Abstract
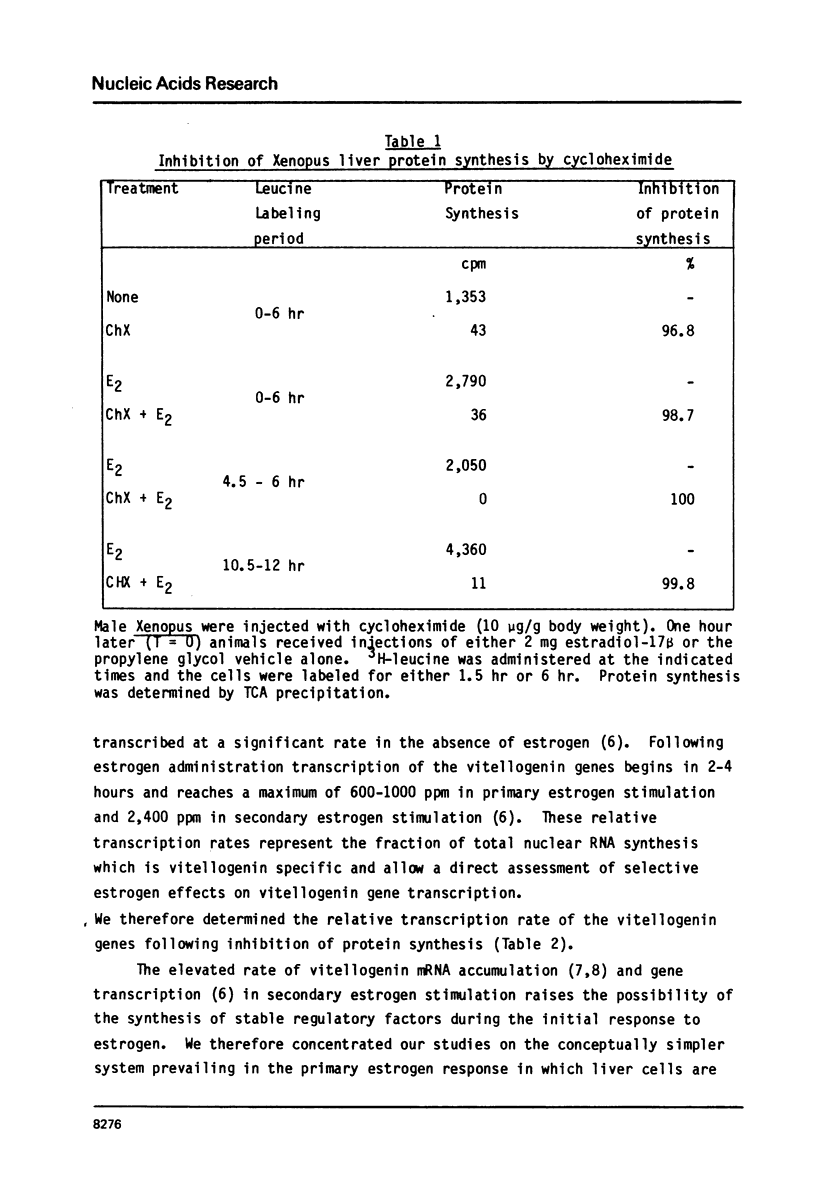
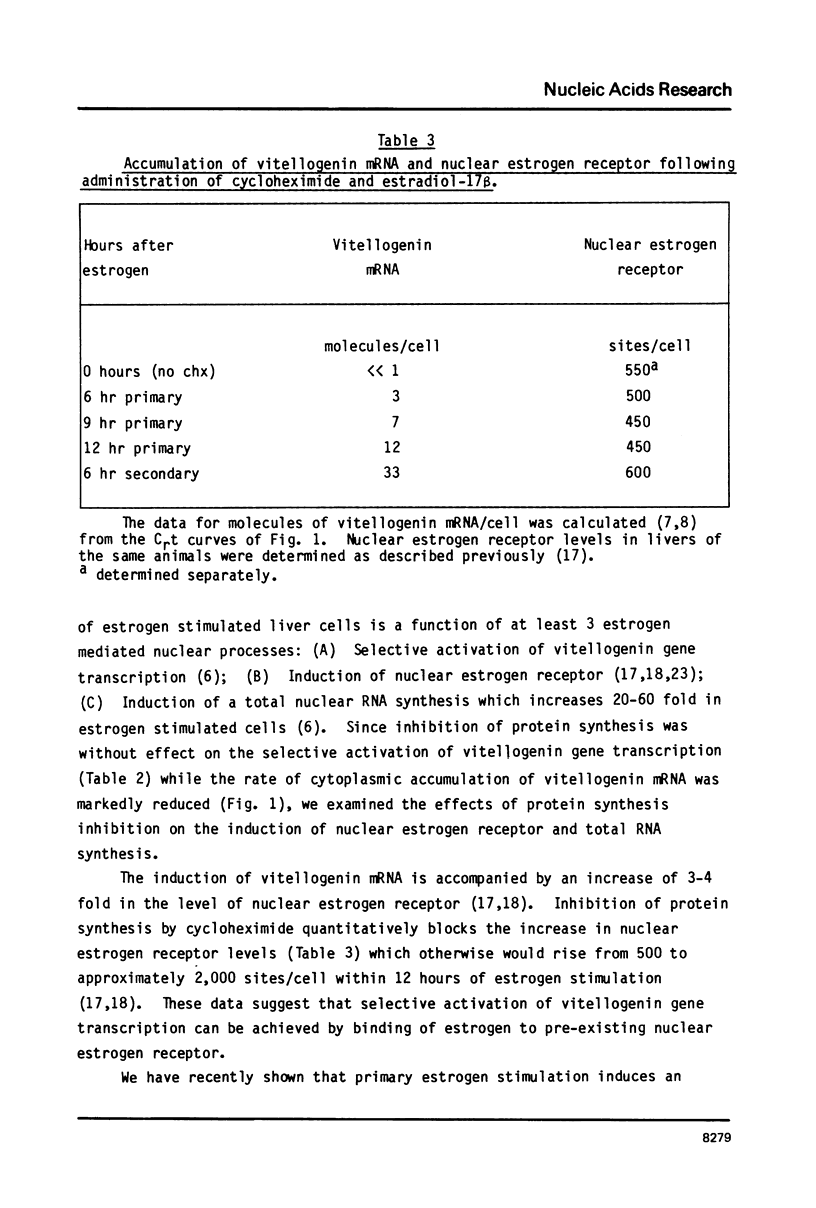
Estrogen induces the synthesis of vitellogenin mRNA by activating transcription of the vitellogenin genes. Quantitative inhibition of liver protein synthesis by cycloheximide does not prevent activation of vitellogenin gene transcription. The relative transcription rate of the vitellogenin genes in estrogen stimulated liver is similar in control and cycloheximide treated animals (800-1000 ppm). Selective estrogen activation of vitellogenin gene transcription therefore represents a direct effect of estrogen on vitellogenin gene transcription which can occur without any change in the cells' protein complement. Two other cellular responses to estrogen, the induction of nuclear estrogen receptor, and an increased rate of total nuclear RNA synthesis, are blocked by cycloheximide administration. Since the overall rate of vitellogenin mRNA synthesis is a function of both the selective estrogen activation of vitellogenin gene transcription which is not blocked by cycloheximide and the increased rate of total nuclear RNA synthesis which is blocked by cycloheximide, the total rate of vitellogenin mRNA synthesis is markedly reduced following cycloheximide administration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Rapid accumulation of vitellogenin messenger RNA during secondary estrogen stimulation of Xenopus laevis. J Biol Chem. 1978 Jul 10;253(13):4521–4524. [PubMed] [Google Scholar]
- Felber B. K., Ryffel G. U., Weber R. Estradiol-induced accumulation of vitellogenin mRNA and secretion of vitellogenin in liver cultures of Xenopus. Mol Cell Endocrinol. 1978 Nov;12(2):151–166. doi: 10.1016/0303-7207(78)90111-9. [DOI] [PubMed] [Google Scholar]
- Green C. D., Tata J. R. Direct induction by estradiol on vitellogenin synthesis in organ cultures of male Xenopus laevis liver. Cell. 1976 Jan;7(1):131–139. doi: 10.1016/0092-8674(76)90263-4. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Brock M. L., Shapiro D. J. The role of estrogen receptor in Xenopus laevis vitellogenin gene expression. Am J Physiol. 1982 Jul;243(1):C1–C6. doi: 10.1152/ajpendo.1982.243.1.E1. