Background: Disulfide-regulated NTPDases from T. gondii are related to the virulence of the parasite.
Results: Crystal structures of the active and inactive state were determined.
Conclusion: The 258–268 disulfide bridge acts like a clamp, which upon reduction allows concerted motions of the subunits and domains of the tetrameric enzyme.
Significance: First molecular model of the disulfide-regulated activation mode.
Keywords: Crystal Structure, Enzyme Mechanisms, Enzyme Structure, Protein Conformation, X-ray Crystallography, Purinergic Signaling, Domain Motion, Kinetic ITC, Nucleotidase, Phosphatase
Abstract
The intracellular parasite Toxoplasma gondii produces two nucleoside triphosphate diphosphohydrolases (NTPDase1 and -3). These tetrameric, cysteine-rich enzymes require activation by reductive cleavage of a hitherto unknown disulfide bond. Despite a 97% sequence identity, both isozymes differ largely in their ability to hydrolyze ATP and ADP. Here, we present crystal structures of inactive NTPDase3 as an apo form and in complex with the product AMP to resolutions of 2.0 and 2.2 Å, respectively. We find that the enzyme is present in an open conformation that precludes productive substrate binding and catalysis. The cysteine bridge 258–268 is identified to be responsible for locking of activity. Crystal structures of constitutively active variants of NTPDase1 and -3 generated by mutation of Cys258–Cys268 show that opening of the regulatory cysteine bridge induces a pronounced contraction of the whole tetramer. This is accompanied by a 12° domain closure motion resulting in the correct arrangement of all active site residues. A complex structure of activated NTPDase3 with a non-hydrolyzable ATP analog and the cofactor Mg2+ to a resolution of 2.85 Å indicates that catalytic differences between the NTPDases are primarily dictated by differences in positioning of the adenine base caused by substitution of Arg492 and Glu493 in NTPDase1 by glycines in NTPDase3.
Introduction
Toxoplasma gondii is an obligate intracellular parasitic protozoan that can infect a wide variety of warm-blooded animals and humans. About one-third of the human world population is infected with the pathogen, but chronic infections are normally asymptotic (1). However, toxoplasmosis can cause dangerous complications for patients with AIDS, developing fetuses, or organ transplant recipients undergoing immunosuppressive treatment (2).
Several microbial pathogens, including T. gondii, express nucleoside triphosphate diphosphohydrolases (NTPDases3; E.C. 3.6.1.5). In vertebrates, cell surface-located homologs (<15% sequence identity) are well established to control purinergic signaling events via degradation of extracellular nucleotides (3–6). In contrast, the exact function of NTPDases from microbial pathogens is less clear (7). In recent years, evidence has been accumulating that the expression of NTPDase genes is required for virulence of many pathogens (7–11). As host ATP exerts a proinflammatory action via P2 receptors and acts as a danger signal, it appears likely that microbial NTPDases interfere with this host response to suppress inflammatory responses and evade immune reactions (7). However, the intracellular location of T. gondii and the millimolar Km values of TgNTPDases argue against a function to interfere with the canonical extracellular purinergic signaling. Alternatively, a role in purine salvage has been proposed because many pathogens, including T. gondii, are purine-auxotroph and need to scavenge adenosine for growth (12).
Two NTPDase genes, TgNTPDase1 (NTPase II) and TgNTPDase3 (NTPase I) can be expressed in T. gondii (7, 8, 13). Both enzymes consist of 628 amino acids, including 15 cysteines (8, 13), and form non-covalent homotetramers in solution. Despite their high sequence identity of 97%, they exhibit significant differences in substrate specificity; NTPDase1 cleaves ATP and ADP about equally well, whereas NTPDase3 is much more specific toward ATP. Also, TgNTPDase3 cleaves ATP at 4.5 times the rate of TgNTPDase1 (8, 14). Caused by a short patch of dissimilar amino acids, these differences in substrate specificity are correlated to the virulence of the parasite; NTPDase1 is found in virulent and avirulent T. gondii strains, whereas NTPDase3 is only found in virulent strains (8, 14). Via antisense RNA inhibition, it was demonstrated that NTPDase3 is required for replication of the parasite inside the host cells, but it appeared not to influence parasite invasion (15). In contrast, pretreatment of parasites with a monoclonal antibody inhibiting both NTPDases significantly decreased the number of infected cells, suggesting that one or both isoforms are involved in invasion of the parasites into host cells (16).
Strikingly, TgNTPDases are produced as inactive proforms that accumulate in the dense granules and are secreted in large quantities (up to 8% of the total protein) into the parasitophorous vacuole that surrounds the parasite after infection (13, 17). In vitro, activation of the dormant enzymes can be achieved by incubation with dithiol compounds. Hence, activation appears to be induced by reduction of a yet unidentified disulfide linkage. How and when exactly the activation occurs in vivo is currently unclear. In cellular assays, activation of NTPDases by exogenously added dithiols leads to rapid depletion of host cell ATP and an increase in host calcium levels and results in an abrupt exit of parasites from cells (18). It has been speculated that T. gondii itself is responsible for the activation of the TgNTPDases (7, 18, 19). Glutaredoxin can activate TgNTPDase3 in vitro, and it may be secreted by the parasite for stimulating exit from host cells (19, 20). In summary, the presence of NTPDases and their activation has been implicated in T. gondii invasion, replication, and egress.
Based on crystal structures, we explain here the activation mechanism of TgNTPDases. The cysteine bridge responsible for suppression of enzyme activity was identified and appears to lock the enzyme in an open, inactive conformation. Reduction of this cysteine bridge induces a pronounced conformational change that involves a contraction of the whole tetramer and closure of the active site clefts. Complex structures with the substrate analog AMPPNP and the product AMP help to explain the different substrate specificities of TgNTPDase1 and -3.
EXPERIMENTAL PROCEDURES
Protein Preparation
The cDNA coding for the mature T. gondii NTPDase1 and -3 were kindly provided by Michael Johnson (University of Technology, Sydney, Australia) and cloned via NdeI- and XhoI-sites into the pET20b(+) vector (Novagen, Madison, WI). The expressed proteins consisted of residues Thr26–Leu628, which corresponds to the mature protein without the signal peptide (8). In addition to these residues, the constructs contained an N-terminal methionine as well as an additional glutamate and a hexahistidine tag at the C terminus.
The proteins were expressed in Escherichia coli Rosetta pLysS cells after induction with 1 mm isopropyl 1-thio-β-d-galactopyranoside in the form of inclusion bodies, which were isolated and purified as described (21). Denatured and reduced NTPDases at a concentration of 3 mg/ml were refolded by rapid dilution into 1.5 liters of 100 mm Tris, pH 8.5, 10% (v/v) glycerol, 1 mm EDTA, 1 mm reduced glutathione, and 0.2 mm oxidized glutathione to a final concentration of 100 μg/ml. After incubation for 7 days at 12 °C, the protein was concentrated 20-fold to ∼70 ml, dialyzed two times against 50 volumes of 20 mm Tris, pH 8.5, and subjected to anion exchange chromatography (5 ml of HiTrap Q HP column, GE Healthcare) using 20 mm Tris/HCl, 1 mm EDTA, pH 8.5, as running buffer. A linear salt gradient from 0 to 2 m potassium acetate over 30 column volumes was applied to elute the protein. The most active fractions were pooled, concentrated, and further purified using size exclusion chromatography (Superdex 200 16/60, GE Healthcare). Purification was carried out on FPLC instruments (Äkta Explorer/Äkta Purifier, GE Healthcare). Protein was concentrated to 10 mg/ml and stored in size exclusion buffer (10 mm Tris, pH 8, 1 mm EDTA, 1 mm NaN3) at −80 °C.
Protein concentrations of purified protein were analyzed using extinctions at 280 nm, and specific extinction coefficients were calculated by the ProtParam tool (see supplemental Table S1) (22).
Activity Assay
The reaction buffer in all activity assays contained 50 mm Hepes/NaOH (pH 7.5) and 20 mm magnesium acetate and was chosen according to previous studies (8, 13, 14, 23). Protein samples were diluted in this buffer supplemented with 100 μg/ml BSA to prevent adhesion of NTPDase to the reaction tube. The reaction was started in microtiter plates by the addition of 280 μl of reaction buffer containing 2 mm ATP or 5 mm ADP and optionally 3 mm DTT to 20 μl of protein sample. Released phosphate was measured colorimetrically using a modified malachite green assay (24).
Preparation of Cysteine to Serine Mutants
Cysteine to serine mutants were prepared using the QuikChange method from Stratagene, and positive clones were validated by sequencing. The sense mutagenesis primer for NTPDase3 had the sequence 5′-GAC CCA GCT AGG AGC ATG ATT GAT GAA TAC GG-3′ for mutagenesis of cysteine 258 to serine (C258S), 5′-GG GTG AAG CAA TCC CGC AAT GAC CTT GCT GG-3′ for C268S, 5′-CTC AAG GAG CTG TCT AGT AAC GAT GAG TTT TTG C-3′ for C341S, 5′-G CAA GGC GGA ATT TCC TCC AAC CCG TG-3′ for C352S, and 5′-G AAG ATC GAG AAC TCC AGT ATA ATC AAA GGA ACC GG-3′ for C433S, containing the respective point mutations (underlined). The proteins were subjected to rapid dilution refolding in a 1.5-ml test scale and without further purification subjected to activity tests.
