Background: In vitro TGF-β differentiates Th17 and Tc17 cells, but TGF-βRIIDN mice display multiorgan autoimmune disorders.
Results: CD4+T cells from TGF-βRIIDN mice are resistant to Th17 cell differentiation, whereas CD8+T cells acquire IL-17-producing phenotype, and IL-17 neutralization or depletion inhibited inflammation in TGF-βRIIDN mice.
Conclusion: Tc17 cell differentiations in vivo are distinct from Tc17 cell differentiations in vitro.
Significance: The Tc17 cell differentiation program is unique to Th17.
Keywords: Cytokine, Inflammatory Bowel Disease, Interleukin, T cell, Transforming Growth Factor Beta (TGFbeta), CD4 T Cell, CD8 T Cell, IL-17, IL-6
Abstract
TGF-β is a pleiotropic cytokine that predominantly exerts inhibitory functions in the immune system. Unexpectedly, the in vitro differentiation of both Th17 and Tc17 cells requires TGF-β. However, animals that are impaired in TGF-β signaling (TGF-βRIIDN mice) display multiorgan autoimmune disorders. Here we show that CD4+ T cells from TGF-βRIIDN mice are resistant to Th17 cell differentiation and, paradoxically, that CD8+ T cells from these animals spontaneously acquire an IL-17-producing phenotype. Neutralization of IL-17 or depletion of CD8+ T cells dramatically inhibited inflammation in TGF-βRIIDN mice. Therefore, the absence of TGF-β triggers spontaneous differentiation of IL-17-producing CD8+ T cells, suggesting that the in vivo and in vitro conditions that promote the differentiation of IL-17-producing CD8+ T cells are distinct.
Introduction
TGF-β is a pleiotropic cytokine that influences nearly all cell types (1). However, in the immune system, TGF-β is widely regarded as a regulatory cytokine (2–6). Thus, TGF-β knock-out mice exhibit hyper-lymphoproliferation and succumb to multiorgan autoimmune disease (7). Similarly, mice expressing a dominant negative TGF-β receptor (TGF-βRIIDN) exhibit spontaneous differentiation of Th cells and display multiorgan autoimmune inflammation, most notably inflammatory bowel disease (IBD)4 (8).
Over the past several years, it has become widely accepted that T helper 17 (Th17) cells play a pathogenic role in a large number of autoimmune and inflammatory diseases (9, 10). Interestingly, TGF-β along with IL-6 are required for the differentiation of this inflammatory Th cell subset (11, 12). It has been shown that TGF-β does not play a direct role in the molecular orchestration of Th17 cell differentiation, but rather facilitates Th17 cell differentiation by inhibiting the differentiation of Th1 and Th2 cells (13). In this context, it appears surprising that transgenic animals carrying a dominant negative form of the TGF-β receptor II under control of the CD4 promoter (CD4-TGF-βRIIDN mice) exhibit multiorgan autoimmune inflammation (14, 15). This led us to examine the role of IL-17 in autoimmune inflammation in TGF-βRIIDN mice. Surprisingly, we found that TGF-βRIIDN mice produce large amounts of IL-17 and IL-6 in the serum, suggesting spontaneous activation of IL-17-producing cells in vivo. Previously, it has been shown that CD4+ T cells from TGF-βRIIDN mice fail to differentiate into Th17 cells (16). Consistent with this finding, we also found that CD4+ T cells isolated from these mice were resistant to Th17 cell differentiation. Instead we found that CD8+ T cells from these animals produced large amounts of IL-17 along with other pro-inflammatory cytokines upon activation. Finally, we showed that neutralization of IL-17 with anti-IL-17 antibodies or depletion of CD8+ T cells with anti-CD8 antibodies dramatically reduced inflammation in these mice. Therefore, TGF-β is not required for the in vivo differentiation of IL-17-producing CD8+ T (Tc17) cells but instead inhibits the differentiation of this cell type. Taken together these results indicate that the conditions that facilitate Tc17 cell differentiation in vivo are distinct from those that promote Tc17 cell differentiation in vitro.
MATERIALS AND METHODS
Mice
C57BL/6 and TGF-βRIIDN transgenic mice (a kind gift by Prof. Ruslan Medzhitov, Yale University, New Haven, CT) on a C57BL/6 background (6–8 weeks of age) were initially purchased from The Jackson Laboratory. Stat6−/−T-bet−/−TGF-βRIIDN mice were generated in our International Centre for Genetic Engineering and Biotechnology (ICGEB) knock-out facility by crossing Stat6−/−T-bet−/− double knock-out with TGF-βRIIDN. All animals were subsequently bred and maintained in the animal facility of the ICGEB, New Delhi, India.
