Abstract
Chemopreventive and antitumor properties of perillyl alcohol (POH) studied preclinically indicate that topical POH inhibits both ultraviolet B (UVB)-induced murine skin carcinogenesis (squamous cell tumor models) and DMBA-induced murine melanoma (transgenic models involving tyrosinase-driven Ras). A previous Phase 1 clinical trial in participants with normal-appearing skin demonstrated that topical POH cream was well tolerated at a dose of 0.76% (w/w). Here we performed a three month, double-blind, randomized, placebo-controlled Phase 2a trial of two different doses of topical POH in individuals with sun-damaged skin. Participants applied POH cream twice daily to each dorsal forearm. Baseline and end-of-study biopsies were taken from each participant to evaluate whether the topical application of POH was effective in reversing actinic damage as evidenced by normalization of quantitative skin histopathologic scores and change in nuclear chromatin pattern measured by karyometric analysis. There was a borderline reduction in the histopathologic score of the lower dose of POH compared to placebo (p = 0.1), but this was not observed in the high dose group. However, in the high dose group, a statistically significant reduction in the proportion of nuclei deviating from normal was observed using karyometric analysis (p< 0.01). There was no statistical significance demonstrated in the lower dose group. No changes were observed in p53 expression, cellular proliferation (by PCNA expression), or apoptosis in either treatment group compared to placebo. These results suggest that while our karyometric analyses can detect a modest effect of POH in sun-damaged skin, improved delivery into the epidermis may be necessary.
Keywords: Chemoprevention, topical perillyl alcohol, karyometry, skin cancer, histopathologic score
Introduction
Perillyl alcohol (POH) is a naturally occurring hydroxylated monocyclic monoterpene, found in the essential oils of a number of plants, including cherries and lavendin. POH, administered systemically, has demonstrated both antitumor and chemopreventive properties that have been studied in both human and animal models. In mouse skin carcinogenesis the mechanism of action of POH includes induction of apoptosis in tumor cells while leaving normal cells unaffected (1). It has been shown in a UVB mouse skin carcinogenesis study that topical perillyl alcohol inhibits the development of squamous cell carcinomas and also inhibits UVB induced AP-1 activation (2). The inhibition of UVB induced AP-1 activation is significant because it has been shown that activation of AP-1 plays a functional role in mouse skin tumor promotion and progression (3). Also, in an in vivo murine model of DMBA-induced melanoma, topical administration of POH resulted in a significant reduction in the proportion of new tumors, suggesting that POH may be a promising chemopreventive agent for the prevention of melanoma (4).
Clinical trial experience with oral POH for cancer treatment has been largely unsuccessful due to GI toxicity resulting from the extremely high doses required for systemic activity. GI toxicities were observed as dose-limiting in a Phase 1 trial of 16 participants with refractory cancer (5). Similar toxicities were observed in Phase 2 trials of orally administered POH in advanced ovarian and metastatic colorectal and metastatic breast cancer, leading to early suspension of study accrual (6–8). Thus, topical application may represent a more promising route of administration for this drug, especially in the context of skin cancer chemoprevention.
We have previously demonstrated in a placebo-controlled Phase 1 trial that a topical POH formulation was well tolerated (9). A cream-based topical formulation of POH (10) in two strengths was developed, at 0.3 % w/w (20mM) and at 0.76 % w/w (50 mM). No severe cutaneous toxicities, systemic toxicities or histopathological abnormalities were observed with a 0.76% w/w cream POH formulation (9). Thirty-two percent of the participants reported mild adverse events at least possibly related to topical cream but there was no significant difference between the numbers or grade of the skin lesions on the POH treated forearms vs. the placebo treated forearm.
The purpose of this randomized, placebo-controlled, double-blind, Phase 2a study was to determine if topical administration of POH cream, applied twice daily to the forearms for three months, could reverse actinic damage as evidenced by normalization of quantitative skin histopathologic scores and change in nuclear chromatin pattern measured by karyometric analysis. In addition changes in epidermal cell proliferation indices, p53 expression and apoptosis rates were also measured using immunohistochemistry in skin biopsies.
Material and Methods
Participant eligibility and recruitment
We recruited 89 participants with sun-damaged forearm skin (as evidenced by at least 2 clinically-diagnosed actinic keratoses (AK) in the treatment areas). Participants were recruited through newspaper, radio and television advertisements, public service announcements, media stories, the Southern Arizona VA Health Care System, the Melanoma/Skin Cancer Screening Program Registry and the dermatology outpatient clinic at the University Medical Center in Tucson. Participants were screened by the study dermatologists to verify eligibility. All participants provided informed consent and research authorization (HIPAA) before initiation of therapy and following a complete explanation of the nature of the study.
