Background: VEGF-A is an important mediator of angiogenesis; however, the role of endogenous VEGF-A in endothelial cells is unclear.
Results: Endogenous VEGF-A maintains expression of VEGFR-2 and other endothelial-specific proteins via transcriptional regulation.
Conclusion: We propose that endogenous VEGF-A maintains endothelial homeostasis.
Significance: Targeting endogenous VEGF-A may complement current anti-angiogenesis therapies and be combined with them as an effective therapeutic tool.
Keywords: Angiogenesis, Endothelial Cell, Hemostasis, Transcription, Vascular Endothelial Growth Factor (VEGF), VEGF-A, VEGFR-2, Endogenous
Abstract
Vascular endothelial growth factor A (VEGF-A) is one of the most important factors controlling angiogenesis. Although the functions of exogenous VEGF-A have been widely studied, the roles of endogenous VEGF-A remain unclear. Here we focused on the mechanistic functions of endogenous VEGF-A in endothelial cells. We found that it is complexed with VEGF receptor 2 (VEGFR-2) and maintains a basal expression level for VEGFR-2 and its downstream signaling activation. Endogenous VEGF-A also controls expression of key endothelial specific genes including VEGFR-2, Tie-2, and vascular endothelial cadherin. Of importance, endogenous VEGF-A differs from exogenous VEGF-A by regulating VEGFR-2 transcription through mediation of FoxC2 binding to the FOX:ETS motif, and the complex formed by endogenous VEGF-A with VEGFR-2 is localized within the EEA1 (early endosome antigen 1) endosomal compartment. Taken together, our results emphasize the importance of endogenous VEGF-A in endothelial cells by regulating key vascular proteins and maintaining the endothelial homeostasis.
Introduction
Vascular endothelial growth factor (VEGF), initially known as vascular permeability factor (1), is the key regulator of physiological and pathological angiogenesis (2–4). VEGF-A, the most-studied isoform of VEGF, plays important roles in endothelial proliferation, migration, and survival (5–7). VEGF-A interacts with VEGF receptor-1 (Flt1) and VEGF receptor-2 kinase insert domain receptor (KDR) tyrosine kinases as well as its co-receptor neuropilin-1 (Nrp-1), all of which are expressed in normal endothelial cells. Upon VEGF-A stimulation, signal transduction events are activated (8), mostly through VEGFR-2. These involve receptor dimerization and autophosphorylation followed by phosphorylation of downstream proteins protein kinase C (PKC), phospholipase Cγ (PLCγ), and phosphatidylinositol 3-kinase (PI3K). Hence, the VEGF receptor-2 plays a major role in VEGF-A-mediated vascular endothelial cell biology (9–11).
Inactivation of only one allele of VEGF-A causes abnormal blood vessel formation and blood-island formation in embryos and leads to lethality on embryonic day (E) 9.5 in mice (12, 13). On the other hand, moderate overexpression of VEGF-A results in severe abnormalities in heart development and also leads to embryonic lethality at E12.5-E14 in mice (14). The VEGF-A level must be tightly controlled to allow normal vasculogenesis and angiogenesis. In this regard, extensive studies about paracrine VEGF-A and its VEGF receptor-2 in pathological situations have been performed in recent decades (15–17).
VEGF-A is one of the most important mediators of pathological angiogenesis, and an anti-VEGF monoclonal antibody, bevacizumab (Avastin), is already used for the first-line treatment of metastatic colorectal cancer, whereas its roles in other tumor types are under investigation (18, 19). However, most studies to date have focused on paracrine VEGF, whereas few have focused on the role of endogenous VEGF-A in non-pathological situations. The physiological functions of autocrine VEGF-A in endothelial cells have been investigated recently in vivo (20), and autocrine VEGF-A was shown to be required for blood vessel homeostasis in the adult. Endothelial cell-specific deletion of VEGF-A in mice caused more than a 50% mortality within 25 weeks of age (20). Importantly, a new transcriptional enhancer regulated by the FOX:ETS motif has been recently discovered and shown to be sufficient to direct expression specifically and exclusively to the developing vascular endothelium (21). However, this transcriptional control has not yet been connected with VEGF-A.
Here we have investigated the cellular mechanism controlled by endogenous VEGF-A in vitro and how endogenous VEGF-A influences VEGFR-2 signaling in endothelial cells. We found that endogenous VEGF-A forms a complex with VEGFR-2 and maintains VEGFR-2 expression and its downstream signaling activity. This complex is partially localized within the early endosome antigen 1 (EEA1)3 endosomal compartment. We further showed that endogenous VEGF-A, unlike the exogenous protein, controls VEGFR-2 transcription, most likely through the FOX:ETS motif. Furthermore, expression of the other endothelial markers, Tie-2 and VE-cadherin, is also controlled by endogenous VEGF-A. We propose that by regulating endothelial-specific protein transcription, endogenous VEGF-A maintains endothelial homeostasis. i.e. the maintenance of the equilibrium of intercellular and intracellular factors/parameters, as such that the proper function of the endothelium should be sustained. Here, lack of intracellular VEGF-A can influence VEGFR-2 expression regardless of outside conditions and eventually influences endothelial functions.
EXPERIMENTAL PROCEDURES
Antibodies and Other Reagents
For Western Blotting
VEGFR-2 and VEGFR-1 primary antibodies and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.); β-actin was purchased from Sigma; p-VEGFR-2 Y1059 and Tie-2 were purchased from Upstate Biologicals (Millipore, Lake Placid, NY); p-VEGFR-2 Tyr-1175, Src, p-Src Tyr-416, Tyr-527, p-paxillin Tyr-118, PLCγ, p-PLCγ Tyr-783, and VE-cadherin were purchased from Cell Signaling; paxillin was purchased from BD Biosciences.
For Immunofluorescence
VEGFR-2 was purchased from Sigma; VEGF-A was purchased from Abcam (Cambridge, MA); EEA1-FITC was purchased from BD Biosciences; VE-cadherin was purchased from Cell Signaling; fluorescein-labeled secondary antibodies were purchased from Invitrogen.
For Chromatin Immunoprecipitation
FoxC2 antibody was purchased from Sigma, and IgG rabbit was purchased from Santa Cruz Biotechnology.
Human recombinant VEGF-A165 was purchased from R&D systems; actinomycin D, pepstatin, and oleoyl-l-α-lysophosphatidic acid sodium salt (LPA) were purchased from Sigma. [3H]thymidine was purchased from PerkinElmer Life Sciences.
Cell Culture
Human Umbilical Vein Endothelial Cells (HUVECs; Lonza, San Diego, CA) were cultured in endothelial basal medium supplemented with EGM-MV Bullet kit (5% fetal bovine serum (FBS), 12 μg/ml bovine brain extract, 1 μg/ml hydrocortisone, and 1 μg/ml GA-1000). Cells of passage 3 or 4 were used throughout all experiments. Plates were always coated by collagen bovine type I (BD Biosciences). Cells were starved overnight (0.1% FBS) before VEGF-A stimulation. Mouse brain microvascular endothelial cells (bEnd.3) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 1% penicillin/streptomycin.
siRNA and shRNA Transfection
Human VEGF-A or control scrambled siRNA were obtained from Qiagen, Inc. (Valencia, CA). siRNA transfection was performed in Opti-MEM media using Oligofectamine (Invitrogen) following the manufacturer's instructions. shRNA for human VEGF-A and control were obtained from Open Biosystems (Huntsville, AL), and lentivirus was prepared as previously described (22, 23). To maintain the primary cell characteristics of HUVECs, we infected cells by shRNA lentivirus, selected by puromycin, and collected lysates 72 h after infection.