To account for amino acid differences, the C268S mutagenesis primer with the sequence 5′-GAA TAC GGG GTG AAG CAT TCC CGC AAT GAC CTT GCT GG-3′ was used to generate TgNTPDase1 C258S/C268S. In the following, the mutation C258S/C268S is indicated as ΔCC.
Isothermal Titration Calorimetry (ITC)
ATPase and ADPase activities were determined at 25 °C in a VP-ITC isothermal titration microcalorimeter (MicroCal, part of GE Healthcare) as described (25). In this work, two buffers were employed that were optimized for low dilution endotherms of the nucleotides. Buffer 1 is identical to the one used in the characterization of rat NTPDase1 (25) and contained 100 mm Tris, 50 mm NaCl, 10 mm CaCl2, 100 μg/ml BSA, adjusted to pH 7.4 with malonic acid. Buffer 2 contained 10 mm MgAc2 instead of CaCl2 to resemble more closely those buffers that have previously been used for characterization of TgNTPDases (8, 13, 14, 23). The molar enthalpy of hydrolysis was determined by subtracting dilution endotherms from heat recordings of experiments with complete hydrolysis of the nucleotide. For the magnesium buffer, the molar enthalpy of hydrolysis was found to be −8.25 ± 1.35 kcal/mol for ATP and −11.17 ± 0.51 kcal/mol for ADP. Values for the calcium buffer were determined previously (25). The catalytic parameters Km and kcat were then determined for the permanently activated variants (see “Results”) by using the multiple injection method (26–28). Substrate was added in a stepwise manner from a 30 mm stock solution in reaction buffer to 0.4 nm (TgNTPD1ΔCC, ATP), 0.4 nm (TgNTPD1ΔCC, ADP), 53.4 pm (TgNTPD3ΔCC, ATP), or 4 nm (TgNTPD3ΔCC, ADP) enzyme equilibrated in the ITC cell. Thermal power data recorded after compensation of dilution endotherms (determined in pilot experiments) were transformed to apparent rate constants using the molar reaction enthalpy and the molar enzyme concentration (25). Experimental data were fitted based on Michaelis-Menten kinetics to derive the kinetic constants kcat and Km using OriginPro 8G.
Crystallization and Derivatization
TgNTPDase3 (Thr26–Leu628) wild type crystallized within 2 days at 19 °C and 8 mg/ml protein concentration by hanging drop vapor diffusion after mixing 1 μl of protein with 0.7 μl of reservoir solution (containing 200 mm ammonium acetate, 25 mm CaCl2, 50 mm sodium cacodylate, pH 6.2, 11% (w/v) PEG 4000) and 0.3 μl of microseeding solution. Microseeds were prepared using Seed Beads (Hampton Research, Aliso Viejo, CA), 500 μl of 200 mm KCl, 10 mm MgCl2, 50 mm sodium cacodylate, pH 6.5, 10% (w/v) PEG 4000, and crystals were grown from this condition.
Heavy atom derivatives were produced by soaking the crystals for 45 min in a new drop with the composition of the original reservoir solution supplemented with 2 mm thiomerosal or cis-dichlorodiammine platinum. The iodide derivative was prepared by soaking of the crystal in the final cryosolution supplemented with 100 mm KI for 30 s and immediate cryocooling in liquid nitrogen.
The AMP Complex Was Produced by Soaking Crystal during Cryoprotection with 20 mm AMP for 2 min
Crystals of the disulfide mutant TgNTPDase3ΔCC in complex with Mg2+-AMPPNP were obtained at 19 °C within 2 days by mixing 1 μl of a 10 mg/ml protein solution (containing 2 mm AMPPNP and 10 mm magnesium acetate) and 1 μl of reservoir solution (100 mm KCl, 10 mm MgCl2, 50 mm Tris, pH 8.5, 30% (w/v) PEG 400). TgNTPDase1ΔCC was crystallized accordingly in 100 mm Tris, pH 8.2, 50 mm MgCl2, 25% (v/v) pentaerythritol propoxylate (17/8 PO/OH).
Data Collection and Structure Determination
X-ray data were collected at 100 K. Cryoprotection was achieved by stepwise transfer to a cryoprotection buffer, which was the reservoir or soaking solution plus 20% (w/v) PEG 200 for TgNTPDase3 and TgNTPDase1ΔCC or 50% (w/v) PEG400 for TgNTPDase3ΔCC. X-ray data sets were collected at BL14.1 and BL14.2 at the Protein Structure Factory/Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung (Berlin, Germany) and beamline ID23–2 at the European Synchrotron Radiation Facility (Grenoble, France). Data sets were processed and scaled using XDS (29) or MOSFLM and SCALA (30) (Table 1). For the data sets of active TgNTPDase1ΔCC and TgNTPDase3ΔCC, many crystals had to be tested to find a suitable specimen for structure analysis because the diffraction spots were diffuse along the short reciprocal unit cell axis c* and were overlapping for most of the crystals.
TABLE 1.
Data collection and refinement statistics (values in parentheses refer to the highest resolution shell)
| TgNTPDase3 apo | TgNTPDase3 thiomerosal | TgNTPDase3 KI | TgNTPDase3 cis-Pt(NH3)2Cl2 | TgNTPDase3−AMP | TgNTPDase3ΔCC ×Mg2+−AMPPNP | TgNTPDase1ΔCC | |
|---|---|---|---|---|---|---|---|
| Data collection | |||||||
| X-ray source | BL14.1/HZB | BL14.1/HZB | BL14.1/HZB | BL14.1/HZB | BL14.1/HZB | BL14.2/HZB | ID23–2/ESRF |
| Wavelength (Å) | 0.91841 | 0.83 | 1.9 | 0.89 | 0.91841 | 0.91841 | 0.8726 |
| Space group | P21 | P21 | P21 | P21 | P21 | C2221 | C2221 |
| Cell dimensions | |||||||
| a (Å) | 89.0 | 88.9 | 89.4 | 88.9 | 89.2 | 72.0 | 73.7 |
| b (Å) | 165.9 | 164.4 | 165.8 | 164.7 | 166.0 | 150.8 | 150.4 |
| c (Å) | 97.5 | 97.6 | 97.3 | 97.3 | 97.9 | 487.1 | 242.4 |
| β (degrees) | 97.0 | 97.1 | 97.4 | 97.1 | 97.2 | 90 | 90 |
| Wilson B factor (Å2) | 21.5 | 55.5 | 72.8 | 51.3 | 33.0 | 49.6 | 42.1 |
| Resolution (Å) | 40.39–2.00 | 54.00–2.90 | 55.22–3.20 | 60.19–2.90 | 43.40–2.20 | 39.00–2.85 | 39.30–2.60 |
| Resolution, last shell (Å) | 2.11–2.00 | 3.06–2.90 | 3.37–3.20 | 3.06–2.90 | 2.30–2.20 | 3.00–2.85 | 2.60–2.50 |
| Unique reflections | 183,360 | 61,578 | 46,364 | 61,544 | 142618 | 61587 | 47598 |
| Average multiplicity | 5.1 (5.0) | 5.1 (5.1) | 5.3 (5.2) | 4.7 (4.6) | 3.0 (2.9) | 3.7 (3.4) | 4.4 (3.9) |
| Completeness (%) | 97.3 (97.9) | 99.9 (99.9) | 100 (100) | 100 (100) | 96.9 (93.3) | 98.3 (94.3) | 99.9 (99.6) |
| 〈I/σI〉 | 10.1 (2.9) | 8.2 (2.9) | 9.3 (3.3) | 8.3 (2.8) | 7.6 (2.0) | 5.0 (2.0) | 11.9 (3.0) |
| Rmerge (%)a | 10.5 (50.8) | 12.9 (43.1) | 13.2 (46.9) | 12.9 (44.6) | 8.1 (48.9) | 16.9 (45.2) | 9.6 (47.4) |
| Rrim (%)a | 11.7 (56.6) | 16.4 (54.7) | 16.9 (59.0) | 16.7 (57.0) | 11.5 (68.9) | 22.7 (60.4) | 10.9 (55.5) |
| Rpim (%)a | 5.0 (24.5) | 7.2 (23.9) | 7.3 (25.8) | 7.7 (26.4) | 6.4 (40.0) | 15.1 (39.7) | 5.1 (27.7) |
| Refinement | |||||||
| Resolution range (Å) | 40.39–2.00 | 43.40–2.20 | 39.00–2.85 | 39.30–2.50 | |||
| Rwork/Rfree (%) | 17.4/20.9 | 18.0/22.8 | 23.4/28.4 | 17.6/22.6 | |||
| No. of amino acids | 2369 | 2371 | 2360 | 1185 | |||
| Water | 1274 | 884 | 0 | 247 | |||
| Ligandsb | 4 | 5 | 8 | 0 | |||
| Average B factor (Å2) | |||||||
| Protein | 31.9 | 41.6 | 20.4 | 36.5 | |||
| Water | 33.8 | 37.2 | 30.8 | ||||
| Ligandsb | 33.0 | 46.8 | 59.4 | ||||
| rmsd bond length (Å)c | 0.009 | 0.010 | 0.010 | 0.010 | |||
| rmsd angles (degrees)c | 1.03 | 1.14 | 1.45 | 1.19 | |||
| Ramachandran plots | |||||||
| Favored (%) | 98.7 | 98.8 | 97.1 | 98.5 | |||
| Allowed (%) | 100 | 99.9 | 100 | 100 | |||
| No. of outliers | 1 | 2 | 0 | 0 | |||
| Protein Data Bank accession code | 4a57 | 4a59 | 4a5a | 4a5b | |||
The structure of TgNTPDase3 was determined by multiple isomorphous replacement with anomalous scattering using data of the iodide, mercury, and platinum derivatives in the program autoSharp (31). Experimental phases were significantly improved with the program DM (32), applying NCS-operators, which were manually determined from the heavy atom positions. An initial model was built by Buccaneer (33) and refined using REFMAC5 (34) and Buster-TNT (35). The structure of the active disulfide mutant TgNTPDase3ΔCC was determined by molecular replacement using PHASER (36). Molecular replacement trials with the tetrameric structure or the monomer as search models failed. A first monomer could be placed by searching for truncated N- and C-terminal domains separately. Additional monomers were then found using this monomer as a search model. Two half-tetramers are present in the asymmetric unit. Full tetramers can be generated by the crystallographic symmetry operators. Given the limited resolution of the active TgNTPDase3ΔCC variant, high NCS restraints were used during refinement. The structure of TgNTPDase1ΔCC was determined with the program MOLREP (37) using a TgNTPDase3ΔCC monomer as a search model. Two monomers are present in the asymmetric unit of the crystals of TgNTPDase1ΔCC, and the full tetramer is generated by the crystallographic symmetry operators. The crystal structure was refined with Buster-TNT (35). Translation/libration/screw (TLS) refinement was employed in refinement for all structures. TLS groups were assigned based on the output of the TLS Motion Determination Home Server (38, 39) and were defined for each chain as follows: I, 35–58; II, 59–256 and 586–629; III, 257–268; IV, 269–394 and 425–585; V, 395–424.
