Abstract
Rationale
Exaggerated startle response is a prominent feature of posttraumatic stress disorder (PTSD) although results examining differences in the acoustic startle response (ASR) between those with and without PTSD are mixed. One variable that may affect ASR among persons with PTSD is smoking. Individuals with PTSD are more likely to smoke and have greater difficulty quitting smoking. While smokers with PTSD report that smoking provides significant relief of negative affect and PTSD symptoms, the effects of smoking or nicotine deprivation on startle reactivity among smokers with PTSD are unknown.
Objectives
The purpose of the current study were to: 1) examine baseline acoustic startle response (ASR) in smokers with and without PTSD under conditions of overnight abstinence; 2) evaluate the effect of smoking on ASR; and 3) evaluate the contextual effects of trauma versus neutral script presentations.
Methods
ASR was measured among 48 smokers with and without PTSD in the context of a 2 (Group: PTSD vs. non-PTSD) X 2 (Context: trauma vs. neutral) X 3 (Smoking Condition: usual brand cigarette vs. denicotinized cigarette vs. no smoking) design.
Results
Effects of modest size indicated: 1) PTSD participants demonstrated higher ASR; 2) compared to non-PTSD participants, PTSD participants reported greater negative affect following a trauma related script; and 3) following a trauma related script and smoking a usual brand cigarette, PTSD participants demonstrated higher ASR.
Conclusions
Although many smokers with PTSD report that smoking reduces PTSD symptoms, results suggest that smoking may actually potentiate or maintain an exaggerated startle response.
Keywords: Acoustic Startle Response, posttraumatic stress disorder, nicotine, cigarette smoking
Posttraumatic stress disorder (PTSD) is a prevalent psychiatric disorder estimated to occur in approximately 10% of the United States population. Psychophysiological symptoms have long been a prominent feature of trauma related disorders (Kardiner 1941) and exaggerated startle response has been codified as PTSD arousal criterion D5 in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association 1994). Startle is characterized by a pattern of muscular reflexes that is produced by a sudden or unexpected intense stimulus (Orr and Roth 2000). In humans, the acoustic startle response (ASR) represents a reflexive response to a high intensity, abrupt auditory stimulus and is commonly measured as a change in electromyogram (EMG) activity resulting from contraction of the orbicularis oculi muscle.
Over 25 studies have quantified ASR magnitude across a variety of PTSD populations (for a review, see Metzger et al. 1999). While meta-analyses of studies comparing the magnitude of startle responses between individuals with and without PTSD provides validation of the DSM-IV PTSD symptom of exaggerated startle, there is significant variation across studies and many studies have failed to provide evidence for exaggerated startle among those with PTSD (Metzger et al. 1999; Pole 2007).
In a review of this literature, Grillon and Baas (2003) explored the complexity of the PTSD startle data and suggested a number of variables (e.g., presence of medications, length of PTSD duration, contextual effects) that may moderate ASR. While a number of studies have suggested that ASR in PTSD participants may be modulated by aversive states (for a review, see Grillon and Baas 2003; Pole 2007), a more recent meta-analysis found no evidence that startle effect sizes were significantly related to whether the study involved a threat condition or other potential moderators including whether or not patients with psychoactive medications were excluded, PTSD symptom severity, trauma types, and types of startle stimuli (Pole 2007).
One potential variable that may affect magnitude of ASR among persons with PTSD that has not been previously evaluated is smoking and nicotine. Smoking is highly prevalent among those with PTSD (Beckham 1999; Lasser et al. 2000) with rates as high as 60% (Beckham et al. 2007). No previous PTSD startle studies have reported smoking rates among participants. While smokers with PTSD report that smoking significantly reduces PTSD symptoms (Beckham et al. 2007), the effects of smoking on ASR among individuals with PTSD who are also regular smokers has not been examined.
Relatively few studies have examined the effects of nicotine on startle magnitude and those that have examined the effects of nicotine on the ASR have primarily been conducted in the context of examining the effects of nicotine on emotional reactivity. It is well established that startle magnitude is modulated by affective processing. Response to a startling acoustic probe is enhanced when participants view unpleasant or negative emotional pictures in comparison with pleasant or neutral pictures (e.g., Vrana et al. 1988; Cinciripini et al. 2006), while startle is reduced during viewing of positive cues (Cuthbert et al. 1996). Startle potentiation by unpleasant stimuli is thought to reflect activation of the defensive motivational system (Lang et al. 1990).
The effects of nicotine on startle modulation among smokers unassessed for psychiatric disorders have been mixed. Some studies have found no effect for nicotine deprivation on neutral context (i.e., no affective stimuli) startle responding (Kumari et al. 1997; Mueller et al. 1998) or to ASR in the presence of negative cues (Geier et al. 2000; Hogle and Curtain 2006; Piper and Curtin 2006). In contrast, other studies have reported that nicotine deprivation among smokers increased ASR across all types of stimuli (Piasecki et al. 2002) and nicotine suppresses ASR to smoking and affective cues in non-psychiatric smokers (Cinciripini et al. 2006).
Data from our laboratory indicate that following exposure to trauma-related cues, smokers with PTSD report more smoking craving, negative affect, and PTSD symptoms (Beckham et al. 2007) and smoking results in significant relief of negative affect and PTSD symptoms (Beckham et al. 2007). Ambulatory data from our research group also indicates that smokers with PTSD are more likely to ad lib smoke following the occurrence of negative affect or a PTSD symptom (Beckham et al. 2005; Beckham et al. 2008). These data suggest that smokers with PTSD may be particularly reactive to specific contexts and their craving to smoke and behavior are related to their symptomatology. The effects of smoking or nicotine deprivation on startle reactivity among individuals with PTSD, however, are unknown.
