Abstract
Ornithine transcarbamylase deficiency (OTCD), the most common and severe urea cycle disorder, is an excellent model for developing liver-directed gene therapy. No curative therapy exists except for liver transplantation which is limited by available donors and carries significant risk of mortality and morbidity. Adeno-associated virus 8 (AAV8) has been shown to be the most efficient vector for liver-directed gene transfer and is currently being evaluated in a clinical trial for treating hemophilia B. In this study, we generated a clinical candidate vector for a proposed OTC gene therapy trial in humans based on a self-complementary AAV8 vector expressing codon-optimized human OTC (hOTCco) under the control of a liver-specific promoter. Codon-optimization dramatically improved the efficacy of OTC gene therapy. Supraphysiological expression levels and activity of hOTC were achieved in adult spfash mice following a single intravenous injection of hOTCco vector. Vector doses as low as 1×1010 genome copies (GC) achieved robust and sustained correction of the OTCD biomarker orotic aciduria and clinical protection against an ammonia challenge. Functional expression of hOTC in 40% of liver areas was found in mice treated with a low vector dose of 1×109 GC. We suggest that the clinical candidate vector we have developed has the potential to achieve therapeutic effects in OTCD patients.
Keywords: Adeno-associated viruses (AAV), liver gene therapy, ornithine transcarbamylase deficiency (OTCD), codon optimization
1. Introduction
Inborn errors of metabolism affecting the urea cycle can trigger hyperammonemia, a life threatening condition that often leads to irreversible cognitive impairment, coma and death [1, 2]. The prevalence of urea cycle disorders is estimated to be at least 1 in 15,000 [3]. Patients with ornithine transcarbamylase deficiency (OTCD), an X-linked disorder, account for nearly half of all cases of inborn errors of urea synthesis, making it a compelling disorder for developing new therapies [4]. Current therapies for OTCD have numerous challenges [5-7]. Patients can be managed with a low protein diet combined with the use of medications that activate alternate nitrogen clearance pathways, however, this regimen does not prevent hyperammonemic crises [6, 7]. Liver transplantation can cure OTCD, although it is limited by availability of donor livers, associated morbidity and mortality of the procedure, and the immunosuppressive drugs that are necessary to prevent rejection of the graft [8-11].
Vectors based on adeno-associated virus (AAV) have shown great potential for sustained expression of therapeutic transgenes. The main advantages of AAV are the attractive safety profiles and the possibility of generating long-term transgene expression without the requirement for chromosomal integration. Successful transduction of hepatocytes has been achieved in a number of animal species, including mice, dogs and non-human primates [12-15]. Among the AAV serotypes, AAV8 has been shown to be the most efficient for liver-directed gene transfer [16, 17], and is currently being evaluated in a clinical trial for hemophilia B [18].
We and others have demonstrated the capacity of AAV-based gene therapy to restore protective levels of liver OTC enzyme activity with a single treatment in spfash mouse, a mouse model of OTCD [19-21]. Systemic delivery of AAV2/8 vectors expressing murine OTC (mOTC) under the control of liver-specific promoters achieved sustained correction. Modifications of the vector and/or transgene cassette, such as the use of a self-complementary (sc) AAV vector, incorporation of Kozak-like sequences or a post-transcriptional regulatory element, dramatically improved the potency of the vector [20, 21]. More recently, Cunningham et al showed that in spfash mice rendered completely deficient in OTC through vector-mediated expression of shRNA that the dose of OTC-expressing AAV vector required to prevent hyperammonemia was five-fold lower than that required to normalize the biomarker for OTCD, orotic aciduria [22].
Progression of a gene therapy product for OTC into the clinic eventually requires pre-clinical evaluation of a vector expressing the human OTC (hOTC) gene. However, previous studies using adenoviral vectors have illustrated the difficulties of expressing sufficient levels of active human OTC in OTCD mice [23, 24]. Using chimeric OTC constructs, Ye et al demonstrated that differences in the human and mouse amino-terminal leader peptides of OTC caused low activity of hOTC in spfash mice [25]. In the current study, we focused on generating an efficient clinical candidate AAV vector for OTC gene therapy in humans. We performed detailed evaluations of the characterizations of the kinetics, stability, and efficacy of the AAV vector in spfash mice.