Structure Analysis
The buried surface area was determined using the program AREAIMOL (30) by subtracting the accessible surface area of the tetramer from the sum of the accessible surface areas of the dimers. The tetramer contact areas were calculated as half of the buried surface area. All molecular figures were generated with the program PyMOL (available on the World Wide Web). For an automatic determination of dynamic domains, the program Dyndom (40) was used. Residues that move together as a rigid body during the conformational change are assigned to a dynamic domain based on a clustering algorithm. A window size of 5 residues was used, and the ratio of interdomain to intradomain displacements was set to a relatively low value of 0.7 for the domain assignment. The large intradomain displacements are due to large changes in the conformation, in particular of residues 257–269 and 459–471.
RESULTS AND DISCUSSION
Crystal Structure of Dormant State of T. gondii NTPDase3
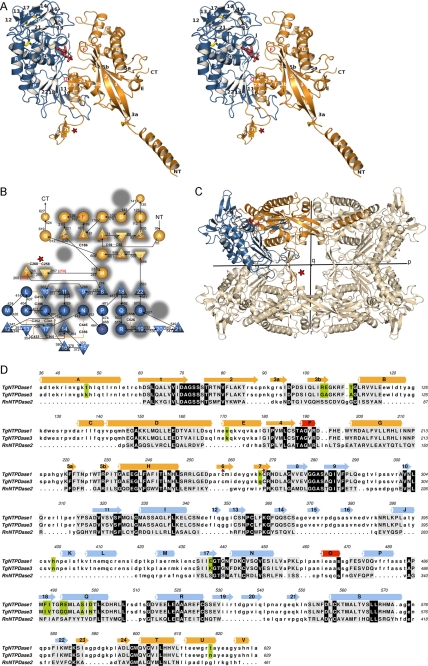
TgNTPDase3 was refolded from purified bacterial inclusion bodies, separated from misfolded protein, and crystallized. The crystal structure was determined at 2.0 Å by the multiple isomorphous replacement with anomalous scattering method using crystals derivatized by thiomerosal, potassium iodide, and cis-Pt(NH3)2Cl2 (Table 1). An almost complete model could be built that includes residues 35–628 of the mature native protein and the artificially introduced Glu629 (Fig. 1). Due to partial disorder, residues 35–39 are missing in some of the chains.
FIGURE 1.
Protein fold of TgNTPDase3. A, stereo view of a TgNTPDase3 monomer in complex with AMP (red). Domains I and II are colored in orange and blue, respectively. Strands are numbered 1–24, and helices are labeled A–V. Cysteines are depicted in yellow. B, topology diagram. Triangles, β-strands; circles, α-helices. The flanking strands 3 and 5 of the first β-sheet are interrupted by β-bulges. Red labeled helices and red marked β-sheet limitations correspond to the topology of the activated variant TgNTPDase3ΔCC. The topology of rat NTPDase2 (41) is underlaid in gray, highlighting the conservation, deletion, and addition of structural elements. C, orientation of the subunit domains in the tetramer. D, structure-based sequence alignment between TgNTPDase1 and -3 and RnNTPDase2 using RAPIDO (42, 52), with 328 aligned residues (root mean square deviation, 3.25 Å) and three defined rigid bodies of 159 residues (root mean square deviation (rigid bodies), 1.59 Å). Aligned residues are shown in capital letters and underlaid in gray. Residues conserved between TgNTPDases and RnNTPDase2 are underlaid in black, and positions that are different between TgNTPDase1 and -3 are highlighted in green. The red star in A–D highlights the activation loop with the disulfide bridge responsible for inactivity of the oxidized form.
The asymmetric unit contains four monomers of TgNTPDase3, and identification of the physiological tetramer is possible without ambiguity. Each subunit of the tetramer shows an arrangement of two structural domains that is in a similar fashion also found in mammalian cell surface NTPDases (41). Domain I is formed from the N-terminal part as well as the very C-terminal region of the protein (residues 36–273 and 586–629). Domain II consists of residues 274–585 (Fig. 1).
A flexible structure-based comparison with RnNTPDase2 (41), the prototypic structure of the NTPDase family, using the program RAPIDO (42) reveals the overall conserved fold, but also new or extended secondary structure elements (Fig. 1, B and D). Several secondary structure elements of domain I are found to be considerably longer than in RnNTPDase2 (41). Most notably, TgNTPDases have a long N-terminal α-helix (A), which sticks out of the monomer. In general terms, the new (e.g. helices A, K, L, and P) or extended (strands 1 and 2, helix D) elements are found to participate directly in tetramer formation or provide the necessary framework. The flanking β-strands 3 and 5 of the central β-sheet of domain I are interrupted by proline-containing β-bulges. These bulges are involved in tetramer formation (strand 5) and the activation mechanism (strand 3) as detailed below.
Tetramer Structure
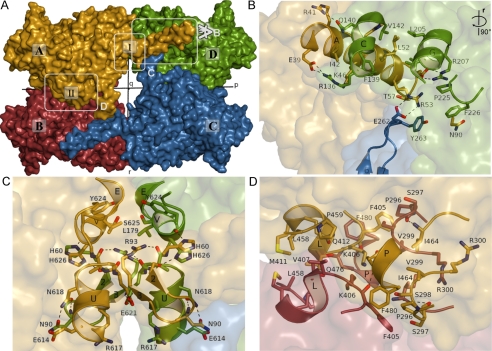
The TgNTPDases form homotetramers with 222 symmetry (a dimer of dimers). In the following discussion, we denote the three perpendicular 2-fold axes as p, q, and r as defined in Fig. 1C and Fig. 2A. The TgNTPDase3 tetramer has dimensions of ∼147 × 101 × 85 Å along the p, r, and q axes, respectively. Mostly hydrophobic interactions account for the formation of the tetramer (Fig. 2). Two major interfaces are involved in oligomerization.
FIGURE 2.
Tetramer interactions of TgNTPDase3. Chains A, B, C, and D of the protein structure are colored in yellow, red, blue, and green, respectively. A, surface representation with the 2-fold symmetry axes p, q, and r (see “Results and Discussion”). Interfaces I and II are indicated. B–D, zoom of different areas of the tetramer interfaces. Interacting residues are shown as sticks and labeled with the residue number. Polar interactions are indicated by dashed lines. B and C, interactions at interface I. For a better view, the molecules in B were rotated 90° around the r axis. D, interface II between chains A and B.
Interface I (Fig. 2, B and C) is formed between the N-terminal domains of chains A and D as well as B and C and symmetrical around the r axis. A large surface contact area of 5334 Å2/tetramer is formed by this interaction. About half of this area (2433 Å2) can be attributed to the N-terminal helix alone, which protrudes about 30 Å into the neighboring monomer and forms interactions with helices C, D, and G as well as residues Pro225 and Phe226 (Fig. 2B). The latter residues are positioned for this interaction as a result of the formation of the β-bulge of strand 5. Helix A is indispensable for the formation of active protein because it was not possible to refold denatured protein into an activatable form from an N-terminally shortened variant (amino acids Leu55 to Leu628; data not shown). In proximity to the N-terminal helix, the C-terminal helices U and V form the remaining contact area of this interface via interactions with symmetry mates of these helices generated by the r dyad (Fig. 2C). In general, hydrophobic interactions are interspersed with hydrogen bonds and salt bridges. Closely adjoining interface I, subunit interactions are formed via the β-hairpin of strands 6 and 7. This interaction appears to be central for the activation mechanism as discussed below.