Luciferase Reporter Assay
pGL3 plasmids containing the corresponding human VEGFR-2 reporter constructs were transfected into HUVECs using Lipofectin according to the manufacturer's instructions (Invitrogen). pRL-TK Renilla luciferase vector was used as the internal control. Briefly, HUVECs were seeded into 24-well plates at 60% confluency. VEGFR-2 promoter construct (0.9 μg) and 0.1 μg of pRL-TK Renilla luciferase vector with 400 nm control or VEGF-A siRNA were diluted into 50 μl of Opti-MEM. Separately, Lipofectin (5 μl) was also diluted into 50 μl of Opti-MEM. Both dilutions were kept at room temperature for 45 min and then mixed together and incubated at room temperature for 15 min. The mixture was finally added to HUVECs already incubated in 400 μl of Opti-MEM. Forty-eight hours after transfection, firefly luciferase and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI) in a LB960 microplate luminometer. In the case of VEGFR-2 enhancer, the vector used was pGL3-promoter. The empty vector or the vector containing the VEGFR-2 enhancer domain was cotransfected with pRL-TK Renilla luciferase vector.
Preparation of Whole Cell Extracts
Cells were washed twice with chilled PBS, lysed by chilled radioimmunoprecipitation assay lysis buffer (50 mm Tris, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 0.5% sodium deoxycholate, 0.1% SDS) with 1% proteinase inhibitor mixture (Sigma) and 1% Halt phosphatase inhibitor mixture (Pierce), incubated on ice for 20 min, and centrifuged at 12,000 rpm at 4 °C for 10 min. The supernatant was collected, and the protein concentration was measured by the Bradford method (Bio-Rad Protein assay).
Western Blotting
Proteins were denatured by adding 6× Laemmli SDS sample buffer and heated for 10 min. Equal amounts of total protein per lane were subjected to SDS gel electrophoresis followed by dry transfer of the protein to a polyvinylidene fluoride membrane. The membrane was blocked by incubation in TBS-T buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 0.05% Tween 20) containing 5% nonfat milk. The primary antibody was diluted in TBS-T containing 5% BSA overnight at 4 °C, and horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) was diluted in TBS-T and incubated for 1 h at room temperature. Immunodetection was performed with the SuperSignal West Pico Substrate (Thermo Scientific, Rockford, IL).
RNA Isolation and Real-time PCR
Total RNAs were extracted using the RNeasy mini kit (Qiagen) and reverse-transcribed by oligo(dT) priming using the iScript cDNA synthesis kit following the manufacturer's instructions (Bio-Rad). Semiquantitative real-time PCR analyses were performed using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green PCR Master Mix (Applied Biosciences). Primers used in real-time PCR were purchased from Qiagen.
Cell Proliferation Assay
HUVECs (2 × 104 cells per well) were seeded into 24-well plates and subjected to siRNA treatment. Thirty-six hours after transfection, cells were starved overnight (0.1% FBS) and then stimulated by VEGF-A165 at 10 ng/ml for 24 h. [3H]thymidine (1 mCi) was added to each well, and 4 h later cells were washed with cold PBS, fixed with 100% chilled methanol, and collected for measurement of trichloroacetic acid-precipitable radioactivity.
Boyden Chamber Migration Assay
Cell migration was detected using the Transwell system (Costar; 6.5-mm wells, 8.0-μm pore size), which allows cells to migrate through pores in polycarbonate membranes. Transwell chambers were coated by collagen bovine type I (BD Biosciences). siRNA-treated cells were starved overnight, then 5 × 104 cells were seeded into the upper chamber of each Transwell in basal medium. Basal medium supplemented with 10 ng/ml of VEGF-A165 was added to the bottom chamber as chemoattractant. Four hours later, the cells were fixed and stained. Each well was photographed, and total cell numbers were counted.
Calcium Release Assay
Levels of intracellular calcium were measured with Fura-2 AM cell permeant (Invitrogen). Briefly, siRNA-treated HUVECs of 70–80% confluency were washed twice with PBS and then gently harvested with 4 ml of collagenase solution containing 0.2 μg/ml collagenase, 0.2 μg/ml soybean trypsin inhibitor, 1 μg/ml BSA, and 2 mm EDTA in PBS. Detached cells were washed once with 2 ml of Ca2+ buffer containing 5 mm KCl, 140 mm NaCl, 1 mm CaCl2, 1 mm MgCl2, 5.6 mm glucose, 0.1% BSA, 0.25 mm sulfinpyrazone, and 10 mm HEPES, pH 7.5. The collected cells were resuspended in Ca2+ buffer containing 1 μg/ml Fura-2 and 0.02% Pluronic F-127 for 30 min at 37 °C covered in aluminum foil. After incubation, the cells were washed again and resuspended with Ca2+ buffer. Aliquots of the Fura-2-loaded cell suspension were placed in quartz cuvettes and stirred throughout the measurement. Fluorescence was measured at room temperature with a dual-wavelength DeltaScan Illumination System (Photon Technology International). Dual excitations at 340/380 nm with an emission at 510 nm were recorded for 1000 s. VEGF-A165 was added to a final concentration of 10 ng/ml at 100 s. The change in cytosolic free calcium ([Ca2+]i) was calculated as the 340/380-nm ratio for the emitted fluorescence using Felix software.
Tube Formation Assay
Human control scrambled or VEGF-A siRNA-treated HUVECs were starved overnight. A 96-well, flat-bottomed plate was coated with 50 μl of Matrigel per well and kept at 37 °C for 1 h to promote gelling. Cells (2 × 104) in 100 μl of starved medium with or without VEGF-A165 at 10 ng/ml were seeded into each Matrigel-coated well. Cells were then incubated at 37 °C in a 5% CO2 humidified atmosphere. The center of each well was photographed every 15 min over 8 h using an Axiovert 200M inverted microscope with Apotome Module (Carl Zeiss) running Axiovision 4.8 software.
Chromatin Immunoprecipitation and PCR
Chromatin immunoprecipitation (ChIP) was performed according to the manufacturer's instructions (Chromatin Immunoprecipitation ChIP assay kit, Millipore). Briefly, after treating cells with siRNA for 48 h, formaldehyde was added directly to cell culture medium at a final concentration of 1% for 10 min at 37 °C. The cross-linking was quenched by adding chilled PBS containing protease inhibitors. Cells were scraped, and the pellet was resuspended in SDS lysis buffer and incubated on ice for 10 min. Sonication was performed on ice to get DNA fragments between 200 and 400 bp. Samples were centrifuged, and supernatants were diluted with ChIP dilution buffer containing protease inhibitors. Nonspecific background was reduced by protein A-agarose-salmon sperm DNA at 4 °C for 30 min. Ten percent of the precleared cell supernatant was kept as input. The supernatant was immunoprecipitated by 5 μg of either IgG control (Santa Cruz) or anti-FoxC2 antibody (Sigma) overnight at 4 °C. Protein A-agarose-salmon sperm DNA was added again to the collected DNA-FoxC2 complex for 1 h at 4 °C. The agarose pellet was then washed by low salt immune complex wash buffer, high salt immune complex wash buffer, LiCl immune complex wash buffer, and Tris-EDTA buffer. DNA fragments were eluted from the agarose beads by elution buffer at room temperature for 15 min. Cross-linking was reversed by 5 m NaCl at 65 °C for 4 h. Eluates were then incubated in buffer containing proteinase K for 1 h at 45 °C to reduce protein background. DNAs were recovered by phenol/chloroform extraction and ethanol precipitation.
PCR mixtures contained 15 ng of input sample or 30 ng of immunoprecipitated DNA sample, 50 ng of each primer, 250 μm (of each) dNTP, 5× Herculase II reaction buffer (Agilent) with 2 mm MgCl2, 4% DMSO, and 1.25 units of Herculase DNA polymerase (Agilent) in a total volume of 25 μl. After 50 cycles of amplification, PCR products were run on a 2% agarose gel and analyzed by ethidium bromide (EtBr) staining. Primers were obtained from Integrated DNA Technologies. Primer sequences are indicated in supplemental Table S1.
