Abstract
There is evidence for a “sensitive period” in respiratory development in rats around postnatal age (P) 12-13d. Little is known about sex differences during that time. The purpose of this study was to assess the effect of sex on breathing development, specifically around the “sensitive period”. We used whole-body plethysmography to study breathing in normoxic, hypoxic and hypercapnic gases in non-anesthetized male and female neonatal rats from P10-P15, juvenile (P30) and young adult (P90) rats. Compared to other neonatal ages, P12-13 male rats had significantly lower ventilation during normoxia, hypoxia, and hypercapnia. Compared to age-matched females, P12-13 male rats had lower ventilation in normoxia and hypoxia and a lower O2 saturation during hypoxia. Circulating estradiol was greater in P12-13 male vs. female rats. Estradiol and ventilatory responses to hypoxia and hypercapnia were negatively correlated in neonatal male, but not female, rats. Our results suggest that P10-P15 includes a critical developmental period in male but not female rats.
1. Introduction
Breathing is a well-regulated process that is essential for life, yet the control of breathing differs with age and gender (Wenninger et al., 2009). Much research has shown that sex steroid hormones (testosterone, estrogen, progesterone) have profound effects on central nervous system development, respiratory plasticity, and the neural control of breathing. Males and females respond differently to ventilatory challenges such as hypoxia and hypercapnia. Furthermore, several human breathing disorders exhibit a gender bias. The prevalence of infant respiratory distress syndrome (IRDS), sudden infant death syndrome (SIDS) and obstructive sleep apnea (OSA) is higher in males than females (Thomas et al., 2006; Mage and Donner, 2004; Redline et al., 1994). At high altitude, pre-menopausal females have a stronger ventilatory drive than males, suggesting females have an increased chemoreflex to hypoxia (Pequignot et al., 1997; Joseph et al., 2000). The peripheral carotid body chemoreceptor initiates the hypoxic ventilatory response (HVR) and contributes to the hypercapnic ventilatory response, protecting the body from hypoxic ischemia and respiratory acidosis. Little is known, however, about sex differences in the development of the peripheral and central respiratory control systems and how these differences contribute to the sexual dimorphism of breathing disorders.
In rodents, the respiratory control system undergoes postnatal development and maturation, including sensitive periods in which respiratory control may be susceptible to challenges (Carroll, 2003; Wong-Riley and Liu, 2005; Liu et al., 2006). In the present study, we used rodents as an animal model to investigate these respiratory “sensitive periods”. The critical period of ventilatory development in rats occurs within the first four weeks of life. A sensitive period has been identified around postnatal days (P) 12-13 when changes are seen in normoxic ventilation and the HVR in rats (Liu et al., 2006). Many studies have focused on changes in carotid body chemoreceptor morphology in response to exposure to low or high levels of oxygen early in postnatal development. Postnatal hypoxia in the first week of life in the rat causes an increase in carotid body volume (Wang and Bisgard, 2005), while postnatal hyperoxia in the first 1-4 weeks of life causes a decrease in carotid body size (Bisgard et al., 2003; Wang and Bisgard, 2005). Exposure to postnatal hyperoxia has been shown to affect the HVR as well as breathing stability in normoxia in neonatal rats (Bavis et al., 2010), and postnatal hypoxia and hyperoxia also affect the HVR in adult rats (Okubo and Mortola, 1990; Bavis et al., 2004; Ling et al., 1996). Taken together these findings suggest the presence of a critical period during early postnatal development of the respiratory control system that has lasting effects on respiratory function.
In brainstem respiratory nuclei, multiple neurotransmitter systems undergo changes during early postnatal development. The Wong-Riley group have described significant changes in several neurotransmitters and receptors in a number of respiratory-related nuclei during development, including a peak in GABAA-1a and GluR2 at P12-13, as well as significant decreases in NMDAR1, NMDAR2A, TPH and SERT at this time point (Liu and Wong-Riley, 2010a,b; Liu and Wong-Riley, 2006, Wong-Riley and Liu, 2005). However, animals were not segregated by sex in their studies. Indeed, few anatomical or physiological studies have focused on sex differences in early postnatal development of the respiratory control system. Schlenker and Hansen reported sex differences in the expression of ER-α and NMDA R1 in the nucleus tractus solitarius (NTS) and hypoglossal nucleus in weanling rats (P24-25) (Schlenker and Hansen, 2006). They also reported that in weanling rats NMDA receptor modulation of breathing is sex specific, as are the densities of NR1 positive neurons in medullary nuclei associated with control of breathing (Schlenker and Hansen, 2007).
The role of sex hormones during the development of the respiratory control system remains unclear. There is a prenatal testosterone surge at day 18-19 of gestation (Weisz and Ward, 1980), and a perinatal testosterone surge within four hours of birth in male rats (Ward et al., 2002). This later hormonal surge causes masculinization and defeminization of the brain (Morris et al., 2004), and occurs at a time when the respiratory control system is likely undergoing key developmental changes.
In the present study we investigated the role of sex hormones in the development of the respiratory control system by assessing the ventilatory response to hypoxia and hypercapnia in neonatal (P10-P15), pubertal (P30) and young adult (P90) male and female rats. We hypothesized that there are sex differences in the chemosensory control of respiration, particularly the ventilatory response to hypoxia and hypercapnia in male vs. female rats during critical periods of respiratory development, and that these differences correlate with levels of sex hormones.
2.0 Methods
Animals
All experiments and animal procedures were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin School of Veterinary Medicine. Pregnant female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) arrived at the School of Veterinary Medicine 13 days after conception (E13). Rat pups were born by natural, vaginal delivery at E21-22. Neonatal rats were studied each day from P10-P15. Juvenile rats were studied at P30 (+4d). Young adult rats were studied at P90 (+15d); female P90 rats were studied one day after estrus as determined by vaginal lavage.