Participants ≥ 18 years of age were recruited from Pima and adjoining Southern Arizona counties. Female participants were required to be postmenopausal or surgically sterile by hysterectomy. Participants had to have sun-damaged skin as judged by the study physician and have at least two AK on both of their dorsal forearms. Clinical criteria for determining sun damage included fine wrinkling, coarse wrinkling, mottling, hyperpigmentation, and a global assessment. Participants were eligible if at least 4 weeks had passed since the last surgical biopsy, surgical excision, or cryotherapy for AK in the test area. Participants were considered eligible if at least six months had passed since topical treatment for AK or any therapeutic approach for SCC or BCC in the test area (e.g. 5-fluorouracil, imiquimod, surgical excisions). Participants were required to discontinue all topical medications to the upper extremities, except for emollients or sunscreens, at least 30 days prior to study entry. Oral multivitamin intake was limited to Dietary Reference Intake values. Participants also agreed to limit sun exposure as much as possible during the three month study. In addition, participants who used citrus peel topically or consumed citrus peel were excluded.
Test agent
POH cream formulations were prepared at two strengths (50 mM, 0.76% w/w and 20mM, 0.3% w/w), formulated as previously described (10, 11). POH cream and matching placebo creams were packaged in 2.0 oz plastic ointment tubes and participants were instructed to store tubes at room temperature and avoid exposure to high temperatures. Clinic personnel and study participants were blinded to the specific contents of tubes to ensure the integrity of the double-blinded study. Adherence to the study medication was evaluated through the weighing of returned tubes and self-monitoring via study diaries.
Trial design
Baseline data (blood, urine, EKG, skin biopsies) were obtained prior to randomization. Participants were randomly assigned by computer to one of three treatment groups (placebo cream, 0.3% POH, or 0.76% POH). Participants were instructed to apply cream twice daily to each dorsal forearm for three months. Additional efficacy evaluations included alterations in patterns of biomarker expression, including p53 expression, cellular proliferation (PCNA) and apoptosis rates (morphologically apoptotic cells) (Table 1). Participants were also instructed to maintain a study diary to record daily time of application and any adverse events. Prestudy requirements included dermatologic exam, blood counts and serum chemistry analysis to rule out any serious underlying disease. In addition an EKG was completed at baseline, steady-state (between 4–6 weeks) and at completion of study to comply with FDA-mandated cardiac monitoring for new drug products.
Table 1.
Schedule of biopsies and endpoints for analysis
| Biopsy | Time | Location | Analysis |
|---|---|---|---|
| 1 | Baseline | R or L forearm | p53, PCNA, apoptosis, histopathology, karyometry |
| 2 | End-of-study | R or L forearm* | p53, PCNA, apoptosis, histopathology, karyometry |
Consistent with Bx #1
Biopsies
From each participant at baseline, and again after 12 weeks (end-of-study), a 4 mm punch biopsy was obtained from sun damaged skin on one dorsal forearm, 10 cm distal to the lateral epicondyle, for dermatopathology, karyometry, and immunohistochemical analysis. The matched end-of-study biopsy was collected at a point 2 cm adjacent to the location of the baseline biopsy, oriented using baseline photographs of the forearm. Biopsies were formalin fixed for analyses.
Histopathological analysis
Four millimeter skin punch biopsies were collected from the lateral posterior forearm, approximately 6 cm distal to the elbow joint in all randomized participants at baseline (BL) and end-of-study. The end-of-study biopsies for histopathology, karyometry and immunohistochemical markers were collected 10 to 18 days after the last dose of test article was applied to allow for resolution of any local site reactions, since any acute inflammation could confound these results. Biopsies were evaluated in a blinded fashion by one study dermatopathologist (P.S.) and scored according to a histopathologic scoring system previously described (12). Formalin-fixed biopsies were stained by routine H&E. The total histopathologic score was the sum of 4 criteria including basal or suprabasilar pleomorphism (atypia); inflammation; hyperkeratosis; and parakeratosis. Change in the score for paired biopsies was calculated by subtracting the baseline score from the end-of-study score. The high and low dose treatment groups were compared to the placebo treated group.
Immunohistochemistry
Immunohistochemical staining was done using peroxidase (p53) or alkaline phosphatase avidin with a fast red chromagen (PCNA) and hematoxylin counterstain (Ventana Medical Systems, Tucson, AZ) on an automated VMS 320 immunostainer (Ventana Medical Systems) at 1:100 dilutions. Anti-PCNA PC10 and anti-p53 (Calbiochem) were used at a 1:200 dilution in antibody diluent (Ventana Medical Systems). Negative controls (to assure lack of nonspecific staining) were run on each sample by substituting antibody diluent for the antibody. Controls included in each run included tonsil tissue for PCNA and T47D human breast cancer cells for p53 to assure proper staining and to assess variability of staining intensity. Tissue sections were analyzed by ImagePro Plus software (Media Cybernetics, Silver Springs, MD), a Leica DMR microscope (Leica, Westzlar, Germany), and a Sony 3 CCD color video camera (Sony, Tokyo, Japan). The percentage positive area per 40× field was determined for each biopsy. Three representative 4× fields were used for image analysis and the entire epidermis was included so all basal and suprabasal cells were analyzed
Apoptosis by morphology
The number of apoptotic cells was assessed based on morphology (i.e., condensed and/or pyknotic nuclei, eosinophilic cytoplasm, formation of apoptotic bodies) on H&E per 100 basal keratinocytes. The entire biopsy was used for each apoptosis assessment.
