Abstract
Objective
To test the hypothesis that variability in SNCA Rep1, a polymorphic dinucleotide microsatellite in the promoter region of the gene encoding α-synuclein, modifies the association between head injury and Parkinson’s disease (PD) risk.
Methods
Participants in Farming and Movement Evaluation (FAME) and Study of Environmental Association and Risk of Parkinsonism using Case-Control Historical Interviews (SEARCH), two independent case-control studies, were genotyped for Rep1 and interviewed regarding head injuries with loss of consciousness or concussion prior to PD diagnosis. Logistic regression modeling adjusted for potential confounding variables and tested interaction between Rep1 genotype and head injury.
Results
Consistent with prior reports, relative to medium-length Rep1, short Rep1 genotype was associated with reduced PD risk (pooled odds ratio (OR) 0.7, 95% confidence interval (CI) 0.5-0.9), and long Rep1 with increased risk (pooled OR 1.4, 95%CI 0.95-2.2). Overall, head injury was not significantly associated with PD (pooled OR 1.3, 95%CI 0.9-1.8). However, head injury was strongly associated with PD in those with long Rep1 (FAME OR 5.4, 95%CI 1.5-19; SEARCH OR 2.3, 95%CI 0.6-9.2; pooled OR 3.5, 95%CI 1.4-9.2, p-interaction 0.02). Individuals with both head injury and long Rep1 were diagnosed 4.9 years earlier than those with neither risk factor (p = 0.03).
Interpretation
While head injury alone was not associated with PD risk, our data suggest head injury may initiate and/or accelerate neurodegeneration when levels of synuclein are high, as in those with Rep1 expansion. Given the high population frequency of head injury, independent verification of these results is essential.
INTRODUCTION
Despite several decades of research, established genetic and environmental risk factors of Parkinson’s disease (PD) are rare and explain only a small proportion of cases. Rather, most PD is thought to have multifactorial etiology, with both genetic and environmental factors contributing.1
At least 10 epidemiologic studies over the past 25 years reported associations of mild-to-moderate head injury with increased risk of PD,2-11 but others failed to find such an association.12-17 Inconsistency could be due to differences in diagnostic methods, definition of head injury, or other methodological issues. Alternatively, it could reflect differential susceptibility to effects of head injury in genetically heterogeneous study populations. Because more than 2 million traumatic brain injuries occur annually in the U.S.,18 clarification of the relationship between head injury and PD is essential.
The central role of α-synuclein protein in both familial and sporadic PD is well established. A universal component of Lewy pathology, it is clearly implicated in PD etiology. Duplications and triplications of wild-type SNCA, the gene encoding α- synuclein, have been identified in typical and early onset PD, respectively, strongly suggesting SNCA over-expression is directly related to PD pathogenesis.19 Moreover, expansion of Rep1, a polymorphic mixed-dinucleotide repeat in the SNCA promoter region that increases expression in both animal models20 and humans21, 22 is associated with elevated risk for sporadic PD.23, 24
Head injury has also been shown to increase and modify α-synuclein in animal models and humans, possibly due to axonal shear injury and nitrative stress. 25-28 Thus, we hypothesized that variability in Rep1 genotype might modify the association between head injury and PD risk and tested this hypothesis in two well-characterized case-control populations.
METHODS
Participants were drawn from two case-control studies of PD: FAME (Farming and Movement Evaluation) and SEARCH (Study of Environmental Association and Risk of Parkinsonism using Case-Control Historical Interviews). Analyses were conducted in each population separately and in pooled samples.
Subject Ascertainment
FAME is a case-control study nested in the Agricultural Health Study (AHS).29 The AHS is a prospective study of private pesticide applicators (mostly farmers) and their spouses recruited in 1993-97 in Iowa and North Carolina (n = 84,742).30 Participants were identified from AHS data releases P1REL0506 and AHSREL06 (http://aghealth.nci.nih.gov/). Cases: AHS cohort members suspected to have PD were identified by self-report. As part of FAME, neurologists assessed suspect case subjects at home. Assessments included a standardized neurological history, examination and scripted videotaped assessment of parkinsonism. Final diagnosis was by consensus of two movement disorder specialists using all available information including medical records, applying NINDS/UK Brain Bank criteria.31, 32 Controls: Potential control subjects were identified by stratified random sampling of all non-demented AHS participants not suspected to have PD, and were frequency-matched to case subjects by age, gender, and state (Iowa or North Carolina) at a ratio of approximately three per case. Neurologists or technicians trained by neurologists conducted control assessments. Technician-assessed controls with possible parkinsonism were re-assessed by neurologists. 88% of “suspected” cases and 71% of eligible controls participated, and a total of 115 case and 383 control subjects were enrolled.