2.1 Plethysmography
Ventilatory studies of P10-P15 neonatal rats were conducted in a 1 liter whole body, flow through (~1.2 L/min) plethysmograph (25.9°C ± 0.66°C). Prior to placing an awake rat pup in the plethysmograph, rectal temperature was recorded and a non-invasive neck pulse oximeter (STARR Life Sciences Corp., Oakmont, PA, USA) was secured to the animal. Pulse rate and arterial oxygen saturation (SpO2) were continuously measured and recorded throughout the study via the pulse oximeter. After 10-20 minutes of quiet normoxic breathing (21% O2), rats were exposed to a 10 minute hypoxic challenge (12% O2, balance N2), followed by 20 minutes of normoxic recovery, then a 10 minute hypercapnic challenge (7% CO2, 21% O2). After hypercapnic exposure, animals were removed from the plethysmograph and rectal temperature and weight were recorded. Chamber temperature and humidity were measured with a standard digital thermometer and hygrometer, and recorded during the last minute of each gas exposure. Tidal volume and breathing frequency were measured with a pressure transducer (Validyne Engineering Corp., Northridge, CA, USA) and recorded via a data acquisition program (DATAQ Instruments, Inc., Akron, OH, USA). The system was calibrated by ten 0.2ml injections of air into the plethysmograph chamber via a syringe following each animal’s study. Data from the last 4 minutes of each gas or normoxic exposure were analyzed.
Ventilatory studies of P30 and P90 rats were conducted in a 5 liter whole body, non-flow-through plethysmograph. The entire study was carried out as described above, with the exception that during the first six minutes of each gas exposure a tube delivered gas into the chamber. The chamber was then sealed and ventilation was recorded for the last four minutes of each exposure. The system was calibrated by ten 0.4ml injections of air into the plethysmograph chamber via a syringe following each animal’s study.
2.2 Perfusion and Hormone Measurement
Following each plethysmography study, rats were anesthetized with isoflurane. Prior to perfusion, blood was drawn from the heart for sex hormone level measurement. Blood samples were centrifuged, and serum was immediately frozen at −80°C. After collection of all serum samples, 17β estradiol, progesterone, and testosterone levels were analyzed by ELISA (IBL, Hamburg, Germany; 17β estradiol, Cat. #IB79103, sensitivity 0.0–2000 pg/ml; progesterone, Cat. #IB79105, sensitivity 0.00–40 ng/ml; testosterone, CAT. #IB79106, sensitivity 0.00-16 ng/ml). Tissues (brainstem and carotid body) were harvested for later analysis.
2.3 Statistical analysis
Ventilation and oxygen saturation data from the last four minutes of each gas exposure were binned into 3-second samples and averaged. Averages were compared using two-way repeated-measures ANOVA with a Tukey t-test for multiple comparisons. Averages of ventilation and estradiol concentrations were used for regression analysis. For all analyses, P < 0.05 was considered significant. Data were normalized to 100g of body weight and segregated into age groups P10-11, P12-13, P14-15, P30 and P90.
3. Results
3.1 Ventilation in normoxia
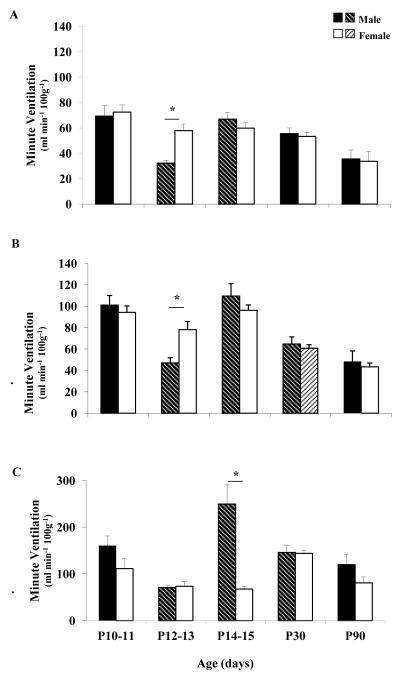
Weight and body temperature data for all ages of male and female rats are shown in Table 1. During eupneic ventilation, there were significant age-related changes in the early neonatal period (P10-15) in male rats. P12-13 male rats had significantly lower ventilation and tidal volume compared to P10-11 and P14-15 male rats, as well as compared to P12-13 female rats (Figure 1A). In contrast, there were no significant age-related changes in ventilation in female rats during normoxia. Breathing frequency in normoxia decreased significantly with age (P30 and P90) in both male and female rats, though frequency in male vs. female rats was not significantly different at any age. At all ages studied and in both sexes, O2 saturation was greater than 95% in normoxia (data not shown).
Table 1. Body Weight, Temperature and Ventilation in Room Air (RA), Hypoxia (Hx) and Hypercapnia (CO2) in neonatal, prepubertal and young adult male and female rats.
Shown are the number of animal in each group (n), body temperature (Tb) before and after study, plethysmograph temperature, ventilation, tidal volume, and breathing frequency, in male and female rats at P10-11, P12-13, P14-15, P30 and P90. Values are means ± SEM.
| Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | P10-11 | P12-13 | P14-15 | P30 | P90 | P10-11 | P12-13 | P14-15 | P30 | P90 | |
| n | 14 | 16 | 14 | 6 | 7 | 14 | 19 | 15 | 11 | 7 | |
| Initial Tb °C | 34.8±0.3 | 36.0±0.1 | 36.4±0.2 | 37.8±0.2 | 37.0±0.3 | 34.9±0.3 | 35.9±0.1 | 36.6±0.2 | 37.7±0.1 | 37.8±0.3 | |
| box temp °C | 26.1±0.1 | 25.5±0.1 | 25.5±0.3 | 23.6±0.2 | 23.0±0.2 | 25.9±0.1 | 26.4±0.5 | 25.8±0.3 | 23.5±0.2 | 22.4±0.2 | |
| weight (g) | 22.7±1.1 | 29.3±1.1 | 35.9±1.4 | 108.6±2.6*† | 387.2±0.0*† | 21.3±1.0 | 26.1±1.0 | 33.3±1.1 | 90.1±1.9* | 222.6±7.2* | |
| RA | Minute Ventilation (ml/min/100g) | 69.4±8.3 | 32.2±2.4*† | 66.9±5.1* | 55.5±4.4 | 35.5±7.1 | 72.5±5.7 | 57.9±5.2 | 59.9±4.7 | 53.4±3.3 | 33.7±7.6 |
| Tidal Volume (ml/br) | 0.47±0.06 | 0.23±0.02*† | 0.47±0.04* | 0.46±0.03 | 0.38±0.07 | 0.50±0.04 | 0.40±0.04 | 0.44±0.04 | 0.44±0.02 | 0.43±0.04 | |
| Breathing Frequency (br/min) | 150±3.8 | 141±3.4 | 146±4.3 | 121±4.3* | 86±6.3* | 147±5.7 | 150±5.2 | 143±6.8 | 120±3.3* | 79±4.7* | |
| Hx | Minute Ventilation (ml/min/100g) | 101.0±8.9 | 47.0±4.9*† | 109.5±11.6* | 64.7±6.6* | 48.4±9.8 | 94.3±5.9 | 78.1±7.5 | 96.2±5.0 | 60.8±3.2* | 43.4±3.0 |
| Tidal Volume (ml/br) | 0.56±0.06 | 0.27±0.03*† | 0.58±0.59* | 0.47±0.04 | 0.39±0.07 | 0.52±0.04 | 0.44±0.04 | 0.54±0.04 | 0.44±0.22 | 0.45±0.04 | |
| Breathing Frequency (br/min) | 185±7.6 | 178±3.8 | 190±6.7* | 136±7.3* | 120±7.1† | 186±8.2 | 180±6.3 | 185±7.4 | 138±7.1* | 98±4.9* | |
| CO2 | Minute Ventilation (ml/min/100g) | 160.6±20.6 | 70.7±5.1* | 249.4±41.2*† | 145.9±14.7* | 95.9±22.7 | 111.2±21.5 | 85.4±10.2 | 120.1±6.1 | 143.9±6.8 | 80.6±12.9 |
| Tidal Volume (ml/br) | 1.03±0.11† | 0.41±0.03* | 1.06±0.14*† | 0.76±0.08 | 0.60±0.12 | 0.69±0.17 | 0.53±0.04 | 0.64±0.04 | 0.73±0.04 | 0.59±0.05 | |
| Breathing Frequency (br/min) | 155±7.8 | 173±7.6* | 226±8.1† | 193±3.3 | 147±0.1* | 171±13.3 | 166±16.3 | 193±12.1 | 198±3.9 | 133±15.5* | |
| Final Tb °C | 34.7±0.4 | 36.1±0.2 | 36.6±0.3 | 37.5±0.1 | 37.4±0.2 | 34.8±0.3 | 36.0±0.1 | 36.5±0.2 | 37.4±0.1 | 37.5±0.2 | |
P<0.05 vs. previous age group of the same sex
P<0.05 vs. age-matched female
Figure 1. Minute ventilation in normoxia, hypoxia and hypercapnia.
Ventilation during normoxia (A), hypoxia (B) and hypercapnia (C) in male (black bars, shaded black bars) and female (white bars, shaded white bars) rats at postnatal ages 10-11, 12-13, 14-15, 30 and 90 days. Asterisk indicates statistically significant sex differences (P< 0.05). Shaded bars indicate a statistically significant difference compared to the previous age group of the same sex (P<0.05).
3.2 Ventilatory response to hypoxia
During hypoxia (FIO2 = 12%), there were significant age-related changes in the early neonatal period (P10-15) in male rats. Ventilation and tidal volume were significantly lower in P12-13 male rats compared to P10-11 and P14-15 male rats, and also compared to age-matched female rats (Figure 1B; Table 1). In contrast, there were no significant age-related changes in ventilation or tidal volume in neonatal female rats during hypoxia. Breathing frequency in hypoxia decreased significantly by P30 in both sexes, with a significant decrease in ventilation in P30 male and female rats. Additionally, P90 male rats had a significantly greater breathing frequency during hypoxia than female rats. Because of a lower tidal volume in P90 male rats (not statistically different from P90 female rats), ventilation was not different between P90 male and female rats.
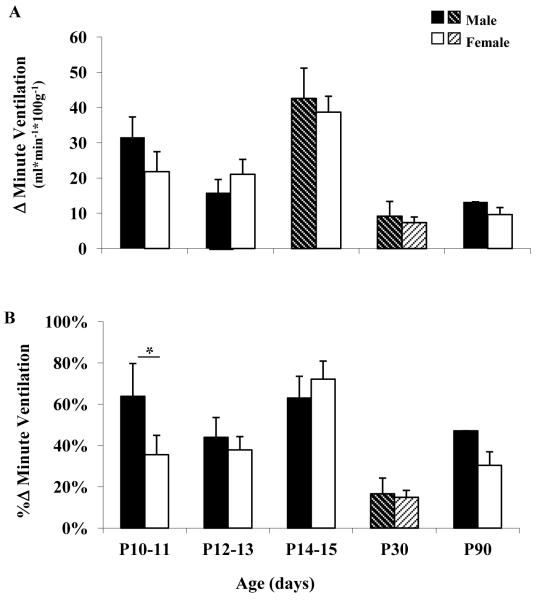
Figure 2 shows the average change in ventilation (ml/min/100g; Figure 2A) and percent change in ventilation (Figure 2B) for each age group. At P14-15 the change in ventilation increased significantly in male rats, and then decreased significantly by P30. In female rats, there was also a significant decrease in ventilation at P30 (Figure 2A). There were no sex differences in the change in ventilation at any age (Figure 2A). When assessing the percent change in the ventilation, a sex difference was evident only at the youngest age group (P10-11), with male rats having a significantly greater percent increase in ventilation compared to female rats (Figure 2B). It is noteworthy that within the three neonatal age groups, male rats had a pronounced nadir in the ventilatory response to hypoxia at P12-13 which was not apparent in female rats.
Figure 2. Ventilatory response to hypoxia.
Ventilatory response to hypoxia reported as the change (Δ) in ventilation (A), and the percent change (%Δ) in ventilation (B) in male (black and black shaded bars) and female (white and white shaded bars) rats at postnatal ages 10-11, 12-13, 14-15, 30 and 90 days. Asterisk indicates statistically significant sex difference (P< 0.05). Shaded bars indicate a statistically significant difference (P<0.05) compared to the previous age group of the same sex.