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McKnight G. S. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978 Jun;14(2):403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel G. U. Synthesis of vitellogenin, an attractive model for investigating hormone-induced gene activation. Mol Cell Endocrinol. 1978 Dec;12(3):237–246. doi: 10.1016/0303-7207(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Tata J. R. Vitellogenin gene expression in male Xenopus hepatocytes during primary and secondary stimulation with estrogen in cell cultures. Cell. 1981 Mar;23(3):741–746. doi: 10.1016/0092-8674(81)90437-2. [DOI] [PubMed] [Google Scholar]
- Shapiro D. Steroid hormone regulation of vitellogenin gene expression. CRC Crit Rev Biochem. 1982 Mar;12(3):187–203. doi: 10.3109/10409238209108706. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G., de Vellis J., Inglish D., Hsu C. Y., Moylan R. D. Involvement of cyclic amp and protein synthesis in catecholamine refractoriness. Adv Cyclic Nucleotide Res. 1978;9:33–52. [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Knowland J. Synthesis of vitellogenin in cultures of male and female frog liver regulated by estradiol treatment in vitro. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3172–3175. doi: 10.1073/pnas.72.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L. J., Osborne J. A., Hentschel C. C., Tilly R. Parenchymal cells purified from Xenopus liver and maintained in primary culture synthesize vitellogenin in response to estradiol-17 beta and serum albumin in response to dexamethasone. Dev Biol. 1979 Jun;70(2):479–499. doi: 10.1016/0012-1606(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Westley B., Knowland J. An estrogen receptor from Xenopus laevis liver possibly connected with vitellogenin synthesis. Cell. 1978 Oct;15(2):367–374. doi: 10.1016/0092-8674(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Westley B., Knowland J. Estrogen causes a rapid, large and prolonged rise in the level of nuclear estrogen receptor in Xenopus laevis liver. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1167–1172. doi: 10.1016/0006-291x(79)91531-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]