2. Materials and Methods
2.1. Codon optimization, vector construction and production
The initial codon optimization of human OTC cDNA was performed by GenScript using proprietary OptimumGene™ codon optimization technology (GenScript, Piscataway, NJ). The DNA sequences were examined and further modified to eliminate potential alternative reading frames from internal out of frame ATG with the size equal to or greater than 9 peptides. The final codon-optimized human OTC cDNA (hOTCco) was synthesized by GenScript. pAAVsc.TBG.hOTCwt and pAAVsc.TBG.hOTCco were constructed by replacing the mOTC coding sequencing with wild-type (WT) hOTC (hOTCwt) or hOTCco cDNA, respectively, in a plasmid derived from the previously described pAAVsc.TBG.mOTC1.3 with the intron disrupted [19-21]. The two vector preps (AAV2/8sc.TBG.hOTCwt and AAV2/8sc.TBG.hOTCco) used in the initial comparison study (described in Fig. 1) were purified by two rounds of cesium chloride gradient centrifugation, as previously described [17]. Vectors used in the rest of the study were produced by a scaled production method based on polyethylenimine (PEI) transfection and purified from supernatant or total lysate by iodixanol gradient centrifugation as described [26]. Genome titers [genome copies (GC)/ml] of AAV vectors were determined by real-time PCR using primer and probe sets targeting the TBG promoter (forward primer 5′-AAACTGCCAATTCCACTGCTG-3′, reverse primer 5′-CCATAGGCAAAAGCACCAAGA-3′, probe 6FAM–TTGGCCCAATAGTGAGAACTTTTTCCTGC–TAMRA), and using a linearized plasmid as the standard. The forward primer is located 400bp downstream of the 5′ closed hairpin. Fagone et al recently reported that the quantitative PCR (Q-PCR) method could significantly underestimate the titer of scAAV vectors, especially when the PCR primers were close to the closed hairpin of the scAAV vector [27]. We remeasured the titer of one lot of AAV2/8sc.TBG.hOTC covector using a primer and probe set targeting the polyA region (1900bp downstream of the 5′ closed hairpin), and the genome titer was 1.1-fold of the original titer, which was within the intra-assay error of Q-PCR.
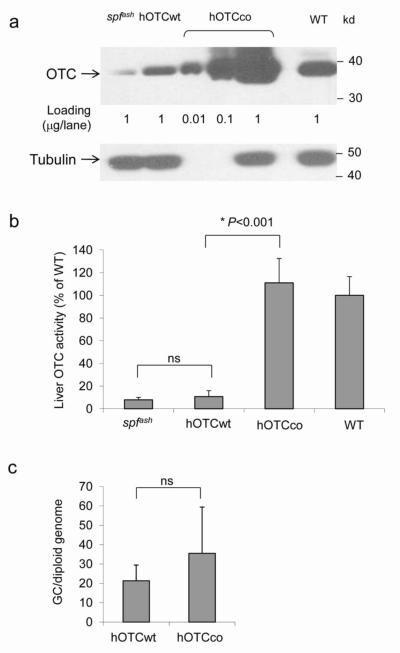
Figure 1. Dramatic improvement of OTC expression levels and activity in liver by codon optimization of the hOTC gene.
Adult male spfash mice were injected intravenously with 1×1011 GC of AAV2/8sc.TBG.hOTCwt or AAV2/8sc.TBG.hOTCco vectors. Fourteen days after vector treatment, liver was harvested. (a) Western analysis to detect OTC protein in the liver lysates from untreated and vector-treated spfash mice, and WT mouse. (b) Liver OTC activity following vector treatment. OTC activity levels are presented as percentages of the mean level in WT mice (n=5; Mean ± S.D.). The OTC activities in mice treated with AAV2/8sc.TBG.hOTCwt (hOTCwt) were not statistically different (ns) from untreated spfash mice. (c) Vector genome copies in liver.*P<0.001, student t test.
2.2. Animal studies
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Spfash mice were maintained at the Animal Facility of the Translational Research Laboratories at the University of Pennsylvania as described previously [19]. Three to six months old spfash mice and their normal littermates were used in the studies. Vectors were administered by intravenous (i.v.) injection via the tail vein.
2.3. Measurement of urinary orotic acid
Urine samples were collected before and at various time points after vector treatment for orotic acid analysis as previously described [19].
2.4. OTC enzyme activity assay
OTC enzyme activity was measured using a liquid chromatography mass spectrometry stable isotope dilution method to detect the formation of citrulline normalized to [1,2,3,4,5-13C5] citrulline (98% 13C). The method is adapted from a previously developed assay for detection of N-acetylglutamate synthase activity [28]. Slivers of fresh frozen liver were weighed and briefly homogenized in buffer containing 10 mM HEPES, 0.5 % Triton X-100, 2.0 mM EDTA and 0.5 mM DTT. Volume of homogenization buffer was adjusted to obtain 50 mg/ml tissue. Enzyme activity was measured using 250 μg liver tissue in 50 mM Tris-acetate, 4 mM ornithine, 5 mM carbamyl phosphate, pH 8.3. Enzyme activity was initiated with the addition of freshly prepared 50 mM carbamyl phosphate dissolved in 50 mM Tris-acetate pH 8.3, allowed to proceed for 5 minutes at 25 °C and quenched by addition of an equal volume of 5 mM 13C5-citrulline in 30%TCA. Debris was separated by 5 minutes of microcentrifugation, and the supernatants were transferred to vials for mass spectroscopy. Ten μL of sample was injected into an Agilent 1100 series LC-MS under isocratic conditions with a mobile phase of 93% solvent A (1 ml trifluoroacetic acid in 1 L water):7% solvent B (1ml trifluoroacetic acid in 1L of 1:9 water/acetonitrile). Peaks corresponding to citrulline [176.1 mass:charge ratio (m/z)] and 13C5-citrulline (181.1 m/z) were quantitated, and their ratios were compared to ratios obtained for a standard curve of citrulline run with each assay. Samples were normalized to either total liver tissue or to protein concentration determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
2.5. OTC histochemistry and immunostaining
Histochemical demonstration of OTC enzyme activity and immunostaining of OTC protein in liver sections were performed as previously described [21, 24]. The primary antibody to detect hOTC was a custom rabbit polyclonal antibody provided by Hiroki Morizono’s laboratory at the Children’s National Medical Center.
