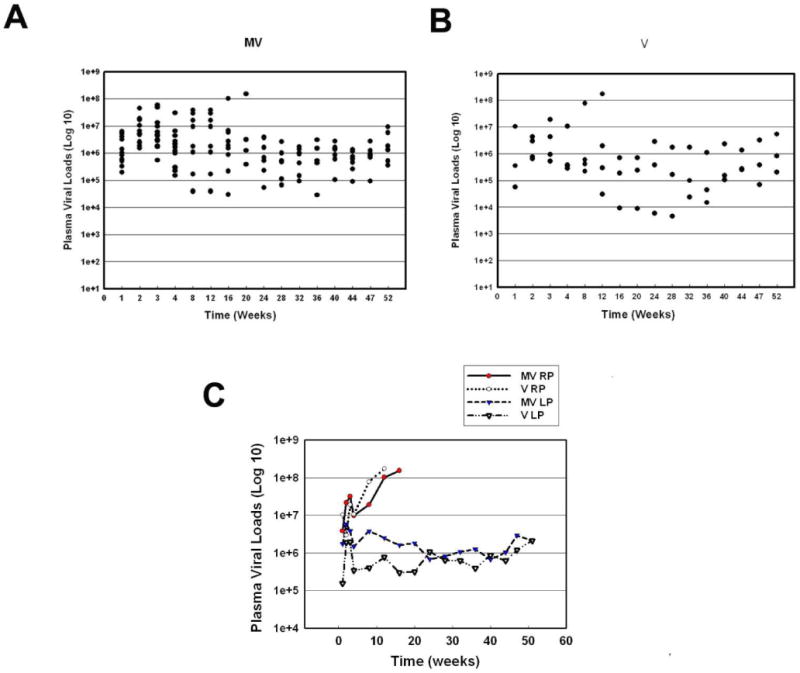
(A) Scattergram expressing viral loads in plasma of M + V animals at various time points PI. (B) Scattergram expressing viral loads in plasma of V animals at various time points PI. (C) Average virus load in the plasma from MV RP, V RP, MV LP and V LP groups over time.
2) Viral RNA in CSF
Viral RNA became detectable in CSF by week 1 following virus inoculation. Viral titers in the CSF of all animals at week 4 PI attained a level of at least 103 copies/ml (Figure 4A&B). Three of the twelve M+V animals had CSF viral titers of 107 copies/ml indicating a high degree of neuroinvasion in these animals; of note, two of these three were RPs and had to be euthanized. Intriguingly, CSF viral loads in the M+V animals were at least two logs higher than in the V animals at weeks 4 and 5 PI. Only C3, the lone RP in the V group, showed a high virus load of 1.8 × 108 copies/ml before being euthanized at week 12 PI (Figure 4B). Overall, the average CSF viral load of RPs in the M+V group at week 4 PI were at least 1-2 log higher than the RPs in the V group, 3-4 log higher than the LPs in the V group, and at least a half a log higher than the LPs in the M+V group (Figure 4C).
Figure 4. Viral load in CSF of SIV-infected monkeys treated with morphine compared to those without morphine.
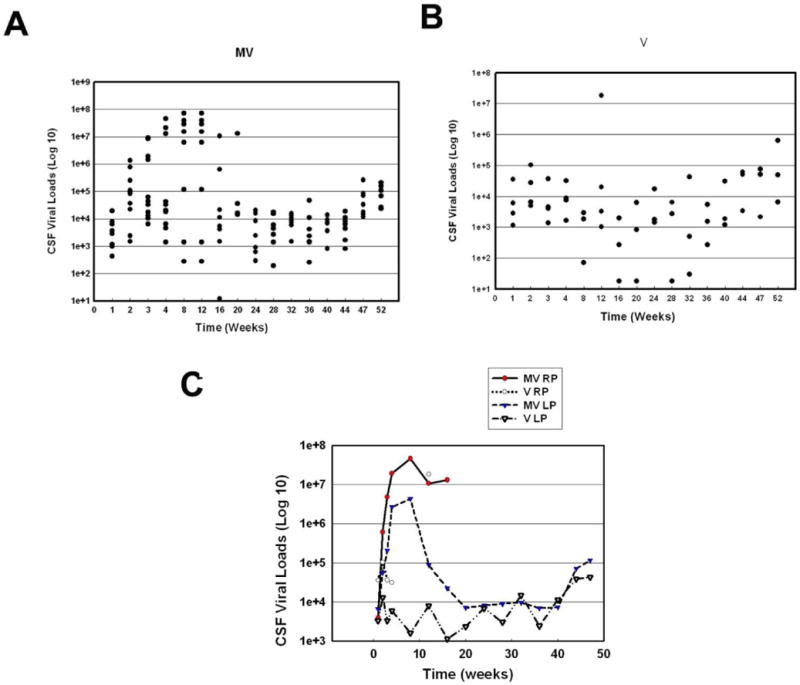
(A) Scattergram expressing viral loads in CSF of M + V animals at various time points PI. (B) Scattergram expressing viral loads in CSF of V animals at various time points PI. (C) Average of virus load in the CSF from MV RP, V RP, MV LP and V LP groups over time.