SEARCH is a case-control study of PD and parkinsonism conducted in eight North American movement disorders centers between July 2004 and May 2007.33 Cases: Non-demented case subjects were consecutively enrolled in six centers and convenience sampled in two. Diagnostic evaluations conducted by enrolling investigators (movement disorder specialists) included neurological history and examination, assessment of cognitive status and response to therapy. All SEARCH case subjects included in the present study met NINDS/UK Brain Bank criteria for PD.31,32 Controls: Control subjects without neurodegenerative disorders or dementia were frequency-matched to cases by age, gender and location. To minimize bias related to demographic or socioeconomic differences, controls were primarily non-blood relatives (68%) or acquaintances (15%) referred by patients in the clinical practice of the enrolling physicians. The remainder had other non-patient relationships with referring clinics (7%) or were recruited using a commercial list of telephone numbers matching on case subjects’ zipcodes (10%). A total of 519 case and 511 control subjects were enrolled. Blood was available for 172 control subjects, because most controls were not evaluated in person. Demographic characteristics were similar in controls with and without blood collection.
Human subjects
FAME and SEARCH were approved by institutional review boards (IRBs) of all participating institutions. All participants provided written informed consent.
Data collection
Exposure assessments
Exposure assessment methods were identical in FAME and SEARCH. Trained interviewers at the Parkinson’s Institute used standardized computer-assisted telephone interviews (CATI) to collect demographic and risk factor information including history of head injury, use of tobacco and other data, such as lifetime occupational and residential histories. If a participant was deceased or cognitively impaired at interview, a proxy respondent was recruited. Head injury was defined as an affirmative response to the question “Have you ever had a head injury where you lost consciousness or were diagnosed with a concussion by a doctor.” The number of head injuries and presence or absence of loss of consciousness for each injury was recorded. Tobacco use was defined as smoking at least one cigarette daily for six months or longer. Exposures were assessed until a reference age, defined as diagnosis age for cases, and median case diagnosis age in corresponding gender-, state/center- and age-specific strata for controls.
Genotyping
DNA was extracted from venous blood.34 Genotyping of SNCA Rep1 allele length variants was conducted by genomics cores at Mayo Clinic, Jacksonville (FAME), and the University of California, San Francisco (SEARCH), using similar methods and common reference allele length.23, 35 A mixed dinucleotide marker [(TC)x(TT)1(TC)y(TA)z(CA)w] in the 5′ upstream region of SNCA represents the Rep1 genomic region (8,748 bp upstream of exon 1; accession no.U46895; D4S3481). Published and optimized primers were used to amplify the region: NACPRep1- F (5′-CCTGGCATATTTGATTGCAA-3′) and NACPRep1- R (5′-GACTGGCCCAAGATTAACCA-3′). Sizing of PCR products (267bp, ref seq chr4:90986062-90986328) was performed on an ABI 310 using Genescan software or ABI 3730 using GeneMapper (Applied Biosystems, Inc, Foster City, CA).
Statistical Analyses
We compared subject characteristics within and between study populations using Fisher’s exact test or Pearson’s chi-square statistic for categorical data and independent t-tests or Mann-Whitney-Wilcoxon rank-sum tests for continuous data. Between-study covariate heterogeneity was tested using Cochran’s statistic. Deviation from Hardy Weinberg equilibrium (HWE) was tested with Pearson’s chi-square statistic. Associations between head injury, Rep1 allele length and PD were tested using unconditional logistic regression. To control for potential confounding, we included reference age (tertiles), gender, ethnicity (non-Hispanic white or other), and cigarette smoking in all models. 97% of FAME participants were non-Hispanic white, and we considered 6 subjects with missing race/ethnicity to be non-Hispanic white. Because SEARCH had a higher proportion of non-white subjects (12%), we excluded three participants with missing race/ethnicity. In sensitivity analyses, we examined whether adjusting for pesticide use (ever/never applied), educational level or proxy informant status changed inferences, and excluded head injuries that occurred within five and 10 years of the reference date to rule out reverse causation. We also performed analyses restricted to non-Hispanic whites and men.
For logistic regression modeling, we systematically chose a parsimonious coding of SNCA Rep1 genotype that would permit inclusion of all available genotyping data. Rep1 allele length was coded as “short” (257bp or 259bp), “medium” (261bp) or “long” (263bp or 265bp), resulting in six possible genotype combinations (Table 1). The parsimonious model that fit best was a “dominant” model, wherein the effect of a single short or long allele was dominant over a medium allele. The reference genotype was medium/medium (M_M). We tested interaction using likelihood-ratio tests comparing regression models with and without two multiplicative-interaction terms for head injury (yes, no) × S* genotype and head injury × L* genotype. We also conducted analyses of pooled observations from both studies. This model additionally included a study variable (FAME or SEARCH) and, after examination of potential covariate heterogeneity, an interaction term for smoking x study (p < 0.2). Additional analyses considered an alternative Rep1 genotype coding employed by other investigators,36 which defined three genotype classes: 259* for 259_259 or 259_261; 261_261 (reference); and 263* for 261_263, or 263_263, excluding other allele combinations. This model excludes the 259_263 genotype, because the alleles have opposing effect on PD risk. Analyses were conducted with SAS version 9.1.3 (SAS Institute, Cary, NC) and SPSS version 12.0 (SPSS Inc., Chicago, IL).
Table 1.