3.3 Oxygen saturation during hypoxia
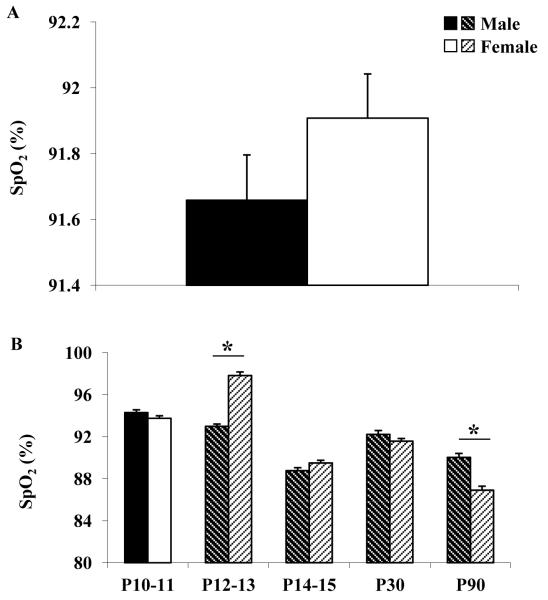
A pulse oximeter was used to monitor the arterial oxygen saturation of hemoglobin (SpO2) throughout each ventilatory study. Figure 3 shows the average SpO2 during the last four minutes of hypoxic gas exposure in all rats studied, from ages P10-P90 in males vs. females (Figure 3A), as well as for each age group (Figure 3B). While the overall average SpO2 during hypoxia for all rats studied was not significantly different between males and females, there were significant changes in O2 saturation throughout development in both sexes. Important to note is that at P12-13, female rats maintained a significantly higher SpO2 during hypoxia than male rats, despite having similar ventilatory responses (change ventilation and percent change in ventilation; Figure 2). Conversely, male rats had a significantly higher SpO2 than females at P90, which was possibly due to in part a greater breathing frequency in P90 males vs. females.
Figure 3. Oxygen saturation during hypoxia.
A. Arterial oxygen saturation (SpO2) during hypoxia (Hx) in male (black bar) and female (white bar) rats at all ages studied (P10-P90). B. Arterial oxygen saturation (SpO2) during hypoxia in male (black and black shaded bars) and female (white and white shaded bars) rats at postnatal ages 10-11, 12-13, 14-15, 30 and 90 days. Asterisk indicates statistically significant sex difference (P< 0.05). Shaded bars indicate a statistically significant difference (P<0.05) compared to the previous age group of the same sex.
3.4 Ventilatory response to hypercapnia
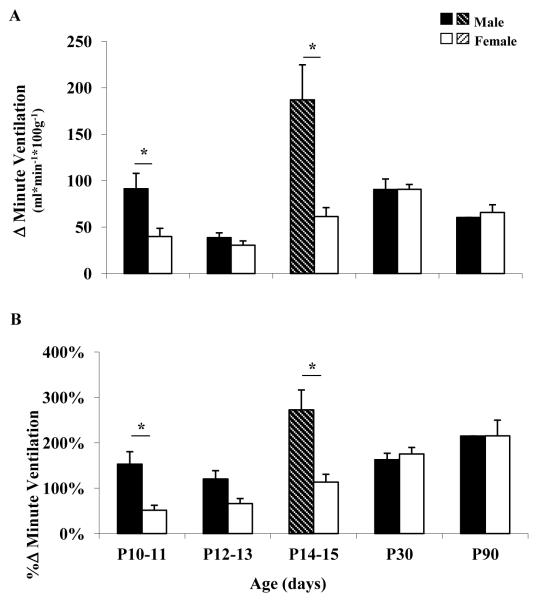
During hypercapnia there were significant age-related changes in the early neonatal period (P10-15) in male rats. Ventilation, tidal volume and breathing frequency decreased significantly at P12-13 (vs. P10-11), and then increased significantly at P14-15 (vs. P12-13) (Figure 1C; Table 1). These abrupt age-related changes in breathing were not evident in age-matched female rats. Additionally, change in ventilation and percent change in ventilation increased significantly at P14-15 (vs. P12-13) in male but not in female rats (Figure 4).
Figure 4. Ventilatory response to hypercapnea.
Ventilatory response to hypercapnia reported as the change (Δ) in ventilation (A), and the percent change (%Δ) in ventilation (B) in male (black and black shaded bars) and female (white and white shaded bars) rats postnatal ages 10-11, 12-13, 14-15, 30 and 90 days. Asterisk indicates statistically significant sex differences (P< 0.05). Shaded bars indicate a statistically significant difference (P<0.05) compared to the previous age group of the same sex.
During hypercapnia there were significant sex differences in the early neonatal period (P10-15). Male rats had significantly greater change in ventilation and percent change in ventilation compared to female rats at P10-11 and at P14-15 (Figure 4A and 4B, respectively). However, there were no sex differences in the change in ventilation and the percent change in ventilation at P12-13.
3.5 Sex hormones
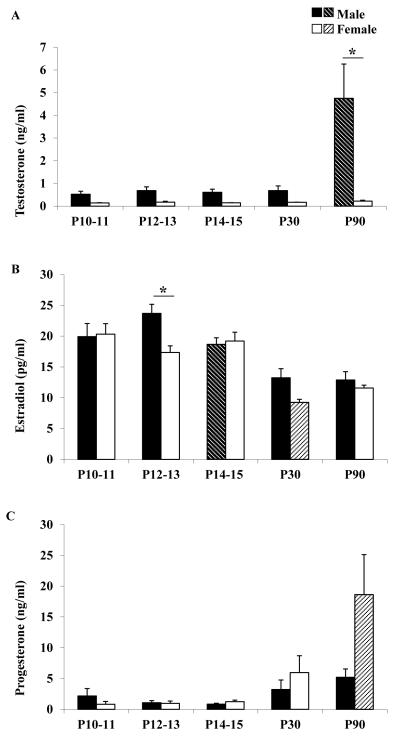
Circulating testosterone, estradiol, and progesterone levels for each age group are shown in Figure 5. Testosterone (Figure 5A) and progesterone (Figure 5C) levels remained low throughout early development in male and female rats, but by young adulthood (P90) testosterone levels increased significantly in male rats, and progesterone levels increased significantly in female rats. Most notable was the change in estradiol concentration that occurred in male rats during early development. Estradiol concentration in male rats peaked at P12-13 and thereafter decreased significantly (Figure 5B). Additionally, estradiol concentration in male rats at P12-13 was significantly higher than in age-matched female rats. In female rats, there were no significant age-related changes in estradiol concentration until P30 when they showed a significant decrease.