The objectives of the current pilot study were to evaluate the effects of nicotine deprivation and trauma related imagery on ASR among smokers with PTSD. While the majority of the work examining affective modulation of startle has relied on a picture-presentation paradigm (e.g., Vrana et al. 1988), the current study aimed to extend this work using a script-driven imagery paradigm (Pitman et al. 1987). Script-driven imagery has been shown to significantly differentiate PTSD from non-PTSD participants in terms of both valence (e.g., ratings of fear, anxiety, guilt), arousal (e.g., heart rate, skin conductance; Orr et al. 1993), and smoking craving (Beckham et al. 2004). Using a 2 (Group: PTSD vs. non-PTSD) X 2 (Context: trauma vs. neutral) X 3 (Smoking Condition: usual brand cigarette vs. denicotinized cigarette vs. no smoking) design, the purposes of the study were to examine in smokers with and without PTSD: 1) baseline ASR under conditions of overnight abstinence; 2) the effects of smoking and nicotine on ASR; and 3) the effects of context (trauma vs. neutral) alone and in combination with smoking. We included a denicotinized cigarette condition in order to evaluate the sensory and behavioral aspects of smoking separate from the effects of nicotine. Specifically, we hypothesized that: 1) ASR would be significantly higher in participants with PTSD; 2) nicotine deprivation would potentiate the ASR; and 3) a PTSD X context interaction where smokers with PTSD would demonstrate (a) increased PTSD symptoms and negative affect; and (b) potentiated ASR in a trauma script condition compared to a neutral script condition.
Method
Participants
Participants were 18 smokers with PTSD and 30 smokers without PTSD. Participants were eligible if they were generally healthy, not currently seeking treatment for nicotine dependence, and current cigarette smokers, reporting consumption of at least 15 cigarettes/day with afternoon expired CO concentrations of at least 15 ppm.
Participants were excluded if they had major unstable medical problems or if they used any other forms of nicotine. Candidates who met DSM-IV criteria for schizophrenia, current manic episode, lifetime but not current PTSD, or current substance abuse/dependence were excluded. Since depression is independently associated with increased smoking (Kassel et al. 2003; Kassel and Unrod 2000) and can affect startle (Grillon and Baas 2003), smokers with current major depressive disorder were excluded.
Based on the summary provided by Grillon and Baas (2003) regarding which medications affect startle response, individuals who were prescribed benzodiazepines (including diazepam, alprazolam, lorazepam, and oxazepam), clonidine, or amitryptiline were excluded. Individuals who exhibited deficits in hearing within the 500 to 3,000 Hz range as assessed through audiologic screening with a Welch Allyn device were excluded (Grillion et al. 1998).
Trauma history and PTSD diagnosis were evaluated using the Clinician Administered PTSD Scale (CAPS; Blake et al. 1995) and the Trauma Life Events Questionnaire (TLEQ; Kubany et al. 2000). The Structured Clinical Interview for DSM-IV Diagnosis (SCID; First et al. 1994) was administered to assess other Axis I disorders (kappa across 9 raters for diagnoses was .96).
Smoking History, Nicotine Dependence, and Symptom Measures
In addition to a general smoking history questionnaire, participants completed the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al. 1991) in order to assess nicotine dependence. PTSD and mood symptoms were measured using the Davidson Trauma Scale (DTS; Davidson et al. 1997) and the Positive Affect and Negative Affect Schedule (PANAS; Watson et al. 1988a; Watson et al. 1988b). The DTS is a commonly used self-report measure of PTSD symptom severity with excellent reliability and validity (Davidson et al. 1997; McDonald et al. 2009). From the DTS, a score for re-experiencing, avoidance and numbing, and hyperarousal symptoms were calculated for each measurement occasion. The PANAS is a 10-item positive affect and 10-item negative affect scale designed to measure positive and negative affect with demonstrated reliability and validity (Watson et al. 1988a; Watson et al. 1988b).
Tobacco Use Biomarkers and Denicotinized Cigarettes
Expired carbon monoxide (CO) concentrations were monitored at various times over the course of the study to: a) verify smoking status and b) verify compliance with study requirements (e.g., overnight abstinence); CO was measured using a handheld CO monitor (piCO Smokerlyzer, Bedfont Scientific, Ltd., UK). The criterion for overnight abstinence was based on a previously validated formula (Rose and Behm 2004) that takes into account baseline CO levels. In instances in which overnight abstinence was not confirmed, participants were rescheduled to attend the study visit after completing overnight abstinence. Quest brand denicotinized cigarettes that contain at most .05mg of nicotine per cigarette were used in the nicotine-free condition. Participants received either a regular or menthol Quest cigarette depending on their normal smoking brand.
Screening and Script Development
Following a screening session (for which participants were paid $65), eligible participants completed a script development session and received $100. Scripts were developed as outlined by the Pitman research group (Orr et al. 1990; Orr et al. 1993; Pitman et al. 1990; Pitman et al. 1987; Pitman et al. 1989; Shalev and Rogel-Fuchs 1993) and as used in our previous work on smoking and PTSD (Beckham et al. 2007). Six individualized scripts portraying actual experiences from the smoker’s past were composed, including the three most traumatic events the participant could recall, as well as three neutral experiences. The use of three trauma and neutral scripts allows responses to be pooled across sessions for subsequent analyses.