Interface II (Fig. 2D) is formed along the p axis between the C-terminal domains of chains A and B as well as C and D with a contact area of 3000 Å2/tetramer. The interface includes residues of loop 289–301 (between β-strands 9 and 10), the region 405–412 (partially involved in α-helix L), and α-helix P. Based on visual inspection, interface II is estimated to be relatively loose, lacking specific polar interactions and strong hydrophobic contacts.
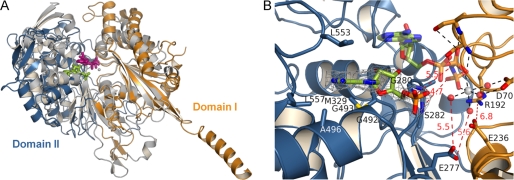
Structure of Dormant State of TgNTPDase3 Represents Open, Inactive Conformation
Superposition of TgNTPDase3 and RnNTPDase2 (41) shows that the cleft between the two domains is more open in the microbial enzyme as a result of a domain rotation of about 12° (Fig. 3). This leads to a relative orientation of the catalytic residues that would not allow productive binding of nucleotides, as shown by superposition with the complex structure of RnNTPDase2 with Ca2+-AMPPNP (41) (Fig. 3B). Gly280 of TgNTPDase3 is expected to bind to the β/γ-bridging oxygen of ATP, but the observed distance of 5.5 Å of this residue to the substrate is 2.5 Å longer than the corresponding hydrogen bond in RnNTPDase2 (41). Furthermore, Ser282 cannot bind to the γ-phosphate group of ATP because this distance of 4.7 Å is 1.5 Å longer compared with the bond in RnNTPDase2 (41). In addition, Glu277 cannot position the metal-coordinating water molecules, and the salt bridge between Arg192 and the catalytic base Glu236 cannot be formed as a result of the open active site cleft in TgNTPDase3. The latter interaction was found to be important for correct positioning of the catalytic base (41, 43, 44). These findings indicate that in the dormant form of TgNTPDases, the open state may be stabilized, and activation might be achieved by induction of a domain closure motion.
FIGURE 3.
Comparison of TgNTPDase3 to the mammalian cell surface NTPDase2. A, superposition of RnNTPDase2 (gray; bound Ca2+-AMPPNP gray/violet) and TgNTPDase3 (orange/blue; bound AMP green). Residues 45–58 and 115–127 for domain I of RnNTPDase2 were aligned with corresponding residues 70–83 and 181–193 of TgNTPDase3. B, close-up view of the active site. An omit electron density map (Fo − Fc × ϕc, contoured at 3 σ) of AMP is shown in gray. Active site ligands from the superimposed RnNTPDase2 structure are shown with half-transparency. Amino acids involved in binding of the base and residues expected to interact with the phosphate groups of ATP or ADP are indicated. A selection of polar interactions that occur in the productive substrate binding mode of RnNTPDase2 are shown as dashed lines and drawn red when distances are too long for the formation of hydrogen bonding or salt bridge interaction. The figure illustrates that domain II that binds the nucleoside moiety has to rotate toward domain I for the formation of a productive substrate binding mode.
A complex structure with the substrate fragment AMP shows that the position of the nucleoside binding site is conserved between RnNTPDase2 (41) and TgNTPDase3. However, weaker and fewer contacts are made to the nucleoside in TgNTPDase3. This may explain the much larger Km values of TgNTPDases for nucleotides compared with their mammalian orthologs (8, 21). In TgNTPDase3, the adenine base is sandwiched between Leu553 and the peptide bond between Gly492 and Gly493. In contrast, in RnNTPDase2, the nucleobase is bound between the large planar side chain groups of Arg394 and Tyr350 (41). Leu557 in TgNTPDase3 lies in the plane of the nucleobase, but in contrast to Tyr398 in RnNTPDase2 (41), it cannot form direct or indirect hydrogen bonds with it. The hydrophobic binding pocket for the base is further formed by Gly492 and Ala496.
Identification of Cystine Linkage Involved in Enzyme Activation
The crystal structure of TgNTPDase3 revealed the so far unknown disulfide pattern of TgNTPDases. They contain 15 cysteines (Fig. 1 and supplemental Fig. S1) that form seven disulfide bonds (1, Cys59-Cys88; 2, Cys258-Cys268; 3, Cys341-Cys352; 4, Cys356-Cys445; 5, Cys365-Cys433; 6, Cys396-Cys413; 7, Cys526-Cys558) and one free cysteine (Cys186). The free cysteine is located on β-strand 4 and is relatively buried inside the molecule and isolated from other cysteines. Mutation of the free cysteine Cys186 yielded a variant that retained ∼75% of the activity of the wild type after activation with DTT or tris(2-carboxyethyl)phosphine (data not shown). Sequence alignments using all available NTPDase sequences (Protein Families Database, PFAM 01150) shows that disulfides 4 and 7 are strictly conserved in the NTPDase family. These disulfides are required for correct folding of NTPDases, as determined by mutations to serine residues for mammalian NTPDases (45, 46).
Knowing the disulfide pattern of TgNTPDases, cysteine mutations were introduced to identify the disulfide responsible for enzyme activation. Conserved disulfide bonds were not considered for mutagenesis experiments. Variants C258S/C268S, C341S/C352S, and C433S of TgNTPDase3 were prepared. In particular, the disulfide bridge Cys258-Cys268 was chosen as a preferred target due to its location in an exposed loop between the monomers.
Subsequent activity assays with protein out of small scale refolding batches identified variant C258S/C268S to be active with and without the addition of DTT, whereas no activity at all was measured for the two other variants. This cystine bridge is formed between the strands of a β-hairpin that protrudes between domains I and II (Fig. 1A) and reaches across the internal void of the tetramer to interact with the diametrically positioned monomer close to interface I (Figs. 1C and 2B). Because the kinetic characterization (see below) of the C258S/C268S variant demonstrated that this disulfide bridge is solely responsible for the activation of the enzyme by thiol compounds, variants of other disulfide bridges were not characterized in this study.
To gain further insight into catalysis and the activation mechanism of TgNTPDases, large scale refolding and purification of C258S/C268S variants were carried out for TgNTPDase1 and -3 (further referred to as TgNTPDase1ΔCC and -3ΔCC). The permanently active variants were subjected to kinetic analysis and crystallization trials.
Kinetic Characterization
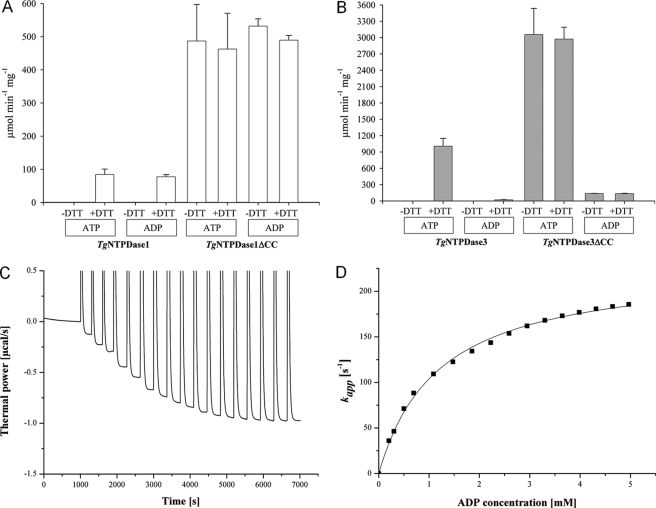
The activity of the ΔCC variants was found to be ∼3–6-fold higher for ADP and ATP than that of the DTT-activated wild-type enzymes (Fig. 4, A and B; see supplemental Table S2). The addition of DTT did not raise the activity of the ΔCC mutants any further. Thus, mutagenesis of the C258/C268 linkage can fully activate TgNTPDases without the requirement of further activation by thiol reagents. These findings strongly indicate that the reduction of this disulfide bridge alone is responsible for the activation of the wild-type enzymes.
FIGURE 4.
Kinetic analysis of enzyme activation and specificity toward ATP and ADP. A and B, specific activity ± S.D. (indicated as error bars) for TgNTPDase variants with 2 mm ATP or 5 mm ADP and optionally with the addition of 3 mm DTT according to a malachite green assay. Reactions were carried out for 10 min at room temperature. Please note the different scale in A and B. C, ITC-based enzyme assay. Titration of TgNTPDase1ΔCC with ADP as substrate. 3 × 5 μl, 2 × 10 μl, and 12 × 20 μl of a 30 mm ADP were added to 0.4 nm TgNTPDase1ΔCC in a magnesium reaction buffer with reequilibration times of 300 to 350 s. D, calculation of turnover rates from the data in C.
Hence, the generation of permanently activated variants allowed us for the first time to measure kcat and Km values unbiased by incomplete activation of the latency-associated disulfide bridge or partial nonspecific reduction of other structurally important disulfide bonds. To derive the kinetic constants, we utilized a novel calorimetric activity assay (25). Briefly, the heat release from hydrolysis of the energy-rich anhydride bonds was measured in an isothermal titration calorimeter (Fig. 4C). This assay allowed the determination of Km and kcat with high accuracy in a single experiment (Fig. 4D, Table 2). No labeling of substrates or separation of substrates and products is required.
TABLE 2.