Figure 5. Sex hormone levels.
Testosterone (A), Estradiol (B) and Progesterone (C) levels in male (black and black shaded bars) and female (white and white shaded bars) rats postnatal ages 10-11, 12-13, 14-15, 30 and 90 days. Asterisk indicates statistically significant sex differences (P< 0.05). Shaded bars indicate a statistically significant difference (P<0.05) compared to the previous age group of the same sex.
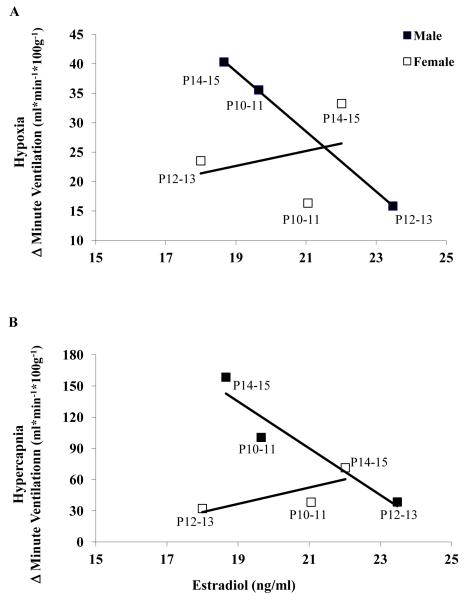
Because the emphasis of this paper is on breathing development during the neonatal period, we reported correlations of circulating estradiol concentration and ventilatory responses during the aforementioned developmental period. There was a striking negative correlation between estradiol concentration and both the hypoxic (Figure 6A) and hypercapnic (Figure 6B) ventilatory responses in male, but not female, rats at early postnatal ages (P10-11, P12-13, P14-15). In male rats, the correlation between estradiol concentration and ventilation was R2 = 0.999 in hypoxia and in hypercapnia the correlation was also high (R2 = 0.908). However, compared to neonatal male rats, there was no correlation between estradiol and ventilation in hypoxia (R2 = 0.0975) and a less robust correlation in hypercapnia in neonatal female rats (R2 = 0.613).
Figure 6. Correlation between serum estradiol concentration and ventilatory responses to hypoxia and hypercapnia.
A. Correlation between serum estradiol levels and change (Δ) in ventilation in hypoxia in P10-11, P12-13, and P14-15 male and female rats. There is a strong negative correlation in male rats (R2 = 0.999) whereas in female rats there is no correlation (R2 = 0.098). B. Correlation between serum estradiol levels and change (Δ) in ventilation in hypercapnia in P10-11, P12-13, and P14-15 male and female rats. There is a strong negative correlation in male rats (R2 = 0.908) whereas in female rats there is a less robust correlation (R2 = 0.613).
4. Discussion
The key findings of this study are: 1) there are sex differences in breathing during the developmental “sensitive period”, postnatal days 12-13 in rats; 2) male rats undergo changes in ventilatory responses to both hypoxia and hypercapnia during early development (P10-P15), whereas female rats show little or no change with age in ventilatory responses to these stimuli; 3) there are sex differences in O2 saturation in hypoxia during the “sensitive period”; 4) at P12-13, there is a transient peak in the circulating estradiol concentration in male rats which is significantly greater than the estradiol concentration in age-matched female rats; 5) there is a strong correlation between circulating estradiol concentration and ventilatory responses to hypoxia and hypercapnia in neonatal male but not female rats. Our results support the observation that a critical period in the development of the respiratory control system exists at P12-13 (Liu et al., 2006). Additionally, our results suggest that P12-13 is a distinct transition period within respiratory development in male but not female rats.
4.1 Normoxic Ventilation
Our data are not in complete agreement with previous findings. Whereas Liu et al. (2006), reported a significant increase in ventilation, tidal volume and breathing frequency in room air in P12 vs. P13, we found a significant age-related decrease in ventilation and tidal volume (but not breathing frequency) at P10-11 vs. P12-13 in male but not in female rats (Table 1; Figure 1). Moreover, we found that ventilation and tidal volume were significantly lower in P12-13 male vs. female rats (Table 1). It appears that Liu et al. (2006) included both sexes in their study although the proportion of male:female pups is unknown. Were we to average our data on ventilation and tidal volume from male and female rats, this would still not show an increased resting ventilation or tidal volume at P13. Although we grouped data from P12 and P13 animals, resting ventilation from animals at P12 did not differ significantly from those at P13 (P=0.993). Consistent with our findings, Liu et al. (2006) found a significant decrease in tidal volume at P12. However, since they report no change in breathing frequency, there was no significant change in ventilation at P12. It also appears that much of the change in ventilation reported by Liu et al. was due to a significant increase in breathing frequency at P13. We found no such change in breathing frequency with age during the early neonatal period. We also report a significant increase in normoxic breathing (ventilation and tidal volume) from P12-13 vs. P14-15 in male but not female rats, which was not reported by Liu et al., (2006). Why their rats showed a sharp increase in ventilation at P13, while our rats showed a significant decrease, could be due to strain differences and/or previous hypoxic exposure. The rats used in our study were naive to hypoxia whereas those in the Liu et al. study had been exposed to hypoxia on two or more occasions.
4.2 Hypoxic ventilatory response
Distinct differences in the hypoxic ventilatory response were also observed at P12-13 in male rats. Similar to normoxic breathing, during hypoxia P12-13 male rats had significantly lower ventilation than at any other age (Table 1; Figure 1). Additionally, ventilation in P12-13 male rats was significantly lower than age-matched female rats. In no other age group were sex differences in hypoxic ventilation evident. In a recent study by Rotem-Kohavi et al. (2011), they found sex differences in burst onset of neurons in the pre-Botzinger Complex from neonatal brainstem slices following re-oxygenation after hypoxic exposure. In slices from P10-12 male rats, the time to first burst following re-oxygenation was significantly longer compared to slices from female rats, suggesting that neurons in female slices recover more quickly from hypoxia at this age. These sex differences were not found in neurons in slices at younger ages. While the studies by Rotem-Kohavi et al. (2011) report post hypoxic ventilatory-related bursting activity and our studies measured ventilation in hypoxia, it is noteworthy that in both studies there is evidence of a “sensitive period” during respiratory development as well as sex differences in this “sensitive period”, such that females respond more favorably following exposure to hypoxia..