Reagents
Fluorochrome-conjugated antibodies against IFN-γ (XMG1.2) and IL-17 (TC11-18H10) and unconjugated antibodies to CD3 (145.2C11), CD28 (37.51.1), CD4 (GK1.5), and CD8 (TIB105) were purchased from BD Biosciences. Antibodies to cytokines (IL-4, IL-6, IL-12, IFN-γ, and TGF-β) were purchased from R&D Systems. IL-17 (MM17F3)-neutralizing antibodies were purchased from eBioscience.
Detection of Cytokines
Cytokine levels in serum and culture supernatants were determined by multiplexed bead array immunoassay using the Luminex technology (Bio-Plex; Bio-Rad Laboratories).
Differentiation of Th Cell Subsets
Naive CD25−CD62LhiCD44lo CD4+ T cells were sorted from lymph nodes by using the FACSDiva software (BD Biosciences). These cells were differentiated under Th1- or Th2-polarizing conditions. In brief, 106 CD4+ lymphocytes/ml were activated with 1 μg/ml anti-CD3 antibodies bound to plastic and 2 μg/ml soluble anti-CD28 antibodies. 10 ng/ml IL-12 and 10 μg/ml anti-IL-4 antibodies were included in Th1 cultures, whereas 5 ng/ml anti-IL-4 antibodies, anti-IL-12 antibodies, and anti-IFN-γ antibodies (each at 10 μg/ml) were supplied for Th2 cultures. After 24 h, IL-2 was added to all cultures. Cells were divided at a 1:4 ratio after 3 days and allowed to rest under the described cytokine culture conditions in the absence of anti-CD3 and anti-CD28 antibodies for another 2 days. Differentiation of Th17 cells was performed as described previously (11, 12). In brief, FACS-sorted naive CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 antibodies in the presence of 5 ng/ml TGF-β, 20 ng/ml IL-6, or both. Cultures were terminated after different time points for analysis of mRNA expression and cytokines produced in the supernatants. All cell cultures were maintained in RPMI 1640 medium supplemented with 2 mm l-glutamine, 50 μm 2-mercaptoethanol, 10% heat-inactivated FBS, and 10 mm gentamycin.
Isolation of CD8+ T Cells from Splenocytes
For the isolation of CD8+ T cells from spleen, we prepared single cell suspensions and then centrifuged the cells. Next, we lysed red blood cells by red blood cell (RBC) lysis buffer and washed the cells twice with RPMI-1640 supplemented with 10% FCS. We resuspended the cells in a minimal volume (50 μl) of chilled 10% RPMI 1640, added 30 μl of magnetic-activated cell sorting beads, and incubated for 45 min at 4 °C.
Flow Cytometric Analysis
Spleens were isolated from mice, macerated by frosted slides in 10% RPMI 1640 (Invitrogen), and made into a single cell suspension. RBCs were lysed with RBC cell lysis buffer and washed with 10% RPMI 1640. The cells were counted, and 1 × 106 cells were used for surface staining. Cells were washed twice with PBS and stained with antibodies directed against surface markers with fluorescently labeled anti-cytokine antibodies. For intracellular cytokine staining, cells were treated with 50 ng/ml phorbol myristate acetate and 750 ng/ml ionomycin with 10 μg/ml brefeldin A (Sigma-Aldrich) added during the last 6 h of culture. Cells were washed twice with PBS and resuspended in a permeabilization buffer (Cytofix/Cytoperm kit; BD Biosciences), stained with fluorescently conjugated antibodies, and washed again with PBS. Fluorescence intensity of fluorochrome-labeled cells was measured by flow cytometry (FACSCantoTM II, BD Biosciences). FACSDiva was used for acquiring the cells, and final data analysis was performed by FlowJo (Tree Star).
Antibody Treatment
For the depletion of CD8-expressing cells, we injected mice intravenously (50 μg/mouse/dose) with anti-CD8 antibody twice per week.
Histology
For histology, pieces of intestine and pancreas were washed thoroughly in PBS and fixed in periodate-lysine-paraformaldehyde. Tissues were embedded in paraffin, and 5–6-μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) and examined microscopically.
Statistics Analysis
All data were derived from at least three independent experiments. Statistical analyses were conducted using the SPSS10 software, and values were presented as mean ± S.D. Significant differences between the groups were determined by analysis of variance followed by Tukey's multiple comparison test (SPSS software). A value of p < 0.05 was accepted as an indication of statistical significance.
