Background: Nel is a multimodular glycoprotein and plays important roles in neural development and functions.
Results: The N-terminal thrombospondin-1 domain is involved in multimer formation and heparin- and retinal axon-binding. Cysteine-rich domains bind to and inhibit retinal axons.
Conclusion: Different molecular interactions and functions are mediated by distinct domains of Nel.
Significance: The findings provide insights into how Nel exerts diverse functions.
Keywords: Axon, Extracellular matrix proteins, Heparin-binding protein, Neurite outgrowth, Protein domains, Protein-protein interactions, Nel
Abstract
Nel (neural epidermal growth factor (EGF)-like molecule) is a multimeric, multimodular extracellular glycoprotein with heparin-binding activity and structural similarities to thrombospondin-1. Nel is predominantly expressed in the nervous system and has been implicated in neuronal proliferation and differentiation, retinal axon guidance, synaptic functions, and spatial learning. The Nel protein contains an N-terminal thrombospondin-1 (TSP-N) domain, five cysteine-rich domains, and six EGF-like domains. However, little is known about the functions of specific domains of the Nel protein. In this study, we have performed structure-function analysis of Nel, by using a series of expression constructs for different regions of the Nel protein. Our studies demonstrate that the TSP-N domain is responsible for homo-multimer formation of Nel and its heparin-binding activity. In vivo, Nel and related Nell1 are expressed in several regions of the mouse central nervous system with partly overlapping patterns. When they are expressed in the same cells in vitro, Nel and Nell1 can form hetero-multimers through the TSP-N domain, but they do not hetero-oligomerize with thrombospondin-1. Whereas both the TSP-N domain and cysteine-rich domains can bind to retinal axons in vivo, only the latter causes growth cone collapse in cultured retinal axons, suggesting that cysteine-rich domains interact with and activate an inhibitory axon guidance receptor. These results suggest that Nel interacts with a range of molecules through its different domains and exerts distinct functions.
Introduction
Nel (neural epidermal growth factor (EGF)-like)3 is a multimodular extracellular glycoprotein that has structural similarities to thrombospondin-1. Nel was first isolated from a chicken cDNA library and was so named because it contains EGF-like domains and is strongly expressed in neural tissues (1, 2). Subsequently, two related genes were identified in mammals and termed Nell (Nel-like) 1 and 2 (3, 4). Based on sequence similarities, Nell2 appeared to be the mammalian ortholog of chicken Nel. Chicken Nell1 has not yet been identified. In this report, we refer to chicken Nel and mammalian Nell2 as Nel.
The Nel gene is predominantly expressed in the developing and adult nervous system (1, 2, 4–6). Nel has been shown to play crucial roles in development and functioning of the nervous system. In the developing chicken nervous system, Nel promotes differentiation of motor and sensory neurons and stimulates mitogenesis in dorsal root ganglia (7). Nel also promotes survival of embryonic cortical and hippocampal neurons in vitro (8). In addition, we have recently shown that Nel inhibits retinal axon outgrowth and induces growth cone collapse and axon retraction, indicating that Nel can act as an inhibitory axon guidance molecule (9). In the adult mouse brain, targeted disruption of the Nel gene results in significant enhancement of long term potentiation in the dentate gyrus, suggesting that Nel is a negative regulator of neuronal activity (10). Interestingly, Nel mutant mice show impairment of spatial learning, further suggesting that Nel plays important roles in regulation of synaptic plasticity in the hippocampus (11). No specific cell surface receptors have yet been identified for Nel.
The Nel genes (Nel and related Nell1) encode multimodular proteins and belong to (i) the laminin G/TSP-N/pentraxin supergene family (12), (ii) the chordin-like cysteine-rich domain family (13), and (iii) the EGF-like domain family (14). Structurally, Nel and Nell1 contain, from the N terminus to the C terminus, a cleavable signal peptide, an N-terminal thrombospondin-1 (TSP-N) domain, two cysteine-rich domains that have structural similarities to chordin and von Willebrand factor C domain, six EGF-like domains, and three additional cysteine-rich domains. Secreted Nel and Nell1 proteins exist as homo-trimers in solution and have heparin-binding activity (4). However, little is known about the functions of specific domains of Nel.
In this study, we have conducted structure-function analyses of Nel, by using a series of expression constructs for specific domains. We show that homo-multimer formation of Nel is mediated by the TSP-N domain. Interestingly, Nel and Nell1 are expressed with partly overlapping patterns in several regions of the developing mouse nervous system. When co-expressed in culture cells, Nel and Nell1 can form hetero-multimers through the TSP-N domain. In contrast, thrombospondin-1 does not appear to form hetero-complexes with Nel or Nell1. The TSP-N domain is also responsible for heparin-binding activity of Nel. Whereas both the TSP-N domain and cysteine-rich domains can bind to retinal axons in vivo, only cysteine-rich domains induce growth cone collapse in cultured retinal axons, suggesting that cysteine-rich domains are involved in binding to and activation of an inhibitory axon guidance receptor on retinal axons. These results suggest that Nel interacts with a range of molecules and exerts distinct functions through its different domains.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Construction of an expression vector for chicken Nel-AP was described previously (9). For expression of a myc-tagged Nel (Nel-myc), the protein-coding region of chicken Nel cDNA (GenBankTM accession number NM_001030740.1, nucleotides 118–2565) was amplified by PCR from the Nel-AP expression plasmid with artificial 5′-NotI and 3′-XhoI sites and was inserted between the NotI and XhoI sites of the pCMV-Tag1 vector (Agilent Technologies, Santa Clara, CA).