Experimental Sessions
Table 2 outlines the procedures used in each of the six experimental sessions (scheduled approximately 3 days apart) that crossed the two trauma context conditions and three smoking conditions. Payment for each session was $100. Sessions began between 8 and 9 am. Smokers were required to be overnight abstinent from cigarette smoking, alcohol, and marijuana. Breath samples were used to assess compliance. Participants then completed initial measurements and symptom questionnaires (PTSD symptoms and mood). Following the initial assessment, participants were offered a standardized breakfast and sensors necessary for the EMG were attached.
Table 2.
Experimental Nicotine Abstinence/Smoking Challenge Sessions
| Time (Minutes) | Procedures |
|---|---|
| 0 – 15 | Compliance monitoring (e.g., CO measurement, breath alcohol); PTSD symptom, and mood questionnaires |
| 15 – 25 | Breakfast |
| 25 – 30 | Attach sensors and demonstration |
| 30-40 | Baseline ASR: 1 block of 10 trials |
| 40-45 | Smoking condition: Usual brand, Denicotinized cigarette, or No smoking) |
| 46 | Script driven imagery: Trauma or Neutral scripts |
| 47 – 48 | ASR Measurement: 1 block of 3 trials |
| 49 | Repeat Script |
| 50-51 | ASR measurement: 1 block of 3 trials |
| 52-53 | PPI measurement |
| 54-60 | CO measurement; PTSD symptom and mood questionnaires |
One block of 10 baseline trials of ASR was collected. Participants then completed one of three smoking conditions (nicotine, denicotinized, and no cigarette). Participants were asked to smoke a cigarette (usual brand or denicotinized) through a smoking topography device (Plowshare Technologies, Inc.; Rose et al. 1983). In the no-cigarette condition, participants were instructed to sit quietly for 5 minutes. Following the smoking condition, scripts were presented. Three of the sessions involved presentation of a neutral script and three included presentation of a trauma script. Smoking condition and script type were counterbalanced with the order randomly assigned.
Next, ASR was measured using two blocks of three trials. After the first block of three trials, the same script was presented again followed by the second block of three ASR trials. Participants were instructed to keep their eyes open, listen carefully and to imagine the scene as it was described as the scripts were presented. Participants than completed a series of pre-pulse inhibition (PPI) trials. For all sessions, participants completed a final set of symptom response questionnaires (e.g., craving, mood, and PTSD symptoms) and a breath CO to verify smoke inhalation. All testing took place in a ventilated, sound attenuated room outfitted with visual and auditory participant monitoring systems.
Assessment of Acoustic Startle Response
Electromyographic (EMG) activity of the orbicularis oculi muscle was used as an index of the ASR. The ASR task was presented binaurally through headphones (Sony model MDR-V600). All stimuli were broadband white noise. The startle stimulus was 100 dB (A) (50 ms; instantaneous rise time). EMG was collected from Ag/AgCl electrodes filled with isotonic gel placed on the skin above the orbicularis oculi, directly under the pupil and 10 mm lateral. A grounded electrode was attached to the forehead. Signal was online amplified (x 1000) and bandpass filtered (5k Hz to 1.0 Hz). Signals were digitized at 250 Hz, passed to a PC-based BIOPAC MP100 data acquisition workstation, and saved to disk. All auditory stimuli (ASR task and scripts) were digitally recorded and/or generated using Adobe Audition (formally Cool Edit Pro). Stimuli were presented using a computer soundcard and amplified using a low-noise sound studio quality headphone amplifier. Signal levels were calibrated to a standardized tone prior to each session with a sound pressure meter fitted with a headphone adapter.
Data Reduction and Analyses
Raw EMG data were integrated (root mean square method) prior to scoring. Responses were considered valid if: 1) the trial was scorable (i.e., high signal/noise ratio; no artifact) and 2) a deflection was observed in 20-120 ms following startle onset. Mean baseline (25 ms pre-startle onset) amplitude and peak (i.e., maximum) response amplitude were recorded for all valid trials and all responses were baseline corrected. All participants were included in analyses.
Mean startle response amplitude was modeled as the primary outcome measure. Response amplitudes for the 10 baseline habituation trials were averaged at each of the six sessions. For response habituation analyses, responses from each trial were averaged across the six sessions to yield 10 time-points. To assess response amplitudes during the experimental phase (i.e., in response to smoking and script manipulations), ASR responses were averaged across the two blocks of three startle probes within each visit.
As startle data were not normally distributed, data were log-transformed before analysis. Hypotheses examining startle amplitude were tested using a series of longitudinal regression models using SAS PROC GENMOD to estimate generalized estimating equations under an assumed Gaussian distribution; working correlation matrices were specified as exchangeable. In analyses of the experimental phase, responses from the habituation trials were included as a covariate. This approach has several methodological advantages over the computation of a difference score between blocks (Mulligan and Wiesen 2003) which is an alternative method to assess adaptation across blocks (Cinciripini et al. 2006). Because of overfitting concerns, modeling was restricted to the three primary design variables: diagnostic status (PTSD versus control), smoking condition (abstinence, denicotinized, or usual brand), and script (trauma versus neutral) with response amplitude from the habituation phase and time since trauma as the only covariates. For each outcome measure, three models were estimated: main effects; first order (all two-way interactions); and second order (the three-way interaction). In some instances specified contrasts were tested using appropriate post hoc procedures. Models presented below represent the highest order of model significance. Rate (habituation) models were estimated as above using longitudinal regression for the 10-trial baseline data. The model included a covariate for trial, a proxy variable denoting diagnostic status and an interaction term crossing the latter two. Our interest was in determining if the rate of response attenuation occurred differentially by diagnostic status (i.e., did the coefficient associated with the interaction term between trial and diagnosis differ significantly from zero).