While the change in ventilation and the percent change in ventilation during hypoxia in male rats at P12-13 did not decrease significantly compared to P10-11 rats, there was a distinct nadir in the change in ventilation at P12-13 within animals aged P10-15 (Figure 2A, B). Additionally, there was a significant increase in the change in ventilation at P14-15 in male rats by comparison with P12-13 rats (Figure 2A). Liu et al. (2006) also reported a significant decrease in ventilation at P13 with a rebound at P14. However, it is not clear what proportion of the animals in their study were male or female.
During adolescence (P30), both male and female rats had significant decreases in the ventilatory response to hypoxia by comparison with P10-15 animals that began to rebound by early adulthood (P90). This sudden drop in the hypoxic response around the time of the onset of hormonal surges, (as evidenced by a subtle increase in circulating progesterone levels in both male and female rats) likely reflects the influence of sex hormones on ventilatory control (Skatrud et al., 1978; Strohl et al., 1981; Tatsumi et al., 1991; Tatsumi et al., 1994; Behan et al., 2003; Netzer et al., 2003; Zabka et al., 2005).
4.3 Oxygen Saturation
Bruder et al., (2008) reported an ~80% O2 saturation in P8 rats exposed to 8% O2 for 1 h using the same instrumentation described in this study. Thus, the average SpO2 of 91.7 in 12% O2 is reasonable. During hypoxic gas exposure, the arterial oxygen saturation of hemoglobin (SpO2) was significantly lower in male vs. female rats at the P12-13 critical period of respiratory development (Figure. 4). The lower SpO2 could be due in part to the significantly lower ventilation (tidal volume) in P12-13 male rats (Table 1). Alternatively, the lower SpO2 could reflect an innate difference in the oxygen carrying capacity of hemoglobin. Sex differences in O2 carrying capacity have been reported in humans (Lewis et al., 1986) and fish (Clark et al., 2009). Hemoglobin in men had a greater O2 carrying capacity than that of women, but the opposite was reported in fish. A lower SpO2 in P12-13 male rats resulting from, or combined with, a lower ventilatory response to hypoxia could lead to a decreased delivery of oxygen to tissues and hypoxic ischemia. In contrast, female rats had a significant increase in SpO2 at P12-13 despite showing no age-related change in their ventilatory response to hypoxia. Taken together, these data highlight the fact that there are sex differences in the response to moderate hypoxia during a brief period of postnatal respiratory development.
We recognize that metabolic changes are important when evaluating ventilatory responses in small mammals (Mortola et al., 1994). Although we did not measure metabolic rate, in a study by Liu et al., (2008) metabolic rates in hypoxia in (presumably male and female) neonatal rats increased progressively with age. However, on a day-to-day basis the increases were not significantly different from one another, even during the critical period (P12-13). Additionally, Mortola et al. (1994) reported no sex difference in the change in metabolic rate during hypoxia, and stated that “specific ventilation changed with body weight with the same proportionality as metabolic rate did”. In a study by Sant’Anna and Mortola (2003) where ventilation and metabolic rate were measured in neonatal rat pups exposed to prolonged cold, they concluded that “the development of thermal or respiratory control appears to be rather insensitive to postnatal alterations in metabolic rate.” Thus, given there were no significant differences in body weight between the neonatal animals studied here, we believe that the contribution of metabolic rate to ventilation was similar among the neonatal animal groups.
4.4 Estradiol and breathing
It is important to note that the sex hormone concentrations measured in this study reflect circulating hormone concentrations, and that concentrations of sex hormones at the local level (within respiratory nuclei or the carotid body) may be different. Nonetheless, it is plausible to consider how an increase in circulating estradiol levels in P12-13 male rats might affect the control of breathing. In adult mammals the postulated rhythm generator is comprised of a GABAA-dependent reciprocal inhibition between two medullary nuclei (Fregosi, et al., 2004; Mellen et al., 2003; Rybak et al., 2007). GABAA receptor subunit composition in the rostral ventral respiratory group (rVRG) in rats has been shown to change during pregnancy, when sex hormones are at a physiological peak (Stang et al., 2011). If GABAA receptor expression was altered on bulbospinal respiratory neurons, changes in ventilation might result (Zuperku and McCrimmon, 2002). Developmental changes in both GABA and GABAA receptor subunits have been described in several respiratory nuclei in rats at P12-13 (Liu and Wong-Riley, 2006). How GABA and sex hormones (specifically estradiol) might interact to affect cell development was recently reviewed by McCarthy (2011). She suggested that hypoxia/ischemia induces cell death via Ca++-mediated excitotoxicity following increased extracellular glutamate and GABA release. The magnitude of the Ca++ transient is increased in the presence of elevated estradiol, leading to an increased GABA release during hypoxia/anoxia which might exacerbate cell damage/death. If this mechanism is engaged in respiratory nuclei of P12-13 male rats during the rise in estradiol levels (Figure 4), neurons could undergo damage/death, leaving the system susceptible to compromise or failure.
Measurement of brainstem sex hormone concentrations as well as measurement of sex hormone and GABA receptors on respiratory-related nuclei in neonatal male and female rats studied here will provide insight in determining if this mechanism is plausible.
4.5 Hypercapnic ventilatory response
The developmental changes in the response to hypercapnia were somewhat similar to changes reported in hypoxia. In CO2, male but not female rats had significantly decreased hyperpnea at P12-13 with a significant rebound by P14-15. Interestingly, male rats had more robust response to CO2 at P10-11 and at P14-15 compared to female rats (Figure 2). In both the hypercapnia and hypoxia ventilatory responses (change in ventilation), male rats underwent volatile changes around the “sensitive period”, whereas female rats had a more stable response. By puberty and into young adulthood, male and female responses to CO2 (and hypoxia) were similar. The latter findings are in agreement with a previous study of F344 male and female rats aged 3-4 months (Wenninger et al., 2009).