SNCA Rep1 Genotype Parameterization
| Allele lengths | Genotype coding | Dominant codinga |
|---|---|---|
| 259_259 | Short/short | S* |
| 259_261 | Short/medium | S* |
| 257_263, 259_263 or 259_265 | Short/long | S*_L* |
| 261_261 | Medium/medium | M_M, reference |
| 261_263 or 261_265 | Medium/long | L* |
| 263_263, 263_265 or 265_265 | Long/long | L* |
short and long alleles are coded as dominant over a medium allele
RESULTS
DNA was available for 100 case and 371 control subjects in FAME, and 418 case and 172 control subjects in SEARCH. Rep1 genotype could be assigned for 97.2% and 99.0% of subjects, respectively. Complete genotyping and interview data were available for 89 cases and 329 controls in FAME, and 387 cases and 159 controls in SEARCH. Demographic characteristics were similar in subjects with and without complete data (data not shown). Compared with FAME, SEARCH participants were more likely to be female, non-white, and have a post-secondary education (Table 2). At enrollment, FAME cases had a longer PD duration than SEARCH cases (7.5 vs. 2.8 years), and were approximately five years older, although reference age was similar. Rep1 allele frequencies were comparable in both studies and satisfied Hardy-Weinberg equilibrium (p > 0.7).
Table 2.
Subject Characteristics
| FAME | SEARCH | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Number | 89 | 329 | 387 | 159 |
|
| ||||
| Reference age Mean (SD), range |
61.5 (9.1), 45-87 |
61.7 (7.4), 45-80 |
61.6 (10.1), 30-86 |
61.5 (10.0), 32-91 |
|
| ||||
| Enrollment age Mean (SD), range |
69.0 (8.0), 48-87 |
69.3 (8.1), 42-88 |
64.4 (9.9), 30-87 |
65.0 (9.6), 37-92 |
|
| ||||
| Years from reference date until interview Mean (SD), range |
8.5 (5.3) 0-23 |
8.3 (4.5) 0-22 |
3.0 (2.0) 0-9 |
3.7 (1.3), 0-7 |
|
| ||||
| Male (%) | 65 (73%) | 247 (75%) | 229 (59%) | 97 (61%) |
|
| ||||
| Non-Hispanic White | 86 (97%) | 322 (98%) | 340 (88%) | 139 (87%) |
|
| ||||
| Education, Mean years (SD) | 12.4 (2.4) | 12.7 (2.1) | 14.9 (2.1) | 14.7 (2.1) |
|
| ||||
| Head injury (%) | 20 (22%) | 58 (18%) | 97 (25%) | 36 (23%) |
| 1 head injury | 19 (21%) | 48 (15%) | 69 (18%) | 32 (20%) |
| > 1 head injury | 1 (1%) | 10 (3%) | 28 (7%) | 4 (3%) |
|
| ||||
| -with unconsciousness | 18 (21%) | 48 (15%) | 75 (19%) | 26 (17%) |
|
| ||||
| Age at first head injury, Mean (SD), range | 28.6 (15.7), 8-67 |
25.2 (18.8), 2-73 |
22.3 (15.3), 2-65 |
18.1 (14.2), 2-76 |
|
| ||||
| Years first head injury preceded reference date, Mean (SD), range |
30.1 (15.4), 1-54 |
37.3 (21.0), 1-72 |
37.1 (16.5), 2-73 |
42.9 (15.5), 4-77 |
|
| ||||
| Cigarette smoker (%) | 21 (24%) | 120 (37%) | 150 (39%) | 70 (44%) |
|
| ||||
| Proxy respondent (%) | 13 (15%) | 4 (1.2%) | 0% | 0% |
|
| ||||
| -proxies reporting a head injury | 1 | 2 | n/a | n/a |
|
| ||||
| Rep1 Allele frequencies | ||||
| 257 | 0 | 0 | 1 (0.1%) | 0 |
| 259 | 38 (21.3%) | 171 (30.0%) | 184 (23.8%) | 98 (30.8%) |
| 261 | 118 (66.3%) | 433 (65.8%) | 519 (67.1%) | 198 (62.3%) |
| 263 | 21 (11.8%) | 52 (7.9%) | 66 (8.5%) | 20 (6.3%) |
| 265 | 1 (0.6%) | 2 (0.3%) | 4 (0.5%) | 2 (0.6%) |
|
| ||||
| Rep1 Dominant Genotype^ | ||||
| S* | 29 (32.6%) | 129 (39.2%) | 140 (36.1%) | 78 (49.0%) |
| M_M | 39 (43.8%) | 149 (45.3%) | 182 (47.0%) | 61 (38.4%) |
| L* | 18 (20.2%) | 37 (11.2%) | 48 (12.4%) | 15 (9.4%) |
| S*_L* | 3 (3.4%) | 14 (4.3%) | 17 (4.4%) | 5 (3.1%) |
Coding defined in Table 1
Abbreviations: S* = short/short or short/medium; M_M = medium/medium; L* = medium/long or long/long
Head injuries were more frequently reported in SEARCH than FAME (24.3% vs. 18.6%, p = 0.04), but rates of head injury with unconsciousness were similar (18.7% vs. 16%). A greater proportion of SEARCH cases reported more than one head injury (7% vs. 1%; p = 0.04). In both studies, injuries were significantly more common in men (SEARCH: 29.8% of men, 16.4% of women, p < 0.001; FAME: 22.1% of men, 8.5% of women, p = 0.003).
