Abstract
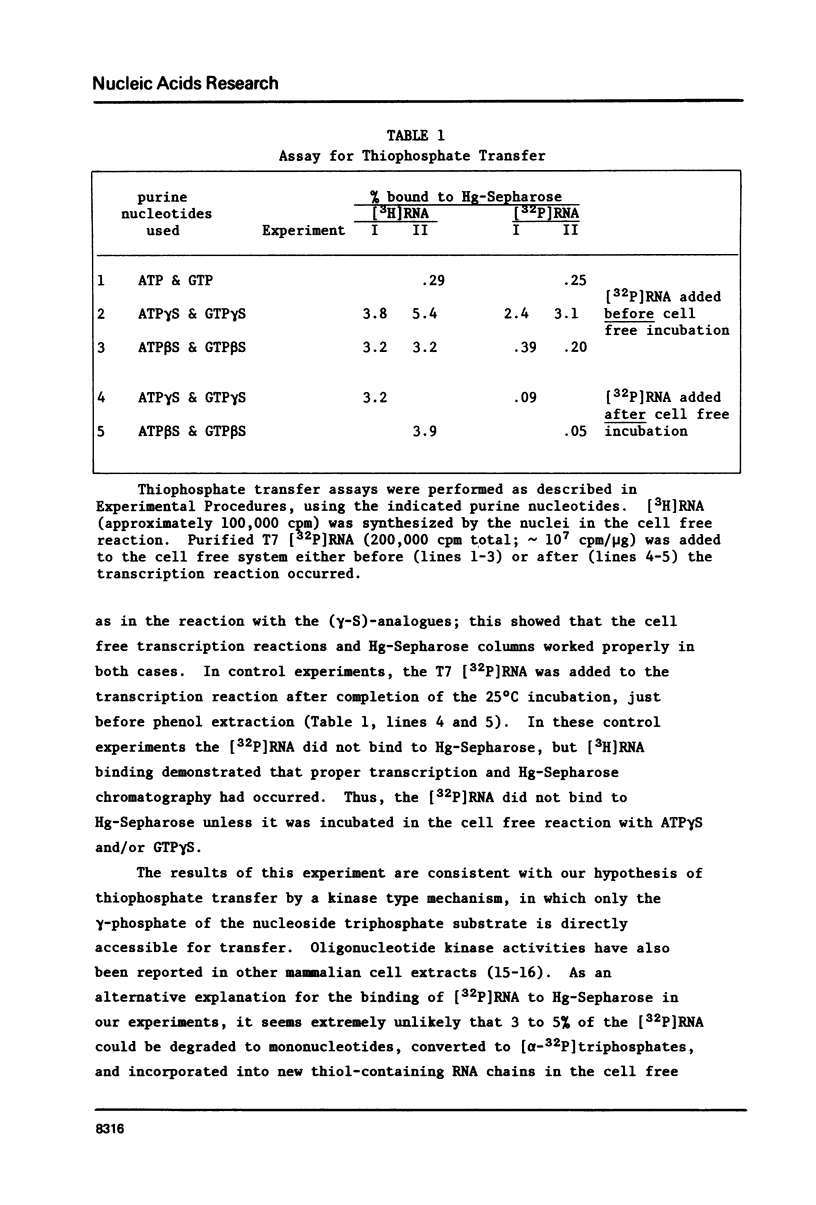
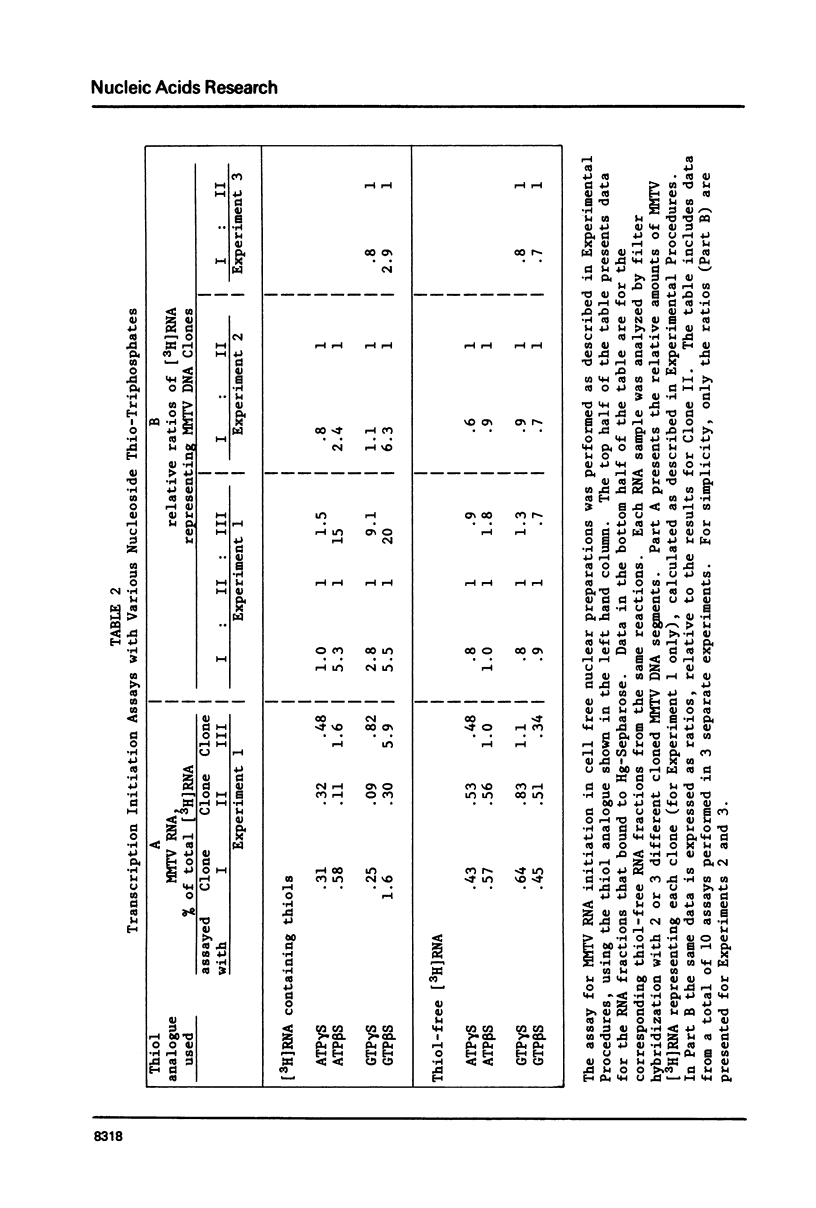
RNA chains initiated with nucleoside (beta-S)triphosphates and (gamma-S)triphosphates retain the thiol groups and can be separated from thiol-free RNA by chromatography on mercury-Sepharose. Thiol-containing mouse mammary tumor virus (MMTV) RNA synthesized by preparations of nuclei from virus-infected cells was quantitated by nucleic acid filter hybridization. With ATP beta S and GTP beta S, region-specific initiation of MMTV RNA chains was detected in the cell free system. However, with ATP gamma S and GTP gamma S, region-specific initiation was not clearly demonstrable. The nuclear preparations can also transfer thiol groups, presumably in the form of thiophosphate, from ATP gamma S or GTP gamma S onto preexisting RNA molecules; little or no thiol-transfer occurs with the two (beta-S)-analogues. The thiophosphate transfer activity apparently interferes with the measurement of RNA chain initiation with ATP gamma S and GTP gamma S.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Romaniuk P. J., Eckstein F. Synthesis and characterization of diastereomers of guanosine 5'-O-(1-thiotriphosphate) and guanosine 5'-O-(2-thiotriphosphate). Biochemistry. 1982 Apr 27;21(9):1983–1989. doi: 10.1021/bi00538a002. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Bankhurst A. D., Mason S., Warner N. L. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973 Feb;110(2):431–438. [PubMed] [Google Scholar]