In Situ Proximity Ligation Assay (PLA)
Cells were fixed in 4% paraformaldehyde on ice for 30 min and thereafter subjected to in situ PLA using the Duolink Detection kit (Olink Bioscience, Uppsala, Sweden) according to the manufacturer's instructions. Briefly, slides were blocked, incubated with anti-VEGFR-2 (Sigma) and anti-VEGF (Abcam) antibodies, and then incubated with PLA probes, which are secondary antibodies (anti-mouse and anti-rabbit) conjugated to unique oligonucleotides.
Circularization and ligation of the oligonucleotides was followed by an amplification step. The products were detected by a complementary fluorescently labeled probe. Slides were mounted using Vectashield (Vector Laboratories, Inc., Burlingame, CA) and evaluated using an LSM 510 v3.2SP2 Live Confocal Laser Scanning Microscope (Carl Zeiss).
Immunofluorescence and Confocal Microscopy
Cells were seeded on coverslips and treated by control scrambled or VEGF-A siRNA. Forty-eight hours after transfection, cells were rinsed with Dulbecco's PBS (D-PBS; 8.1 mm Na2HPO4, 1.2 mm KH2PO4, pH 7.2, 138 mm NaCl, 2.7 mm KCl, 0.9 mm CaCl2, and 0.5 mm MgCl2) at room temperature and fixed with 2.5% formaldehyde in 0.1 m Pipes, pH 6.95, 3 mm MgSO4, and 1 mm EGTA for 20 min. After rinsing by D-PBS, cells were permeabilized by 0.1% Triton X-100 in D-PBS for 2 min and rinsed again by D-PBS. Cells were blocked by D-PBS containing 4% BSA at 37 °C for 1 h and then incubated with primary antibodies (1–2 μg/ml) for 2 h at 37 °C. After washing with D-PBS, cells were incubated with the appropriate fluorescein-labeled secondary antibody (10 μg/ml) for 1 h at 37 °C. Cells were washed again and mounted on a glass slide in mounting reagent (Vectashield with DAPI, Vector Laboratories). An LSM 510 v3.2SP2 Live confocal laser scanning microscope (Carl Zeiss) with Axiovert 100M (Carl Zeiss) with a c-Apochromat 100× oil immersion or 60× water immersion objective was used to analyze immunostained cells and to capture representative images. Confocal images were converted to 8-bit grayscale. Images obtained were exported by AxioVision software (Carl Zeiss) in TIF format.
mRNA Stability Assay
VEGFR-2 mRNA stability was estimated by utilizing actinomycin D (Sigma). siRNA-treated cells were incubated with actinomycin D (7.5 μg/ml) for 0, 1, 2, 4, and 6 h. mRNAs were then extracted and reverse-transcribed into cDNA, and real-time PCR was performed as described above.
Cloning
Wild-type VEGF-A165 and signal peptide-deleted VEGF-A165 has been cloned into the retroviral vector pMMp. VEGFR-2 enhancer domain containing the motif FOX:ETS has been cloned into pGL3-promoter vector.
Cell Apoptosis Assay
Cells were washed, resuspended in the staining buffer, and stained with propidium iodide and annexin V-FITC (BioVision, Mountain View, CA). Stained cells were analyzed by fluorescence-activated cell sorting (FACSCalibur, BD Biosciences).
Statistical Analysis
The Student's t test was used to determine the statistical significance of the data obtained and to compare the means between groups. A p value of <0.05 represented a statistically significant difference.
RESULTS
Endogenous VEGF-A Controls VEGFR-2 Expression and VEGFR-2 Downstream Signaling
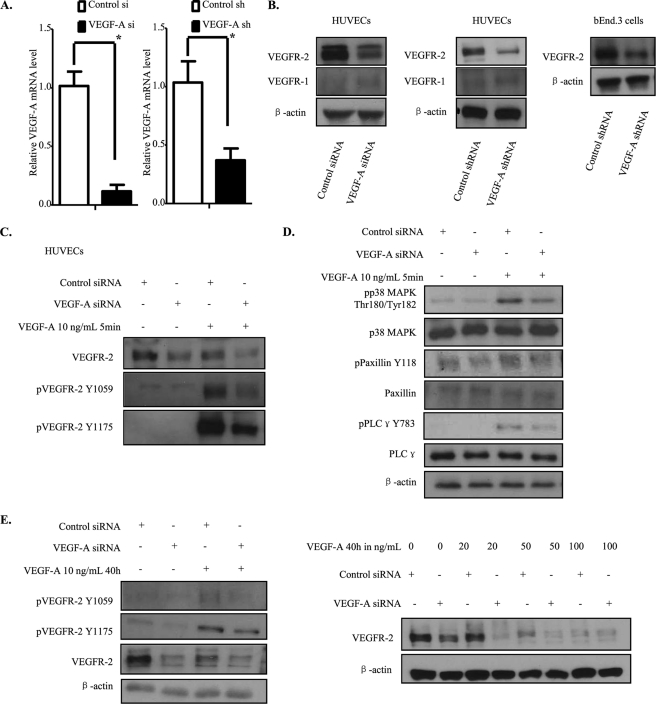
To investigate the mechanistic functions displayed by endogenous VEGF-A in endothelial cells, we utilized siRNA to knock down endogenous VEGF-A in HUVECs. Knockdown effectiveness was verified by real-time PCR (Fig. 1A). Surprisingly, we observed a considerable decrease in the VEGFR-2 protein level compared with control siRNA-treated cells, whereas the VEGFR-1 level was unchanged in endogenous VEGF-A knockdown cells (Fig. 1B). To confirm the validity of our findings and exclude any possible off-target effects for the siRNA utilized, these experiments were repeated with an shRNA lentivirus expression system that targets VEGF-A sequences that are independent of those targeted by the siRNA, and the same results were observed (Fig. 1, A and B). To further corroborate our findings, we utilized another endothelial cell line, bEnd.3, which is a polyoma middle T-transformed mouse brain capillary endothelial cell line. As reported in Fig. 1B, VEGFR-2 down-regulation also occurred in bEnd.3 cells when endogenous VEGF-A was knocked down by shRNA targeting the mouse VEGF-A sequence.
FIGURE 1.
Knockdown of endogenous VEGF-A down-regulates VEGFR-2 expression and its downstream signaling activation. A and B, HUVECs were treated with control or VEGF-A siRNA. Forty-eight hours after transfection, cells were lysed and analyzed by Western blot. For shRNA, HUVECs or bEnd.3 cells were infected by lentivirus for 24 h, selected by puromycin for 48 h, and lysed. For mRNA analysis, mRNA were extracted and then reverse-transcribed into cDNA. The latter was used for real-time PCR. C and D, siRNA-transfected HUVECs were starved overnight and stressed by VEGF-A165 at 10 ng/ml for 10 min and then quenched and lysed. E, HUVECs were treated by control or VEGF-A siRNA. Eight hours later, VEGF-A165 was added into the culture medium, and the cells incubated for 40 h. The cells were then lysed and analyzed by Western blot.
We further evaluated the consequences of endogenous VEGF-A knockdown on VEGFR-2 downstream signaling activation. First of all, we observed a basal phosphorylation of VEGFR-2 as well as its downstream signaling proteins, including PLCγ, Src, and paxillin in control siRNA-treated cells without any exogenous VEGF-A stimulation (see supplemental Fig. 1A), as previously reported (20, 24). In addition, in VEGF-A knockdown cells, the decrease in VEGFR-2 protein level resulted in a marked attenuation of the VEGFR-2 tyrosine phosphorylation level, which was accompanied by a decrease in VEGFR-2 downstream signaling components activation, such as PLCγ, p38 MAPK, and paxillin (see supplemental Fig. 1A).
We then examined the VEGFR-2 response to exogenous VEGF-A stimulation. A significant down-regulation of VEGFR-2 phosphorylation after exogenous VEGF-A treatment was observed in endogenous VEGF-A knockdown cells compared with control siRNA-treated cells, which can be explained by the decrease in VEGFR-2 protein level that we observed (Fig. 1C). Activation of some VEGFR-2 downstream signaling response markers, such as PLCγ, p38 MAPK, and paxillin was also impaired (Fig. 1D).