2.6. Western blot analysis
Western analysis to detect hOTC expression in liver lysate was performed as previously described [21]. The primary antibody to detect hOTC was a custom rabbit polyclonal antibody provided by Hiroki Morizono’s laboratory at the Children’s National Medical Center. Liver lysates (10 μg/lane) were also blotted and probed by an anti-tubulin antibody (Abcam, Cambridge, MA).
2.7. Ammonia challenge
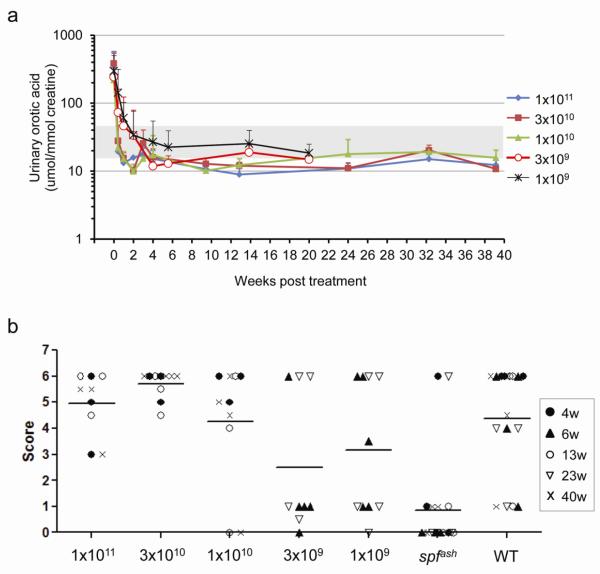
As a nitrogen-load challenge test, mice were injected intraperitoneally with a 0.75 M NH4Cl solution at the dose of 7.5 mmolkg−1. Behavioral measurements were assessed as previously described [21], and the scoring scale was slightly modified [21, 29] (Table 1). A complete normal mouse would score as 6. Untreated spfash mice and the normal littermates were challenged at the same time as controls.
Table 1.
Behavioral scoring scale
| Category | Score | Description of behavior |
|---|---|---|
| Ataxia | 3 | Normal gait |
| 2 | Abnormal gait | |
| 1 | Unable to support itself | |
| 0 | Death | |
| Response to sound | 3 | No response to sound, normal behavior |
| 2 | Twitching of extremities | |
| 1 | Jumping | |
| 0 | Moribund; unable to right itself |
2.8. Quantification of vector genomes in liver
Vector genomes in liver were quantified by real-time PCR (TaqMan Universal Master Mix, Applied Biosystems, Foster City, CA) as described previously [30]. Primer sequences and PCR conditions are described above in section 2.1. The PCR conditions were set at 1 μg total cellular DNA as template, 300 nM primers, and 200 nM probe each. Cycles were for 10 min at 95 °C, 40 cycles of 15 seconds at 95 °C, and 1 min at 60 °C. A value of 1.5×105 GC per 1 μg DNA was calculated to represent one GC per diploid genome. Each test sample was also spiked in with 106 copies of linearized plasmid DNA to ensure there were no inhibition effects from the test sample.
2.9. Morphometric analysis of OTC expression in liver
The relative area of liver sections occupied by transduced hepatocytes was determined on sections processed both for OTC immunostaining and for OTC enzyme activity staining. A representative image was taken with a 10x objective from every liver section under identical camera and microscope settings for each staining method. ImageJ software (Rasband 1997–2006; National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) was used to threshold the images (i.e., to mark areas of hepatocytes staining positive for OTC protein or enzyme activity) and then to determine the percentage of the selected area per total image area. Reported are the average percentage values for each group.
3. Results
3.1. Significant improvement of OTC protein expression levels and activity by codon optimization of hOTC cDNA
To generate an efficient clinical candidate vector for gene therapy of OTCD in humans, we first performed codon-optimization on human OTC cDNA, using Genscript’s OptimumGene™ codon optimization technology. The optimized cDNA sequences were examined and further modified to eliminate potential alternative reading frames (ARFs) from internal non-in-frame ATG sequences that could theoretically encode peptides of 9 or more amino acids in length. This measure was taken to avoid potential cytotoxic T lymphocyte (CTL) responses to transgene products generated from ARFs [31]. Both wild-type human OTC cDNA (hOTCwt) and codon optimized human OTCcDNA (hOTCco) were cloned into a previously established AAV2/8sc.TBG.mOTC vector [21], replacing the murine OTC cDNA. OTC protein expression levels and OTC activity were evaluated in the liver of spfashmice 14 days after i.v. injection of 1×1011 GC of AAV2/8sc.TBG.hOTCwt or AAV2/8sc.TBG.hOTCco vectors. The extent of gene transfer based on resident vector genomes was not statistically different between the two groups (Fig. 1c). Western analysis demonstrated 100-fold higher expression of hOTC from the hOTCco vector as compared to the hOTCwt vector, reaching levels in slight excess of those seen in WT mice (Fig. 1a). An assessment of OTC enzyme activity generally correlated with the OTC Western blot experiments (Fig. 1b) although OTC protein was more elevated than OTC enzyme activity when compared to endogenous OTC. When subtracting the background activity levels in the spfash mice, the hOTCco vector resulted in over 33-fold higher activity than the hOTCwt vector.