The longitudinal data of plasma and CSF RNA replicates were analyzed by mixed-effects models. The distributions of RNA replicates were skewed to the right and were compared on their logarithms assuming log-normal distributions. CSF viral load data from both the M+V and V groups showed no significant differences between the two groups at any time point except for the 8 week PI (p=0.022) (Figure 4C). Similarly, when comparing CSF viral loads in the RPs of the M+V versus the V group (p=0.21, p=0.17 respectively) or LPs in the M+V versus the V group (p=0.79, p=0.78 respectively) there were no significant differences in group-by-time interactions (data not shown). Though comparisons of the viral titers in the CSF between the two groups were not significant, there was a trend towards less control of viral replication in the M+V group, particularly in the CSF, during the first few weeks PI.
Evaluation of CD4+T cells and CD8+T cells in infected macaques
SIV R71/17E is an R5 virus that mainly targets memory CD4+ T cells in the spleen, lymph nodes and gut-associated lymphoid tissue (GALT) resulting in a precipitous loss of these cells during the early phase of the infection. Though the largest pool of activated, terminally differentiated memory CCR5+CD4+T cells resides in the mucosal tissues, peripheral blood was chosen for examination due to its ease of access. The question we wished to answer was whether morphine had an effect on CD4+ and CD8+ T cell numbers. Blood samples from animals at periodic intervals were obtained to determine the T cell subsets. FACS analysis of PBMCs from M+V and V group of animals demonstrated a loss of CD4+ cell numbers in the first few weeks PI in both the M+V and V groups (Figure 5A and B). Average CD4+T cell numbers in the M+V group animals were similar to those of V group animals until week 16 PI. From week 16 PI onwards, the M+V animals demonstrated significantly higher (p<0.0001) CD4+ numbers compared to the V group (Figure 5C).
Figure 5. CD4+T cell counts in PBMC of SIV-infected macaques treated with or without morphine.
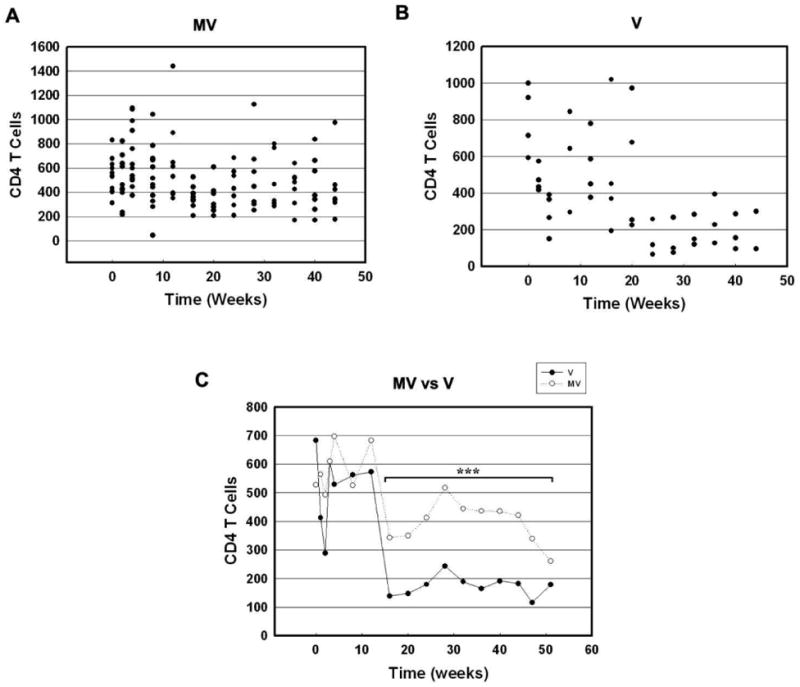
(A) Scattergram expressing CD4+T cells in PBMC of M + V animals at various time points PI. (B) Scattergram expressing CD4+T cells in PBMC of M + V animals at various time points PI. (C) Average of CD4+T cell counts in PBMC from V and MV animals at various time points PI. ***p<0.001 vs V group.