Karyometric analysis of topical drug effects
For the 27 participants treated with the higher dose of POH, 1,330 nuclei were recorded at baseline and 1,945 nuclei at the end-of-study. For the 17 fully evaluable participants receiving the placebo cream, 1,318 nuclei were recorded at baseline, and 1,394 nuclei at the end-of-study. Tissue sections were cut at 5 microns and stained with H&E under carefully controlled conditions, as previously reported (13). High resolution digitized imagery of the histopathologic sections was recorded on a 3 CCD SONY video-microphotometer, equipped with a 100:1, N.A. 1.4 plan apochromatic oil immersion objective (NIKON). Sampling density was adjusted to 6 pixels per linear micron. A 620 nm interference filter was used to enhance contrast. The nuclei in the digitized imagery were segmented by a semiautomated procedure with manual correction where needed. For each nucleus, features descriptive of the texture of the nuclear chromatin pattern were computed. In the feature selection, a statistical difference for two data sets at a significance level of p<0.005 was chosen. This was done in view of the multiple comparisons - for 95 different features - to keep selection of a spuriously significant feature at a chance of less than 1 in 200, and as a compromise in order not to increase the chances of a type II error, i.e., unnecessarily rejecting a useful feature. For a skin biopsy to be evaluable, a target of 100 stained non-overlapping nuclei per biopsy was set for the analysis, randomly selected after pathologist review and demarcation of the areas to be imaged.
Statistical Analysis
Histopathologic scoring and biomarker analyses were conducted using Stata version 10. To determine if histopathologic scores varied by treatment group at baseline the Kruskal-Wallis test was used. Changes in histopathologic scores for treated sun-damaged skin were compared between any treatment group and the placebo group using the Wilcoxon rank sum test.
The values for p53 and PCNA expression were derived by averaging the three highest staining 40× fields for each biopsy. Apoptosis measures were calculated by counting the number of apoptotic cells and basal cells and then standardizing the results to number of apoptotic cells per 100 basal cells. The Wilcoxon rank sum test was used to compare change in each measure between the treatment groups and the placebo group. Proportions of adverse events in different groups were compared using Fisher's exact test.
In the karyometric analyses, to determine differences for the karyometric features the Kruskal Wallis test (14) and ambiguity function of Genchi and Mori (15) were used. Wilks' Lambda was used to evaluate the difference between the four major features selected from the stepwise linear discriminant algorithm. A reduction in the proportion of nuclei assigned to the baseline data set was used as a measure of efficacy of the drug. The statistical significance of such a reduction was established based on the Fleiss Tables (16). The Kolmogorov-Smirnov statistic was used to determine the deviation from the “normal” in the end of study biopsy nuclei.
Results
Participant characteristics
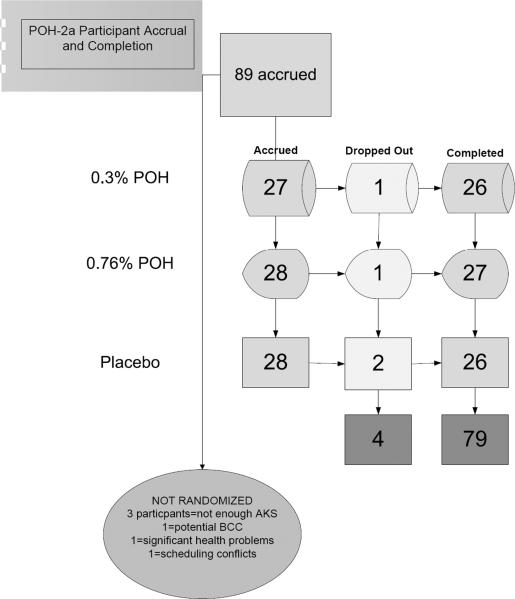
Eighty-nine participants were accrued and 83 participants were randomized (Figure 1) with a total of 79 participants completing the study. Table 2 shows the age and gender distribution of participants within the three groups (placebo, 0.3% POH, and 0.76% POH). There was no statistically significance difference in gender (Pearson χ2= 0.95, p = 0.62) or age (F(3,79) =0.49, p=0.69) between the three groups. All participants had sun-damaged skin with at least two clinically diagnosed and visible AK on each of their dorsal forearms. Baseline variables including age, gender, histological score and number of AKs were all well balanced (no statistically significant differences) across the three study groups. To determine adherence, the number of drug applications actually administered to the skin were calculated as a percentage of the total prescribed number of applications, and the weight of the drug actually consumed was calculated as a percentage of the total weight of drug expected to be consumed under the protocol. No statistically significant difference in either of these two adherence measures was observed across the three study groups (data not shown).