RESULTS AND DISCUSSION
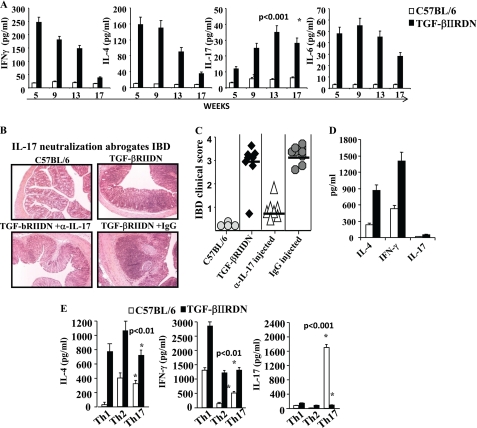
Previously, it was reported that CD4-TGF-βRIIDN animals exhibit multiorgan autoimmune inflammation and spontaneous differentiation of Th1 and Th2 cells (14, 15). In recent years, IL-17, an inflammatory cytokine predominantly produced by Th17 cells, has been implicated in many autoimmune disorders. Differentiation of Th17 cells requires TGF-β (11, 12). Thus, we determined production of IFN-γ, IL-4, and IL-17, as the representative cytokines of Th1, Th2, and Th17 cells, respectively, by Th cells from TGF-βRIIDN mice. We found that young animals had increased levels of the characteristic Th1 and Th2 cytokines in their serum when compared with wild type littermates (Fig. 1A), which is in agreement with our previous study (13). However, the levels of these cytokines gradually decreased as the animals aged (Fig. 1A). Surprisingly, we observed that the levels of IL-17 in the serum gradually increased (Fig. 1A) as the animals aged and started to show evidence of intestinal inflammation. To investigate whether the elevated IL-17 levels were responsible for the observed inflammation in the intestine, we injected anti-IL-17 antibodies into these animals twice a week starting at 4 weeks of age. Interestingly, we found that inhibition of IL-17 dramatically abrogated inflammation in the intestine (Fig. 1, B and C). These observations suggested that IL-17 plays an important role in the development of intestinal inflammation in TGF-βRIIDN mice. However, it is important to note that treatment with anti-IL-17 antibodies could not completely abrogate inflammation, which is consistent with prior studies indicating that factors in addition to IL-17 contribute to intestinal inflammation (17).
FIGURE 1.
IL-17 plays a critical role in intestinal inflammation in TGF-βRIIDN mice. A, wild type or TGF-βRIIDN animals were bled at the indicated ages, and inflammatory cytokines were measured by multiplex assays. A representative experiment is shown of three independent experiments with a total of 18 animals per group. B, animals were injected with anti-IL-17 antibodies or control Ig as described under “Materials and Methods.” Intestines were harvested, fixed in paraformaldehyde, and sectioned on 5-μm glass slides using cryo-sectioning. Sections were stained with H&E. Note that anti-IL-17 antibodies dramatically abrogate inflammation in the intestine. A representative experiment is shown from 30 slides prepared from six animals. C, quantitation of IBD. IBD was quantified from 30 slides of six animals in a blinded fashion. Scores presented are the mean of all individual scores. D, cytokines produced by CD4+ T cells derived from wild type or TGF-βRIIDN mice activated on plate-bound anti-CD3 and anti-CD28 antibodies. E, cytokines produced by Th cells that differentiated from CD4+ T cells from wild type or TGF-βRIIDN animals and were subjected to Th1, Th2, and Th17 differentiation conditions.
Next, we determined the source of IL-17 in TGF-βRIIDN animals at the onset of inflammation. We isolated CD4+ T cells, activated these cells with plate-bound anti-CD3 plus anti-CD28 antibodies, and determined the cytokines produced. We observed that these cells produced high amounts of IL-4 and IFN-γ, but failed to produce IL-17 (Fig. 1D). We further tested whether these cells could be differentiated into polarized Th1, Th2, or Th17 cells under appropriate culture conditions. Surprisingly, we found that for all culture conditions tested, CD4+ T cells derived from TGF-βRIIDN mice produced high amounts of IL-4 and IFN-γ, but IL-17 was not produced under any of these conditions (Fig. 1E). Taken together, these observations suggested that CD4+ T cells are not the relevant cellular source of IL-17, which is responsible for the observed intestinal inflammation in TGF-βRIIDN animals.