Next we sought to determine the effect of morphine on CD8+T cell numbers in plasma following infection with SIV. As shown in Figure 6A and B, CD8+T cell numbers increased in the first few weeks PI in both M+V and V groups of animals. The average CD8+T cell numbers in the M+V group were significantly higher (p<0.0007) at week 4 and week 16 onwards (Figure 6C). Interestingly, however, there was no significant difference in the CD4/CD8 cell ratios between the two groups of macaques (Figure 6D).
Figure 6.
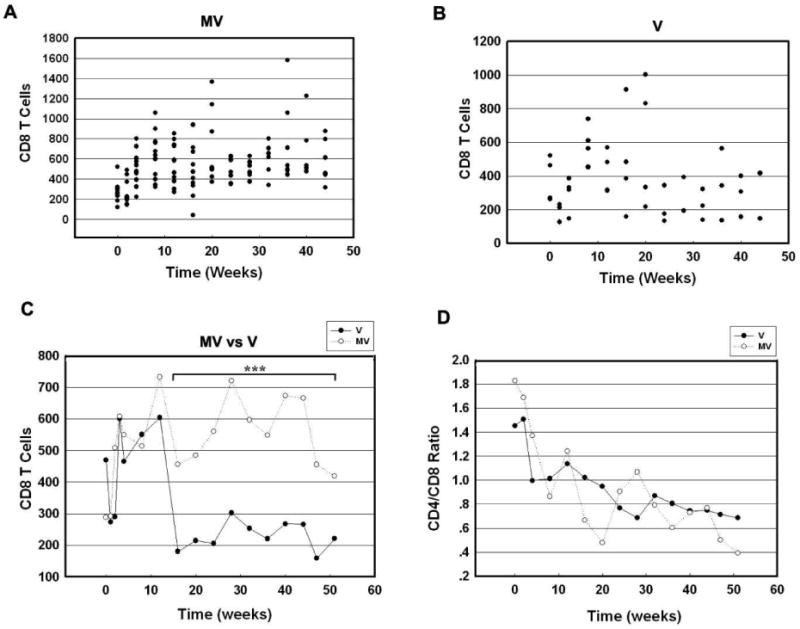
CD8+T cell counts in PBMC of SIV-infected macaques treated with or without morphine. (A) Scattergram expressing CD8+T cells in PBMC of M + V animals at various time points PI. (B) Scattergram expressing CD8+T cells in PBMC of M + V animals at various time points PI. (C) Average of CD8+T cell counts in PBMC from V and MV animals at various time points PI. (D) CD4/CD8 ratios of averaged cell counts from V and MV animals at various time points PI. ***p<0.001 vs V group.
Brain tissue viral burden
Prior to the euthanasia, all the animals were deeply anesthetized, exsanguinated, and perfused with saline solution to minimize the contamination of tissues with viral RNA from plasma. In order to determine the effect of morphine on viral burden in different regions of the brain, RNA was isolated from six regions: basal ganglia, brain stem, frontal cortex, parietal cortex, occipital cortex and cerebellum. Real-time RT-PCR for SIV gag was then conducted on all of these regions. Among the RP animals, those with encephalitis (A1, B4 B5, and C3) demonstrated significantly higher viral burdens in all the brain regions examined ranging from 107 - 108 copies compared with 104 - 105 copies in rapid progressors without encephalitis (macaque B6, Figure 7A, red dots). In contrast, SIV gag copy numbers in LP (A3, A4, C1, C2, C4) were much lower ranging from 103 to 105 with the exception of macaque A2 that exhibited copy numbers around 106 (Figure 7B, red dots).