Consistent with prior reports, relative to homozygous medium genotype (M_M), short Rep1 genotype (S*) was associated with lower PD risk, and long Rep1 genotype (L*) tended to increase risk (Table 3) (pooled p-trend for genotype = 0.0003). PD diagnosis age was similar across genotypes (data not shown). The first head injury occurred on average more than 30 years before reference age. Though risk was modestly elevated, overall, head injury was not significantly associated with PD in FAME, SEARCH, or the pooled sample. Associations were similar when considering only injuries that resulted in loss of consciousness. In the pooled model, there was a non-significant trend toward increasing risk with number of head injuries (OR for 1 head injury 1.2, 95%CI 0.8-1.7; OR for >1 head injury 1.7, 95%CI 0.8-3.6; p-trend = 0.13). Mean diagnosis age was younger in subjects with a head injury (59.4, standard error (SE) 1.0 with head injury; 62.4, SE 0.5 without head injury; p = 0.006, adjusted for gender, race, smoking).
Table 3.
Risk-Factor-Associated Odds Ratios for PD (95% Confidence Interval)a
| Variable | FAME (n = 418) |
SEARCH (n = 546) |
Pooledb (n = 964) |
|---|---|---|---|
| Cigarette smoking | 0.5 (0.3-0.9) | 0.8 (0.6-1.2) | 0.7 (0.5-0.98) |
| Head injury | 1.5 (0.8-2.6) | 1.2 (0.7-1.8) | 1.3 (0.9-1.8) |
| -with unconsciousness | 1.6 (0.9-3.0) | 1.2 (0.7-2.0) | 1.3 (0.9-2.0) |
| Rep1 S* (vs. M_M) | 0.7 (0.4-1.2) | 0.7 (0.4-0.95) | 0.7 (0.5-0.9) |
| Rep1 L* (vs. M_M) | 1.6 (0.9-2.9) | 1.3 (0.7-2.3) | 1.4 (0.95-2.2) |
Multivariable logistic models adjusted for age, gender, race, smoking, head injury
Additionally adjusted for study
Abbreviations: S* = short/short or short/medium; M_M = medium/medium; L* = medium/long or long/long
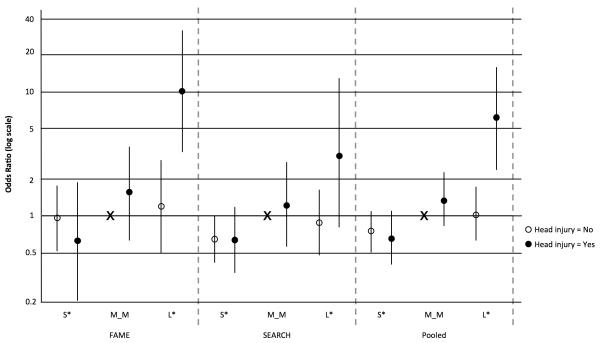
The effect of head injury varied markedly with Rep1 genotype (Tables 4, 5). Using “dominant” genotype coding (Table 1), we observed greater than multiplicative (i.e., synergistic) interaction in FAME, with head injury strongly associated with PD only in those with L* genotype (OR 5.4, 95%CI 1.5-19). A similar, though non-significant, interaction was also seen in SEARCH (OR, 2.3; 95% CI, 0.6-9.2). In the pooled analysis, head injury was associated with increased risk in those with L* genotype (OR 3.5, 95%CI 1.4-9.2 overall; OR 4.1, 95%CI 1.5-11.1 in non-Hispanic whites), but had minimal effect in those with other genotypes. The combined risk of PD associated with Rep1 L* genotype and head injury was more than 6 times that of M_M genotype and no head injury (Fig 1). Results were similar in models that included education or a global variable for pesticide exposure, excluded proxy respondents, or excluded head injuries within five or 10 years of reference date (data not shown). Results were also similar in analyses restricted to men, or when models included a head injury x study interaction term. The strength of interaction was even greater using an alternative Rep1 coding that excluded subjects with rare variants (257 or 265) or genotypes combining short and long alleles.
Table 4.
Head Injury Frequency Stratified by Rep1 Genotype
| Rep1 Genotype | FAME (n = 418) |
SEARCH (n = 546) |
Pooled (n = 964) |
|||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| S* | 4/29 | 26/129 | 33/140 | 19/78 | 37/169 | 45/207 |
| M_M | 8/39 | 24/149 | 46/182 | 14/61 | 54/221 | 38/210 |
| L* | 8/18 | 4/37 | 16/48 | 2/15 | 24/66 | 6/52 |
| S_L | 0/3 | 4/14 | 2/17 | 1/5 | 2/20 | 5/19 |
Abbreviations: S* = short/short or short/medium; M_M = medium/medium; L* = medium/long or long/long
Table 5.