Analyses of self-report measures including indices of PTSD symptoms and affect were conducted using SAS PROC MIXED. All analyses were conducted with SAS version 9.1. Given uneven distribution of males and females across groups, Wilcoxon nonparametric tests were used to examine wither gender was associated with startle magnitude. Results indicated that gender was not associated with ASR, z = 1.03, n.s.
Results
Participant Characteristics
Characteristics of the cohort at baseline are presented in Table 2. Gender was the only demographic factor differing between the cohorts with a substantially higher proportion of female participants in the PTSD group. For descriptive purposes, Table 1 also includes a categorization of primary traumas identified by each group during the CAPS interview.
Table 1.
Participant Demographic, Diagnostic and Smoking Measures
| Variable | PTSD (N=18) | Control (N=30) | Test Statistic |
|---|---|---|---|
| Age [M(SD)] | 4.61 (10.58) | 42.53 (8.72) | F(1,46)=−0.54, n.s. |
| SES (Hollings) | 60.44 (9.02) | 59.59 (9.66) | F(1,45)=−0.09, n.s. |
| Education [M(SD)] | 11.39 (1.91) | 12.63 (2.53) | F(1,46)=3.24, n.s. |
| Gender (% Female) | 56% (10 of 18) | 17% (5 of 30) | χ21= 7.92, p=.005 |
| Race | |||
| % African American | 67% (12 of 18) | 83% (25 of 30) | |
| % minority | 67% (12 of 18) | 87% (26 of 30) | χ21= 2.73, n.s. |
| % Married | 18% | 23% | χ21=0.21, n.s. |
| % Veteran | 33% (6 of 18) | 37% (11 of 30) | χ21=0.05, n.s. |
| % Currently Employed | 56% (10 of 18) | 33% (10 of 30) | χ21=2.29,n.s. |
| PTSD-DTS | 59.78 (33.41) | 14.83 (17.90) | 36.99, p<.0001 |
| Months since trauma | 202.86 (185.58) | 183.23 (150.61) | F(1,42)=0.15, n.s. |
| Trauma Types | |||
| None | 0% (0 of 18) | 13% (4 of 30) | χ27= 12.96, n.s. |
| Death/Illness of Loved One | 28% (5 of 18) | 27% (8 of 30) | |
| Childhood Sexual Abuse | 28% (5 of 18) | 7% (2 of 30) | |
| Childhood Physical Abuse | 0% (0 of 18) | 7% (2 of 30) | |
| Combat related traumas | 17% (3 of 18) | 4% (1 of 30) | |
| Adult Physical Violence | 17% (3 of 18) | 23% (7 of 30) | |
| Adult Sexual Assault | 6% (1 of 18) | 0% (0 of 30) | |
| Motor Vehicle Accident | 6% (1 of 18) | 20% (6 of 30) | |
| % MDD (Lifetime) | 72% | 17% | χ21=14.81, p=.0001 |
| CO level at Screen | 22.72 (7.83) | 25.67 (12.75) | F(1,46) = 0.78, n.s. |
| Number of Cigs | 19.94 (5.55) | 20.79 (6.97) | F(1,46) = 0.19, n.s. |
| FTND | 6.61 (1.46) | 6.13 (1.98) | F(1,46) = 0.79, n.s. |
| PreCO | 9.47 (5.26) | 12.43 (6.30) | F(1,46)= 2.80, n.s. |
| PostCO | 12.63 (5.28) | 15.32 (6.13) | F(1,46)= 2.40, n.s. |
Baseline Startle
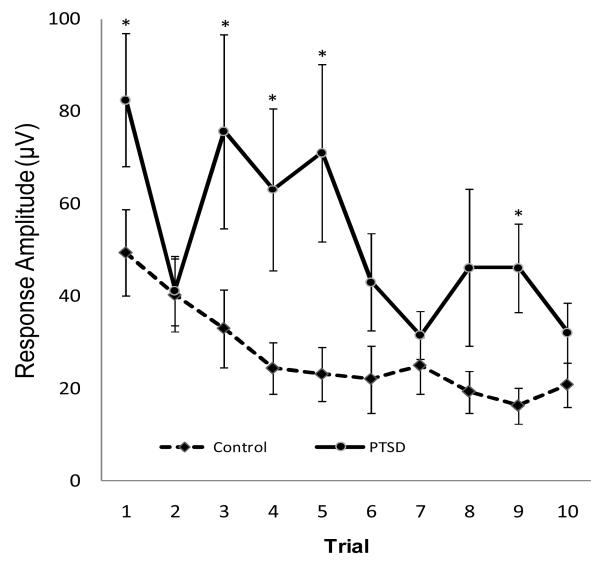
Group differences in baseline ASR responses and rates of habituation across the 10 trials are presented in Figure 1. Results indicated that the covariate of time since trauma was significantly related to magnitude of ASR across trials (χ21 = 4.24; p =.039) where longer time since trauma was associated with a decreased startle response. As predicted, response amplitude averaged over trials was significantly higher for participants with a diagnosis of PTSD (χ21 = 7.79; p < 0.005). Analyses examining habituation revealed a main effect of trials averaged over diagnosis (χ21 = 27.12; p < 0.0001) with ASR response attenuating at an approximate rate of 11% per trial. The interaction term crossing diagnosis with trials, however, was not statistically significant (χ21 = 1.56; p = 0.2114), indicating that the rate of habituation did not differ significantly between those with and without PTSD.
Figure 1.
Baseline Startle and Habituation by Group
Note. *p < .005. Error Bars indicate +/− 1 standard deviation.