Figure 1.
Flowchart of study accrual
Table 2.
Gender and Age of participants
| Treatment Group | Female | Male | Total | |
|---|---|---|---|---|
| PLACEBO | Average Age (sd) | 63.9 (8.4) | 68.1 (10.4) | 66.9 (9.9) |
| N | 8 (29%) | 20 (71%) | 28 (100%) | |
| 0.3% POH | Average Age (sd) | 66.1 (11.6) | 69.7 (10.4) | 68.8 10.6 |
| N | 7 (26%) | 20 (74%) | 27 (100%) | |
| 0.76% POH | Average Age (sd) | 68.0 (7.4) | 67.6 (11.6) | 67.6 (10.9) |
| N | 5 (18%) | 23 (82%) | 28 (100%) | |
|
| ||||
| Total | Average Age (sd) | 65.7 (9.1) | 68.4 (10.7) | 67.7 (10.4) |
| N | 20 | 63 | 83 | |
Histopathologic scoring
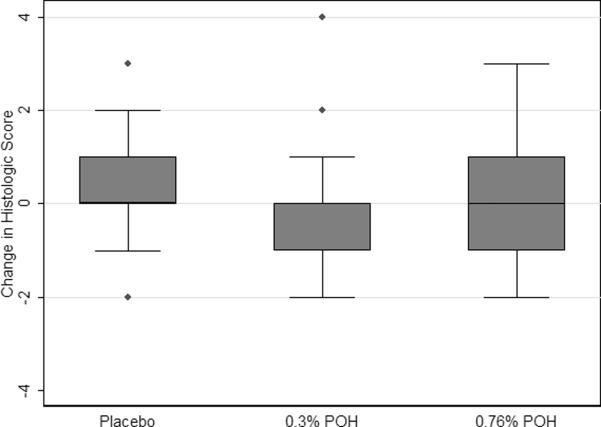
Baseline and end-of-study biopsies of sun-damaged skin were evaluated by the study dermatopathologist and scored according to a histopathologic scoring system previously described (12). This score reflects the presence or absence of two criteria (hyperkeratosis and parakeratosis) and the severity (absent, mild/moderate or severe) of two more criteria (pleomorphism and inflammation). Scoring of the biopsies taken at baseline did not vary by treatment (p=0.42). Figure 2 shows the box plot of distribution of the change in histopathologic score changes for biopsies taken from sun-damaged skin by treatment group. Table 3 contains the statistical analyses of these data. The score change in the lower dose POH group approached a statistically significant difference vs. the placebo group (p=0.10), which may be meaningful given the relatively small sample size. However, there was not a statistically significant difference between the high dose POH group and placebo (p=0.41).
Figure 2.
Box plots of change in histopathologic score for sun-damaged skin by treatment. A borderline significant difference in score was detected between the low dose POH group and the placebo group (p=0.1005), while the score change in the high dose group was not statistically significantly different from the placebo group (p=0.4124).
Table 3.
Average change in histopathologic score by treatment group
| Treatment | Type of Change in Histopathologic Score | ||||||
|---|---|---|---|---|---|---|---|
| Group | Skin | N | Mean* | median | Min | Max | sd |
| Placebo | SD skin | 26 | 0.4 | 0 | −2.0 | 3.0 | 1.3 |
| 0.3% POH | SD skin | 26 | −0.1 | 0 | −2.0 | 4.0 | 1.3 |
| 0.76% POH | SD skin | 27 | 0.1 | 0 | −2.0 | 3.0 | 1.4 |
| Total | SD skin | 79 | 0.1 | 0 | −2.0 | 4.0 | 1.3 |
mean change from baseline
Biomarker expression
No statistically significant differences by treatment group were apparent in the sun-damaged skin for change in either p53 expression, cellular proliferation (as measured by PCNA expression), or apoptosis (data not shown).
Karyometric analysis
For the samples in the high dose POH group, the results from analysis using the Kruskal Wallis test (14) and the ambiguity function of Genchi and Mori (15, 17) indicated the presence of statistically significant differences for more than 20 karyometric features, at p-values < 0.005. The ambiguity function values, though, suggested poor discrimination potential for nearly all of them. Table 4 lists the best of these features, with their mean values, the percent difference for the baseline and end-of-study data sets, and the ambiguity function value. An ambiguity value of 1.0 indicates no discrimination. All features are given in relative, arbitrary units. All of the features listed in Table 4 had a p-value of less than 0.005.
Table 4.