Head Injury × Rep1 Interaction. Genotype-Specific Risk Associated with a Head Injury, Relative to No Head Injury (Odds Ratio (95% CI))a
| Genotype | FAME | SEARCH | Pooledd | |||
|---|---|---|---|---|---|---|
| All subjects (n = 418) |
Non- Hispanic Whites (n = 408) |
All subjects (n = 546) |
Non- Hispanic Whites (n = 479) |
All subjects (n = 964) |
Non- Hispanic Whites (n = 887) |
|
|
Dominant
modelb |
||||||
| S* | 0.5 (0.1-1.5) | 0.5 (0.1-1.5) | 0.9 (0.5-1.7) | 0.7 (0.4-1.2) | 0.8 (0.4-1.3) | 0.7 (0.4-1.2) |
| M_M | 1.8 (0.8-4.3) | 1.8 (0.7-4.2) | 1.3 (0.7-2.5) | 1.1 (0.6-2.3) | 1.5 (0.9-2.6) | 1.3 (0.8-2.3) |
| L* | 5.4 (1.5-19) | 5.8 (1.6-21) | 2.3 (0.6-9.2) | 3.1 (0.6-15) | 3.5 (1.4-9.2) | 4.1 (1.5-11) |
| p-interaction | 0.022 | 0.021 | 0.45 | 0.25 | 0.022 | 0.008 |
|
| ||||||
|
Alternative
modelfc |
||||||
| 259* | 0.7 (0.2-2.1) | 0.6 (0.2-2.0) | 1.0 (0.5-1.9) | 0.8 (0.4-1.5) | 0.9 (0.5-1.5) | 0.7 (0.4-1.3) |
| 261_261 | 1.5 (0.6-3.8) | 1.6 (0.6-3.9) | 1.2 (0.6-2.4) | 1.1 (0.6-2.3) | 1.3 (0.7-2.3) | 1.3 (0.8-2.3) |
| 263* | 8.4 (2.0-36) | 8.2 (1.9-35) | 3.5 (0.7-18) | 3.3 (0.6-17) | 6.3 (2.0-20) | 5.9 (1.9-19) |
| p-interaction | 0.018 | 0.018 | 0.30 | 0.21 | 0.006 | 0.006 |
Adjusted for age, gender, race, and smoking
S* = short/short or short/medium; M_M = medium/medium; L* = medium/long or long/long;
Excludes offsetting short and long allele combinations and rare alleles (257 and 265); 259* = 259_259 or 259_261; 263* = 261_263 or 263_263
Additionally adjusted for study and smoking*study
Figure 1. PD Risk Associated with Joint Occurrence of Head Injury and Rep1 Genotypea.
aAdjusted for age, gender, race, and smoking
X represents Rep1 M_M without head injury as reference category
Abbreviations: S* = short/short or short/medium; M_M = medium/medium; L* = medium/long or long/long
Mean PD diagnosis age in pooled analyses was significantly younger in subjects with L* genotype and head injury (57.7, SE 2.2) compared to all others (61.8, SE 0.5; p = 0.047) or to those with neither L* nor head injury (62.6, SE 0.6; p = 0.03).
DISCUSSION
We found that the effect of head injury was highly dependent on SNCA Rep1 genotype, with ORs ranging from 2.3 to 8.4 in those carrying a long Rep1 variant, depending on the model and study population. In addition, PD diagnosis age averaged four to five years younger in those with both head injury and expanded Rep1 genotype. Finding similar relationships in two unrelated study populations supports our hypothesis of biological interaction between head injury and α-synuclein. In contrast, the modest, non-significant association between head injury and PD overall suggests heterogeneity at the SNCA Rep1 locus may be one factor underlying the inconsistency of prior epidemiologic studies.
Animal models of head injury recapitulate many fundamental pathophysiological processes relevant to PD etiology, including protein accumulation and aggregation,37-39 increased striatal levels of nitrated α-synuclein,25 and altered proteosomal function.40 In human post-mortem studies, α-synuclein accumulation was observed after severe head injury,30, 31 possibly due to impaired axonal transport or as a direct response to axonal sheer stress.25, 27, 41, 42 Because aggregated extracellular α-synuclein activates microglia,43 and activated microglia enhance α-synuclein aggregation,44 one could envision a self-perpetuating “feed-forward” cycle of synuclein upregulation, aggregation and microglial activation resulting from head injury. Enhanced production and accumulation of α-synuclein, the major component of Lewy bodies and Lewy neurites, is a risk factor for PD.19, 45-48 Expansion of SNCA Rep1 increases striatal α-synuclein expression in transgenic mice,20 is associated with increased blood and brain levels in human post-mortem studies,21, 22 and increases PD risk.23, 24 Thus, our results are consistent with the hypothesis that a self-perpetuating neurodegenerative cycle initiated by head injury is more likely to occur and/or persist when levels of synuclein are high, and that head injury may aggravate degenerative processes in an environment already stressed by synuclein overexpression.