Kinetic constants determined for ADP and ATP hydrolysis of TgNTPDase1ΔCC and TgNTPDase3ΔCC in magnesium or calcium buffer using the ITC-based enzyme assay
| kcat | Km | kcat/Km | Ratio (kcat/Km) ATP/(kcat/Km)ADP | |
|---|---|---|---|---|
| s−1 | mm | s−1/mm | ||
| TgNTPDase1ΔCC | ||||
| ADP/Mg2+ | 238 ± 14 | 1.17 ± 0.05 | 204 ± 15 | 0.40 |
| ATP/Mg2+ | 276 ± 27 | 3.4 ± 0.5 | 81 ± 15 | |
| ADP/Ca2+ | 333 ± 15 | 1.30 ± 0.16 | 255 ± 34 | 0.57 |
| ATP/Ca2+ | 349 ± 22 | 2.4 ± 0.2 | 150 ± 20 | |
| TgNTPDase3ΔCC | ||||
| ADP/Mg2+ | 18 ± 1 | 1.25 ± 0.15 | 15 ± 2 | 202 |
| ATP/Mg2+ | 2290 ± 74 | 0.8 ± 0.2 | 3000 ± 800 | |
| ADP/Ca2+ | 13.2 ± 0.3 | 2.3 ± 0.2 | 5.8 ± 0.6 | 177 |
| ATP/Ca2+ | 588 ± 52 | 0.572 ± 0.009 | 1030 ± 90 | |
We measured substrate turnover in two buffers: 1) 100 mm Tris/malonate, 50 mm NaCl, 10 mm CaCl2 (pH 7.4) and 2) 100 mm Tris/malonate, 50 mm NaCl, 10 mm MgAc2 (pH 7.4). Substrate was titrated to the enzyme from a 30 mm solution in reaction buffer (see the example in Fig. 4, C and D). Table 2 shows the magnesium buffer to be preferred for TgNTPDase3ΔCC, especially for the hydrolysis of ATP, whereas for TgNTPDase1ΔCC, the measurements indicate slightly higher activities in CaCl2 buffer.
Km values were found to be in the low millimolar range. Specificity constants for TgNTPDase3ΔCC show a 202-fold (Mg2+) or 177-fold (Ca2+) higher specificity constant (kcat/Km) for ATP over ADP (Table 2). The preference of TgNTPDase3ΔCC for ATP is also shown in the malachite green assay (Fig. 4B) and is in agreement with the kinetic data of the wild-type enzymes activated by thiol reduction (8). TgNTPDase1 was described as hydrolyzing ATP and ADP equally well (8), which could be confirmed in the malachite green assay for TgNTPDase1 and TgNTPDase1ΔCC, whereas ITC-based saturation kinetics indicate a slight preference of TgNTPDase1ΔCC for ADP.
Activation Mechanism
Insight into the activation mechanism was gained from crystal structures of TgNTPDase1ΔCC at 2.5 Å resolution and TgNTPDase3ΔCC in complex with Mg2+-AMPPNP at 2.85 Å. Due to mutation of Cys258 and Cys268 to serines and the full activation of these protein variants, these structures represent the reduced state of TgNTPDases.
In the following, we will refer to the extended β-hairpin loop carrying the disulfide bridge responsible for regulation of enzyme activity as the “activation loop” (Fig. 1). Comparison of the structures of TgNTPDase3 and TgNTPDase3ΔCC reveals large conformational rearrangements of the activation loop, the relative position of the subunits of the tetramer and of the two domains of each subunit (Fig. 5 and supplemental Movie S1). These rearrangements were also observed in the absence of bound substrate for TgNTPDase1ΔCC. This finding demonstrates that the conformational switch results from the opening of the disulfide linkage and not from substrate binding.
FIGURE 5.
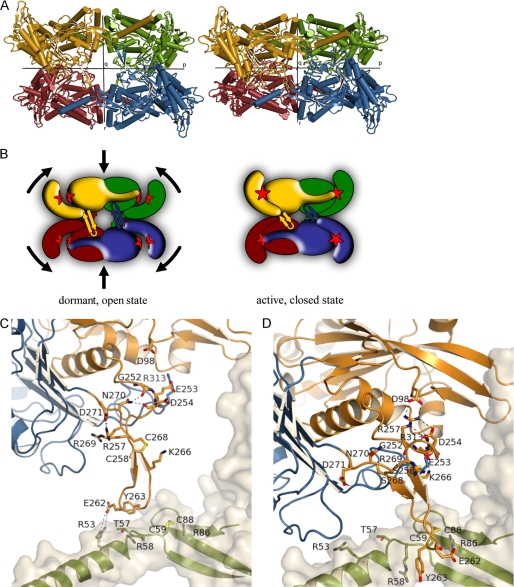
Structural changes upon enzyme activation (see also supplemental Movie S1). A, tetrameric structures of TgNTPDase3 (left) and TgNTPDase3ΔCC (right). The chains are colored as in Fig. 2. B, schematic representation of the conformational switch shown in A after reduction of Cys258-Cys268, which is located in the β-sheet between the monomers. Reduction of the activation loop (β-hairpin) results in tetramer contraction, domain closure, and establishment of the correct active site (red star) geometry. C and D, conformational change and interactions of the activation loop. Shown is the loop of monomer A in the same orientation as in A. Domains I and II are colored in orange and blue, respectively. The schematic and sticks of the neighboring subunit are colored in green with a light gray protein surface. Shown are all residues involved in interactions either in the open/inactive form (C) or in the closed/activated conformation (D). The hydroxyl groups of Ser258 and Ser268 in the crystal structure of TgNTPDase3ΔCC (D) are colored yellow to indicate the positions of the free cysteines in the reduced activated enzyme.
Global Changes
The dimensions of the tetramer shrink along the r axis by ∼13 Å. The accessible surface area decreases from 94,391 Å2 for the inactive form (TgNTPDase3) to ∼85,200 Å2 for the activated variant (TgNTPDase3ΔCC). The largest contribution to this change comes from the increase of the contact area at tetramerization interface II (along the p axis) by around 800 Å2. In contrast, the total contact area at interface I (around the r axis) hardly changes.
The contraction of the whole tetramer results mainly from a movement of the two dimers A/D and B/C toward each other (see Fig. 5, A and B). This approximation is accompanied by a relative rotation of the dimer pairs around the r axis by ∼10°. Due to their strong interactions across interface I, the domain I pairs of A/D and B/C move together as rigid bodies. In parallel to the convergence of the domain I pairs, domain II of all monomers experiences a rotational movement, which closes the active site cleft between domains I and II. Therefore, the inactive and active states can also be described as open and closed conformations, respectively.
Changes at the Activation Loop
The movements of the subunits and domains are triggered by a conformational change of the activation loop. In the inactive state, residues 257–260 and 266–269 form two antiparallel β-strands, and the 258–268 disulfide bridge acts as a clamp to hold the two strands together, thus stabilizing this loop conformation. The activation loops of all four monomers show an approximately parallel orientation along the r axis. By contacting the diametrically positioned subunits at the p interface, the four loops push apart the A/D and B/C dimers in a concerted fashion (Fig. 5, A–C).
Upon reduction, the β-hairpin is destabilized at the proximal region, and a winding up movement can be observed in the region just prior to the hairpin. The β-strands are shortened, and the cysteines form the first and last residues of the two β-strands. In the Ramachandran plot, Arg257 and Arg269 undergo large shifts from the β-strand to the α-helical region (see supplemental Fig. S2). Both of their guanidino groups are translated by more than 16 Å. Arg269 is oriented toward domain II in the dormant state and becomes fully solvent-exposed upon activation. Arg257 forms a weak salt bridge to Asp271 before activation. Upon reduction, two salt bridges are formed with Asp254 and Asp98. Asp98 is exposed from the bulge in β-strand 3 (Fig. 5D), highlighting the importance of this unusual structural element for activation. Notably, analysis with the program SSBOND (47) shows that despite the fact that Cys258 and Cys268 remain part of the β-hairpin, the distance of their Cβ atoms in the active state is too large for the formation of a disulfide bond. Hence, the wound-up loop conformation cannot be formed in the oxidized enzyme.
The shortening of the activation loop is accompanied by a rotational movement of the loop of ∼45° relative to domain I. Although the tip of the activation loop (Glu262, Tyr263) remains bound to the diametrically opposed monomer, the mode of interaction is altered. In the inactive state, a polar interaction with Arg53 and a hydrophobic interaction with Thr57 can be observed. Upon activation, the loop slides along helix A to stop close to the exposed cysteine bridge Cys59-Cys88. The aromatic ring of Tyr263 now stacks to the side chain of Arg58, and its backbone oxygen forms a hydrogen bond with Cys59. The backbone carboxyl oxygen of Glu262 forms a hydrogen bond with Arg86 (Fig. 5D).
Changes at Interface II
By reduction of the Cys258-Cys268 disulfide bridge, the tetramer interface along the p axis changes. The loop between β-strands 9 and 10 (residues 287–301) interacts in the inactive state mainly with helix K, Phe405, and Lys406 as well as residues 462–464. After activation, it slides along the neighboring monomer to interact with residues Phe194, Glu196, Trp197, and Asp200, partially involved in helix G (see supplemental Fig. S3). Furthermore, activation of the enzyme induces the formation of two additional helices, F and O. Helix F is located close to the active site (see below). Helix O is formed at the interface across the p axis by residues 464–468, which are part of a loop in the inactive state.