Karyometric features
| Feature ID | Mean value | |||
|---|---|---|---|---|
| Baseline | End-of-study | % difference | Ambiguity value | |
| # 307 run percentage | 783.4 | 695.3 | −11.2% | 0.981 |
| #001 total O.D. | 0.740 | 0.665 | −10.2% | 0.981 |
| #002 rel. nuclear area | 35.2 | 31.5 | −10.5% | 0.988 |
| #275 run length 5–6 | 4.2 | 3.6 | −14.3% | 0.987 |
| #321 # dense staining pixels | 603.3 | 548.7 | −9.1% | 0.990 |
| #303 short run emphasis | 0.51 | 0.52 | +2.0% | 0.986 |
| #014 pixel O.D. Int. 0.7 | 13.8 | 12.4 | −10.1% | 0.981 |
| #314 pixel OD clumpiness | 0.72 | 0.71 | −1.4% | 0.995 |
The data sets treated with the higher dose were each divided into two halves by assigning alternate nuclei to the first or the second halves. The first half data sets from the baseline and the end-of-study nuclei were used as a training set, with 668 and 995 nuclei. These data were submitted to a stepwise linear discriminant algorithm. It selected four features from the set listed in Table 4. The values following the feature ID number represent the standardized coefficients in the discriminant function. These selected features include the run percentage (# 307, 1.196), short run emphasis (# 303, −0.823), the relative frequency of occurrence dn/n in the pixel O.D. histogram interval from O.D. 0.70– 0.79 (# 014, 0.271), and the relative nuclear area (# 002, 0.662). Wilks' Lambda was reduced to 0.9595, i.e., only by 4.1%. The proportion of nuclei assigned to the baseline data set, i.e., the set with presumably greater deviation from normal, was reduced by 15.3%. For the sample size of 668 nuclei in the smaller data set this is a significant reduction, at p<0.01, and power of test of 0.99. The same discriminant function was next applied to the second half data sets. The reduction in the number of nuclei assigned to the baseline data set here was 19.1%.
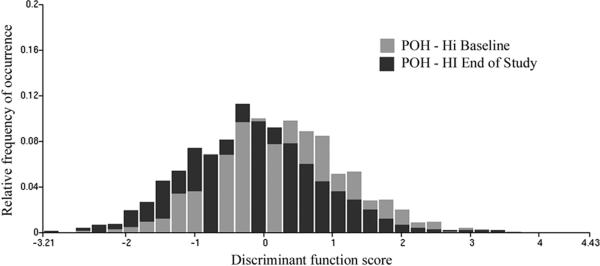
For the combined data sets, of 1,330 and 1,945 nuclei, the classification resulted in a reduction from 55.9% to 39.5%, i.e. by 16.4%. For the sample sizes involved, this reduction is highly significant at p<0.01, and power of the test of 0.99. The distribution of discriminant function scores is shown in Figure 3. There is a notable shift to the left on the score axis, indicating less deviation from “normal” in the end-of-study biopsy nuclei. The two distributions were statistically significantly different, as shown by the Kolmogorov-Smirnov statistic. The Dmax value was 0.179288, and the D alpha value was 0.0546, which resulted in a p<0.0001. Application of the discriminant function to the placebo treated end-of-study data set, of 1,394 nuclei, resulted in a reduction of the proportion of nuclei assigned to the baseline data set from 55.9% to 53.4%, (i.e., by only 1.5%).
Figure 3.
Distribution of discriminant function scores for the high dose POH samples at baseline and at end-of-study.
Discriminant function scores for the three treatment groups at baseline and end-of-study are shown in Table 5. As indicated above, only one discriminant function was used throughout this study, and the same function was applied in all comparisons. The function compounds, as a linear combination, the values of the four features listed above (Table 4). It is evident that there is a significant shift for the group treated with the high dose of POH, with no overlap of confidence limits between baseline and end-of-study. The baseline and end-of-study values for the low dose group are statistically indistinguishable, as are the values for the placebo treated group. Also, there are no statistically significant differences between baseline groups.
Table 5.
Discriminant function scores determined by karyometric analysis of biopsies from sun damaged skin.