4.6 Mechanisms and implications
Within the neonatal age groups (P10-11, P12-13, P14-15), the nadir in ventilation in male rats at P12-13 suggests a sex-specific critical period of decreased respiratory output during normal eupneic breathing. Our results also suggest that in male rats, P12-13 is a time at which there is less robust ventilation in normoxia and a less robust hypoxic hyperpnea compared to P12-13 female rats, or to male rats at any other age. These data suggest that the occurrence of a severe hypoxic ventilatory challenge during this critical period could have deleterious consequences for male rats if they failed to maintain the threshold of ventilation required for normal delivery of oxygen to vital organs including the brain. Indeed, hypoxic challenges in the early developmental period (P4) have been shown to result in changes in diaphragm muscle composition in neonatal rats, although breathing was normal in these animals at P13-14 (van Heerden et al., 2011).
P12-13 male rats, due to their elevated estradiol levels, may have a natural susceptibility that makes them prone to exogenous ventilatory challenges (see Discussion 4.4). The mechanism(s) by which estradiol may be exerting its effects, especially within this narrow time frame, and the potential site(s) of action (peripheral and central chemoreceptors, bulbo-spinal neurons, diaphragm etc.) warrants further investigation.
Similar mechanisms may also exist in human male infants that lead to an increased susceptibility to ventilatory challenges during critical periods in respiratory development, and contribute to the striking gender bias in respiratory disorders. In an analysis of human infant mortality due to respiratory causes, Mage and Donner (2004) reported an approximately 50% male excess. A critical period of susceptibility in human infant mortality due to SIDS also exists around 3-4 months of age (Hoffman et al., 1988). Respiratory death characterized by a 50% male excess includes infant respiratory distress syndrome (IRDS), sudden infant death syndrome (SIDS), respiratory tract obstruction or suffocation, respiratory infections and accidental drowning (Mage and Donner, 2004). The consistent male excess in a wide variety of respiratory disease etiologies suggests a common terminal hypoxic condition (Mage and Donner, 2004). The significantly lower SpO2 in mild hypoxia in P12-13 male rats compared to female rats reported here may support this hypothesis.
Gender bias in respiratory disorders is most likely due to a complex interplay of physiological and molecular factors in respiratory-related structures during the critical period of respiratory development. Sex differences in systemic levels of the sex steroid hormones testosterone, estrogen and progesterone in could have widespread effects on all aspects of the respiratory control. In a simple hormonal model, the increased prevalence of respiratory disorders in males vs. females could be caused by either the inhibitory effects of estradiol (circulating or derived from testosterone) and/or testosterone, or a protective effect of progesterone on ventilatory drive. In short, sex hormones act on various neurotransmitter systems in the brain and carotid body. There is evidence from work by Joseph et al. (2002) and McCarthy (2011) that domaminergic, glutamitergic and gabaminergic receptors are affected by sex steroid hormones. Future studies will evaluate the aforementioned receptors in the animals studied here.
Finally, we hypothesize that the prenatal and perinatal testosterone surges, which can be metabolized to estradiol in the brain by the enzyme aromatase, affect the development of the respiratory control system, and as a consequence, influence the gender bias in respiratory disorders. In future studies we will eliminate prenatal testosterone surges via injection of an androgen receptor antagonist in the pregnant dam, and assess the ventilatory response to normoxic, hypoxic and hypercapnic gas exposure in neonatal control vs. testosterone-negative rats
Summary 4.7
Compared to age-matched females, P12-13 male rats had lower ventilation in normoxia and hypoxia and a lower O2 saturation during hypoxia. Additionally, there was a striking negative correlation between circulating estradiol levels and ventilatory responses to hypoxia and hypercapnia in male but not in female neonatal rats. Our data support the hypothesis that a “sensitive period” in breathing development exists in male, but not female, neonatal rats. Understanding the mechanisms by which sex hormones influence the development of the respiratory control system may help inform future therapeutic treatments of respiratory disorders.
We examined sex differences in breathing in rats during postnatal days 10-15.
>In hypoxia, P12-13 male rats had lower ventilation and O2 saturation vs female rats.
>At P12-13, male rats had higher estradiol concentration vs. female rats.
>In male rats, estradiol and hypoxic ventilation had a strong negative correlation.
>P12-13 is a sensitive period of breathing development in male but not in female rats.