Finally, to determine whether exogenous VEGF-A could replace the endogenous protein and restore VEGFR-2 expression levels, we maintained exogenous VEGF-A in the culture medium for 40 h after siRNA treatment (Fig. 1E). As expected, despite the presence of exogenous VEGF-A, the VEGFR-2 protein level was still down-regulated upon endogenous VEGF-A depletion and this even in the presence of high concentration of exogenous VEGF-A. These results demonstrate clearly that endogenous VEGF-A is necessary for maintaining the VEGFR-2 protein level.
Endogenous VEGF-A Depletion Inhibits Major VEGFR-2-regulated Endothelial Functions
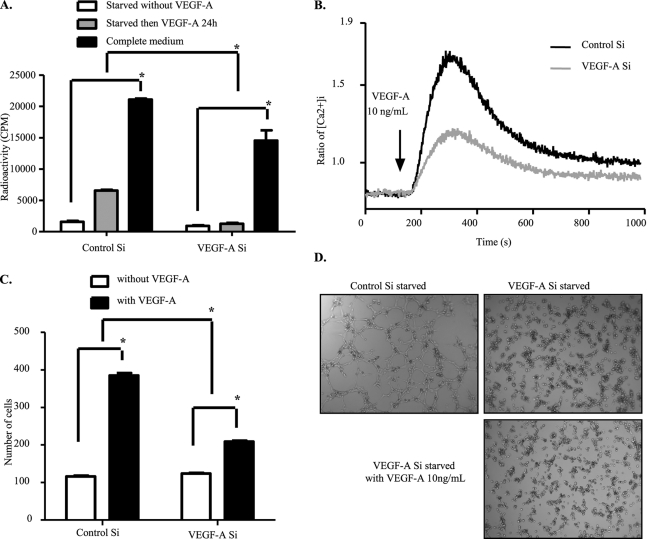
Because VEGFR-2 phosphorylation at key tyrosine residues is necessary for VEGF-A signaling, we next investigated how some important functions controlled by VEGFR-2 signaling in endothelial cells were affected by knockdown of endogenous VEGF-A. Phosphorylation of VEGFR-2 at Tyr-1175 has been shown to play a critical role in PLCγ/p44/42-MAPK activation and proliferation of endothelial cells (25). Intracellular calcium release is also controlled by PLCγ/PKC activation (26). In addition, phosphorylation of Tyr-1175 is needed for PI3K activation, and the adaptor protein Shb-mediated migration (27). In this regard, we first evaluated VEGF-A-induced cell proliferation and calcium release in HUVEC cells with or without endogenous VEGF-A expression. As shown in Fig. 2, A and B, endogenous VEGF-A knockdown impaired endothelial cell proliferation as well as intracellular calcium release in response to exogenous VEGF-A. This result can be explained by a decrease in VEGFR-2 protein level and in VEGFR-2 tyrosine 1175 phosphorylation-induced downstream signaling.
FIGURE 2.
Endothelial functions displayed by VEGFR-2 are impaired upon depletion of VEGF-A. A, shown is the reduced proliferation capacity of endogenous VEGF-A knockdown cells. HUVECs were transfected with control or VEGF-A siRNA for 48 h, and cell proliferation was then measured by [3H]thymidine incorporation assay. B, reduction in calcium release upon VEGF-A stimulation is shown. HUVECs were transfected with control or VEGF-A siRNA. Forty-eight hours later cells were digested and loaded with Fura-2. Intracellular calcium release upon VEGF-A stress was followed for 1000 s. C, cell migration capacity was affected by endogenous VEGF-A silencing. siRNA-treated cells were starved overnight. Forty-eight hours after transfection, cells were seeded into the upper chambers of Transwells and allowed to migrate into the bottom chambers containing VEGF-A165 (10 ng/ml) as the chemoattractant. Four hours later, cells were fixed and photographed for counting. D, tube formation was inhibited by depletion of endogenous VEGF-A. siRNA-transfected HUVECs were starved overnight, then seeded onto solidified Matrigel. VEGF-A165 (10 ng/ml) was then added into the culture medium, and tube formation was followed for 8 h. The data represented here are the average of three independent experiments. All bars represent means ± S.D. of three experiments. *, p < 0.05.
Besides the tyrosine 1175 site, phosphorylation of VEGFR-2 at the tyrosine 951 site is also important for VEGF-A-induced cell migration through binding to TSad and complex formation with Src (11, 28). Another component important for endothelial cell migration is focal adhesion kinase and its substrate paxillin, which can also be phosphorylated by Src (29, 30). We then examined the impact of endogenous VEGF-A knockdown on endothelial cell migration by using the Boyden chamber assay. siRNA-mediated endogenous VEGF-A knockdown effectively impaired migration of endothelial cells toward the chemoattractant VEGF-A (Fig. 2C).
Furthermore, VEGFR-2 activation is known to be necessary for endothelial cell tube formation (31). We, therefore, assessed the consequence of endogenous VEGF-A knockdown on endothelial cell morphogenesis within the extracellular matrix. In control siRNA-treated cells, tubular structures formed very quickly after seeding the cells on Matrigel, whereas endogenous VEGF-A knockdown clearly inhibited HUVECs tube formation, as shown in Fig. 2D and supplemental Movies 1–3. Although cells were still able to migrate randomly, branching formation was completely inhibited. Moreover, treatment with VEGF-A exogenously could not restore the endogenous VEGF-A lacking phenotype. We believe that VEGFR-2 down-regulation and the subsequent decrease in downstream signaling are the main causes of these functional impairments. Therefore, the presence of endogenous VEGF-A is indispensable for sustained VEGFR-2 signaling.
We finally examined cell apoptosis, and in vitro endogenous VEGF-A knockdown does not lead to HUVEC cell apoptosis (see supplemental Fig. 1B).
VEGFR-2 Transcription Is Altered by Endogenous VEGF-A Knockdown
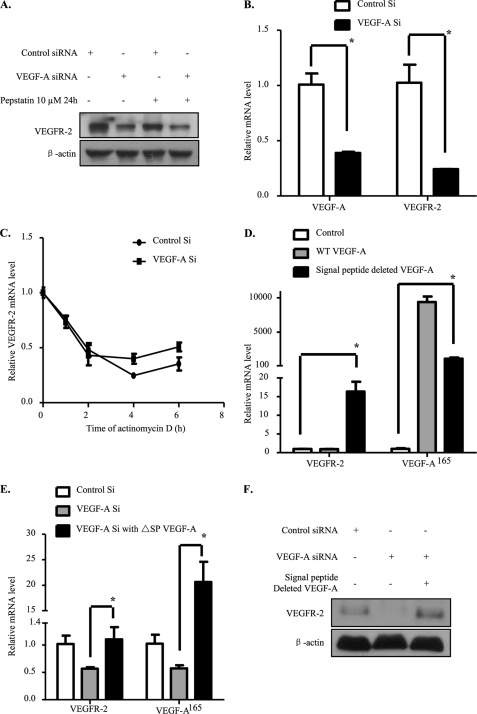
We further explored the mechanism causing the decrease in VEGFR-2 protein level upon depletion of endogenous VEGF-A. The VEGFR-2 down-regulation we observed might be due to VEGFR-2 protein degradation or mRNA down-regulation. Previous reports showed that exogenous VEGF-A could induce VEGFR-2 protein degradation, most likely through the lysosomal pathway (32). We used pepstatin to inhibit lysosomal degradation and found that the VEGFR-2 decrease caused by endogenous VEGF-A knockdown could not be restored (Fig. 3A). While in the presence of exogenous VEGF-A, degradation of VEGFR-2 caused by accelerated VEGFR-2 endocytosis and lysosomal degradation could be inhibited by pepstatin (supplemental Fig. 1C). Proteasomal degradation of VEGFR-2 has also been reported (33, 34). Thus MG132 was used to examine whether inhibition of proteasomal degradation can inhibit down-regulation of VEGFR-2. Interestingly, MG132 treatment could not reverse VEGFR-2 down-regulation caused by endogenous VEGF-A knockdown. In addition to that, the decrease in VEGFR-2 level was even more accentuated in the presence of MG132 (data not shown). This observation is in agreement with a previous report showing that proteasomal inhibitors could affect both transcription and mRNA stability of VEGFR-2 in HUVECs (35).