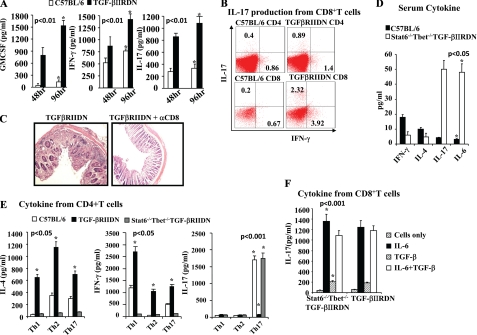
It has been well established that T cells are required for the development of IBD (18) Because CD4+ T cells were not the pathogenic source of IL-17 in TGF-βRIIDN animals, we tested whether CD8+ T cells from these animals produced IL-17. We isolated CD8+ T cells, activated these cells with plate-bound anti-CD3 and -CD28 antibodies, and measured cytokine production. We observed that CD8+ T cells produced large amounts of IL-17 as well as other pro-inflammatory cytokines (Fig. 2, A and B). Therefore, these observations suggested that CD8+ T cells in these animals spontaneously differentiated to an IL-17-producing phenotype. It is well known that IL-17 is produced by T cells as well as several other innate cell types (19). Therefore, to test whether CD8+ T cells are the relevant cellular source of IL-17, we treated TGF-βRIIDN animals with depleting anti-CD8 antibodies twice a week (50 μg/mouse/dose) starting at 4 weeks of age. We analyzed inflammation in the gut at 12 weeks of age. We found that TGF-βRIIDN mice treated with anti-CD8 antibodies had profoundly reduced clinical scores of intestinal inflammation when compared with mice treated with isotype control antibodies (Fig. 2C). Therefore, these observations suggested that CD8+ T cells are the predominant source of IL-17 that induces intestinal inflammation in TGF-βRIIDN mice.
FIGURE 2.
CD8+ T cells are the predominant source of IL-17 that induces intestinal inflammation in TGF-βRIIDN mice, and unlike Th17 cells, in vivo differentiation of Tc17 cells requires neither TGF-β nor IL-4 or IFN-γ. A, cytokines produced by CD8+ T cells derived from wild type and TGF-βRIIDN mice. B, FACS analysis to show IL-17 production by CD8+ T cells from TGF-βRIIDN mice. C, histological sections to show that anti-CD8 antibodies profoundly reduced clinical scores of intestinal inflammation in TGF-βRIIDN mice. The experiment shown is representative of three independent experiments (four mice in each group). D, serum cytokines in C57BL/6 and Stat6−/−T-bet−/−TGF-βRIIDN mice at the indicated ages. E, cytokines from differentiated CD4+ T cells from C57BL/6, TGF-βRIIDN, and Stat6−/−T-bet−/−TGF-βRIIDN mice. F, IL-17 production by CD8+ T cells from TGF-βRIIDN and Stat6−/−T-bet−/−TGF-βRIIDN mice. The experiment shown is representative of three independent experiments (six mice in each group).
Previously, we have reported that TGF-β is dispensable for the molecular orchestration of Th17 cell differentiation but instead inhibits Stat6 and T-bet induction, which facilitates the differentiation of Th17 cells (13). Therefore, we tested Tc17 differentiation in Stat6−/−T-bet−/−TGF-βRIIDN animals. As shown in Fig. 2D, these animals produced very large amounts of IL-17 in their serum. Next, we compared IL-17 production in CD4+ T cells derived from TGF-βRIIDN and Stat6−/−T-bet−/−TGF-βRIIDN mice (Fig. 2E). Consistent with our findings above, CD4+ T cells from TGF-βRIIDN animals were resistant to Th17 cell differentiation but produced high amounts of IL-4 and IFN-γ irrespective of the differentiation condition used (Fig. 2E). In sharp contrast, CD4+ T cells derived from Stat6−/−T-bet−/−TGF-βRIIDN mice were unable to differentiate into IL-4- or IFN-γ-producing cells but readily differentiated into IL-17-producing cells (Fig. 2E). Next, we tested IL-17 production by CD8+ T cells in these animals. We found that CD8+ T cells from TGF-βRIIDN and Stat6−/−T-bet−/−TGF-βRIIDN animals produced similar amounts of IL-17 (Fig. 2F). Therefore, unlike Th17 cells, in vivo differentiation of Tc17 cells requires neither TGF-β nor IL-4 or IFN-γ. Our previous study indicated that TGF-β does not play a direct role in Th17 cell differentiation but rather facilitates Th17 cell differentiation by inhibiting T-bet and Stat6 expression (13). These findings are in agreement with prior studies showing that production of IFN-γ in CD8+ T cells is independent of T-bet expression (20). However, these findings contrast with prior in vitro studies, which provided evidence that culture of naive CD8+ T cells with TGF-β and IL-6 facilitates the differentiation of Tc17 cells (21).