3.2. The dose of vector correlated with liver OTC activity and gene transfer
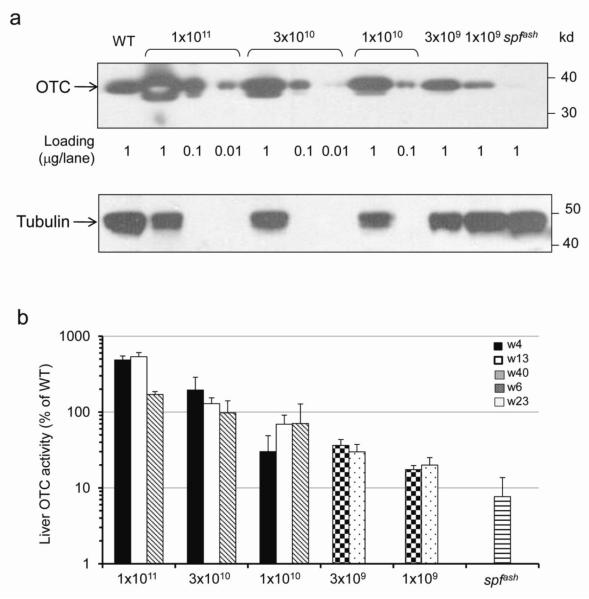
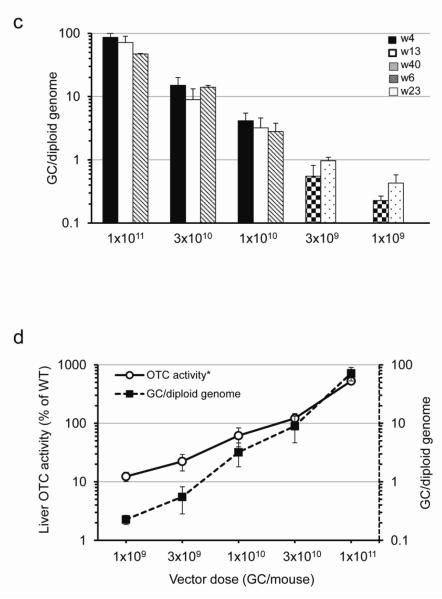
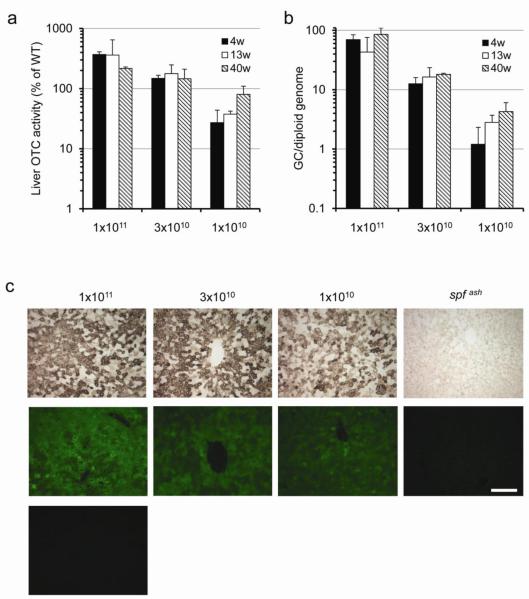
To further characterize the activity of the hOTCco vector, a dose escalation study was carried out in spfash mice, varying doses half logs between 1×109 to 1×1011 GC. The dose of vector correlated with transgene-derived OTC protein as assessed by Western analysis on liver lysates (Fig. 2a). Mice treated with 1×1010 GC of the hOTCco vector had OTC protein levels close to WT mice. Liver OTC activity levels also correlated well with vector doses. Mice treated with 3×1010 GC of the hOTCco vector had OTC activity levels close to WT mice (Fig. 2b). Vector genome copies in liver also correlated well with vector doses with close to 1 copy of the vector genome per diploid genome detected in the liver of mice treated with 3×109 GC of vector (Fig. 2c). A linear correlation was observed between vector genome copies in liver and an incremental increase in liver OTC activity levels in the vector-treated mice (Fig. 2d). Except for the 1×1011 GC dose group, which had a 2-fold reduction of vector genomes in liver and a 3-fold reduction in liver OTC activity between week 4 and week 40, stable gene transfer and stable gene expression was observed in all the other four dose groups for the duration of the experiment, which spanned 40 weeks (high dose groups) or 23 weeks (low dose groups; Figs. 2b and c).
Figure 2. Dose correlated OTC protein expression levels and vector genomes in liver.
Livers were harvested from vector-treated spfash and untreated control mice at 4, 13, and 40 weeks (1×1011, 3×1010 and 1×1010 GC groups) or 6 and 23 weeks (3×109 and 1×109 GC groups) post vector treatment. (a) Western blot analysis on liver lysates from WT, vector-treated (4 or 6 weeks post) or untreated spfash mice (1 μg, 0.1 μg or 0.01 μg protein per lane). Representative samples from each dose group are shown. (b) Dose-correlated liver OTC activity at different time points post vector administration. Mean ± S.D. are shown (n=3-5 per group). (c) Dose-correlated vector genome copies in the liver of treated mice at different time points post vector administration. Mean ± S.D. are shown (n=3-5 per group). (d) Dose response showing the correlation of vector genome copies and liver OTC activity levels (*background activity in the spfash mice was subtracted). Data for 1×1011, 3×1010 and 1×1010 GC groups were based on week 13, and data for 3×109 and 1×109 GC groups were based on week 23. Mean ± S.D. are shown (n=3-5 per group).