FIGURE 3.
VEGFR-2 decrease is due to transcriptional down-regulation. A, VEGFR-2 down-regulation was not due to lysosomal degradation. siRNA-transfected HUVECs were treated with pepstatin at 10 μm for 24 h. Forty-eight hours after transfection cells were lysed and analyzed by Western blot. B, the VEGFR-2 mRNA level was decreased. Forty-eight hours after siRNA transfection, HUVECs were harvested for mRNA extraction. cDNA was obtained by reverse transcription, and real-time PCR was performed. C, mRNA stability was unaffected. Forty-eight hours after siRNA transfection, cells were treated with 7.5 μg/ml actinomycin D at different time points, the mRNA was harvested, and the procedure was repeated. The data represented here are the average of three independent results. D, a non-secretive form of VEGF-A up-regulates VEGFR-2 mRNA level. HUVECs were infected by retroviral overexpression system, and 48 h later mRNAs were extracted. Real-time PCR were realized with reverse-transcribed cDNA. E and F, HUVECs were first transfected by siRNA and 6 h later a retrovirus overexpressing non-secretive form of VEGF-A was added. 72 h after siRNA treatment, mRNAs were extracted for real-time PCR or cells were lysed for Western blot. All bars represent the means ± S.D. of three experiments. *, p < 0.05.
Next, we performed real-time PCR and found that the VEGFR-2 mRNA level was down-regulated as well (Fig. 3B). With this result, either VEGFR-2 mRNA stability or transcription could be the cause of the decrease in VEGFR-2 mRNA level. To rule out the possibility that VEGFR-2 mRNA stability was affected, actinomycin D was used at indicated time points to inhibit transcription. Our results showed that VEGFR-2 mRNA degradation rates of HUVECs were similar in the control siRNA and endogenous VEGF-A siRNA-treated groups (Fig. 3C). Thus, our data demonstrate that VEGFR-2 mRNA down-regulation was not due to decrease in mRNA stability. Therefore, endogenous VEGF-A modulates VEGFR-2 transcription.
Endogenous VEGF-A Differs from Paracrine/Secreted VEGF-A
To further confirm our hypothesis, we used a retroviral system to overexpress wild-type and the non-secretive form (signal peptide deleted) of VEGF-A. As expected, the non-secretive form of VEGF-A could strongly up-regulate VEGFR-2 mRNA level, whereas the wild-type VEGF-A could not (Fig. 3D). Then the overexpression system was used to rescue VEGFR-2 decrease caused by endogenous VEGF-A knockdown. We effectively observed a rescue in VEGFR-2 mRNA level 3 days after siRNA treatment (Fig. 3, E and F). In consequence, we have demonstrated clearly that secreted/paracrine VEGF-A plays different roles than the endogenous protein.
Endogenous VEGF-A Regulates VEGFR-2 Transcription by Altering FoxC2 Binding to the FOX:ETS Motif
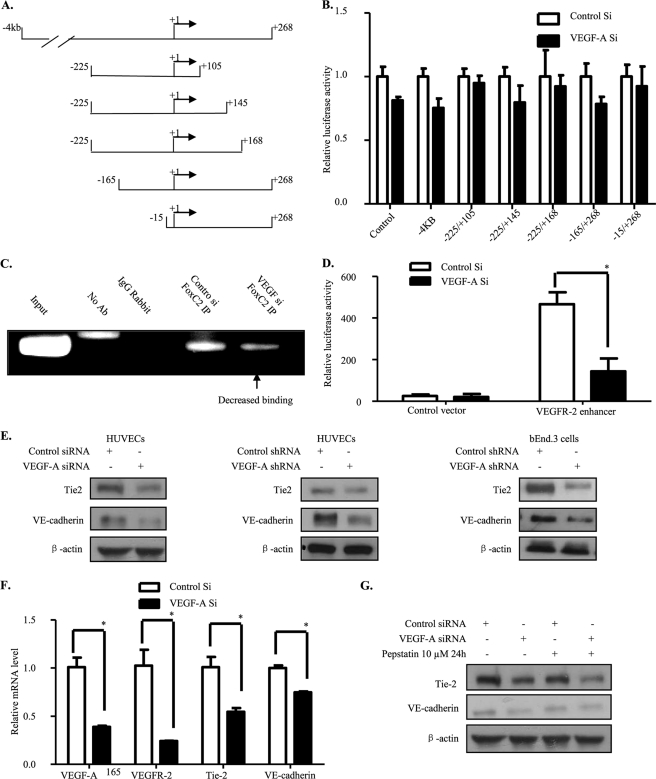
We then looked for the mechanism through which endogenous VEGF-A modifies VEGFR-2 transcription. In past years it has been shown that exogenous VEGF-A controls VEGFR-2 transcription by a positive feedback loop through the VEGFR-2 promoter region (36). To investigate whether endogenous VEGF-A modulates VEGFR-2 transcription through its promoter region, we performed a luciferase assay using constructs containing different VEGFR-2 promoter deletion mutants. It has been shown that 4 kb of the VEGFR-2 5′-flanking sequence induce a high level of luciferase activity in endothelial cells (37) (Fig. 4A). Strikingly, we found that VEGFR-2 transcription through its promoter region was not significantly changed by endogenous VEGF-A knockdown in HUVECs (Fig. 4B).
FIGURE 4.
FoxC2 binding to the FOX:ETS motif is decreased. A, shown is a representation of VEGFR-2 promoter deletion mutants used in our studies. B, VEGFR-2 promoter activation was unaffected by deletions. Different VEGFR-2 promoter deletion-mutant luciferase constructs with Renilla luciferase vector and control or VEGF-A siRNA were co-transfected into HUVECs. Forty-eight hours later, firefly and Renilla luciferase activities were measured. C, binding of FoxC2 to the FOX:ETS motif was decreased by endogenous VEGF-A knockdown. Forty-eight hours after siRNA transfection, HUVECs were subjected to chromatin immunoprecipitation by anti-FoxC2 antibody. Immunoprecipitated DNA fragments were used to perform PCR. D, pGL3-promoter empty vector or containing VEGFR-2 enhancer constructs with Renilla luciferase vector and control or VEGF-A siRNA were co-transfected into HUVECs. Forty-eight hours later, firefly and Renilla luciferase activities were measured. E–G, VE-cadherin and Tie-2 levels were also down-regulated. The same procedure as for VEGFR-2 in Fig. 3 was followed. All bars represent means ± S.D. of three experiments. *, p < 0.05.
The FOX:ETS motif has been recently reported to positively regulate several endothelial cell markers through their transcription. This motif is localized within the intronic region and is distinct from the classic promoter region of VEGFR-2. The FOX:ETS motif is bound and activated by the Forkhead protein, FoxC2, and the Ets protein Etv2 (21). We, therefore, tested whether the FOX:ETS motif is responsible for the VEGFR-2 down-regulation that we observed by performing chromatin immunoprecipitation with an anti-FoxC2 antibody in HUVECs. We found that FoxC2 bound strongly to the VEGFR-2 FOX:ETS motif in control siRNA-treated samples and that this binding was decreased by endogenous VEGF-A knockdown, as shown in Fig. 4C.
In addition, we cloned the VEGFR-2 enhancer domain containing the FOX:ETS motif into pGL3-promoter vector. Luciferase assay was performed (Fig. 4D) and indicated that endogenous VEGF-A knockdown effectively impaired the enhancing role played by the FOX:ETS motif in VEGFR-2 transcription. Thus, endogenous VEGF-A controls VEGFR-2 transcription through a new mechanism in which FoxC2 binding to the FOX:ETS motif is altered. This mechanism differs completely from the classic VEGFR-2 promoter control exerted by exogenous VEGF-A.