In addition to direct effects on synuclein, head injury could lead to a neurodegenerative process such as PD through a number of other biologically plausible mechanisms. Mild-to-moderate closed-head injury induces an inflammatory cascade that begins within minutes and may persist for months.49, 50 Blood-brain barrier breakdown is an early response, followed rapidly by altered blood flow, edema and leukocyte infiltration.51-53 Interleukin-1, interleukin-6, tumor necrosis factor-α, cyclooxygenase-2, and other inflammatory cytokines are upregulated,54 and microglia activated.55 Head injury also disrupts mitochondrial function, increasing free radical production and lipid peroxidation,56, 57 elevating cytosolic calcium and activating calpain,58 which may enhance accumulation of cytotoxic synuclein species.59, 60 Glutamate excitotoxicity and nitric oxide synthase (iNOS) induction simultaneously increase metabolic demands, further taxing already stressed neurons and glia.28, 61 Each of these mechanisms is thought to be important in PD pathogenesis.62-64 Consistent with the systemic pathology of PD, moderate to severe head injury can increase systemic inflammation and impact numerous organ systems outside of the central nervous system,65-67 although systemic effects of mild head injury are not well studied.
PD risk has been inconsistently associated with head injury in epidemiologic studies.2-17 In addition to genetic heterogeneity, possible explanations include different diagnostic methods, varying definitions of head injury, recall bias, confounding by other factors such as smoking, or other environmental exposures. Our reliance on retrospective data could have resulted in exposure misclassification. In particular, recall bias—the predilection of case subjects to report exposures due to heightened awareness or concern—has been suggested as an explanation for associations with head injury.16, 17 Bias could also result from differential access to medical care due to socioeconomic status or geographic location. However, because neither recall nor access to medical care would be expected to vary by Rep1 genotype, these potential biases are not likely to explain our findings.
Use of proxy respondents in FAME could also have introduced reporting bias, but results were not altered when these subjects were excluded from analyses. Furthermore, proxy respondents were less likely to report a head injury and, because proxy respondents were over-represented among cases, this would attenuate rather than inflate associations. Head injury was more frequently reported in SEARCH, especially among women, suggesting a systematic difference in head injury reporting between the studies. However, results were similar in both populations, and inclusion of a head injury × study interaction term in pooled models or restriction to men did not alter results. Another inherent limitation of this work is reliance on prevalent cases, potentially resulting in survival bias. Although this possibility cannot be fully excluded in FAME, survival bias is unlikely in SEARCH, in which disease duration averaged three years. Reverse causation (i.e., head injuries resulting from functional deficits in early pre-diagnosed PD) is another concern, but findings were unaltered in analyses that excluded injuries within five or 10 years of diagnosis. It is conceivable that expanded Rep1 could cause lifelong alterations in motor function predisposing to head injury, but we are not aware of animal or human data to support this. Finally, and most importantly, although the interaction was not statistically significant in SEARCH, the relationship between head injury, Rep1 and PD was similar in two unrelated study populations, arguing against a chance association.
We observed an average latency of 30 years between head injury and PD diagnosis, similar to prior studies. This lag suggests the passage of time may be required for the neurodegenerative cascade to unfold fully in susceptible individuals. At least 2.5 million head injuries occur annually in the U.S., resulting in 1.7 million healthcare visits.18 Because population variability in SNCA Rep1 is also common—10% of controls carried the long Rep1 genotype in the present studies—if our results are confirmed, the proportion of PD risk attributable to the combination of head injury and Rep1 allele expansion in the general population could be substantial. For example, based on an observed frequency of head injury and L* genotype co-occuring in 1.2% of controls, and an OR of 6.8 in subjects with both risk factors relative to the remainder of the population, 7% of PD in the general population could potentially be attributable to co-occurrence of head injury and L* genotype.68 Although these observations require replication, this large “at-risk” population could be targeted for prospective study and potential therapeutic intervention.
Acknowledgements
The authors wish to thank Dr. Aaron Blair for guidance related to the AHS cohort, Drs. Meike Kasten, Anabel Chade, Hubert Fernandez, Franca Cambi and Amanda Deligtisch for examination fieldwork, Dr. Matthew Farrer for sample genotyping and review of the manuscript, Dr. Marie Richards and Mr. Benjamin Priestly for statistical support, Ms. Jennifer Wright for editorial assistance, and the participants of the FAME and SEARCH studies. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, NIEHS grants (Z01- ES044007 and Z01-ES049030), NCI grant (Z01-CP010119), NIEHS grants R01-ES10803 and U54 ES012077, the Michael J. Fox Foundation, Parkinson’s Unity Walk, and James and Sharron Clark. SEARCH was supported by an unrestricted grant from a group of current and former manufacturers of welding consumables awarded to The Parkinson’s Institute.