Electron density of the structures of the inactive form of TgNTPDase3 indicates a partial opening of the Cys258-Cys268 disulfide bonds. Based on the obvious differences between the protein crystallized in the inactive state and the structure of the permanently active variant, we assume that the partial opening of the bridge in the inactive structure is caused by radiation damage during data collection. This assumption is supported by the relatively strong radiation sensitivity of the crystals (data not shown). The Cys258-Cys268 disulfide bridge of the activation loop is probably more easily reduced than the other disulfide bridges, as indicated by the time course of activation and deactivation of the enzymes by thiol reagents (18). This cysteine linkage might thus also open first by radiation damage. An alternative explanation would be that the Cys258-Cys268 bridges are partially open in the purified enzyme due to a high redox potential. However, the closed conformation, as observed in the TgNTPDase3ΔCC structure, forms only upon reduction of all four Cys258-Cys268 disulfide bridges due to a cooperative movement of the four subunits upon activation.
Function of Activation Loop
From the present data, it is not clear whether the function of the activation loop is purely inhibitory in its oxidized state (forcing the monomers and domains apart), activating in its reduced state (drawing the domains together), or a mixture of both. Replacement of the whole loop (residues Glu253–Arg269) by a short flexible linker (sequence GG) gave rise to a variant that was active without the addition of reducing agents but had only strongly diminished activity of 8 units/mg (data not shown). A possible explanation is that the reduced loop indeed stabilizes the enzyme in the active state. However, the activation loop might also be necessary to support a yet uncharacterized domain motion during catalysis, which has been observed for other NTPDases (25). Although it remains unclear how the activation is realized in nature, we note that the exposed position of the four activation loops makes them well accessible for an activating reductase (20).
The soluble, monomeric NTPDase from the bacterium Legionella pneumophila is lacking a β-hairpin loop between the two structural domains (48). However, a putative membrane interaction loop is found in the membrane-anchored mammalian NTPDases at a position corresponding to the activation loop of TgNTPDases (Fig. 1B)(41). This membrane interaction loop is considered to insert in or interact with the cell membrane and thereby influence catalytic properties (41). Functional studies analyzing the importance of the membrane interaction loop in mammalian enzymes are still missing. For these enzymes, a movement of the two protein domains has been suggested to be involved in the effects of the membrane attachment on the kinetic properties (49).
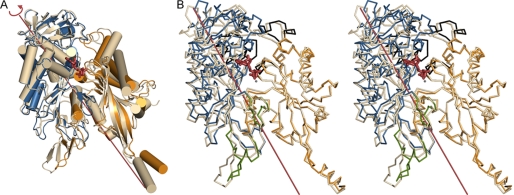
Domain Motion Involved in Enzyme Activation
The movement of the subunits within the TgNTPDase tetramer is coupled to a movement of the two protein domains of each subunit (Fig. 6). Shortening of the activation loop generates a drawing force along r onto its flanking domains. Domain I forms a sturdy interaction with domain I of a neighboring monomer (interface I), and these domain dimers are free to follow the drawing force generated by the activation loop. Domain II, however, cannot follow this movement along r because it bumps at interface II into domain II of its neighbor. To concede to the drawing force, domain II evades into a tilting motion relative to domain I, leading to closure of the active site clefts (Fig. 5, A and B).
FIGURE 6.
Domain motion upon enzyme activation: comparison between TgNTPDase3 (chain C (light gray)) and TgNTPDase3ΔCC (chain B, domain I (orange) and domain II (blue)). A, superposition of the structures based on domain II. AMPPNP (red) marks the position of the active site. B, stereo view of Dyndom assignment of the dynamic domains. The interdomain screw axis (12° rotation, 0.03 Å translation) and AMPPNP are shown in red. The structures are superimposed based on domain I. Residues assigned to neither of the two domains are colored in green. Regions undergoing significant internal displacements are colored in black.
For an unbiased determination of the rigid domains, the program Dyndom was used (40). Residues that move together as a rigid body during the conformational change are automatically assigned to a dynamic domain. All four subunits of TgNTPDase3 were compared with the four subunits in TgNTPDase3ΔCC. Although relatively small ratios of interdomain to intradomain displacements of 0.63–1.01 were observed, consistent and plausible results were obtained for all comparisons concerning the presence of two domains that mostly matched the structural domains described in the legend to Fig. 1. The interdomain rotation angles vary from 5.6 to 12.3°, and the translational components of the screw operations are small (between 0.0 and 1.0 Å). The largest difference of the domain orientation was observed for chain C of TgNTPDase3 and chain B of TgNTPDase3ΔCC (Fig. 6B).
The line depicted in Fig. 6B marks the orientation of the rotation axis. Based on the angle between the rotation axis and the line connecting the domain centers, the movement is characterized as the percentage closure motion between 0% (pure twist motion) and 100% (pure closure motion). For the comparison between chains C and B, the axis is characterized by 27% closure motion. For all comparisons, the percentage closure motion varies from 12 to 57% (i.e. it resembles more a twist motion). For most of the comparisons, the rotation axis runs approximately parallel to helix T of the domain interface. Helices H and T, which are located between the domains, are consistently assigned to domain I.
The differences in the magnitude of the interdomain rotation angle obtained for a comparison of the structures of TgNTPDase3 and TgNTPDase3ΔCC are mostly due to different domain orientations of the four subunits of the inactive form of TgNTPDase3, with rotation angles varying between 2.8 and 4.7°. In contrast, the four subunits in TgNTPDase3ΔCC and TgNTPDase1ΔCC are more similar to each other, with a maximum interdomain rotation angle of 1.2°.
Superposition of the C-terminal domains (Fig. 6A) and N-terminal domains (Fig. 6B) alone shows that the domains move largely as rigid bodies with the following exceptions. In the N-terminal domain, the loop between helices B and C and the loop between helices F and G undergo an internal displacement to move away from the domain interface, where they are in contact with residues of the C-terminal domain, in particular with the region 395–424, including helices L and K. As a result, the latter region of the C-terminal domain also shows significant internal displacements to accommodate the domain movement (Fig. 6B). In addition, the activation loop (residues 252–272) and the loop 586–597 between the two domains show large independent motions and are assigned to neither of the two domains.
The domain-domain reorientation described above induces the following secondary structure changes in the active site cleft. Helix F is formed and contributes to the correct arrangement of the phosphate binding pocket by positioning of Thr188 and Ala189, which coordinate the γ-phosphate group of the substrate and the nucleophilic water. Arg192 moves closer to the nucleophilic base Glu236 to position it by formation of a salt bridge.
Domain Motion in Actin/hsp70/Sugar Kinase Superfamily
A domain motion has been shown to be involved in the catalytic cycle of many of the enzymes of the actin/hsp70/sugar kinase superfamily, with hexokinase being a prototypical example for a substrate-induced domain closure motion (50). A domain movement of rat NTPDase1 is characterized based on a comparison of four independent conformers in the crystal (25). The assignment of dynamic domains is similar to the results obtained for TgNTPDases with the exception that helix H and parts of helix T are assigned to the C-terminal domain in the mammalian enzyme. These helices are located at the domain interface, and the interdomain rotation axis passes nearby. Studies on the 28.1° domain motion of a yeast hexokinase showed that the domain motion in this enzyme can be described as a movement of the two β-sheets against the central α-helices (51), which form a third dynamic domain together with nearby residues. From the combination of movements of the N-terminal domain against helix T and of the C-terminal domain against helix H, more complex motions may arise in T. gondii. Recently, the structure of an NTPDase from the bacterium L. pneumophila was determined in an open conformation (48). Although an AMPPNP complex was obtained, the density for the ribose and the phosphate tails was weak and indicative of multiple unproductive binding modes. Hence, a domain closure movement resulting in active site reorganization is likely to take place in the L. pneumophila enzyme.
It should be noted that for the other enzymes of the actin/hsp70/sugar kinase fold mentioned above, the domain motion has usually been inferred from a comparison of the same enzyme in different environments in the crystal and is most likely necessary for the catalytic activity but not part of an activation mechanism (50, 51). It is currently unclear if a domain motion is necessary for the catalytic action of TgNTPDases (in the activated form) and if the domain reorientation of TgNTPDases obtained from a comparison of TgNTPDase3 and TgNTPDase3ΔCC would be similar to such a putative motion. However, it appears likely that the open conformation observed for TgNTPDase3 is also accessible for the activated enzyme form with a reduced 258–268 disulfide bridge.
Substrate Binding Mode
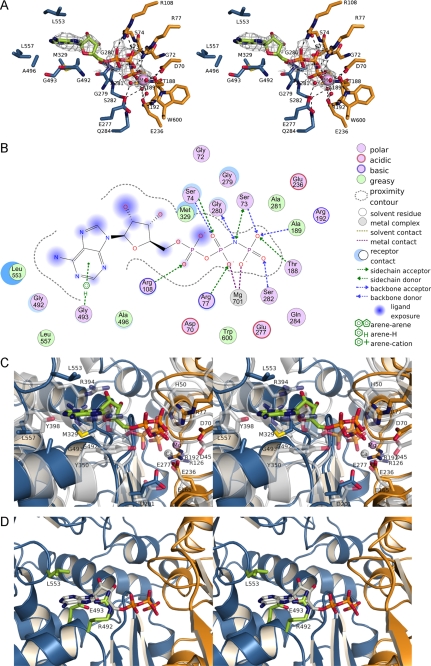
Crystallization and structure determination of TgNTPDase3ΔCC in complex with Mg2+-AMPPNP allowed us to further substantiate our understanding of the activation mechanism of TgNTPDases and to characterize the substrate binding mode and catalytic mechanism (Fig. 7, A and B). The ATP analog is located in the cleft between the two domains. Superposition of RnNTPDase2 (41) indicates a similar arrangement of the domains and the active site (Fig. 7C). The positions of the terminal phosphate groups and the apyrase conserved region loops now correspond closely to that of the AMPPNP complex structure of RnNTPDase2 (41), indicating that the structure of TgNTPDase3ΔCC represents a competent substrate binding mode. Residues from both phosphate binding loops (Gly72–Ser74 and Gly280–Ser282) can now bind simultaneously to the nucleotide. Although water molecules are not resolved in the present structure, the side chain of Glu277 is now within a reasonable distance to position a water molecule in the coordination sphere of the metal ion. Arg192 and Glu236 were too far apart from each other in the inactive state but now can form a salt bridge (Fig. 7C).