Furthermore, this FOX:ETS motif has been shown to be common to several endothelial-specific markers (21) (supplemental Fig. 1D), and we therefore also examined expression of some of these markers. Interestingly, we found that VE-cadherin and Tie-2 expression were also down-regulated in HUVECs as well as in bEnd.3 cells after depletion of endogenous VEGF-A (Fig. 4E). Like the decrease in VEGFR-2 protein level, the down-regulation also resulted from a decrease in mRNA levels (Fig. 4F), whereas their lysosomal degradation was unaffected (Fig. 4G).
VE-cadherin is an endothelial-specific adhesion molecule located at junctions between endothelial cells, and VE-cadherin has been shown to be required for vascular remodeling and morphogenesis (38). As the VE-cadherin protein level was also decreased, we stained endothelial cells with an antibody directed against VE-cadherin to determine how the VE-cadherin presence on cell membranes was affected. In comparison to control siRNA-treated cells, a strong attenuation of VE-cadherin on endothelial cell membranes occurred in endogenous VEGF-A knockdown cells (supplemental Fig. 2). This VE-cadherin down-regulation is also a factor contributing to a decrease in both cell migration and tube formation.
In summary, our results show that depletion of endogenous VEGF-A inhibits binding of FoxC2 to the FOX:ETS enhancer domain of VEGFR-2, thus down-regulating VEGFR-2 transcription. However, how FoxC2 binding to the FOX:ETS enhancer domain is disrupted needs further clarification. FoxC2 belongs to the Forkhead family, and these proteins are activated by phosphorylation and subsequently move into the cell nucleus to control transcription of target proteins (39). Currently, phosphorylation of FoxC2 has not been clearly demonstrated, and the mechanism controlling FoxC2 nuclear-cytoplasmic shuttling remains unknown.
Endogenous VEGF-A Forms a Complex with VEGFR-2 and Is Partially Localized within the EEA1 Endosomal Compartment
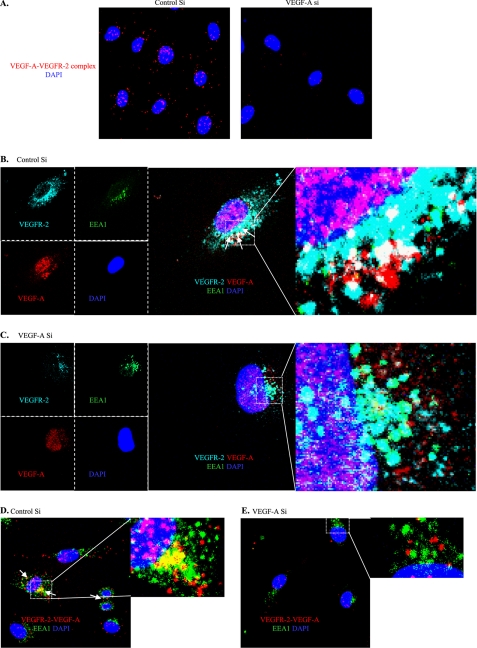
We then examined the cellular localization of endogenous VEGF-A and searched for a possible link to VEGFR-2 transcription. Binding between exogenous/paracrine VEGF-A and VEGFR-2 on the plasma membrane has been studied in detail (10, 40). However, whether endogenous VEGF-A binds to VEGFR-2 is still unknown. We used in situ PLA to examine whether or not there was a direct complexation between these two proteins (41). As shown in Fig. 5A, complexation clearly occurred. Our results, therefore, establish a previously unknown phenomenon in which endogenous VEGF-A binds to VEGFR-2, most likely intracellularly, and maintains a basal VEGFR-2 downstream signaling activation level.
FIGURE 5.
Endogenous VEGF-A binds to VEGFR-2 and is partially localized within the EEA1 endosomal compartment. A, endogenous VEGF-A binds to VEGFR-2. In situ PLA was performed for VEGFR-2 and VEGF-A on siRNA-transfected HUVECs. B–E, shown is co-distribution of the VEGF-A-VEGFR-2 complex with EEA1. White arrows indicate colocalization zones. B and C, immunofluorescence was performed with HUVECs for VEGF-A (red), VEGFR-2 (far red), and EEA1 (green). D and E, in situ PLA of VEGFR-2-VEGF-A was co-stained with EEA1-FITC.
Previous reports have shown that VEGFR-2 is maintained in intracellular pools, such as the EEA1 endosomal compartment (42, 43). We, therefore, examined by immunofluorescence whether the VEGF-A-VEGFR-2 complex is found in this compartment.
Co-distribution of the VEGF-A-VEGFR-2 complex with EEA1 was clearly observed in control siRNA-treated HUVECs (Fig. 5, B and D), whereas in VEGF-A siRNA-transfected HUVECs, this colocalization was strongly decreased (Fig. 5, C and E). VEGFR-2 co-localization with EEA1 is in agreement with previous reports showing an internal VEGFR-2 pool localized within the EEA1 endosomal compartment in quiescent cells. However, the role of this co-localization was unclear (42). The VEGFR-2 presence in the EEA1 endosomal compartment probably maintains the VEGFR-2 basal activation level that we observed. That would not be surprising, as it has also been reported that internalized VEGFR-2 retains signaling activity (44). The important difference is that in our case the VEGF-A was endogenous and initially present in the endothelial cells and differs from internalized exogenous VEGF-A.
Finally, the VEGF-A-VEGFR-2 complex and EEA1 co-distribution suggests a link to the VEGFR-2 transcription that we found. The presence of VEGF-A-VEGFR-2 complex within the EEA1 endosomal compartment might send a signal into the nucleus that controls VEGFR-2 transcription. However, this hypothesis needs further evaluation.
DISCUSSION
The functions of paracrine VEGF-A in non-pathological and pathological angiogenesis are currently well known, whereas autocrine VEGF-A has been reported to be crucial for vascular homeostasis (20). However, the mechanistic functions of endogenous VEGF-A in endothelial cells remain unclear. In this context, we report for the first time that endogenous VEGF-A forms a complex with VEGFR-2 in endothelial cells, most likely intracellularly, and maintains a basal activation level of VEGFR-2 regulated signaling. Importantly, this endogenous VEGF-A functions differently from the paracrine/secreted VEGF-A. Furthermore, our studies also revealed a previously unknown function of endogenous VEGF-A; it is required to maintain VEGFR-2 transcription by altering FoxC2 binding to the lately discovered FOX:ETS motif (21). This transcriptional control is unrelated to the VEGFR-2 promoter and cannot be exerted by exogenous VEGF-A, which also might regulate VEGFR-2 transcription but by a positive feedback loop through the VEGFR-2 promoter (36). It has been reported that exogenous VEGF regulates expression of VEGFR-2 and VEGF itself through other mechanisms such as LPA1 receptor, H2O2, podocin, estradiol, and DLL4/Hey2 (45–50). We have verified in this instance that LPA1 receptor mRNA level was unchanged (supplemental Fig. 1E) and that endogenous VEGF-A knockdown still down-regulated VEGFR-2 expression even by adding LPA at different doses (supplemental Fig. 1F). Our results obtained using signal peptide-deleted VEGF-A exclude the possibility that exogenous or secreted VEGF-A controls VEGFR-2 mRNA level. Therefore, the novel finding of our studies is that endogenous VEGF-A controls VEGFR-2 transcription by a different mechanism than exogenous VEGF-A.