References
- 1.Tanner CM. Etiology: The Role of Environment and Genetics. In: Factor SA, Weiner WJ, editors. Parkinson’s Disease: Diagnosis and Clinical Management. 2nd ed Demos Medical Publishing; New York: 2008. pp. 387–405. [Google Scholar]
- 2.Stern MB. Head trauma as a risk factor for Parkinson’s disease. Mov Disord. 1991;6(2):95–7. doi: 10.1002/mds.870060202. [DOI] [PubMed] [Google Scholar]
- 3.Semchuk KM, Love EJ, Lee RG. Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993 Jun;43(6):1173–80. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- 4.De Michele G, Filla A, Volpe G, et al. Environmental and genetic risk factors in Parkinson’s disease: a case-control study in southern Italy. Mov Disord. 1996 Jan;11(1):17–23. doi: 10.1002/mds.870110105. [DOI] [PubMed] [Google Scholar]
- 5.Seidler A, Hellenbrand W, Robra BP, et al. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: a case-control study in Germany. Neurology. 1996 May;46(5):1275–84. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 6.Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for Parkinson’s disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998 Aug-Oct;19(4-5):709–12. [PubMed] [Google Scholar]
- 7.Taylor CA, Saint-Hilaire MH, Cupples LA, et al. Environmental, medical, and family history risk factors for Parkinson’s disease: a New England-based case control study. Am J Med Genet. 1999 Dec 15;88(6):742–9. [PubMed] [Google Scholar]
- 8.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology. 2003 May 27;60(10):1610–5. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 9.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006 Jul;60(1):65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 10.Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007 Oct;64(10):666–72. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai CH, Lo SK, See LC, et al. Environmental risk factors of young onset Parkinson’s disease: a case-control study. Clin Neurol Neurosurg. 2002 Sep;104(4):328–33. doi: 10.1016/s0303-8467(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha NE, Stokes L, Schoenberg BS, et al. A case-control study of twin pairs discordant for Parkinson’s disease: a search for environmental risk factors. Neurology. 1986 Feb;36(2):284–8. doi: 10.1212/wnl.36.2.284. [DOI] [PubMed] [Google Scholar]
- 13.Morano A, Jimenez-Jimenez FJ, Molina JA, Antolin MA. Risk-factors for Parkinson’s disease: case-control study in the province of Caceres, Spain. Acta Neurol Scand. 1994 Mar;89(3):164–70. doi: 10.1111/j.1600-0404.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 14.Martyn CN, Osmond C. Parkinson’s disease and the environment in early life. J Neurol Sci. 1995 Oct;132(2):201–6. doi: 10.1016/0022-510x(95)00148-u. [DOI] [PubMed] [Google Scholar]
- 15.McCann SJ, LeCouteur DG, Green AC, et al. The epidemiology of Parkinson’s disease in an Australian population. Neuroepidemiology. 1998;17(6):310–7. doi: 10.1159/000026185. [DOI] [PubMed] [Google Scholar]
- 16.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spangenberg S, Hannerz H, Tuchsen F, Mikkelsen KL. A nationwide population study of severe head injury and Parkinson’s disease. Parkinsonism Relat Disord. 2009 Jan;15(1):12–4. doi: 10.1016/j.parkreldis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 19.Ross OA, Braithwaite AT, Skipper LM, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008 Jun;63(6):743–50. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronin KD, Ge D, Manninger P, et al. Expansion of the Parkinson disease-associated SNCA-Rep1 allele upregulates human alpha-synuclein in transgenic mouse brain. Hum Mol Genet. 2009 Sep 1;18(17):3274–85. doi: 10.1093/hmg/ddp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs J, Tichopad A, Golub Y, et al. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008 May;22(5):1327–34. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
- 22.Linnertz C, Saucier L, Ge D, et al. Genetic regulation of alpha-synuclein mRNA expression in various human brain tissues. PLoS One. 2009;4(10):e7480. doi: 10.1371/journal.pone.0007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006 Aug 9;296(6):661–70. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 24.Kay DM, Factor SA, Samii A, et al. Genetic association between alpha-synuclein and idiopathic Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008 Oct 5;147B(7):1222–30. doi: 10.1002/ajmg.b.30758. [DOI] [PubMed] [Google Scholar]
- 25.Uryu K, Giasson BI, Longhi L, et al. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol. 2003 Nov;184(1):214–24. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- 26.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007 Dec;208(2):185–92. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newell KL, Boyer P, Gomez-Tortosa E, et al. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol. 1999 Dec;58(12):1263–8. doi: 10.1097/00005072-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Liu PK, Robertson CS, Valadka A. The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain after brain injury. Ann N Y Acad Sci. 2002 May;962:226–41. doi: 10.1111/j.1749-6632.2002.tb04071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner C, Kamel F, Ross G, et al. Rotenone, Paraquat and Parkinson’s Disease. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002839. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996 Apr;104(4):362–9. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 32.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999 Jan;56(1):33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 33.Tanner CM, Ross GW, Jewell SA, et al. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol. 2009 Sep;66(9):1106–13. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- 34.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrer M, Maraganore DM, Lockhart P, et al. alpha-Synuclein gene haplotypes are associated with Parkinson’s disease. Hum Mol Genet. 2001 Aug 15;10(17):1847–51. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- 36.McCulloch CC, Kay DM, Factor SA, et al. Exploring gene-environment interactions in Parkinson’s disease. Hum Genet. 2008 Apr;123(3):257–65. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 37.Iwata A, Chen XH, McIntosh TK, Browne KD, Smith DH. Long-term accumulation of amyloid-beta in axons following brain trauma without persistent upregulation of amyloid precursor protein genes. J Neuropathol Exp Neurol. 2002 Dec;61(12):1056–68. doi: 10.1093/jnen/61.12.1056. [DOI] [PubMed] [Google Scholar]