Figure 7. Virus RNA titer in different brain regions of SIV-infected macaque with or without morphine.
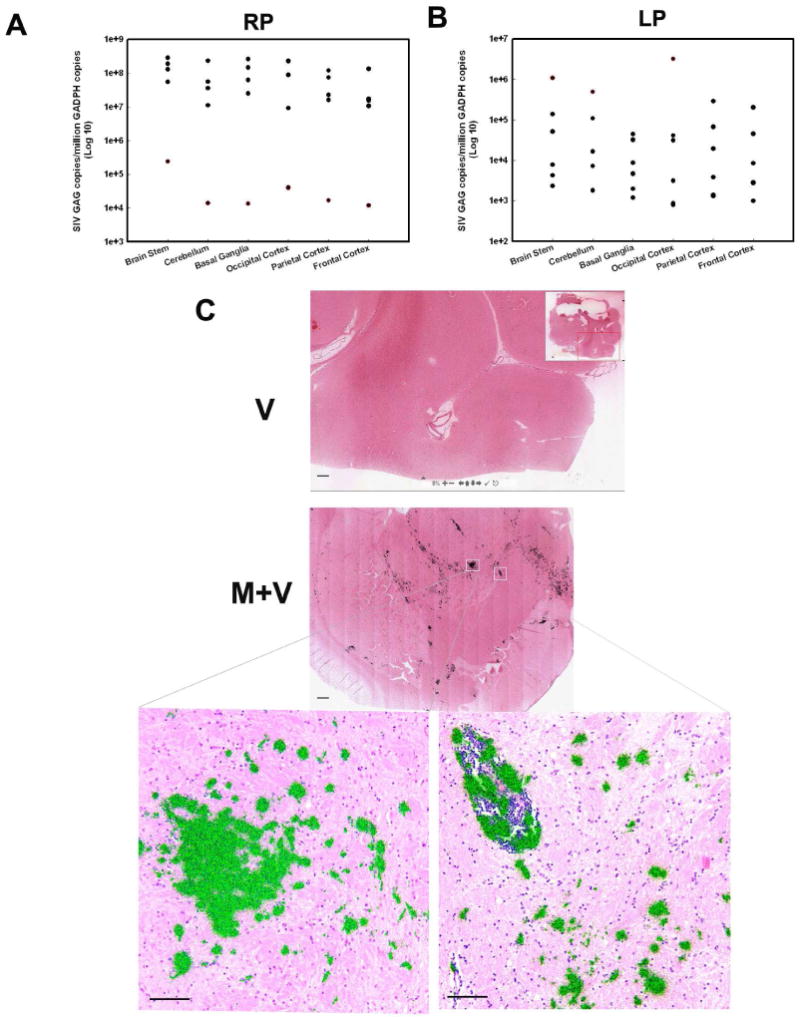
(A) Scattergram expressing viral loads in different brain region of RP animals at various time points PI. Black dots: A1, B4, B5, C3; Red dots: B6. (B) Scattergram expressing viral loads in different brain regions of LP animals at various time points PI. Black dots: A3, A4, C1, C2, C4; Red dots: A2. (C) SIV mRNA in the basal ganglia of macaque brain. In situ hybridization was used to identify SIV RNA in macaque brain sections from M+V and V group. The sections were hybridized to 35S-labelled SIV antisense probes. After washing, digestion with RNases, the sections were coated with nuclear track emulsion, exposed, and developed. When the radioautographs are illuminated with epipolarized and transmitted light, the light reflected from the silver grain imparts a greenish yellow color to cells that have relatively high levels of SIV-1 RNA. (Scale bar=500 μm for upper two panels; Scale bar=100 μm for lower two panels).
Furthermore, in situ hybridization was used to compare the SIV mRNA levels in M+V and V group. Very faint signal for SIV mRNA was observed in animals from V group; in contrast in morphine treated animals SIV mRNA was strongly evident as determined by 35S-labeled SIV specific riboprobes (Figure 7C).