| Baseline Mean (95% CI) |
End of Study Mean (95% CI) |
|
|---|---|---|
| POH (High) | 0.2492 (0.1982, 0.3002) | −0.1705 (−0.2145, −0.1265) |
| POH (Low) | 0.2367 (0.1786, 0.2948) | 0.2747 (0.2200, 0.3293) |
| Placebo | 0.2392 (0.1822, 0.2962) | 0.1498 (0.0998, 0.1997) |
Response in individual participants
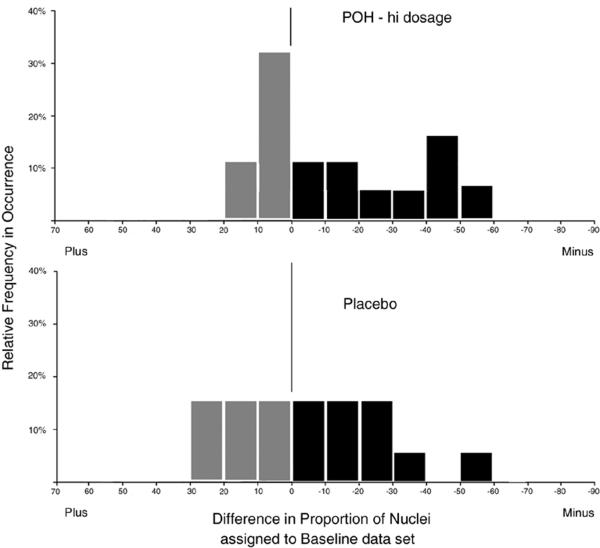
For the high dose POH group, a total of 3,275 nuclei were recorded, 1,330 at baseline and 1,945 at end-of-study. There were 27 cases, but a paired comparison was available for only 18 cases. For most of these cases the full target sample size of 100 nuclei had not been reached. The average number of nuclei per case was 78 nuclei. Figure 4 shows the histogram of relative number of cases plotted over a scale of proportion of nuclei, decreased or increased, respectively, at the end-of-study. The large peak around the zero mark reflects case-to-case sampling error. In the lower half of Figure 4 the same plot is shown for the placebo treated group. Here, no paired comparison was possible, as the high dose baseline cases were different from the placebo treated cases. The differences were computed from a scrambled list, and represent an estimate of the difference distribution, adjusted to the same percentage as presented in the top portion of Figure 4. In this study the difference in proportion of nuclei assigned to the baseline data set was defined as a measure of efficacy. The large sample size of nuclei available for establishing overall efficacy of the intervention provided significance at p<0.01 and a power of the test of 0.99. For the average sample size of 75 nuclei per case, a reduction in the proportion of nuclei from 55% to 30% would be needed to establish statistical significance at p<0.05, and power of the test of 0.80 for efficacy in an individual case.
Figure 4.
Histograms showing relative frequency of occurrence of cases with specific differences in proportion of nuclei assigned to the baseline data sets, comparing the high dose POH group (upper panel) to the placebo group (lower panel).
Low dose topical administration of POH
In the low dose group, no statistically significant effect could be established. The discriminant function derived in the high dose group was applied to the end-of-study data set from the low dose treated samples. It resulted in a decrease of nuclei assigned to the baseline data set by only 0.2%, from 55.9% to 55.7%, with samples sizes of 1,330 and 1,657 nuclei, respectively. In the low dose POH baseline and the low dose POH end-of-study data sets, the percentage of nuclei applied to the baseline data set increased by 0.6%, from 55.1% to 55.7%, with samples sizes of 1,346 and 1657 nuclei, respectively.
Clinical Response and Safety Evaluation
Participants kept a daily diary during the study, which included time of application and any adverse events. Each subject recorded the time and onset and resolution of any such events, and events were rated as mild, moderate or severe. Diaries were reviewed by clinic personnel at each visit.
Of the total events reported in the study, 66% were possible or probably related to study treatment, as rated by the study physician. Only one event in the high dose group was rated definitely related to study treatment. This event was tenderness in the test area which was rated as “mild” in severity. Table 6 shows the skin-related events by participant. The most frequent event occurring in the treatment groups was `rash, redness, erythema' rated “mild” or “moderate” in 54% of participants in the high dose group and in 56% of participants in the low dose group. The placebo group reported the same type of event in 43% of the participants. There was no statistically significant difference in the proportion between treatment and placebo groups.
Table 6.
Skin related events by participants
| Symptom/Sign | Severity | Placebo (n=28) | 0.30% POH (n=27) | 0.76% POH (n=28) |
|---|---|---|---|---|
| RASH, REDNESS, ERYTHEMA** | No Rash | 16 (57%) | 12 (44%) | 13 (46%) |
| MILD | 11 (39%) | 14 (52%) | 15 (54%) | |
| MODERATE | 1 (4%) | 1 (4%) | 0 (0%) | |
|
| ||||
| FLAKING, CRUSTING | No Flaking | 23 (82%) | 20 (74%) | 23 (82%) |
| MILD | 5 (18%) | 7 (26%) | 4 (14%) | |
| MODERATE | 0 (0%) | 0 (0%) | 1 (4%) | |
|
| ||||
| BURNING OR STINGING | No Burning | 25 (89%) | 24 (89%) | 27 (96%) |
| MILD | 2 (7%) | 3 (11%) | 1 (4%) | |
| MODERATE | 1 (4%) | 0 (0%) | 0 (0%) | |
|
| ||||
| PRURITUS | No Pruritus | 22 (79%) | 23 (85%) | 23 (82%) |
| MILD | 6 (21%) | 3 (11%) | 4 (14%) | |
| MODERATE | 0 (0%) | 1 (4%) | 1 (4%) | |
Discussion
We performed a Phase 2a dose-finding and efficacy trial of topically administered POH in a cream formulation having previously documented suitable skin tolerability in a Phase 1 study (9). Trial participants had sun-damaged skin with at least two clinically diagnosed AK on each dorsal forearm to document at least a moderate level of risk for non-melanoma skin cancer. There were multiple primary endpoints (i.e. normalization of quantitative skin histopathologic scores and skin nuclear chromatin pattern) and secondary endpoints (i.e. skin biopsy PCNA, apoptosis, and p53 biomarkers indices).