Experimental Phase: Script Effects on Affect and PTSD Symptoms
Analyses first examined the effects of the script-driven imagery procedure on PTSD symptoms and negative affect. Three separate models were estimated testing respectively for main effects, first order interactions, and second order interactions for each of the self-report outcomes. In no instance was model fit significantly improved by the addition of a term denoting the three-way interaction between group, context, and smoking condition. In only two instances was model fit improved by the addition of a two-way interaction term as described below.
Examining ratings of negative affect from the PANAS, results indicated a significant PTSD X context interaction. As shown in Table 3, there was no difference in levels of negative affect between neutral and trauma contexts among non-PTSD participants. Among PTSD participants, however, the trauma context was associated with greater negative affect.
Table 3.
Effect of Context on Negative Affect and PTSD Symptoms
| Non-PTSD | PTSD | ||||
|---|---|---|---|---|---|
| Outcome | Neutral M (StdE) |
Trauma M (StdE) |
Neutral M (StdE) |
Trauma M (StdE) |
Effects |
| Negative Affect (PANAS) |
13.91 (0.62) |
13.27 (0.54) |
14.92 (0.74) |
16.54 (0.97) |
PTSD X Context F (1,225) = 4.94, p = .027 |
| PTSD B symptoms |
3.13 (0.56) |
4.97 (0.80) |
14.60 (1.63) |
16.49 (1.56) |
PTSD F (1,49) = 24.85, p < .0001 |
| Context F (1,225) = 5.04, p = .0257 |
|||||
| PTSD X Smoking F (1,225) = 3.72, p = .0257 |
|||||
| PTSD C symptoms |
5.35 (0.95) |
6.53 (1.11) |
25.38 (2.37) |
27.06 (2.34) |
PTSD F (1,49) = 29.78, p < .0001 |
| Context F(1,230) = 3.75, p = .054 |
|||||
| PTSD D symptoms |
5.09 (0.79) |
6.47 (0.89) |
18.90 (1.79) |
18.62 (1.69) |
PTSD F (1,49) = 22.29, p < .0001 |
Analyses examining PTSD symptom clusters revealed a main effect for group in all three PTSD symptom clusters (see Table 3) where as expected, smokers with PTSD reported higher PTSD symptoms than smokers without PTSD. Additionally, PTSD re-experiencing symptoms (DSM-IV Cluster B) were significantly higher in the trauma script condition than in the neutral condition. There was a similar but non-significant effect for PTSD avoidance symptoms (DSM-IV Cluster C), where avoidance symptoms tended to be higher in the trauma script condition in comparison to the neutral condition. Results also revealed a PTSD X Smoking interaction for PTSD Cluster B symptoms, where symptoms were lower in the denicotinized condition compared to the abstinence condition , t(225) = -2.3, p = .02.
Experimental Effects on Acoustic Startle Response
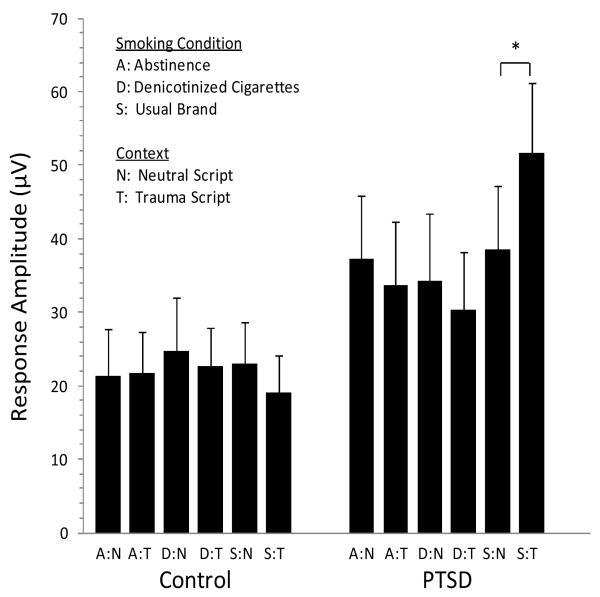
Effects of smoking and script type on mean response amplitude in each group are presented in Figure 2. As noted above, three separate models were estimated testing respectively for main effects, first order interactions, and second order interactions. Time since trauma was not significantly related to ASR during the experimental trials. Group and Context (script) were marginally significant when tested as main effects, both having a positive effect on mean response amplitude; no first order (two-way) interactions were significant. As shown in the second order model in Table 4, however, Smoking, Context, and Group crossed to form a three-way interaction acting together to significantly enhance response amplitude (Wald χ21 = 8.35; p =.01). That is, for participants with a PTSD diagnosis in the (usual brand) smoking condition, response amplitude was significantly higher after the presentation of a trauma script. The specific post hoc contrast evaluating response amplitude comparing the trauma script to the neutral script condition among the PTSD group following smoking a usual brand cigarette was significant (χ21 = 6.43; p < 0.01).
Figure 2.
Effects of Smoking Condition, Context, and Group on Mean Acoustic Startle Response AmplitudeNote. *p < .005. Error Bars indicate +/− 1 standard deviation.
Note. Figure shows the significant three-way Smoking X Context X Group interaction (p =.01). *The specific post hoc contrast comparing the trauma script to the neutral script condition among the PTSD group following smoking a usual brand cigarette was significant (p < 0.01).
Table 4.