Localization of viral gp120 in brain macrophages
Macrophage infiltration is one of the hallmark features of SIV encephalitis. Based on our findings demonstrating increased virus burden in the brains and CSF of morphine-dependent SIV-infected animals, we next wanted to examine whether morphine dependence resulted in increased macrophage infiltration in the CNS and, if so, were these cells also the source of SIV. Paraffin embedded serial sections from the basal ganglia region of M+V and V groups of macaques were immunostained for both viral envelope protein gp120 and macrophage marker CD68. As shown in Figure 8A, in macaque C3 (RP with mild encephalitis, V group), cells positive for the viral antigen are detectable, however, relatively few infiltrating macrophages were identified in these sections. On the other hand, brain sections of the RPs belonging to the M+V group (A1, B4, B5 and B6) demonstrated increased viral antigen primarily in cells that were CD68 positive and that were likely the newly migrating macrophages lining the blood vessels. Increased numbers of infiltrating monocytes/macrophages were observed in both the perivascular cuff and the parenchymal locations in the brains of M+V animals compared with the virus alone group of macaques (Figure 8B).
Figure 8. Localization of viral gp120 in the brain macrophages.
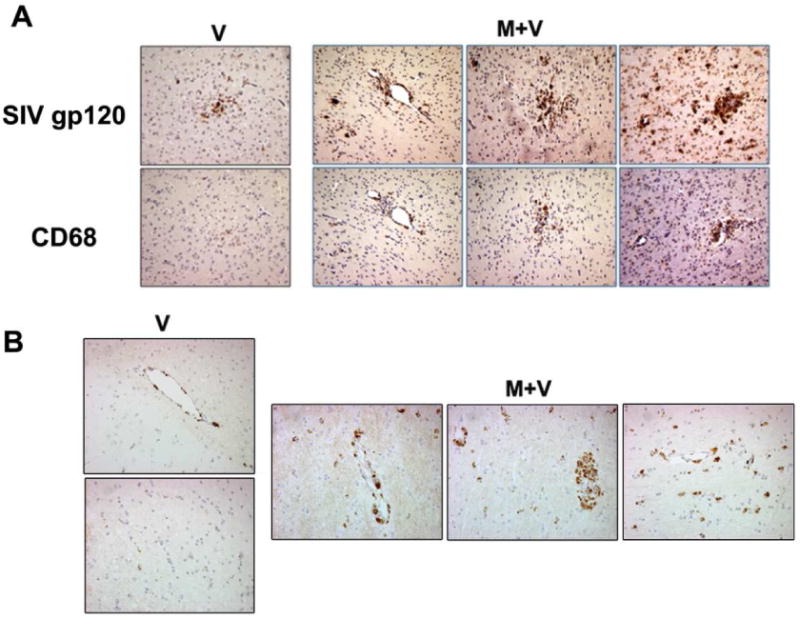
(A) Morphine treated macaques (M+V) showed more viral antigen and CD68+ macrophages in the basal ganglia compared to the SIV-treated (V) animal. The CD68 positive cells corresponded to regions that are also positive for the viral antigen gp120 indicating that macrophages constitute the majority of infected cells. (B) Representative image for CD68 staining in the basal ganglia section from V and M+V groups.
Discussion
The influence of concurrent drug abuse in HIV-infected individuals is a burning issue, as drug abusers are reported to have higher rates of both HIV encephalopathy (HIVE) and HIV-associated neurocognitive disorder (HAND) compared to infected non-drug abusers (Martinez et al., 1995; Bell et al., 1996; Chiesi et al., 1996; Goodkin et al., 1998; Nath et al., 2001). Neuropathologically, drug abusers tend to demonstrate increased neuroinflammation as evidenced by microglial activation, astrocytosis and increased virus burden in the CNS. While SIV infection in macaques has proved to be an excellent model of HIVE, studies on the impact of morphine on SIVE have been controversial. For example, some studies have demonstrated that morphine exposure increased the severity and rate of HIV disease progression (Kumar et al., 2004). In contrast, other studies have pointed to the protective effects of morphine on the progression of HIV infection (Donahoe and Vlahov, 1998; Kapadia et al., 2005; Donahoe et al., 2009). Furthermore, in another study by Marcario et al, it was concluded that morphine not only did not affect the plasma and CSF viral titers, it also did not increase the incidence or progression of neurological disease compared with the untreated, virus-infected animals (Marcario et al., 2008). The present study was an effort to resolve conclusively the role of morphine in SIV neuropathogenesis. This question was examined in Indian rhesus macaques that were divided into two groups and conditioned to either morphine or saline four times per day for 9 weeks prior to inoculation with SIVmacR71/17E. Animals were then followed up to a period of 32 weeks. We used this model to determine the effects of morphine on viral neuropathogenesis.