Our interest in POH as a topical chemopreventive agent for skin cancer was fueled by positive preclinical studies, documenting considerable activity in both UVB-induced murine SCC and melanoma (2, 4). There is increasing interest in the development of topically administered agents that could slow the progression of moderately or severely sun-damaged skin to non-melanoma skin cancer and the strong preclinical chemopreventive activity and excellent tolerability of topically administered POH make it a strong candidate for further clinical development.
As reported, there was a borderline difference that approached statistical significance in the change in sun-damaged dorsal forearm biopsy histopathologic score between study participants treated with placebo cream versus those treated with 0.3% (low-dose) POH; however, the high dose (0.76%) POH group did not show a similar trend. This was surprising, given the strong preclinical results; and the apparent lack of dose-dependence indicates that either the drug is not effective or the pharmacokinetic profile (i.e., the residence time of the drug at the site of action) was not suitable. However, in contrast to the results obtained using the histopathologic scoring system, dose dependent effects on reduction of nuclear abnormalities were observed using karyometric analysis. This supports the hypothesis that the negative histopathologic results were due to inadequate exposure of the drug to its target, most likely due to sub-optimal pharmacokinetic properties of the formulation.
Using karyometric analysis, we observed that the higher dose of POH (but not the lower dose of POH) was associated with a statistically significant reduction in the proportion of skin biopsy nuclei deviating from normal (i.e. p<0.01 with a power of test of 0.99). In case-by-case comparisons, it was difficult in this study to establish the statistical significance of the high dose POH versus placebo, due to the limited sample sizes of nuclei recorded for each participant. Of those participants treated with topically administered higher dose POH, 31% had a statistically significant reduction in proportions of nuclei deviating from normal, versus just 17% in the placebo group. This trend to less deviation from normal may very well represent a beneficial cancer preventive effect that will continue to be studied in future clinical trials using karyometric analysis as a biomarker endpoint.
Neither p53, PCNA, nor apoptosis were affected by the high or low dose POH concentrations administered topically twice daily over three months. These negative biomarker results match those observed in our prior positive skin cancer chemoprevention studies of oral vitamin A and topical difluoromethylornithine (18–20). On the other hand, karyometric analysis of nuclear chromatin abnormality in baseline and end-of-study sun-damaged skin once again has proven useful as an “integrating biomarker” of response to chemopreventive agent activity (13, 18–23).
The only adverse event that occurred frequently was a mild or moderate local reaction (termed rash, redness and/or erythema) that occurred at a rate of 56% and 54% for the low and high dose POH dose, respectively. However, this reaction may very well be due to the vehicle cream since the placebo group reported this same type of event in 43% of participants and this proportion is not statistically significantly different from that observed in the treatment groups. Similarly, the Phase 1 trial also demonstrated a lack of any serious adverse events (9) and no significant differences in the mild, study-related adverse events between treatment and placebo groups.
Obviously, topical POH cannot be effective as a skin cancer chemoprevention agent if it penetrates poorly into epidermal skin, or (possibly) if it rapidly penetrates the skin transdermally. An ongoing direction in our research program includes both in vivo preclinical and clinical projects to compare the skin penetration of the parent POH topical formulation and a novel prodrug topical formulation, engineered specifically to concentrate POH in the epidermis (24, 25). Development of true pharmacokinetic profiles (concentration vs. time curves) of percutaneous absorption and transdermal penetration of topically administered drugs in the context of a trial such as this one are impractical due to the excessive numbers of biopsies that would be required. Development of a prodrug of POH engineered with appropriate lipophilicity will allow for ex vivo human skin models to be developed that can compare the skin pharmacokinetics of parent POH and POH prodrugs to ensure adequate residence time of the active form of the drug.
In conclusion, the results of this study suggest that topically administered POH at 0.76% twice daily for 3 months has a modest effect in reducing nuclear chromatin abnormality in moderately to severely sun-damaged skin. Further studies of prodrug formulations of POH may result in a more active formulation of this novel topical agent that could be utilized in future non-melanoma skin cancer chemoprevention studies.