Parameter Estimates of GEE Analysis Examining ASR response in the Experimental Phase
|
95% Confidence Interval |
Wald Statistics for Type III GEE Analysis |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | − | + | df | χ 21 | p |
| Intercept | −1.924 | 0.379 | −2.666 | −1.182 | |||
| Premean (log) | 0.698 | 0.055 | 0.591 | 0.805 | 1 | 163.92 | <.0001 |
| Months Since Trauma | 0.000 | 0.001 | −0.001 | 0.002 | 0.42 | .519 | |
| Group (PTSD vs. Control) | 0.420 | 0.378 | −0.320 | 1.160 | 1 | 0.53 | .768 |
| Smoking | 2 | 3.15 | .076 | ||||
| Usual Brand | 0.199 | 0.290 | −0.369 | 0.766 | |||
| Denicotinized | 0.117 | 0.314 | −0.499 | 0.733 | |||
| Abstinence | 0 | 0 | 0 | 0 | |||
| Context (Trauma vs. Neutral) | 0.686 | 0.290 | 0.117 | 1.255 | 1 | 5.14 | .077 |
| Group X Smoking | 2 | 1.79 | .18 | ||||
| Group*Usual Brand | −0.336 | 0.393 | −1.107 | 0.435 | |||
| Group*Denicotinized | 0.030 | 0.511 | −0.972 | 1.032 | |||
| Group*Abstinence | 0 | 0 | 0 | 0 | |||
| Context*Group | −0.621 | 0.442 | −1.488 | 0.245 | 1 | 1.98 | .37 |
| Context X Smoking | 2 | 0.14 | .71 | ||||
| Context*Usual Brand | −0.828 | 0.381 | −1.576 | −0.081 | |||
| Context*Denicotinized | −0.460 | 0.369 | −1.183 | 0.262 | |||
| Context*Abstinence | 0 | 0 | 0 | 0 | |||
| Context X Group X Smoking | 2 | 8.35 | .015 | ||||
| Context*Group*Usual Brand | 1.324 | 0.524 | 0.297 | 2.351 | |||
| Context*Group*Denicotinized | 0.311 | 0.705 | −1.070 | 1.692 | |||
| Context*Group*Abstinence | 0 | 0 | 0 | 0 | |||
Discussion
Consistent with expectations, smokers with PTSD demonstrated elevated baseline ASR under conditions of overnight abstinence in comparison to smokers without PTSD. PTSD and non-PTSD participants demonstrated similar rates of habituation across the initial 10 baseline trials. In contrast, results examining the effects of Group, Context (neutral vs. trauma scripts), and Smoking Condition (abstinence vs. denicotinized cigarette vs. usual brand cigarette) during the experimental trials provided partial support for hypotheses, as discussed below.
Although previous research examining the effects of nicotine deprivation in non-psychiatric smokers on ASR has been mixed, there is some evidence that nicotine deprivation may be associated with increases in startle amplitude in comparison to startle probes initiated during a satiated state (Piasecki et al. 2002). In the context of the larger literature examining affective modulation of the ASR, startle has been consistently enhanced when participants are exposed to negative affective stimuli (Cook et al. 1992; Vrana et al. 1988). As nicotine deprivation in smokers is typically associated with increased aversive withdrawal symptoms, it was expected that nicotine deprivation would lead to greater ASR amplitude in comparison to conditions where smokers consumed a usual brand cigarette. Results indicated, however, that there was no main effect for smoking condition. Thus, on average, continued deprivation was not associated with increased ASR in comparison to those that smoked a usual brand cigarette. Further, there were no significant two-way interactions between smoking condition and group. These results are consistent with findings reported by Cinciripini and colleagues (Cinciripini et al. 2006) who reported that nicotine deprivation was associated with suppressed startle in the presence of cigarette cues, presumably because of the activation of an appetitive system, but failed to enhance startle responding to negative cues.
While there was no support for the hypothesis that nicotine deprivation would be associated with increased startle, results were consistent with the hypothesized PTSD X Context interactions on measures of negative affect and startle magnitude. Ratings of negative affect after the Context manipulation indicated that the trauma scripts were associated with higher levels of negative affect compared to the neutral script conditions, but only among those smokers with PTSD. Trauma scripts were also associated with an increase in PTSD re-experiencing symptoms (DSM-IV Cluster B). Further, results demonstrated a group x context x amoking interaction where trauma scripts appeared to potentiate the ASR in comparison to a neutral script, but only among PTSD participants after they had smoked a usual brand cigarette.
Consistent with many previous investigations examining affective modulation of startle in PTSD (e.g., Hamm et al. 1997; Vrana et al. 1988) the current study employed a series of habituation trials before the experimental trials. In general, startle amplitude during the experimental trials was lower than that observed during the 10-baseline trials (see Figure 1 and 2). Although the magnitude of startle responses is expected to decrease over time, valence effects associated with affective modulation of the startle response (e.g., negative > neutral) typically remain intact (Cinciripini et al. 2006). Current study results provide evidence that idiopathic trauma scripts were associated with increased negative affect among smokers with PTSD. Idiopathic trauma scripts also resulted in increased ASR among smokers with PTSD, but only after smoking a usual brand cigarette. In this three way interaction, ASR levels approached the higher level demonstrated in the first few trials of the baseline habituation trials among PTSD participants. While this finding might suggest that nicotine in combination with a threatening trauma context may synergistically potentiate startle, it may also suggest that individuals with PTSD in a threatening context display a relative deficit in long-term habituation of startle after smoking. While either interpretation would be a significant finding, further research is needed to explore whether smoking a regular brand cigarette or trauma related stimuli potentiate startle among PTSD smokers.
Our previous work has suggested that individuals with PTSD who are regular smokers and who are exposed to a trauma-related context, report significant reductions in negative affect and PTSD symptoms following smoking (Beckham et al. 2007). Results from the current study, however, suggest that smoking may actually potentiate or maintain a maladaptive response, i.e., exaggerated acoustic startle. It is possible that smoking may serve to reduce negative affect in some contexts while serving to maintain other PTSD symptoms, i.e., startle. Consistent with this idea, many previous studies have identified that although smokers report that smoking a cigarette is relaxing, smoking appears to increase physiological arousal including raising basal levels of stress hormones, heart rate and blood pressure (Kassel et al. 2003).