Morphine treatment resulted in increased plasma viral loads accompanied by a trend towards increased mortality with a subset of animals succumbing to disease as early as 5 weeks PI. Intriguingly, almost 42% of the morphine animals succumbed to disease rapidly following infection compared with 25% of animals in the virus alone group. This group of animals was hence designated as rapid progressors and the remaining group as long-term progressors. Interestingly, rapid progression of disease correlated with increased CSF and brain virus burdens. Most of these animals also demonstrated SIVE with formation of microglial nodules, and increased macrophage trafficking. In addition to increased mortality, morphine exposure also resulted in significant retardation of weight gain in the animals. Loss of weight gain in the morphine group could likely be attributable to lower food intake by these animals. Intriguingly, support for this notion comes from studies by Anghel et. al. demonstrating changes in genes related to food intake in mice exposed to short and long-term morphine exposure (Anghel et al., 2010).
In both groups of animals, as expected, levels of circulating CD4+T cells precipitously dropped within the first few weeks of infection, although, the total cell numbers in the morphine group were higher than the virus alone group. This could likely be due to the anti-apoptotic effects of morphine. The CD4:CD8 ratio however, did not differ between the two groups.
Interestingly, morphine exposure appeared to potentiate both plasma and CSF viral loads in rapid progressors, which appeared at least a log higher in the morphine versus the virus group. Virological data from plasma and CSF provides a unique opportunity to study the factors associated with the onset of encephalitis. In the present study, it was clear that both the plasma and CSF viral RNA concentrations correlated with the impending CNS disease. What was even more surprising was that the rise in virus concentrations in the CSF of morphine treated rapid progressors occurred very quickly, within a period of 4-12 weeks, and was a predictor of encephalitis. Animals with encephalitis demonstrated increased viral titers in the brains.
Encephalitis is often characterized by extensive replication of the virus in marcrophages in the brain(Koenig et al., 1986). This is accompanied by continuous recruitment of monocytes into the brain. Inside the brain, monocytes begin to differentiate into macrophages by expressing CD16 before crossing the blood brain barrier. In the brain, the recruited macrophages become viral factories resulting in enhanced virus replication. What was novel in this study was that morphine dependence resulted in increased macrophage infiltration and increased virus replication in the CNS. Furthermore, these macrophages also appeared to be the source of SIV as evidenced by the fact that brain sections from morphine-treated animals demonstrated increased CD68 positive cell infiltrate in the CNS that were most likely the newly migrating macrophages lining the blood vessels. These cells were also the source of the viral antigen in the brain.
Evaluation of findings from our study with those reported earlier on the biological role of morphine exemplify some similarities and some dissimilarities. In agreement with the previously published report by Marcario et al, demonstrating increased virus in the brain, our findings also show a trend towards increased virus accumulation in the CNS. However, contrary to our findings, these authors reported no change in plasma or CSF titers or in survival rates of animals exposed to morphine (Marcario et al., 2008). In our study morphine treated animals developed inflammatory encephalitis, characterized by focal accumulations of macrophages in the white matter. Unlike these authors, we failed to demonstrate demeyelinating lesions in the CNS in response to morphine. Studies by Suzuki et al had observed earlier that morphine caused up-regulation of the expression of the CCR5 co-receptor in macropahges and T cells (Suzuki et al., 2002). Similar to these findings, we have also previously shown that morphine up-regulates CCR5 in microglia (Bokhari et al., 2009) and rhesus macrophages (data not shown). The CCR5 co-receptor is the major receptor used by R5 SIV and HIV for entry into macrophages. Enhancement of expression of the receptor would, therefore, predict augmented virus replication in the cells. Studies by Cheung et al, have shown that morphine was critical in enhancing disease during late stage of infection (Chuang et al., 1993). These reports are in agreement with the trends observed in our findings that morphine caused enhanced virus replication and accumulation of macrophages, leading to earlier onset of late-stage disease. The caveat in this study is that morphine-mediated effects are not statistically significant; however, similar to some of the previously published studies, our findings offer the suggestion of a trend toward increased numbers of rapid progressors in the morphine-treated group. Contrary to these findings, reports by Donahoe et al, have actually demonstrated a protective role of morphine in macaques. These reports could explain the lack of statistical significance observed in our study. It could be speculated that the differences between these controversial studies could be attributed to one or more parameters including Mamu genotyping (not done in this study) of animals, drug delivery routes and viral strain used. While our studies do not clarify the controversy in the field, they do lend credence to morphine-mediated potentiation of virus replication.