3.3. Evaluation of transduction efficiency by immunostaining and OTC histochemical staining
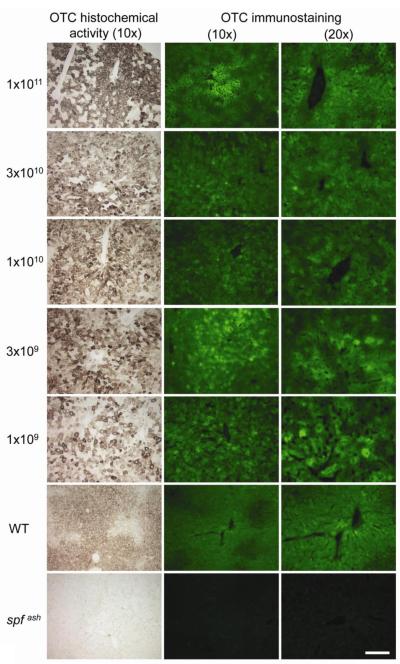
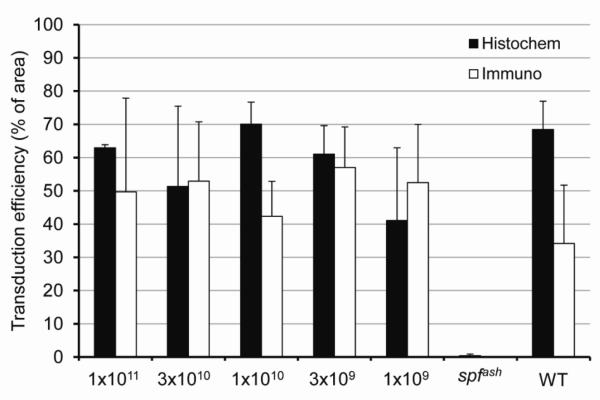
For OTC gene therapy to be effective, it is desirable to have a broad transduction of hepatocytes. To evaluate transduction efficiency, liver sections were analyzed by both immunostaining for OTC protein expression and histochemical staining for OTC activity. As shown in Fig. 3a, there was a general consistency between the immunostaining and OTC histochemical staining results, although OTC histochemical staining appeared to be somewhat more sensitive than the immunostaining. There was no clear dose-correlated effect on transduction efficiency based on morphometric analyses; high transduction was achieved throughout the entire range of doses (Fig. 3b). Between doses 1×1011 and 3×109 GC, transduction efficiency as measured by histochemical staining varied between 50-70%. At the lowest dose of 1×109 GC, 40% of the liver areas were positive by OTC histochemical staining. We estimated that at a dose of 1×109 GC, each transduced hepatocyte contained a single copy of vector genome. This was based on the vector genome copy analysis which showed an average of 0.4 copies per diploid genome.
Figure 3. High levels of OTC protein expression and activity in the liver of spfash mice treated with AAV2/8sc.TBG.hOTCco.
(a) Liver was harvested at 40 weeks (1×1011, 3×1010 and 1×1010 GC groups) or 23 weeks (3×109 and 1×109 GC groups) post vector treatment for histochemical staining of OTC activity (left panels, 20x) and immunostaining of hOTC protein (middle and right panels, 10x and 20x, respectively). Representative pictures from each dose group are shown. Scale bar: 200 μm (left and middle panels) and 100 μm (right panel). (b) Morphometric analysis of liver transduction efficiency based on OTC histochemical staining (Histochem) and immunostaining of OTC protein (Immuno). N=3-5, mean ± S.D.
3.4. Robust and sustained correction of OTCD in spfash mice following a single intravenous injection of AAV2/8sc vector
Phenotype correction in the hOTCco vector-treated spfash mice was evaluated by the correction of the biomarker for OTCD, urinary orotic aciduria, and by the clinical responses of the mice to an NH4Cl challenge. Three days after a single i.v. injection of AAV2/8sc.TBG.hOTCco vector at the dose of 1×1011, 3×1010, and 1×1010 GC (higher dose groups), urinary orotic acid levels in the treated mice were normalized to WT levels (Fig. 4a). The lower dose groups (3×109 and 1×109 GC) took slightly longer to achieve complete correction of orotic aciduria. Once achieved, the correction was stable and sustained throughout the study (40 weeks for the higher dose groups, and 23 weeks for the lower dose groups). NH4Cl challenges were performed at various time points after vector treatment. The higher dose groups had similar responses as WT mice after an NH4Cl challenge, while the lower dose groups had improved responses but lower scores than the WT mice (Fig. 4b).
Figure 4. Dose response study on the efficacy of AAV2/8sc.TBG.hOTCco vector.