In addition, we also observed a decrease in VE-cadherin and Tie-2 protein levels when exogenous VEGF-A is down-regulated. Tie-2 is expressed principally in vascular endothelium, is a tyrosine kinase activated upon angiopoietin binding, and plays a critical role in angiogenesis (51). VE-cadherin is an endothelial-specific adhesion molecule located at the junctions between endothelial cells and plays important roles in endothelial cell adhesion (52). Its expression, localization, and signaling are also critical in angiogenesis (53, 54). Down-regulation of these important endothelial-specific proteins most likely occurs through the same mechanism, that is, by altering FoxC2 binding to the FOX:ETS enhancer domain. The transcriptional control exerted by endogenous VEGF-A on these endothelial-specific markers might also be a mechanism by which autocrine VEGF-A helps in maintaining cell homeostasis. However, the mechanism by which endogenous VEGF-A modified FoxC2 binding to the FOX:ETS enhancer domain remains enigmatic, and future studies are needed to answer this question.
We have shown for the first time that endogenous VEGF-A forms a complex with VEGFR-2 within the EEA1 endosomal compartment. This partial co-distribution may be correlated with the transcriptional control exerted by endogenous VEGF-A on VEGFR-2 expression. To date, very little information is available linking endocytic proteins to the nuclear transcription machinery (55). Other unknown components may also be involved in the same complex in which endogenous VEGF-A, VEGFR-2, and EEA1 are members. These components might enhance FoxC2 translocation into the nucleus or might alter the binding of FoxC2 to the FOX:ETS motif of diverse endothelial markers.
Some endocytic proteins like β-arrestin-1 and APPL (adaptor protein, phosphotyrosine interaction, PH domain, and leucine zipper containing) were found in the nucleus (56, 57) and might be involved in VEGFR-2 transcription by endogenous VEGF-A. APPL has also been correlated with GIPC/synectin in the control of TrkA trafficking and signaling (58), and GIPC is another important protein involved in VEGFR-2 trafficking (59). We also knocked down GIPC in HUVECs and found that the VEGFR-2 mRNA level was unmodified (data not shown). Thus, APPL might be a new and important endocytic component in the VEGFR-2 signaling loop. Future studies should be able to delineate the precise mechanism through which endogenous VEGF-A controls VEGFR-2 transcription.
In conclusion, we have shown that endogenous VEGF-A binds to VEGFR-2 and is required for maintaining a basal VEGFR-2 expression, which is reflected by its phosphorylation and downstream signaling. Endogenous VEGF-A maintains endothelial cell homeostasis by controlling VEGFR-2 transcription in vitro, whereas the complex formed by endogenous VEGF-A with VEGFR-2 is partially localized within the EEA1 endosomal compartment. This co-distribution might be related to the transcriptional control exerted by endogenous VEGF-A on VEGFR-2. Our studies have, therefore, introduced significant new elements concerning the role of endogenous VEGF-A in pathophysiological situations. Based on these findings and other reports, we propose a model in which both exogenous and endogenous VEGF-A exerts control on VEGFR-2 transcription and activation. In the case of exogenous VEGF-A, a series of precisely orchestrated steps tightly controls VEGFR-2 trafficking, degradation, and downstream signaling, leading to neoangiogenesis, whereas endogenous VEGF-A binds to VEGFR-2 intracellularly in the EEA1 endosomal compartment and maintains endothelial cell homoeostasis by promoting VEGFR-2 transcription. Hence, although exogenous VEGF-A is the key target of anti-angiogenic therapeutics, endogenous VEGF-A is also important and should be taken into account. Our results raise the prospect that pharmacological manipulations targeting endogenous VEGF-A may complement current therapies and be combined with them as an effective therapeutic tool.
Supplementary Material
Acknowledgment
We thank James E. Tarara of the Electron Microscopy Core Facilities at the Mayo Clinic Cancer Center for advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL070567, CA78383, and CA150190. This work was also supported by a generous gift from Bruce and Martha Atwater.

This article contains supplemental Figs. S1 and S2, Table S1, and Movies 1–3.
- EEA1
- early endosome antigen
- VEGFR
- VEGF receptor
- HUVEC
- human umbilical vein endothelial cell
- LPA
- oleoyl-l-α-lysophosphatidic acid sodium salt
- VE
- vascular endothelial
- GIPC
- GAIP C terminus interacting protein
- PLA
- proximity ligation assay.
REFERENCES
- 1. Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 [DOI] [PubMed] [Google Scholar]
- 2. Dvorak H. F., Nagy J. A., Feng D., Brown L. F., Dvorak A. M. (1999) Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 237, 97–132 [DOI] [PubMed] [Google Scholar]
- 3. Nagy J. A., Vasile E., Feng D., Sundberg C., Brown L. F., Manseau E. J., Dvorak A. M., Dvorak H. F. (2002) VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb. Symp. Quant. Biol. 67, 227–237 [DOI] [PubMed] [Google Scholar]
- 4. Pettersson A., Nagy J. A., Brown L. F., Sundberg C., Morgan E., Jungles S., Carter R., Krieger J. E., Manseau E. J., Harvey V. S., Eckelhoefer I. A., Feng D., Dvorak A. M., Mulligan R. C., Dvorak H. F. (2000) Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 80, 99–115 [DOI] [PubMed] [Google Scholar]
- 5. Benjamin L. E., Keshet E. (1997) Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors. Induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc. Natl. Acad. Sci. U.S.A. 94, 8761–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrara N., Gerber H. P., LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 7. Watanabe Y., Lee S. W., Detmar M., Ajioka I., Dvorak H. F. (1997) Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene 14, 2025–2032 [DOI] [PubMed] [Google Scholar]
- 8. Dvorak H. F. (2005) Angiogenesis. Update 2005. J. Thromb. Haemost. 3, 1835–1842 [DOI] [PubMed] [Google Scholar]
- 9. Guo D., Jia Q., Song H. Y., Warren R. S., Donner D. B. (1995) Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem. 270, 6729–6733 [DOI] [PubMed] [Google Scholar]
- 10. Shibuya M., Claesson-Welsh L. (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 312, 549–560 [DOI] [PubMed] [Google Scholar]
- 11. Zeng H., Sanyal S., Mukhopadhyay D. (2001) Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J. Biol. Chem. 276, 32714–32719 [DOI] [PubMed] [Google Scholar]
- 12. Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., Declercq C., Pawling J., Moons L., Collen D., Risau W., Nagy A. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 [DOI] [PubMed] [Google Scholar]
- 13. Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J., Moore M. W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 [DOI] [PubMed] [Google Scholar]
- 14. Miquerol L., Langille B. L., Nagy A. (2000) Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 127, 3941–3946 [DOI] [PubMed] [Google Scholar]
- 15. Dvorak H. F. (2006) Discovery of vascular permeability factor (VPF). Exp. Cell Res. 312, 522–526 [DOI] [PubMed] [Google Scholar]
- 16. Mukhopadhyay D., Zeng H., Bhattacharya R. (2004) Complexity in the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) receptors signaling. Mol. Cell Biochem. 264, 51–61 [DOI] [PubMed] [Google Scholar]
- 17. Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) VEGF receptor signaling in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 18. Ferrara N., Hillan K. J., Novotny W. (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 333, 328–335 [DOI] [PubMed] [Google Scholar]
- 19. Jubb A. M., Harris A. L. (2010) Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 11, 1172–1183 [DOI] [PubMed] [Google Scholar]