Acknowledgements
This research was supported by the Merial Veterinary Student Scholars Program (HMH), NIH R01AG18760 (MB), and the Parker B. Francis Family Foundation (JMW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bairam A, Montadon G, Joseph V, Lajeunesse Y, Kinkead R. Enhancement of the breathing frequency response to hypoxia by neonatal caffeine treatment in adult male rats: The role of testosterone. Respir Physiol Neurobiol. 2009;165:261–265. doi: 10.1016/j.resp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Fuller DD, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol. 2004;557:645–660. doi: 10.1113/jphysiol.2004.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Young KM, Barry KJ, Boller MR, Kim E, Klein PM, Ovrutsky AR, Rampersad DA. Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats. J Appl Physiol. 2010 Sep;109(3):796–803. doi: 10.1152/japplphysiol.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003 Jul 16;136(2-3):249–63. doi: 10.1016/s1569-9048(03)00086-7. Review. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Olson EB, Jr, Wang Z-Y, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 weeks of postnatal hyperoxia. J Appl Physiol. 2003;95:946–952. doi: 10.1152/japplphysiol.00985.2002. [DOI] [PubMed] [Google Scholar]
- Bruder ED, Taylor JK, Kamer KJ, Raff H. Development of the ACTH and corticosterone response to acute hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol. 2008 Oct;295(4):R1195–203. doi: 10.1152/ajpregu.90400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL. Obstructive sleep-disordered breathing in children: new controversies, new directions. Clin Chest Med. 2003;24:261–282. doi: 10.1016/s0272-5231(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Clark TD, Hinch SG, Taylor BD, Frappell PB, Farrell AP. Sex differences in circulatory oxygen transport parameters of sockeye salmon (Oncorhynchus nerka) on the spawning ground. J Comp Physiol. 2009 Jul;179(5):663–71. doi: 10.1007/s00360-009-0349-1. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Luo Z, Iizuka M. GABAA receptors mediate postnatal depression of respiratory frequency by barbiturates. Respir Physiol Neurobiol. 2004 Jun 25;140(3):219–30. doi: 10.1016/j.resp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Damus K, Hillman L, Krongrad E. Risk factors for SIDS. Results of the National Institute of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13. doi: 10.1111/j.1749-6632.1988.tb37230.x. [DOI] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Pequignot J, Sempore B, Cottet-Emard JM, Dalmaz Y, Favier R. Spielvogel H, Pequignot JM, 2000. Gender differentiation of the chemoreflex during growth at high altitude: functional and neurochemical studies. Am J Physiol Regul Integr Comp Physiol. 278:R806–816. doi: 10.1152/ajpregu.2000.278.4.R806. [DOI] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, Pequignot JM. Dopaminergic metabolism in carotid bodies and high-altitude acclimatization in female rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R765–773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Kamon E, Hodgson JL. Physiological differences between genders. Implications for sports conditioning. Sports Med. 1986 Sep-Oct;3(5):357–69. doi: 10.2165/00007256-198603050-00005. 1986. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MTT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J Appl Physiol. 2009 Apr;106(4):1212–22. doi: 10.1152/japplphysiol.90949.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neuroscience. 2010a Dec 15;171(3):637–54. doi: 10.1016/j.neuroscience.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol. 2010b Apr 1;518(7):1082–97. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage DT, Donner M. The fifty percent male excess of infant respiratory mortality. Acta Paediatrica. 2004;93:1210–1215. [PubMed] [Google Scholar]
- McCarthy MM. What can development teach us about menopause? Brain Res. 2011 Mar 16;1379:109–18. doi: 10.1016/j.brainres.2010.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003 Mar 6;37(5):821–6. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Matsuoka T, Saiki C, Naso L. Metabolism and ventilation in hypoxic rats: effect of body mass. Respir Physiol. 1994 Jul;97(2):225–34. doi: 10.1016/0034-5687(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breath. 2003 Mar;7(1):25–9. doi: 10.1007/s11325-003-0025-8. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol. 1990;259:R836–41. doi: 10.1152/ajpregu.1990.259.4.R836. [DOI] [PubMed] [Google Scholar]
- Pequignot JM, Spielvogel H, Caceres E, Rodriguez A, Sempore B, Pequignot J, Favier R. Influence of gender and endogenous sex steroids on catecholaminergic structures involved in physiological adaptation to hypoxia. Pflugers Arch. 1997;433:580–586. doi: 10.1007/s004240050317. [DOI] [PubMed] [Google Scholar]
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- Rotem-Kohavi N, Garcia AJ, Zanella S, Ramirez JM. Gender influence on in vitro rhythmogenesis from the preBotzinger complex. FASEB Journal. 2011;25:1074–5. [Google Scholar]
- Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165:201–20. doi: 10.1016/S0079-6123(06)65013-9. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Anna GM, Mortola JP. Thermal and respiratory control in young rats exposed to cold during postnatal development. Comp Biochem Physiol A Mol Integr Physiol. 2003 Feb;134(2):449–59. doi: 10.1016/s1095-6433(02)00321-5. 2003. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006 Dec 6;1123(1):89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Hansen SN. Comparison of NMDA modulation of breathing and NR1 expression in medullary nuclei of weanling male and female rats. Respir Physiol Neurobiol. 2007 Mar 15;155(3):203–12. doi: 10.1016/j.resp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA, Kaiser DG. Ventilatory response to medorxyprogesterone acetate in normal subjects: time course and mechanism. J Appl Physiol. 1978 Jun;44(6):393–44. doi: 10.1152/jappl.1978.44.6.939. [DOI] [PubMed] [Google Scholar]
- Stang KM, Hengen KB, Nelson NR, Johnson S, Behan M. GABAA receptor plasticity on respiratory neurons during pregnancy produces barbiturate insensitivity. FASEB Journal. 2011;25:111–13. [Google Scholar]
- Strohl KP, Hensley MJ, Saunders NA, Scharf SM, Brown R, Ingram RH., Jr. Progesterone administration and progressive sleep apneas. JAMA. 1981 Mar 27;245(12):1230–2. [PubMed] [Google Scholar]
- Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Influences of gender and sex hormones on hypoxic ventilatory response in cats. J Appl Physiol. 1991 Nov;71(5):1746–51. doi: 10.1152/jappl.1991.71.5.1746. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Effects of testosterone on hypoxic ventilatory and carotid body neural responsiveness. Am J Respir Crit Care Med. 1994;149:1248–1253. doi: 10.1164/ajrccm.149.5.8173766. [DOI] [PubMed] [Google Scholar]
- Thomas MR, Marston L, Rafferty GF, Calvert S, Marlow N, Peacock JL, Greenough A. Respiratory function of very prematurely born infants at follow up: influence of sex. Arch Dis Child Fetal Neonatal Ed. 2006 May;91(3):F197–201. doi: 10.1136/adc.2005.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerden ES, Brackett DG, Leso JI, Dmitrieff EF, Bavis RW. Chronic hyperoxia alters the hyperoxic ventilatory response and diaphragm muscle fiber composition in neonatal rats. The FASEB Journal. 2011;25:1111–5. [Google Scholar]
- Wang Z-Y, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol. 2005;149:181–190. doi: 10.1016/j.resp.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ward OB, Ward IL, Denning JH, French JA, Hendricks SE. Postparturitional testosterone surges in male offspring of rats stressed and/or fed ethanol during late pregnancy. Horm Behav. 2002;41:229–235. doi: 10.1006/hbeh.2001.1746. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Olson EB, Jr, Cotter CJ, Thomas CF, Behan M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J Appl Physiol. 2009 May;106(5):1522–8. doi: 10.1152/japplphysiol.90802.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005 Mar 1;563(Pt 2):557–68. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuperku EJ, McCrimmon DR. Gain modulation of respiratory neurons. Respir Physiol Neurobiol. 2002 Jul;131(1-2):121–33. doi: 10.1016/s1569-9048(02)00042-3. [DOI] [PubMed] [Google Scholar]