In summary, our findings suggest that morphine dependence in SIV infected macaques results in increased mortality in a subset of animals that succumb to disease very rapidly. This group of animals also exhibited increased monocytic infiltration and virus replication in the CNS, thereby leading to speculation that morphine facilitates monocyte transmigration. The mechanism of increased monocyte influx could be attributed to various factors. It is likely that morphine exposure results in development of a migratory phenotype with the acquisition of the Lcy marker in monocytes as has been demonstrated for West Nile virus (Getts et al., 2008). Alternatively, morphine could also disrupt the blood brain barrier through increased expression of the chemokine MCP-1, observed in M+V animlas with encephalitis (data not shown), that is known to induce monocyte transmigration.
Despite the advent of antiretroviral therapy, complications of HIV-1 infection with concurrent drug abuse are an emerging problem. Opiates are well-known to modulate immune responses by preventing the development of cell-mediated immune responses. Their effect on the pathogenesis of HIV-1 infection however, remains controversial. Using the Simian Immunodeficiency Virus/macaque model of HIV pathogenesis, we sought to explore the impact of morphine on disease progression and pathogenesis. Sixteen Rhesus macaques were divided into 2 groups; 4 were administered saline and twelve others morphine routinely. Both groups of animals were then inoculated with SIVmacR71/17E and followed longitudinally for disease pathogenesis. The morphine group (M+V) exhibited a trend towards higher mortality rates and retardation in weight gain compared to the virus alone group. Interestingly, a subset of M+V animals succumbed to disease within weeks post-infection. These rapid progressors also exhibited a higher incidence of other end-organ pathologies. Despite the higher numbers of circulating CD4+ and CD8+T cells in the M+V group, CD4:CD8 ratios between the groups remained unchanged. Plasma and CSF viral load in the M+V group was at least a log higher than the control group. Similarly there was a trend toward increased virus build-up in the brains of M+V animals compared with controls. A novel finding of this study was the increased influx of infected monocyte/macrophages in the brains of M+V animals.
Acknowledgments
This work was supported by grant DA024442 from the National Institutes of Health (SB)
This manuscript is dedicated to the memory of a departed friend and colleague, Dr. Opendra “Bill” Narayan for his passion and love for science.