Still, it is important to note that the nicotine manipulation in the current study preceded the affect-induction task. In our previous work (Beckham et al. 2007), we examined the effects of smoking and nicotine after exposure to a trauma-related context. There has been no research which has examined whether smoking before exposure to a trauma-related stimulus may buffer or potentiate PTSD symptoms and negative affect among PTSD smokers. It is possible that the effects of nicotine might have been different if the nicotine manipulation occurred after affect induction.
It is also possible that nicotine exposure itself may have affected the affect induction in the current study. Because smoking occurred before the exposure to the scripts, nicotine may have enhanced engagement in (minimized avoidance of) the imagery and have changed the depth or nature of the affect induction in some other way. The possibility that nicotine manipulation prior to affect induction may have altered that nature of the imagery stimulus induced by the trauma script is supported in part by the observation that during the nicotine-deprived conditions the PTSD group exhibited relatively smaller (not the expected larger) ASRs during the trauma imagery. That is, when nicotine deprived, the trauma script-associated ASRs in the PTSD group tended to be less than the neutral-script ASRs (see Figure 2). Results examining affect and PTSD symptoms, however, provide less support for this possibility. Examining affect and PTSD symptoms, there were no detected significant effects for smoking condition with one exception among PTSD participants. For PTSD re-experiencing symptoms, smoking (M = 17.36, SD = 2.13) and abstinence (M = 16.61, SD = 1.91) were associated with increased symptoms while smoking denicotinized cigarettes (M = 12.83, SD = 1.79) was associated with relatively lower PTSD re-experiencing symptoms. While this pattern of results is not completely consistent with the idea that nicotine may have enhanced engagement to the trauma script and altered the imagery stimulus, the possibility that individuals with PTSD did not experience comparable affective stimuli across the different smoking conditions cannot be ruled out.
It is also possible that the effects of nicotine on negative affect are moderated by potential contextual effects of trauma stimuli (e.g., internally driven versus externally driven). Gilbert (1995) has proposed a model of the effects of nicotine on attention and affect that suggests that “internally driven processes are more influenced by nicotine than are externally driven processes resulting from potent (unconditioned and high emotion elicitation potential) external stimuli,” (p. 195). This model would suggest that nicotine does not reduce negative affect when the stressor is proximal and intense, as it was in the current study. That is, nicotine does not reduce negative affect when the stressor is externally driven. In the current study, the stressor was externally driven by explicit experimental demands to think about the trauma. Thus, future research might assess whether or not nicotine reduces ASR when the trauma stimuli are less externally (more internally) driven or might assess the effects of nicotine on recovery from trauma induction as assessed by ASR and other indices.
Unlike several studies that have failed to find baseline differences in startle responding between PTSD and non-PTSD groups (for reviews see, Grillon and Baas 2003; Pole 2007) there was a large effect for baseline startle observed in this relatively small sample of PTSD and non-PTSD smokers. This effect was observed under conditions of overnight abstinence from smoking. It is unclear whether satiated smokers with and without PTSD would display similar differences in baseline startle magnitude. Currently, there are no studies that have compared baseline startle responding between deprived and satiated smokers with and without PTSD. Similarly, no studies have examined whether smokers with PTSD exhibit increased startle compared to non-smokers with PTSD. These are potentially helpful questions for future research. Although results from the current study failed to detect a main effect for nicotine deprivation after a series of habituation trials, more research is needed to examine whether this is a function of possible basement effects in ASR following habituation.
The majority of previous work examining affective modulation of startle has relied on a picture-presentation paradigm (e.g., Vrana et al. 1988). The current study aimed to extend this work using a script-driven imagery paradigm (Pitman et al. 1987), which has previously distinguished PTSD from non-PTSD groups (Orr et al. 2003). The script-driven imagery paradigm has the advantage of ensuring close correspondence to idiopathic trauma exposure details, however, a weakness of this approach, is that it is difficult to assess whether trauma scripts are equally aversive across PTSD and non-PTSD groups. Unlike a picture-presentation paradigm where reactivity to a standardized set of pictures is easily assessed and pictures can be chosen such that they are aversive to both groups, results in the current study suggest that the idiopathic trauma-related imagery among non-PTSD participants did not result in higher negative affect in comparison to neutral scripts. As a result, it is not surprising that there was no evidence of affective modulation among the control group, as the trauma scripts did not appear to have a significant effect on negative affective states.
Limitations of this study include the small sample of smokers, which limited the number of potential covariates included in statistical models. Further, results may not generalize to all PTSD smokers. PTSD smokers in this sample had a significantly higher proportion of lifetime depression than non-PTSD smokers. Given that negative emotional symptoms are core aspects of PTSD, attempting to remove variance accounted for by depression or negative affect symptoms from PTSD would omit a defining feature of the construct and might obscure the test of study hypotheses (Feldner et al. 2008). Still, it is unknown to what extent the startle results are specific to PTSD given the high rate of comorbidity between PTSD and other psychiatric disorders. Although there was no evidence that gender was associated with ASR, groups were also unbalanced on gender. Participants were instructed to maintain their usual caffeine intake and thus were not caffeine deprived. Future studies would be strengthened by matching on gender, history of depression, caffeine intake, and trauma history.