In summary, our data establish that TGF-β is not only dispensable but in fact inhibitory to Tc17 cell differentiation in vivo, which differs from the requirements for TGF-β in the differentiation of these cells in vitro. Unlike Th17 cells, differentiation of Tc17 cells is not regulated by Stat6 or T-bet. Therefore, the Tc17 differentiation program is unique.
Footnotes
- IBD
- inflammatory bowel disease
- Tc17
- IL-17-producing CD8+ T cells
- Th17
- IL-17-producing CD4+ T cells.
REFERENCES
- 1. Kim I. Y., Kim M. M., Kim S. J. (2005) Transforming growth factor-β: biology and clinical relevance. J. Biochem. Mol. Biol. 38, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Horwitz D. A., Zheng S. G., Gray J. D. (2008) Natural and TGF-β-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 [DOI] [PubMed] [Google Scholar]
- 3. Mantel P. Y., Schmidt-Weber C. B. (2011) Transforming growth factor-β: recent advances on its role in immune tolerance. Methods Mol. Biol. 677, 303–338 [DOI] [PubMed] [Google Scholar]
- 4. Shevach E. M. (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 [DOI] [PubMed] [Google Scholar]
- 5. Wahl S. M. (2007) Transforming growth factor-β: innately bipolar. Curr. Opin. Immunol. 19, 55–62 [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura A., Muto G. (2011) TGF-β function in immune suppression. Curr. Top Microbiol. Immunol. 350, 127–147 [DOI] [PubMed] [Google Scholar]
- 7. Kulkarni A., Ravi T. J., Brodmerkel G. J., Jr., Agrawal R. M. (1997) Inflammatory myositis in association with inflammatory bowel disease. Dig. Dis. Sci. 42, 1142–1145 [DOI] [PubMed] [Google Scholar]
- 8. Mi S., Li Z., Yang H. Z., Liu H., Wang J. P., Ma Y. G., Wang X. X., Liu H. Z., Sun W., Hu Z. W. (2011) Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J. Immunol. 187, 3003–3014 [DOI] [PubMed] [Google Scholar]
- 9. Hemdan N. Y., Birkenmeier G., Wichmann G., Abu El-Saad A. M., Krieger T., Conrad K., Sack U. (2010) Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev. 9, 785–792 [DOI] [PubMed] [Google Scholar]
- 10. Wilke C. M., Bishop K., Fox D., Zou W. (2011) Deciphering the role of Th17 cells in human disease. Trends Immunol. 32, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 12. Mangan P. R., Harrington L. E., O'Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. (2006) Transforming growth factor-β induces development of the Th17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 13. Das J., Ren G., Zhang L., Roberts A. I., Zhao X., Bothwell A. L., Van Kaer L., Shi Y., Das G. (2009) Transforming growth factor-β is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 206, 2407–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorelik L., Flavell R. A. (2000) Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 [DOI] [PubMed] [Google Scholar]
- 15. Zamiri P., Masli S., Kitaichi N., Taylor A. W., Streilein J. W. (2005) Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis. Sci. 46, 908–919 [DOI] [PubMed] [Google Scholar]
- 16. Veldhoen M., Hocking R. J., Flavell R. A., Stockinger B. (2006) Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 17. Kullberg M. C., Jankovic D., Feng C. G., Hue S., Gorelick P. L., McKenzie B. S., Cua D. J., Powrie F., Cheever A. W., Maloy K. J., Sher A. (2006) IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaser A., Zeissig S., Blumberg R. S. (2010) Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cua D. J., Tato C. M. (2010) Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 [DOI] [PubMed] [Google Scholar]
- 20. Hatton R. D., Weaver C. T. (2003) Immunology. T-bet or not T-bet. Science 302, 993–994 [DOI] [PubMed] [Google Scholar]
- 21. Liu S. J., Tsai J. P., Shen C. R., Sher Y. P., Hsieh C. L., Yeh Y. C., Chou A. H., Chang S. R., Hsiao K. N., Yu F. W., Chen H. W. (2007) Induction of a distinct CD8 Tnc17 subset by transforming growth factor-β and interleukin-6. J. Leukoc. Biol. 82, 354–360 [DOI] [PubMed] [Google Scholar]