- 20. Lee S., Chen T. T., Barber C. L., Jordan M. C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K. P., Iruela-Arispe M. L. (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Val S., Chi N. C., Meadows S. M., Minovitsky S., Anderson J. P., Harris I. S., Ehlers M. L., Agarwal P., Visel A., Xu S. M., Pennacchio L. A., Dubchak I., Krieg P. A., Stainier D. Y., Black B. L. (2008) Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135, 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao Y., Szabolcs A., Dutta S. K., Yaqoob U., Jagavelu K., Wang L., Leof E. B., Urrutia R. A., Shah V. H., Mukhopadhyay D. (2010) Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J. Biol. Chem. 285, 31840–31848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Y., Wang L., Nandy D., Zhang Y., Basu A., Radisky D., Mukhopadhyay D. (2008) Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 68, 8667–8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Nedawi K., Meehan B., Kerbel R. S., Allison A. C., Rak J. (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. U.S.A. 106, 3794–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi T., Yamaguchi S., Chida K., Shibuya M. (2001) A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLCγ and DNA synthesis in vascular endothelial cells. EMBO J. 20, 2768–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakurai Y., Ohgimoto K., Kataoka Y., Yoshida N., Shibuya M. (2005) Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. U.S.A. 102, 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmqvist K., Cross M. J., Rolny C., Hägerkvist R., Rahimi N., Matsumoto T., Claesson-Welsh L., Welsh M. (2004) The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J. Biol. Chem. 279, 22267–22275 [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto T., Bohman S., Dixelius J., Berge T., Dimberg A., Magnusson P., Wang L., Wikner C., Qi J. H., Wernstedt C., Wu J., Bruheim S., Mugishima H., Mukhopadhyay D., Spurkland A., Claesson-Welsh L. (2005) VEGF receptor-2 Tyr-951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 24, 2342–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abedi H., Zachary I. (1997) Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 272, 15442–15451 [DOI] [PubMed] [Google Scholar]
- 30. Le Boeuf F., Houle F., Huot J. (2004) Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J. Biol. Chem. 279, 39175–39185 [DOI] [PubMed] [Google Scholar]
- 31. Yang S., Xin X., Zlot C., Ingle G., Fuh G., Li B., Moffat B., de Vos A. M., Gerritsen M. E. (2001) Vascular endothelial cell growth factor-driven endothelial tube formation is mediated by vascular endothelial cell growth factor receptor-2, a kinase insert domain-containing receptor. Arterioscler. Thromb. Vasc. Biol. 21, 1934–1940 [DOI] [PubMed] [Google Scholar]
- 32. Ewan L. C., Jopling H. M., Jia H., Mittar S., Bagherzadeh A., Howell G. J., Walker J. H., Zachary I. C., Ponnambalam S. (2006) Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting, and degradation in endothelial cells. Traffic 7, 1270–1282 [DOI] [PubMed] [Google Scholar]
- 33. Bhattacharya R., Kang-Decker N., Hughes D. A., Mukherjee P., Shah V., McNiven M. A., Mukhopadhyay D. (2005) Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 19, 1692–1694 [DOI] [PubMed] [Google Scholar]
- 34. Bruns A. F., Herbert S. P., Odell A. F., Jopling H. M., Hooper N. M., Zachary I. C., Walker J. H., Ponnambalam S. (2010) Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic 11, 161–174 [DOI] [PubMed] [Google Scholar]
- 35. Meissner M., Reichenbach G., Stein M., Hrgovic I., Kaufmann R., Gille J. (2009) Down-regulation of vascular endothelial growth factor receptor 2 is a major molecular determinant of proteasome inhibitor-mediated antiangiogenic action in endothelial cells. Cancer Res. 69, 1976–1984 [DOI] [PubMed] [Google Scholar]
- 36. Shen B. Q., Lee D. Y., Gerber H. P., Keyt B. A., Ferrara N., Zioncheck T. F. (1998) Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J. Biol. Chem. 273, 29979–29985 [DOI] [PubMed] [Google Scholar]
- 37. Patterson C., Perrella M. A., Hsieh C. M., Yoshizumi M., Lee M. E., Haber E. (1995) Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J. Biol. Chem. 270, 23111–23118 [DOI] [PubMed] [Google Scholar]
- 38. Gory-Fauré S., Prandini M. H., Pointu H., Roullot V., Pignot-Paintrand I., Vernet M., Huber P. (1999) Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126, 2093–2102 [DOI] [PubMed] [Google Scholar]
- 39. Dejana E., Taddei A., Randi A. M. (2007) Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim. Biophys. Acta 1775, 298–312 [DOI] [PubMed] [Google Scholar]
- 40. Grünewald F. S., Prota A. E., Giese A., Ballmer-Hofer K. (2010) Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim. Biophys. Acta 1804, 567–580 [DOI] [PubMed] [Google Scholar]
- 41. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 42. Gampel A., Moss L., Jones M. C., Brunton V., Norman J. C., Mellor H. (2006) VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartment. Blood 108, 2624–2631 [DOI] [PubMed] [Google Scholar]
- 43. Manickam V., Tiwari A., Jung J. J., Bhattacharya R., Goel A., Mukhopadhyay D., Choudhury A. (2011) Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi-localized t-SNARE syntaxin 6. Blood 117, 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lampugnani M. G., Orsenigo F., Gagliani M. C., Tacchetti C., Dejana E. (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174, 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ptaszynska M. M., Pendrak M. L., Stracke M. L., Roberts D. D. (2010) Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol. Cancer Res. 8, 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang D., Donner D. B., Warren R. S. (2000) Homeostatic modulation of cell surface KDR and Flt1 expression and expression of the vascular endothelial cell growth factor (VEGF) receptor mRNAs by VEGF. J. Biol. Chem. 275, 15905–15911 [DOI] [PubMed] [Google Scholar]
- 47. González-Pacheco F. R., Deudero J. J., Castellanos M. C., Castilla M. A., Alvarez-Arroyo M. V., Yagüe S., Caramelo C. (2006) Mechanisms of endothelial response to oxidative aggression. Protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am. J. Physiol. Heart Circ. Physiol. 291, H1395–H1401 [DOI] [PubMed] [Google Scholar]
- 48. Guan F., Villegas G., Teichman J., Mundel P., Tufro A. (2006) Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am. J. Physiol. Renal Physiol. 291, F422–F428 [DOI] [PubMed] [Google Scholar]
- 49. Hervé M. A., Meduri G., Petit F. G., Domet T. S., Lazennec G., Mourah S., Perrot-Applanat M. (2006) Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J. Endocrinol. 188, 91–99 [DOI] [PubMed] [Google Scholar]
- 50. Hayashi H., Kume T. (2008) Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PloS One 3, e2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward N. L., Dumont D. J. (2002) The angiopoietins and Tie2/Tek. Adding to the complexity of cardiovascular development. Semin. Cell Dev. Biol. 13, 19–27 [DOI] [PubMed] [Google Scholar]
- 52. Harris E. S., Nelson W. J. (2010) VE-cadherin. At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 22, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rabascio C., Muratori E., Mancuso P., Calleri A., Raia V., Foutz T., Cinieri S., Veronesi G., Pruneri G., Lampertico P., Iavarone M., Martinelli G., Goldhirsch A., Bertolini F. (2004) Assessing tumor angiogenesis. Increased circulating VE-cadherin RNA in patients with cancer indicates viability of circulating endothelial cells. Cancer Res. 64, 4373–4377 [DOI] [PubMed] [Google Scholar]
- 54. Yamaoka-Tojo M., Tojo T., Kim H. W., Hilenski L., Patrushev N. A., Zhang L., Fukai T., Ushio-Fukai M. (2006) IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler. Thromb. Vasc. Biol. 26, 1991–1997 [DOI] [PubMed] [Google Scholar]
- 55. Pilecka I., Banach-Orlowska M., Miaczynska M. (2007) Nuclear functions of endocytic proteins. Eur. J. Cell Biol. 86, 533–547 [DOI] [PubMed] [Google Scholar]
- 56. Kang J., Shi Y., Xiang B., Qu B., Su W., Zhu M., Zhang M., Bao G., Wang F., Zhang X., Yang R., Fan F., Chen X., Pei G., Ma L. (2005) A nuclear function of β-arrestin1 in GPCR signaling. Regulation of histone acetylation and gene transcription. Cell 123, 833–847 [DOI] [PubMed] [Google Scholar]
- 57. Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. (2004) APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456 [DOI] [PubMed] [Google Scholar]
- 58. Varsano T., Dong M. Q., Niesman I., Gacula H., Lou X., Ma T., Testa J. R., Yates J. R., 3rd, Farquhar M. G. (2006) GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol. Cell. Biol. 26, 8942–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lanahan A. A., Hermans K., Claes F., Kerley-Hamilton J. S., Zhuang Z. W., Giordano F. J., Carmeliet P., Simons M. (2010) VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell 18, 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.