Despite these limitations, the current study is the first to compare ASR between smokers with and without PTSD. Individuals with PTSD who were regular smokers demonstrated higher baseline ASR under conditions of overnight abstinence than smokers without PTSD. Results demonstrated that a script-driven imagery paradigm using idiopathic trauma scripts is a feasible method to manipulate negative affect and PTSD symptoms for the measurement of ASR for those with PTSD. Results from the three-way interaction between Group, Context, and Smoking suggest that nicotine may potentiate or alternatively inhibit habituation in PTSD smokers in a trauma related context. Thus, nicotine appears to potentiate or maintain a maladaptive response, acoustic startle. It would be interesting in future studies to evaluate whether smoking cessation is associated with improved startle function among those with PTSD.
Acknowledgments
The authors thank the participants for their assistance in this research. This material is based upon work supported in part by R21DA019704, R21CA128965, 2K24DA016388, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development, and Health Services Research and Development. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the United States government.
Footnotes
The authors have no competing interests.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn. American Psychiatric Association; 1994. American Psychiatric Association. [Google Scholar]
- Beckham JC. Smoking and anxiety in combat veterans with chronic posttraumatic stress disorder: A review. J Psychoactive Drugs. 1999;31:103–110. doi: 10.1080/02791072.1999.10471731. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 2007;32:2900–2915. doi: 10.1016/j.addbeh.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, Rose JE. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: A preliminary study. Exp Clin Psychopharmacol. 2005;13:218–228. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Gehrman PR, McClernon FJ, Collie CF, Feldman ME. Cigarette smoking, ambulatory cardiovascular monitoring and mood in Vietnam veterans with and without chronic posttraumatic stress disorder. Addict Behav. 2004;29:1579–1593. doi: 10.1016/j.addbeh.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS. Ad lib smoking in posttraumatic stress disorder: An electronic diary study. Nicotine Tob Res. 2008;10:1149–1157. doi: 10.1080/14622200802123302. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered posttraumatic stress disorder scale. J Trauma Stress. 1995;8:75–80. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam C, Wu X, de Moor CA, Baile WF, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine Tob Res. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cook I, E.W., Davis TL, Hawk LW, Spence EW, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg MA, Mellman T, Beckham JC, Smith RD, Davidson RM, Katz R, Feldman ME. Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychol Med. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ, Monson CM, Bonn-Miller MO, Gibson LE. An examination of anxiety sensitivity as a moderator of the relationship between smoking level and posttraumatic stress symptoms among trauma-exposed adults. Cognitive Therapy & Research. 2008;32:116–132. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research Department; New York, NY: 1994. [Google Scholar]
- Geier A, Mucha R, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model of using pictures of smoking. Psychopharmacol. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilbert DG. Smoking: Individual differences, psychopathology and emotion. Taylor and Francis; 1995. Taylor and Francis. [Google Scholar]
- Grillion C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stess disorder. Psychiatr Res. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and the startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtain JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:1–14. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Kardiner AK. The traumatic neuroses of war. Paul B. Hoeber, Paul B. Hoeber. 1941.
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: Support for the role of nicotine in attentionally mediated anxiolytics. J Abnorm Psychol. 2000;109:161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA, Gray JA. Effect of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle inhibition of the acoustic startle reflex in heathy male non-smokers. Psychopharmacol. 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention and startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Beckham JC, Morey RA, Calhoun PS. The validity and diagnostic efficiency of the Davidson Trauma Scale in military veterans who have served since September 11, 2001. J Anxiety Disord. 2009;23:247–255. doi: 10.1016/j.janxdis.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Berry NJ, Ahern CE, Lasko NB, Pitman RK. Physiologic reactivity to startling tones in women with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:347–352. doi: 10.1037//0021-843x.108.2.347. [DOI] [PubMed] [Google Scholar]
- Mueller V, Mucha RF, Pauli Dependence on smoking and the acoustic startle response in healthy smokers. Pharmacol Biochem Behav. 1998;59:1031–1038. doi: 10.1016/s0091-3057(97)00508-x. [DOI] [PubMed] [Google Scholar]
- Mulligan NW, Wiesen C. Using the analysis of covariance to increase the power of priming experiments. Canadian Journal of Experimental Psychology. 2003;57:152–166. doi: 10.1037/h0087422. [DOI] [PubMed] [Google Scholar]
- Orr SP, Claiborn JM, Altman B, Forgue DF, de Jong JB, Pitman RK, Herz LR. Psychometric profile of posttraumatic stress disorder, anxious and healthy Vietnam veterans: Correlations with psychophysiologic responses. J Consult Clin Psychol. 1990;58:329–335. doi: 10.1037//0022-006x.58.3.329. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK, Investigators HVAP-tSDTS Physiologic responses to sudden loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol. 1993;102 doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Orr SP, Roth WT. Psychophysiological assessment: Clinical applications for PTSD. J Affect Disord. 2000;61:225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Shi FY, Fiore MC, Baker TB. Affective consequences of smoking cues, smoking withdrawal, and smoking availability: A startle probe study. Paper presentation. Society for Research on Nicotine and Tobacco; Savannah, GA. 2002. [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect; An analysis of initial emotional response intensity and voluntary emotion regulation. J Abnorm Psychol. 2006;151:96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Clairborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Steketee GS. Psychophysiological investigations of posttraumatic stress disorder imagery. Psychopharmacology Bulletin. 1989;25:426–431. [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychol Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: Pharmacologic and behavioral treatments. Nicotine Tob Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Rogel-Fuchs Y. Psychophysiology of the posttraumatic stress disorder: from sulfur fumes to behavioral genetics. Psychomatic Med. 1993;55:413–423. doi: 10.1097/00006842-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? J Abnorm Psychol. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorder. J Abnorm Psychol. 1988a;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affects: The PANAS scales. J Pers Soc Psychol. 1988b;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]