Abstract
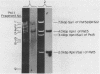
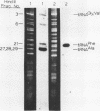
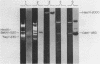
The utility of chemically synthesized deoxyoligonucleotides as hybridization probes for the detection of tRNA genes has been examined. Chloroplast tRNA genes were chosen for this study. Deoxyoligonucleotides complementary to highly conserved regions of chloroplast tRNA genes of both higher plants and Euglena gracilis were chemically synthesized. These synthetic probes have been used to detect tRNA genes by Southern hybridizations to restriction fragments of chloroplast DNAs. This new method of tRNA gene mapping and the oligonucleotides synthesized may be of general application to many chloroplast genomes. This is illustrated by the detection of known and unknown tRNA genes of Euglena gracilis and spinach, and unknown tRNA genes of maize and cucumber chloroplast DNAs. The precise locus and polarity of the Euglena gracilis chloroplast tRNAPhe gene has been determined. We also describe experiments which relate to the effects of the time of hybridization, the stringency of washing, and of base pair mismatches on the hybridization signal.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Brum C. K., Siberklang M., RajBhandary U. L., Hecker L. I., Barnett W. E. The first nucleotide sequence of an organelle transfer RNA: chloroplastic tRNAphe. Cell. 1976 Dec;9(4 Pt 2):717–723. doi: 10.1016/0092-8674(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- El-Gewely M. R., Helling R. B., Farmerie W., Barnett W. E. Location of a phenylalanine tRNA gene on the physical map of the Euglena gracilis chloroplast genome. Gene. 1982 Mar;17(3):337–339. doi: 10.1016/0378-1119(82)90150-0. [DOI] [PubMed] [Google Scholar]
- Graf L., Kössel H., Stutz E. Sequencing of 16S--23S spacer in a ribosomal RNA operon of Euglena gracilis chloroplast DNA reveals two tRNA genes. Nature. 1980 Aug 28;286(5776):908–910. doi: 10.1038/286908a0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Hallick R. B. Restriction endonuclease map of Euglena gracilis chloroplast DNA. Biochemistry. 1977 Apr 19;16(8):1665–1671. doi: 10.1021/bi00627a022. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Noyes B. E., Agarwal K. L. Detection of gastrin-specific mRNA using oligodeoxynucleotide probes of defined sequence. J Biol Chem. 1979 Aug 25;254(16):7472–7475. [PubMed] [Google Scholar]
- Noyes B. E., Mevarech M., Stein R., Agarwal K. L. Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1770–1774. doi: 10.1073/pnas.76.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Gray P. W., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. I. The location of transfer RNA, 5 S, 16 S, and 23 S ribosomal RNA genes. J Biol Chem. 1980 Nov 25;255(22):10991–10996. [PubMed] [Google Scholar]
- Orozco E. M., Jr, Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. I. Physical map of the transfer RNA gene loci. J Biol Chem. 1982 Mar 25;257(6):3258–3264. [PubMed] [Google Scholar]
- Orozco E. M., Jr, Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. II. Nucleotide sequence analysis of a tRNAVal-tRNAAsn-tRNAArg-tRNALeu gene cluster. J Biol Chem. 1982 Mar 25;257(6):3265–3275. [PubMed] [Google Scholar]
- Orozco E. M., Jr, Rushlow K. E., Dodd J. R., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. II. Nucleotide sequence homology between the 16 S--23 S ribosomal RNA spacer and the 16 S ribosomal RNA leader regions. J Biol Chem. 1980 Nov 25;255(22):10997–11003. [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Clone banks of the mung bean, pea and spinach chloroplast genomes. Gene. 1981 Oct;15(1):21–26. doi: 10.1016/0378-1119(81)90100-1. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Orozco E. M., Jr, Lipper C., Hallick R. B. Selective in vitro transcription of Euglena chloroplast ribosomal RNA genes by a transcriptionally active chromosome. J Biol Chem. 1980 Apr 25;255(8):3786–3792. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Hecker L. I., Barnett W. E. Transcriptional origin of Euglena chloroplast tRNAs. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1984–1988. doi: 10.1073/pnas.73.6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of sequences of tRNA genes. Nucleic Acids Res. 1982 Jan 22;10(2):r57–r81. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Tullis R. H., Gutierrez R., Rubin H. Specific detection of human and rabbit glucagon mRNA using a synthetic oligodeoxynucleotide. Biochem Biophys Res Commun. 1980 Apr 14;93(3):941–947. doi: 10.1016/0006-291x(80)91166-3. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]