- 38.Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998 Nov;87(2):359–69. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 39.Smith DH, Chen XH, Nonaka M, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999 Sep;58(9):982–92. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Yao X, Liu J, McCabe JT. Alterations of cerebral cortex and hippocampal proteasome subunit expression and function in a traumatic brain injury rat model. J Neurochem. 2008 Jan;104(2):353–63. doi: 10.1111/j.1471-4159.2007.04970.x. [DOI] [PubMed] [Google Scholar]
- 41.Siebert H, Kahle PJ, Kramer ML, et al. Over-expression of alpha-synuclein in the nervous system enhances axonal degeneration after peripheral nerve lesion in a transgenic mouse strain. J Neurochem. 2010 Aug;114(4):1007–18. doi: 10.1111/j.1471-4159.2010.06832.x. [DOI] [PubMed] [Google Scholar]
- 42.Gaetz M. The neurophysiology of brain injury. Clin Neurophysiol. 2004 Jan;115(1):4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005 Apr;19(6):533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 44.Wilms H, Rosenstiel P, Romero-Ramos M, et al. Suppression of MAP kinases inhibits microglial activation and attenuates neuronal cell death induced by alpha-synuclein protofibrils. Int J Immunopathol Pharmacol. 2009 Oct-Dec;22(4):897–909. doi: 10.1177/039463200902200405. [DOI] [PubMed] [Google Scholar]
- 45.Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005 Mar;62(3):353–7. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- 46.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003 Sep;4(9):727–38. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 47.McCormack AL, Di Monte DA. Enhanced alpha-synuclein expression in human neurodegenerative diseases: pathogenetic and therapeutic implications. Curr Protein Pept Sci. 2009 Oct;10(5):476–82. doi: 10.2174/138920309789351912. [DOI] [PubMed] [Google Scholar]
- 48.Farrer M, Kachergus J, Forno L, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004 Feb;55(2):174–9. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 49.Gentleman SM, Leclercq PD, Moyes L, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004 Dec 16;146(2-3):97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev. 2005 Apr;48(2):388–99. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 51.Goldman H, Hodgson V, Morehead M, Hazlett J, Murphy S. Cerebrovascular changes in a rat model of moderate closed-head injury. J Neurotrauma. 1991 Summer;8(2):129–44. doi: 10.1089/neu.1991.8.129. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Dayem HM, Abu-Judeh H, Kumar M, et al. SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin Nucl Med. 1998 May;23(5):309–17. doi: 10.1097/00003072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129(4):1021–9. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 54.Israelsson C, Bengtsson H, Kylberg A, et al. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma. 2008 Aug;25(8):959–74. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- 55.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007 Nov;66(11):989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 56.Frantseva M, Velazquez JL Perez, Tonkikh A, Adamchik Y, Carlen PL. Neurotrauma/neurodegeneration and mitochondrial dysfunction. Prog Brain Res. 2002;137:171–6. doi: 10.1016/s0079-6123(02)37015-8. [DOI] [PubMed] [Google Scholar]
- 57.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000 Oct;17(10):843–55. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- 58.Kampfl A, Posmantur RM, Zhao X, Schmutzhard E, Clifton GL, Hayes RL. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: implications for pathology and therapy: a review and update. J Neurotrauma. 1997 Mar;14(3):121–34. doi: 10.1089/neu.1997.14.121. [DOI] [PubMed] [Google Scholar]
- 59.Norris EH, Giasson BI. Role of oxidative damage in protein aggregation associated with Parkinson’s disease and related disorders. Antioxid Redox Signal. 2005 May-Jun;7(5-6):672–84. doi: 10.1089/ars.2005.7.672. [DOI] [PubMed] [Google Scholar]
- 60.Dufty BM, Warner LR, Hou ST, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007 May;170(5):1725–38. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004 Mar;61(6):657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caudle WM, Zhang J. Glutamate, excitotoxicity, and programmed cell death in Parkinson disease. Exp Neurol. 2009 Dec;220(2):230–3. doi: 10.1016/j.expneurol.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 63.Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson’s disease. J Neuroimmune Pharmacol. 2009 Dec;4(4):419–29. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008 Jan;7(1):97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 65.Shohami E, Gati I, Beit-Yannai E, Trembovler V, Kohen R. Closed head injury in the rat induces whole body oxidative stress: overall reducing antioxidant profile. J Neurotrauma. 1999 May;16(5):365–76. doi: 10.1089/neu.1999.16.365. [DOI] [PubMed] [Google Scholar]
- 66.Bansal V, Costantini T, Kroll L, et al. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. 2009 Aug;26(8):1353–9. doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci. 2009;14:3795–813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- 68.Cole P, MacMahon B. Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971 Nov;25(4):242–4. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]