FIGURE 7.
Substrate binding to TgNTPDases. A, AMPPNP bound to the active site of TgNTPDase3ΔCC. Residues involved in the hydrolysis of ATP are shown as sticks and are colored as described in the legend to Fig. 1. Water molecules are not present in the crystal structure and were modeled according to the active site of RnNTPDase2 (41). The omit electron density map (Fo − Fc × ϕc, contoured at 2 σ) of AMPPNP is shown in gray. B, scheme of the interactions of Mg2+-AMPPNP in the active site of TgNTPDase3ΔCC. The size of the blue clouds indicates the solvent accessibility of the ligand atoms. The strength of the interactions to the ligands is indicated by a blue shadow behind the residue. C, superposition of RnNTPDase2 (gray) and TgNTPDase3ΔCC (orange/blue; bound AMPPNP green, Mg2+ purple) on all residues. D, superposition of AMPPNP (gray) from the TgNTPDase3ΔCC-Mg2+-AMPPNP complex on TgNTPDase1ΔCC with putative base binding residues colored in green.
However, in contrast to RnNTPDase2 (41), the conformation of the nucleotide differs in detail, and the position of the base is shifted in TgNTPDase3ΔCC. This results in a more extended conformation of the ATP analog in the microbial enzyme. The extended conformation appears to result from a larger distance of the residues involved in binding of the nucleotide base from the binding site of the terminal phosphate group (Fig. 7C). In contrast, even in an extended conformation, as suggested for RnNTPDase2 (41), ADP cannot optimally bridge the distance between the binding sites for the nucleobase and the terminal phosphate group, explaining the high specificity of TgNTPDase3 for ATP.
As in the complex structure TgNTPDase3-AMP, the adenine base is sandwiched between the planar peptide group of Gly492 and the side chain of Leu553. However, whereas the terminal phosphate group of AMP is pointing out of the active site cleft in crystals obtained for the inactive state, the triphosphate group of AMPPNP is oriented to the active site residues of the N-terminal domain in the active state (see supplemental Fig. S4) as expected for a catalytically competent binding mode.
For the AMP complex structure differences between the mammalian and the microbial enzyme in binding of the nucleoside moiety were described above. Additionally, interactions with the triphosphate group of the nucleotide differ. The side chain of Ser75, corresponding to His50 in RnNTPDase2 (41), is too far away from the substrate analog to be involved in binding. Instead, His50 (41) is functionally replaced by the side chain of Arg77, which interacts with the β-phosphate. In addition, Arg108 interacts electrostatically with the γ-phosphate of the ligand. Asp201, as part of the conserved DXG motif of ACR4 (apyrase conserved region 4) in RnNTPDase2 (41), is substituted by Glu277 in TgNTPDase3, thus forming an EXG motif. Although water molecules are not visible in the crystal structure presented here, we assume that Glu236 functions as a general base to deprotonate a water nucleophile for direct attack of the terminal phosphate group because this residue is similarly positioned as the conserved catalytic base Glu165 in RnNTPDase2 (41).
Insight into Catalytic Differences between TgNTPDase1 and TgNTPDase3
Structures of TgNTPDase1 and -3 in the activated state allow us to characterize the molecular basis for the differences in substrate specificity. Analysis on chimeric constructs had previously shown that a block of 12 residues dictates the substrate specificity for ATP versus ADP (14) (residues 488–499 in Fig. 1). Remarkably, two glycines in TgNTPDase3 residues are replaced by amino acids with relatively bulky side chains (Arg492 and Glu493) in TgNTPDase1 in the immediate vicinity of the base binding site (Fig. 7D). The two side chains adopt conformations that would not support binding of the substrate's nucleobase in the position observed in the TgNTPDase3ΔCC-Mg2+-AMPPNP complex structure (Fig. 7D). We assume that upon nucleotide binding, Arg492 adopts a different conformation. This notion is supported by the weak side chain density of this residue in chain B. Glu493 is located behind the base. The close proximity of Arg492 and Glu493 to the nucleobase suggests that these two residues are predominantly responsible for the different substrate specificities of TgNTPDase1 and -3, perhaps by influencing the distance between the base binding site and the binding site for the terminal phosphate group.
CONCLUSIONS
In conclusion, with this study, we could derive a detailed molecular model for the activation mechanism of TgNTPDases. Activation is initiated by the reduction of the disulfide bond Cys258-Cys268, followed by a conformational change of the activation loop that induces a presumably cooperative movement of the protein subunits of the tetramer and of the two domains of each subunit. The observed conformational change resembles that of a jumping jack, where drawing on the cord (activation loop) induces clapping of the hands (domains I and II). The subunit and domain motions finally result in formation of a competent active site geometry as visualized by the complex structure with an ATP analog. The observed cooperative movement of subunits and domains and the control of these motions by the conformation of the activation loop show that the activation mechanism of TgNTPDases is strongly dependent on the quaternary structure of the enzyme. It is likely to be an important regulatory part of the survival of T. gondii in the host and its controlled egress. The comparative analysis of TgNTPDase1ΔCC and -3ΔCC crystal structures allows us to understand the molecular basis for the significant differences in substrate specificity of the two isoenzymes, which are caused by only 3% sequence deviation but contribute to the formation of virulent versus avirulent T. gondii strains.
Supplementary Material
Acknowledgments
We thank Michael Johnson (University of Technology, Sydney, Australia) for providing cDNA. Furthermore, we thank the staff of beamlines BL14.1 and BL14.2 at PSF/BESSY (Berlin, Germany) and beamline ID23-2 at the European Synchrotron Radiation Facility (Grenoble, France) for beam time and support.
Helmholtz-Zentrum Berlin (Berlin, Germany) is acknowledged for travel support.

This article contains supplemental Table S1, Figs. S1–S4, and Movie S1.
The atomic coordinates and structure factors (codes 4a57, 4a59, 4a5a, and 4a5b) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- NTPDase
- nucleoside triphosphate diphosphohydrolase
- RnNTPDase
- Rattus norvegicus NTPDase
- TgNTPDase
- Toxoplasma gondii NTPDase
- AMPPNP
- adenosine 5′-(β,γ-iminotriphosphate)
- ΔCC
- C258S/C268S mutation
- ITC
- isothermal titration calorimetry
- TLS
- translation/libration/screw.