Adult male spfash mice were injected i.v. with AAV2/8sc.TBG.hOTCco vector at the dose of 1×1011, 3×1010, 1×1010, 3×109 and 1×109 GC. (a) Robust and sustained normalization of urinary orotic acid levels following vector treatment. Shaded area indicates orotic acid levels in normal mice. (b) Outcome of NH4Cl challenge performed at 4, 6, 13, 23, or 40 weeks post AAV vector treatment.
3.5. Equivalent function of human and mouse OTC leader peptides
Previous adenoviral vector-based gene transfer studies have suggested that the differences between mouse and human mitochondrial leader peptide sequences of OTC may lead to inefficient import of hOTC into mouse mitochondria [25]. As shown above, the hOTCco protein expressed from AAV2/8sc.TBG.hOTCco vector was active and functional in mouse liver, indicating that the human leader peptide sequences work well in mice. We formally evaluated the impact of a mouse OTC leader sequence on the expression of the hOTCco enzyme by creating a chimera (mL-hOTCco) and produced the corresponding sc AAV8 vector. Evaluation in spfash mice showed that similar gene transfer efficiencies were achieved with the human and mouse mitochondrial leader peptide sequences. Furthermore, there were no significant differences between hOTCco and mL-hOTCco in terms of liver OTC activity at the 3 vector doses tested (Fig. 5). Similar kinetics of correction of orotic aciduria and responses to an NH4Cl challenge were observed (data not shown). These data demonstrate that the human mitochondrial leader peptide sequence in hOTCco works as efficiently as the mouse leader peptide sequence in the context of codon optimization of hOTC cDNA.
Figure 5. In vivo evaluation of AAV2/8sc.TBG.mL-hOTCco vector.
Spfash mice were injected i.v. with AAV2/8sc.TBG.mL-hOTCco vector at the dose of 1×1011, 3×1010, and 1×1010GC. Livers were harvested at 4, 13, and 40 weeks post vector treatment for measurement of liver OTC activity (a), or vector genome copies in liver (b), or OTC enzyme activity staining (top panel) and immunostaining (middle panel) at 40 weeks post vector administration (c). Immunostaining with naïve rabbit serum is shown at the bottom panel as control. Scale bar: 200 μm.
4. Discussion
AAV8 has shown great potential for liver-directed gene therapy. Efficacy has been demonstrated in many preclinical studies using a number of animal species, and AAV8 is currently being evaluated in a gene therapy trial for hemophilia B [15, 18, 20, 32]. Yet, challenges remain, including pre-existing neutralizing antibodies and immune responses towards AAV-encoded transgene products or AAV capsid proteins [33, 34]. Gene therapy of a metabolic disease such as OTCD presents a more challenging model for gene replacement therapy. Because the gene acts in a cell-autonomous manner (i.e., it can only influence the cell in which it is expressed), therapeutic effects should be directly correlated with the number of target cells that are transduced, rather than with the net level of expression in liver such as with a secreted protein where high expression per cell can overcome low transduction. Furthermore, it appears that hOTCwt mRNA is unstable. Without the addition of the mRNA stabilizing woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) in a helper-dependent adenoviral vector, hOTC mRNA was hard to detect in transduced liver by Northern blot analysis [35]. There was an additional concern in evaluating the efficacy of hOTC gene therapy in mouse models because of the possibility that a species-specific mitochondrial importation signal was required [25].
In the current study, we optimized codon usage in the hOTC cDNA in order to maximize translation and improve mRNA stability, and removed potential ARFs to avoid CTL responses to transgene products generated from ARFs [31]. This single modification step dramatically improved the hOTC protein levels by 100-fold and OTC liver activity by 33-fold (Fig. 1). Sustained and dose-correlated hOTC expression and activity levels were observed in the treated spfash mice. Compared to our previous AAV2/8sc.TBG.mOTC1.3 vector which differs mainly in the cDNA [21], the hOTCco vector was about 10-fold more potent. Liver activity levels in spfashmice treated with 3×109 GC of hOTCco vector were similar to those in mice treated with a 10-fold higher dose of the mOTC1.3 vector. The dose response study showed good correlation of vector doses with OTC protein levels and OTC activity levels. Mice treated with 1×1010 GC of the hOTCco vector had OTC protein levels close to those in WT mice (Fig. 2a), and mice treated with 3×1010 GC of the hOTCco vector had OTC activity levels close to WT mice (Fig. 2b). The fact that slightly higher vector doses were needed to reach WT activity levels than those needed to reach the protein levels suggests that not all expressed protein was fully functional.
The most remarkable aspect of this study was the extraordinarily high level of transduction, as measured by OTC histological assays, throughout a broad range of doses. Between doses 1×1011 and 3×109 GC, transduction efficiency, as measured by histochemical staining, varied between 50-70%. At the lowest dose of 1×109 GC, 40% of the liver areas were positive by OTC histochemical staining. The lack of a clear dose effect by histochemistry and immunostaining could be due to the fact that codon optimization significantly improves hOTC expression in the transduced hepatocytes. This leads to improved sensitivity to detect transduced cells with low vector genome copies. Transduction could be saturated with high vector doses (1×1011 - 1×1010 GC), and therefore transduction efficiency measured by in situ detection methods would not discriminate between low and high dose groups in contrast to OTC enzyme activity on liver lysates measured by mass spectrometry. The fact that not 100% of hepatocytes appear transduced even at the highest dose of 1×1011 GC could be related to the long-term time point of 9 months in this study where initial expression might have been lost in some individual hepatocytes.