References
- Anghel A, Jamieson CA, Ren X, Young J, Porche R, Ozigbo E, Ghods DE, Lee ML, Liu Y, Lutfy K, Friedman TC. Gene expression profiling following short-term and long-term morphine exposure in mice uncovers genes involved in food intake. Neuroscience. 2010;167:554–566. doi: 10.1016/j.neuroscience.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhou P, Ho DD, Landau NR, Marx PA. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi A, Vella S, Dally LG, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F, et al. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Chuang LF, Killam KF, Jr, Chuang RY. Increased replication of simian immunodeficiency virus in CEM ×174 cells by morphine sulfate. Biochem Biophys Res Commun. 1993;195:1165–1173. doi: 10.1006/bbrc.1993.2167. [DOI] [PubMed] [Google Scholar]
- Chuang RY, Suzuki S, Chuang TK, Miyagi T, Chuang LF, Doi RH. Opioids and the progression of simian AIDS. Front Biosci. 2005;10:1666–1677. doi: 10.2741/1651. [DOI] [PubMed] [Google Scholar]
- Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2004;147:28–32. doi: 10.1016/j.jneuroim.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, O';Neil SP, Marsteller FA, Novembre FJ, Anderson DC, Lankford-Turner P, McClure HH. Probable deceleration of progression of Simian AIDS affected by opiate dependency: studies with a rhesus macaque/SIVsmm9 model. J Acquir Immune Defic Syndr. 2009;50:241–249. doi: 10.1097/QAI.0b013e3181967354. [DOI] [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Coussons-Read ME, Dykstra LA, Lysle DT. Macrophage-derived nitric oxide is involved in the depressed concanavalin A responsiveness of splenic lymphocytes from rats administered morphine in vivo. J Immunol. 1994;152:5845–5852. [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Shapshak P, Metsch LR, McCoy CB, Crandall KA, Kumar M, Fujimura RK, McCoy V, Zhang BT, Reyblat S, Xin KQ, Kumar AM. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83:88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41:1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Pohlmann S, Hamacher M, Means RE, Kraus T, Uberla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEM×174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354:192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Li Q, Gebhard K, Schacker T, Henry K, Haase AT. The relationship between tumor necrosis factor and human immunodeficiency virus gene expression in lymphoid tissue. J Virol. 1997;71:7080–7082. doi: 10.1128/jvi.71.9.7080-7082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, Jia F, Adany I, Sun KH, Dhillon S, Zhuge W, Narayan O. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004;173:4100–4107. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- Marcario JK, Riazi M, Adany I, Kenjale H, Fleming K, Marquis J, Nemon O, Mayo MS, Yankee T, Narayan O, Cheney PD. Effect of morphine on the neuropathogenesis of SIVmac infection in Indian Rhesus Macaques. J Neuroimmune Pharmacol. 2008;3:12–25. doi: 10.1007/s11481-007-9085-z. [DOI] [PubMed] [Google Scholar]
- Martinez AJ, Sell M, Mitrovics T, Stoltenburg-Didinger G, Iglesias-Rozas JR, Giraldo-Velasquez MA, Gosztonyi G, Schneider V, Cervos-Navarro J. The neuropathology and epidemiology of AIDS. A Berlin experience. A review of 200 cases. Pathol Res Pract. 1995;191:427–443. doi: 10.1016/S0344-0338(11)80730-2. [DOI] [PubMed] [Google Scholar]
- Marx PA, Chen Z. The function of simian chemokine receptors in the replication of SIV. Semin Immunol. 1998;10:215–223. doi: 10.1006/smim.1998.0135. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEM×174 lymphocytes. J Biol Chem. 2000;275:31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Cheney PD, Raymond LA, Joag SV, Stephens EB, Adany I, Pinson DM, Li Z, Marcario JK, Jia F, Wang C, Foresman L, Berman NE, Narayan O. Morphological correlates of neurological dysfunction in macaques infected with neurovirulent simian immunodeficiency virus. Neuropathol Appl Neurobiol. 1999;25:285–294. doi: 10.1046/j.1365-2990.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Charboneau R, Loh HH, Barke RA. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005;175:6361–6367. doi: 10.4049/jimmunol.175.10.6361. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Gupta S, Charboneau R, Loh HH, Barke RA. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147:78–81. doi: 10.1016/j.jneuroim.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Spijkerman IJ, Langendam MW, Veugelers PJ, van Ameijden EJ, Keet IP, Geskus RB, van den Hoek A, Coutinho RA. Differences in progression to AIDS between injection drug users and homosexual men with documented dates of seroconversion. Epidemiology. 1996;7:571–577. doi: 10.1097/00001648-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang AJ, Chuang LF, Doi RH, Chuang RY. Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry. J Infect Dis. 2002;185:1826–1829. doi: 10.1086/340816. [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J Pharmacol Exp Ther. 1993;267:703–706. [PubMed] [Google Scholar]
- Thorpe LE, Frederick M, Pitt J, Cheng I, Watts DH, Buschur S, Green K, Zorrilla C, Landesman SH, Hershow RC. Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37:1423–1430. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- Tomei EZ, Renaud FL. Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro. J Neuroimmunol. 1997;74:111–116. doi: 10.1016/s0165-5728(96)00213-5. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000a;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000b;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]