Acknowledgments
Grant support: National Cancer Institute, NIH, grants CA027502 and CA023074
References
- 1.Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;55(5):979–83. [PubMed] [Google Scholar]
- 2.Barthelman M, Chen W, Gensler HL, Huang C, Dong Z, Bowden GT. Inhibitory effects of perillyl alcohol on UVB-induced murine skin cancer and AP-1 transactivation. Cancer Res. 1998;58(4):711–6. [PubMed] [Google Scholar]
- 3.Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT, et al. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;1(11):848–54. [PubMed] [Google Scholar]
- 4.Lluria-Prevatt M, Morreale J, Gregus J, et al. Effects of perillyl alcohol on melanoma in the TPras mouse model. Cancer Epidemiol Biomarkers Prev. 2002;11(6):573–9. [PubMed] [Google Scholar]
- 5.Ripple GH, Gould MN, Arzoomanian RZ, et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin Cancer Res. 2000;6(2):390–6. [PubMed] [Google Scholar]
- 6.Bailey HH, Levy D, Harris LS, et al. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern Cooperative Oncology Group Study E2E96. Gynecologic Oncology. 2002;85(3):464–8. doi: 10.1006/gyno.2002.6647. [DOI] [PubMed] [Google Scholar]
- 7.Meadows SM, Mulkerin D, Berlin J, et al. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. International Journal of Gastrointestinal Cancer. 2002;32(2-3):125–8. doi: 10.1385/IJGC:32:2-3:125. [DOI] [PubMed] [Google Scholar]
- 8.Bailey HH, Attia S, Love RR, et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemotherapy & Pharmacology. 2008;62(1):149–57. doi: 10.1007/s00280-007-0585-6. [DOI] [PubMed] [Google Scholar]
- 9.Stratton S, Saboda K, Myrdal PB, et al. Phase 1 study of topical perillyl alcohol cream for chemoprevention of skin cancer. Nutr Cancer. 2008;60(3):325–30. doi: 10.1080/01635580701840391. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Myrdal PB. Development of a perillyl alcohol topical cream formulation. International Journal of Pharmaceutics. 2004;269(2):373–83. doi: 10.1016/j.ijpharm.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Stratton SP, Myrdal PB, Gupta A, Stratton SP, Myrdal PB. An HPLC method for quantitation of perillyl alcohol in a topical pharmaceutical cream formulation. Journal of Pharmaceutical & Biomedical Analysis. 2005;37(3):447–52. doi: 10.1016/j.jpba.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Bozzo P, Saboda K, Einspahr JG, et al. Reliability and validity of a histologic score as a marker for skin cancer chemoprevention studies. Analytical & Quantitative Cytology & Histology. 2003;25(5):285–92. [PubMed] [Google Scholar]
- 13.Bartels PH, Krouse RS, Prasad AR, et al. Actinic damage in histopathologically normal skin. Analytical & Quantitative Cytology & Histology. 2008;30(6):316–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Kruskal W, Wallis W. Use of ranks on one-criterion variance analysis. Journal of American Statistics Association. 1952;47:583–621. Addendum 48:907-11. [Google Scholar]
- 15.Genchi H, Mori K. Evaluation and feature extraction on automatic pattern recognition system. Denki Tsuchin Gakkai Pari (in Japanese) 1965:1. [Google Scholar]
- 16.Fleiss J. Statistical methods fro rates and proportions. Wiley; New York: 1981. [Google Scholar]
- 17.Bartels P, Olson G. Computer analysis of lymphocyte images. In: Catsimpoolas N, editor. Methods of cell separation. Plenum Press; New York: 1980. pp. 1–99. [Google Scholar]
- 18.Alberts D, Ranger-Moore J, Stratton S, et al. Safety and efficacy study of dose intensive oral vitamin A I participants with sun damaged skin. Clinical Cancer Research. 2004;10:1875–80. doi: 10.1158/1078-0432.ccr-03-0188. [DOI] [PubMed] [Google Scholar]
- 19.Alberts DS, Dorr RT, Einspahr JG, et al. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1281–6. [PubMed] [Google Scholar]
- 20.Bartels P, Yozwiak M, Einspahr JG, et al. Chemopreventive Efficacy of Topical Difluoromethylornithine and/or Triamcinolone in the Treatment of Actinic Keratoses Analyzed by Karyometry AQCH. 2009. in press. [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels PH, Yozwiak ML, Bartels HG, et al. Limits of detection of chemopreventive efficacy: karyometry of skin biopsies. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(7):1689–95. doi: 10.1158/1055-9965.EPI-08-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank DH, Kimler BF, Fabian CJ, et al. Digital image analysis of breast epithelial cells collected by random periareolar fine-needle aspirates (RPFNA) from women at high risk for breast cancer taking hormone replacement and the aromatase inhibitor, letrozole, for six months. Breast Cancer Research & Treatment. 2009;115(3):661–8. doi: 10.1007/s10549-008-0274-0. [DOI] [PubMed] [Google Scholar]
- 23.Bartels PH, Fabian CJ, Kimler BF, et al. Karyometry of breast epithelial cells acquired by random periareolar fine needle aspiration in women at high risk for breast cancer. Analytical & Quantitative Cytology & Histology. 2007;29(2):63–70. [PubMed] [Google Scholar]
- 24.Jacobson EL, Kim H, Kim M, et al. A topical lipophilic niacin derivative increases NAD, epidermal differentiation and barrier function in photodamaged skin. Experimental Dermatology. 2007;16(6):490–9. doi: 10.1111/j.1600-0625.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson EL, Kim M, Kim H, Wondrak GT, Jacobson MK. Developing Topical Prodrugs for Skin Cancer Prevention. In: Alberts D, Hess L, editors. Fundamentals of Cancer Prevention. Springer-Verlag; Berlin-Heidelberg: 2005. [Google Scholar]