Morphometric analysis showed that about 40% of the liver areas were positive for OTC expression in mice 23 weeks post treatment with 1×109 GC of the hOTCco vector (Fig. 3). Vector DNA measurement showed that there were on average 0.4 vector DNA copies per diploid genome in the liver of mice treated with 1×109 GC of the hOTCco vector (Fig. 2c).These data indicate that each transduced cell had detectable hOTC expression. This level of transduction has never been achieved based on the commonly used reporter genes, such as β-galactosidase or enhanced green fluorescence protein. The results of experiments using these reporter genes have puzzled researchers in that the vector genomes far exceed the number of transduced cells. Our data suggest that the explanation may simply be the sensitivity of the histological assays. In our case, the combination of extraordinarily high efficiency of the optimized transgene cassette together with highly sensitive histological assays indicates every resident vector genome is transcriptionally active and capable of functional correction. The fact is, however, that at some point the level of expression per cell diminishes below that capable of fully reconstituting the urea cycle, since protection to an NH4Cl challenge was less effective at 1×109 GC than at 1×1010 GC.
The correction of urine orotic aciduria and protection against an NH4Cl challenge by the hOTCco vector was robust and long-lasting, indicating the efficacy and stability of hOTCco gene therapy. Three days after a single intravenous injection of vector at 1×1010 GC or higher, urine orotic acid levels in the treated mice were normalized and sustained throughout the experiment (40 weeks; Fig. 4a). It took 1-2 weeks for the lower dose groups (1×109 and 3×109 GC) to reduce the urine orotic acid levels to normal range.
We were originally concerned about our ability to assess the functionality of the hOTCco vector in the spfash mice due to the previous finding that a species-specific leader peptide sequence is needed for import of the OTC protein from the cytoplasm into the mitochondria [25]. To assess this, we constructed a vector encoding a chimeric construct with the mouse leader peptide (mL-hOTCco) and tested it in the spfash mice. We, in fact, found that there were no detectable differences in the OTC liver activity between mL-hOTCco and hOTCco vectors as measured by both an OTC enzyme assay in liver lysates and by OTC histochemical staining of liver sections. The higher expression levels of hOTC protein from the codon-optimized hOTC vector appear to be sufficient to overcome the species specificity of mitochondrial import and processing previously reported by Ye et al[25]. A previous study using helper-dependent adenovirus expressing hOTC with the help of WPRE also showed long-term efficacy in spfash mice [35]. It is possible that in human hepatocytes, where impeded import of hOTC is not an issue, the efficacy of the hOTCco vector may be even higher than that observed in the mouse system.
In summary, we have demonstrated that codon-optimization is an effective and relatively simple way to achieve dramatic improvements in the efficacy of OTC gene therapy. Such optimization has also been demonstrated with other transgenes such as human clotting factor IX that is being evaluated in a clinical trial [14, 15, 18, 36, 37]. The clinical candidate vector we described here has the potential to achieve therapeutic effects in OTCD patients.
Highlights.
- Codon optimization significantly improves OTC protein expression levels and activity.
- The clinical candidate AAV8 vector has high transduction efficiency and efficacy.
- Robust and sustained correction in OTCD mice by a sc AAV2/8 vector expressing hOTC.
- Detection of functional enzyme from each persisting vector genome.
Acknowledgements
We thank Martin Lock, Shu-Jen Chen, Julio Sanmiguel and Vector Core of Gene Therapy Program for supplying vectors and performing quality control assays. This work was supported in part by the Kettering Family Foundation and the following grants to J.M.W.: P01-HD057247, P01-HL059407, and P30-DK047757.