REFERENCES
- 1. Tenter A. M., Heckeroth A. R., Weiss L. M. (2000) Toxoplasma gondii. From animals to humans. Int. J. Parasitol. 30, 1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montoya J. G., Liesenfeld O. (2004) Toxoplasmosis. Lancet 363, 1965–1976 [DOI] [PubMed] [Google Scholar]
- 3. Handa M., Guidotti G. (1996) Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Biochem. Biophys. Res. Commun. 218, 916–923 [DOI] [PubMed] [Google Scholar]
- 4. Robson S. C., Sévigny J., Zimmermann H. (2006) The E-NTPDase family of ectonucleotidases. Structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasconcelos E. G., Ferreira S. T., Carvalho T. M., Souza W., Kettlun A. M., Mancilla M., Valenzuela M. A., Verjovski-Almeida S. (1996) Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J. Biol. Chem. 271, 22139–22145 [DOI] [PubMed] [Google Scholar]
- 6. Vasconcelos E. G., Nascimento P. S., Meirelles M. N., Verjovski-Almeida S., Ferreira S. T. (1993) Characterization and localization of an ATP-diphosphohydrolase on the external surface of the tegument of Schistosoma mansoni. Mol. Biochem. Parasitol. 58, 205–214 [DOI] [PubMed] [Google Scholar]
- 7. Sansom F. M., Robson S. C., Hartland E. L. (2008) Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol. Mol. Biol. Rev. 72, 765–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asai T., Miura S., Sibley L. D., Okabayashi H., Takeuchi T. (1995) Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J. Biol. Chem. 270, 11391–11397 [DOI] [PubMed] [Google Scholar]
- 9. Berrêdo-Pinho M., Peres-Sampaio C. E., Chrispim P. P., Belmont-Firpo R., Lemos A. P., Martiny A., Vannier-Santos M. A., Meyer-Fernandes J. R. (2001) A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch. Biochem. Biophys. 391, 16–24 [DOI] [PubMed] [Google Scholar]
- 10. Pinheiro C. M., Martins-Duarte E. S., Ferraro R. B., Fonseca de Souza A. L., Gomes M. T., Lopes A. H., Vannier-Santos M. A., Santos A. L., Meyer-Fernandes J. R. (2006) Leishmania amazonensis. Biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp. Parasitol. 114, 16–25 [DOI] [PubMed] [Google Scholar]
- 11. Sansom F. M., Newton H. J., Crikis S., Cianciotto N. P., Cowan P. J., d'Apice A. J., Hartland E. L. (2007) A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell Microbiol. 9, 1922–1935 [DOI] [PubMed] [Google Scholar]
- 12. Schwartzman J. D., Pfefferkorn E. R. (1982) Toxoplasma gondii. Purine synthesis and salvage in mutant host cells and parasites. Exp. Parasitol. 53, 77–86 [DOI] [PubMed] [Google Scholar]
- 13. Bermudes D., Peck K. R., Afifi M. A., Beckers C. J., Joiner K. A. (1994) Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 269, 29252–29260 [PubMed] [Google Scholar]
- 14. Nakaar V., Beckers C. J., Polotsky V., Joiner K. A. (1998) Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol. Biochem. Parasitol. 97, 209–220 [DOI] [PubMed] [Google Scholar]
- 15. Nakaar V., Samuel B. U., Ngo E. O., Joiner K. A. (1999) Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J. Biol. Chem. 274, 5083–5087 [DOI] [PubMed] [Google Scholar]
- 16. Kikuchi T., Nagata T., Furuta T. (2001) Production and characterization of a monoclonal antibody against nucleoside triphosphate hydrolase from Toxoplasma gondii. J. Eukaryot. Microbiol. 2001, (suppl.) 195S–196S [DOI] [PubMed] [Google Scholar]
- 17. Sibley L. D., Niesman I. R., Asai T., Takeuchi T. (1994) Toxoplasma gondii. Secretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp. Parasitol. 79, 301–311 [DOI] [PubMed] [Google Scholar]
- 18. Silverman J. A., Qi H., Riehl A., Beckers C., Nakaar V., Joiner K. A. (1998) Induced activation of the Toxoplasma gondii nucleoside triphosphate hydrolase leads to depletion of host cell ATP levels and rapid exit of intracellular parasites from infected cells. J. Biol. Chem. 273, 12352–12359 [DOI] [PubMed] [Google Scholar]
- 19. Stommel E. W., Ely K. H., Schwartzman J. D., Kasper L. H. (1997) Toxoplasma gondii. Dithiol-induced Ca2+ flux causes egress of parasites from the parasitophorous vacuole. Exp. Parasitol. 87, 88–97 [DOI] [PubMed] [Google Scholar]
- 20. Stommel E. W., Cho E., Steide J. A., Seguin R., Barchowsky A., Schwartzman J. D., Kasper L. H. (2001) Identification and role of thiols in Toxoplasma gondii egress. Exp. Biol. Med. 226, 229–236 [DOI] [PubMed] [Google Scholar]
- 21. Zebisch M., Sträter N. (2007) Characterization of Rat NTPDase1, -2, and -3 ectodomains refolded from bacterial inclusion bodies. Biochemistry 46, 11945–11956 [DOI] [PubMed] [Google Scholar]
- 22. Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D., Bairoch A. (2003) ExPASy. The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asai T., O'Sullivan W. J., Tatibana M. (1983) A potent nucleoside triphosphate hydrolase from the parasitic protozoan Toxoplasma gondii. Purification, some properties, and activation by thiol compounds. J. Biol. Chem. 258, 6816–6822 [PubMed] [Google Scholar]
- 24. Baykov A. A., Evtushenko O. A., Avaeva S. M. (1988) A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171, 266–270 [DOI] [PubMed] [Google Scholar]
- 25. Zebisch M., Schäfer P., Krauss M., Sträter N. (2011) Crystallographic evidence for a domain motion in rat nucleoside triphosphate diphosphohydrolase (NTPDase) 1. J. Mol. Biol., in press [DOI] [PubMed] [Google Scholar]
- 26. Bianconi M. L. (2007) Calorimetry of enzyme-catalyzed reactions. Biophys. Chem. 126, 59–64 [DOI] [PubMed] [Google Scholar]
- 27. Freyer M. W., Lewis E. A. (2008) Isothermal titration calorimetry. Experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods Cell Biol. 84, 79–113 [DOI] [PubMed] [Google Scholar]
- 28. Todd M. J., Gomez J. (2001) Enzyme kinetics determined using calorimetry. A general assay for enzyme activity? Anal. Biochem. 296, 179–187 [DOI] [PubMed] [Google Scholar]
- 29. Kabsch W. (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 [Google Scholar]
- 30. Collaborative Computational Project, Number 4 (1994) The CCP4 suite. Programs for protein crystallography. Acta Crystallogr. D. Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 31. Vonrhein C., Blanc E., Roversi P., Bricogne G. (2007) Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 [DOI] [PubMed] [Google Scholar]
- 32. Cowtan K. (1999) Error estimation and bias correction in phase-improvement calculations. Acta Crystallogr. D Biol. Crystallogr. 55, 1555–1567 [DOI] [PubMed] [Google Scholar]
- 33. Cowtan K. (2006) The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 [DOI] [PubMed] [Google Scholar]
- 34. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 35. Blanc E., Roversi P., Vonrhein C., Flensburg C., Lea S. M., Bricogne G. (2004) Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D Biol. Crystallogr. 60, 2210–2221 [DOI] [PubMed] [Google Scholar]
- 36. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vagin A., Teplyakov A. (1997) MOLREP: An automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 38. Painter J., Merritt E. A. (2006) Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D Biol. Crystallogr. 62, 439–450 [DOI] [PubMed] [Google Scholar]
- 39. Painter J., Merritt E. A. (2006) TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 39, 109–111 [Google Scholar]
- 40. Hayward S., Berendsen H. J. (1998) Systematic analysis of domain motions in proteins from conformational change. New results on citrate synthase and T4 lysozyme. Proteins 30, 144–154 [PubMed] [Google Scholar]
- 41. Zebisch M., Sträter N. (2008) Structural insight into signal conversion and inactivation by NTPDase2 in purinergic signaling. Proc. Natl. Acad. Sci. U.S.A. 105, 6882–6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mosca R., Brannetti B., Schneider T. R. (2008) Alignment of protein structures in the presence of domain motions. BMC Bioinformatics. 9, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang F., Hicks-Berger C. A., Smith T. M., Kirley T. L. (2001) Site-directed mutagenesis of human nucleoside triphosphate diphosphohydrolase 3. The importance of residues in the apyrase conserved regions. Biochemistry 40, 3943–3950 [DOI] [PubMed] [Google Scholar]
- 44. Drosopoulos J. H., Broekman M. J., Islam N., Maliszewski C. R., Gayle R. B., 3rd, Marcus A. J. (2000) Site-directed mutagenesis of human endothelial cell ecto-ADPase/soluble CD39. Requirement of glutamate 174 and serine 218 for enzyme activity and inhibition of platelet recruitment. Biochemistry 39, 6936–6943 [DOI] [PubMed] [Google Scholar]
- 45. Mateo J., Kreda S., Henry C. E., Harden T. K., Boyer J. L. (2003) Requirement of Cys399 for processing of the human ecto-ATPase (NTPDase2) and its implications for determination of the activities of splice variants of the enzyme. J. Biol. Chem. 278, 39960–39968 [DOI] [PubMed] [Google Scholar]
- 46. Ivanenkov V. V., Meller J., Kirley T. L. (2005) Characterization of disulfide bonds in human nucleoside triphosphate diphosphohydrolase 3 (NTPDase3). Implications for NTPDase structural modeling. Biochemistry 44, 8998–9012 [DOI] [PubMed] [Google Scholar]
- 47. Hazes B., Dijkstra B. W. (1988) Model building of disulfide bonds in proteins with known three-dimensional structure. Protein Eng. 2, 119–125 [DOI] [PubMed] [Google Scholar]
- 48. Vivian J. P., Riedmaier P., Ge H., Le Nours J., Sansom F. M., Wilce M. C., Byres E., Dias M., Schmidberger J. W., Cowan P. J., d'Apice A. J., Hartland E. L., Rossjohn J., Beddoe T. (2010) Crystal structure of a Legionella pneumophila ecto-triphosphate diphosphohydrolase, a structural and functional homolog of the eukaryotic NTPDases. Structure 18, 228–238 [DOI] [PubMed] [Google Scholar]
- 49. Grinthal A., Guidotti G. (2006) CD39, NTPDase 1, is attached to the plasma membrane by two transmembrane domains. Why? Purinergic Signal. 2, 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bennett W. S., Jr., Steitz T. A. (1978) Glucose-induced conformational change in yeast hexokinase. Proc. Natl. Acad. Sci. U.S.A. 75, 4848–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuettner E. B., Kettner K., Keim A., Svergun D. I., Volke D., Singer D., Hoffmann R., Müller E. C., Otto A., Kriegel T. M., Sträter N. (2010) Crystal structure of hexokinase KlHxk1 of Kluyveromyces lactis. A molecular basis for understanding the control of yeast hexokinase functions via covalent modification and oligomerization. J. Biol. Chem. 285, 41019–41033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mosca R., Schneider T. R. (2008) RAPIDO. A web server for the alignment of protein structures in the presence of conformational changes. Nucleic Acids Res. 36, W42–W46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weiss M. S. (2001) Global indicators of x-ray data quality. J. Appl. Crystallogr. 34, 130–135 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.