- Hipskind R. A., Reeder R. H. Initiation of ribosomal RNA chains in homogenates of oocyte nuclei. J Biol Chem. 1980 Aug 25;255(16):7896–7906. [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Saiga H., Higashinakagawa T. Properties of in vitro transcription by isolated Xenopus oocyte nucleoli. Nucleic Acids Res. 1979;6(5):1929–1940. doi: 10.1093/nar/6.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Analysis of RNA initiated in isolated mouse myeloma nuclei using purine nucleoside 5'[gamma-S]triphosphates as affinity probes. Cell. 1978 Oct;15(2):615–626. doi: 10.1016/0092-8674(78)90030-2. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Ring J., Yamamoto K. R. Synthesis of mouse mammary tumor virus ribonucleic acid in isolated nuclei from cultured mammary tumor cells. Biochemistry. 1978 Apr 18;17(8):1515–1521. doi: 10.1021/bi00601a025. [DOI] [PubMed] [Google Scholar]
- Sun I. Y., Johnson E. M., Allfrey V. G. Initiation of transcription of ribosomal deoxyribonucleic acid sequences in isolated nuclei of Physarum polycephalum: studies using nucleoside 5'-[gamma-S]triphosphates and labeled precursors. Biochemistry. 1979 Oct 16;18(21):4572–4580. doi: 10.1021/bi00588a018. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Ross S. R., Yamamoto K. R. Mammary tumor virus DNA contains sequences required for its hormone-regulated transcription. Cell. 1981 Dec;27(2 Pt 1):257–266. doi: 10.1016/0092-8674(81)90409-8. [DOI] [PubMed] [Google Scholar]
- Winicov I. RNA phosphorylation: a polynucleotide kinase function in mouse L cell nuclei. Biochemistry. 1977 Sep 20;16(19):4233–4237. doi: 10.1021/bi00638a016. [DOI] [PubMed] [Google Scholar]