Footnotes
Disclosure/conflict of interest J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Batshaw ML, Monahan PS. Treatment of urea cycle disorders. Enzyme. 1987;38:242–250. doi: 10.1159/000469211. [DOI] [PubMed] [Google Scholar]
- [2].Krivitzky L, Babikian T, Lee HS, Thomas NH, Burk-Paull KL, Batshaw ML. Intellectual, adaptive, and behavioral functioning in children with urea cycle disorders. Pediatr Res. 2009;66:96–101. doi: 10.1203/PDR.0b013e3181a27a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, Seashore MR, Lee HS, McCarter RJ, Krischer JP, Batshaw ML. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seminara J, Tuchman M, Krivitzky L, Krischer J, Lee HS, Lemons C, Baumgartner M, Cederbaum S, Diaz GA, Feigenbaum A, Gallagher RC, Harding CO, Kerr DS, Lanpher B, Lee B, Lichter-Konecki U, McCandless SE, Merritt JL, Oster-Granite ML, Seashore MR, Stricker T, Summar M, Waisbren S, Yudkoff M, Batshaw ML. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100(Suppl 1):S97–105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- [6].Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr. 2001;138:S46–54. doi: 10.1067/mpd.2001.111836. discussion S54-45. [DOI] [PubMed] [Google Scholar]
- [7].Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK, Cann HM, Kerr D, Mamunes P, Matalon R, Myerberg D, Schafer IA. Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. N Engl J Med. 1982;306:1387–1392. doi: 10.1056/NEJM198206103062303. [DOI] [PubMed] [Google Scholar]
- [8].Todo S, Starzl TE, Tzakis A, Benkov KJ, Kalousek F, Saheki T, Tanikawa K, Fenton WA. Orthotopic liver transplantation for urea cycle enzyme deficiency. Hepatology. 1992;15:419–422. doi: 10.1002/hep.1840150311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meyburg J, Hoffmann GF. Liver transplantation for inborn errors of metabolism. Transplantation. 2005;80:S135–137. doi: 10.1097/01.tp.0000186905.10088.e5. [DOI] [PubMed] [Google Scholar]
- [10].McBride KL, Miller G, Carter S, Karpen S, Goss J, Lee B. Developmental outcomes with early orthotopic liver transplantation for infants with neonatal-onset urea cycle defects and a female patient with late-onset ornithine transcarbamylase deficiency. Pediatrics. 2004;114:e523–526. doi: 10.1542/peds.2004-0198. [DOI] [PubMed] [Google Scholar]
- [11].Leonard JV, McKiernan PJ. The role of liver transplantation in urea cycle disorders. Mol Genet Metab. 2004;81(Suppl 1):S74–78. doi: 10.1016/j.ymgme.2003.08.027. [DOI] [PubMed] [Google Scholar]
- [12].Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, Kay MA. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- [13].Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr., High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- [14].Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, Davidoff AM. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, Sanmiguel J, Morizono H, Batshaw ML, Wilson JM. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang L, Wang H, Bell P, McCarter RJ, He J, Calcedo R, Vandenberghe LH, Morizono H, Batshaw ML, Wilson JM. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ponder KP. Hemophilia gene therapy: a Holy Grail found. Mol Ther. 2011;19:427–428. doi: 10.1038/mt.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A, Sanmiguel J, Wu D, Bell P, Gao GP, Raper SE, Wilson JM, Batshaw ML. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther. 2006;14:25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [20].Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol Ther. 2009;17:1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L, Wang H, Morizono H, Bell P, Jones D, Lin J, McMenamin D, Yu H, Batshaw ML, Wilson JM. Sustained correction of OTC deficiency in spfash mice using optimized self-complementary AAV2/8 vectors. Gene Ther. doi: 10.1038/gt.2011.111. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cunningham SC, Kok CY, Dane AP, Carpenter K, Kizana E, Kuchel PW, Alexander IE. Induction and prevention of severe hyperammonemia in the spfash mouse model of ornithine transcarbamylase deficiency using shRNA and rAAV-mediated gene delivery. Mol Ther. 2011;19:854–859. doi: 10.1038/mt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morsy MA, Zhao JZ, Ngo TT, Warman AW, O’Brien WE, Graham FL, Caskey CT. Patient selection may affect gene therapy success. Dominant negative effects observed for ornithine transcarbamylase in mouse and human hepatocytes. J Clin Invest. 1996;97:826–832. doi: 10.1172/JCI118482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I, Wilson JM. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- [25].Ye X, Zimmer KP, Brown R, Pabin C, Batshaw ML, Wilson JM, Robinson MB. Differences in the human and mouse amino-terminal leader peptides of ornithine transcarbamylase affect mitochondrial import and efficacy of adenoviral vectors. Hum Gene Ther. 2001;12:1035–1046. doi: 10.1089/104303401750214267. [DOI] [PubMed] [Google Scholar]
- [26].Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic Errors in Quantitative Polymerase Chain Reaction Titration of Self-Complementary Adeno-Associated Viral Vectors and Improved Alternative Methods. Hum Gene Ther. doi: 10.1089/hgtb.2011.104. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morizono H, Caldovic L, Shi D, Tuchman M. Mammalian N-acetylglutamate synthase. Mol Genet Metab. 2004;81(Suppl 1):S4–11. doi: 10.1016/j.ymgme.2003.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ye X, Robinson MB, Pabin C, Quinn T, Jawad A, Wilson JM, Batshaw ML. Adenovirus-mediated in vivo gene transfer rapidly protects ornithine transcarbamylase-deficient mice from an ammonium challenge. Pediatr Res. 1997;41:527–534. doi: 10.1203/00006450-199704000-00012. [DOI] [PubMed] [Google Scholar]
- [30].Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, Sanmiguel JC, Sun X, Wivel NA, Raper SE, Furth EE, Batshaw ML, Wilson JM. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [31].Li C, Goudy K, Hirsch M, Asokan A, Fan Y, Alexander J, Sun J, Monahan P, Seiber D, Sidney J, Sette A, Tisch R, Frelinger J, Samulski RJ. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci U S A. 2009;106:10770–10774. doi: 10.1073/pnas.0902269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, Wilson JM. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- [33].Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL, Wilson JM. Impact of Pre-Existing Immunity on Gene Transfer to Nonhuman Primate Liver with Adeno-Associated Virus 8 Vectors. Hum Gene Ther. doi: 10.1089/hum.2011.031. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mian A, McCormack WM, Jr., Mane V, Kleppe S, Ng P, Finegold M, O’Brien WE, Rodgers JR, Beaudet AL, Lee B. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- [36].Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, Samulski RJ, Monahan PE. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- [37].Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, Davidoff AM. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]