For construction of Nell1 expression vectors, the protein coding region of mouse Nell1 (GenBankTM accession number NM_001037906.2, nucleotides 40–2469) was amplified from mouse embryonic cDNA library by PCR with artificial enzyme sites (5′-EcoRI and 3′-BglII sites for AP tag, 5′-SacI, and 3′-XhoI sites for myc tag), and was inserted between the corresponding restriction enzyme sites of the pCMV-AP vector and pCMV tag1 vector, respectively.
For construction of the expression vectors for AP-tagged Nel domains, Nel cDNA fragments containing the TSP-N domain (TSP, nucleotides 118–924 and 118–987), the first and second cysteine-rich domains (CRN, nucleotides 931–1305), the six EGF repeats (nucleotides 1306–2028), and the third to fifth cysteine-rich domains (CRC, nucleotides 2029–2556) were individually amplified by PCR with 5′-PstI and 3′-MluI sites and inserted between the corresponding restriction enzyme sites of the pCMV-Nel-AP vector (9). The Nel ΔTSP-AP vector was constructed by replacing the HindIII-HindIII fragment of Nel CRN-AP with that of Nel-AP. For construction of the Nel TSP-EGF-AP vector, a cDNA fragment encoding the TSP-N and EGF-like domains of Nel (nucleotides 118–924 plus 1306–2028) was created with 5′-MluI and 3′-HindIII sites by overlap extension PCR and inserted between the corresponding restriction enzyme sites of the pCMV-AP vector.
The coding region of thrombospondin-1 was PCR-amplified using pcDNA3 mTSP1 (a gift of Paul Bornstein; Addgene plasmid 12405 (Addgene (Cambridge, MA))) as a template and with artificial NotI/HindIII sites (for the AP tag) and MluI/HindIII sites (for the myc tag), and inserted into the pCMV-AP and pCMV tag1 vector, respectively.
Expression and Purification of Fusion Proteins
AP fusion proteins were expressed and purified basically as previously described (9), except that a calcium phosphate co-precipitation method was used for transfection and 4 m MgCl2 for elution. After elution, the proteins were dialyzed against cold PBS and then concentrated by using Amicon Ultra columns (30K, Millipore, Billerica, MA).
Immunoprecipitation and Immunoblot Analysis
HEK293T cells were transfected with combinations of AP- and myc-tagged expression constructs. After 4–6 days, conditioned media were collected, buffered with 10 mm HEPES (pH 7.0), filtered (0.45-μm pore size PVDF membrane (Millipore, Billerica, MA)), and incubated with anti-human placental AP-agarose (A2080, Sigma-Aldrich) or anti-c-myc agarose (A7470, Sigma-Aldrich) at 4 °C. After washing with HBS (150 mm NaCl, 20 mm HEPES, pH 7.0), the agarose was boiled at 92 °C for 5 min in Laemmli sample buffer with or without 2-mercaptoethanol. Proteins were separated by SDS-PAGE (5% polyacrylamide gel) and transferred onto PVDF membranes (Bio-Rad). The filters were blocked with a blocking buffer (5% skimmed milk either in a Tris buffer (25 mm Tris (pH 7.4), 150 mm NaCl, 0.1% Tween 20) or in a phosphate buffer (PBS containing 0.05% Tween 20)), and treated with sheep anti-AP antibody (AF5905, R&D Systems, Minneapolis, MN) or mouse anti-myc antibody (M5546, Sigma-Aldrich) and then with HRP-conjugated anti-sheep IgG (HAF016, R&D Systems) or anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Detection was performed using the ECL Plus system (GE Healthcare) or RapidStep ECL (Merck, Darmstadt, Germany) reagent. For analysis of cell lysates, cells were treated in the cell lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 1 mm Na3VO4, 1% Triton X-100), and the soluble fraction was incubated with antibody-conjugated agarose.
Heparin Binding Assay
Conditioned media containing individual AP fusion proteins or control AP were incubated with 20 μl (50% slurry) of heparin-Sepharose (GE Healthcare) at 4 °C overnight. After washing with the washing buffer (50 mm Tris, pH 7.5, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 1 mm Na3VO3, 1% Triton X-100), proteins were eluted with the washing buffer containing 500 mm NaCl. Eluted proteins were boiled at 92 °C for 5 min with Laemmli sample buffer containing 2-mercaptoethanol, and then subjected to SDS-PAGE and immunoblot analysis as described under “Immunoprecipitation and Immunoblot Analysis.”
Chick Embryos
Fertilized White Leghorn chicken eggs were purchased from Henry Stewart (England) and incubated at 38 °C until use.
RNA in Situ Hybridization
To make RNA probes, a portion of mouse Nell1 (GenBankTM accession number NM_001037906.2, nucleotides 773–1460) and Nel (GenBankTM accession number NM_016743.2, nucleotides 1000–1693) cDNA was PCR-amplified and subcloned into the pBluescript SK(+) vector (Agilent, Santa Clara, CA) between the EcoRI and NotI sites. RNA in situ hybridization was performed as described previously (9) by using digoxigenin-labeled probes.
Affinity Probe in Situ of Embryonic Chicken Tecta
An affinity probe in situ using AP-tagged full-length protein and different domains of Nel was performed essentially as previously described (15). Briefly, tecta were dissected out from day 17 or day 18 chick embryos and incubated with AP fusion proteins or control AP for 2 h at room temperature. After washing, the tecta were fixed with 65% (v/v) acetone and 8% (v/v) formalin in 20 mm HEPES for 2.5 min, washed, and heated for 100 min at 65 °C to inactivate endogenous APs. Binding of fusion proteins was detected by incubation with 5-bromo-4-choloro-3-indolyl phosphate/nitro blue tetrazolium.
Growth Cone Collapse Assays
Growth cone collapse assays were performed essentially as described previously (16). Retinal explants were prepared from day 6 or day 7 chick embryos and cultured for 24–36 h on glass coverslips coated with 100 μg/ml laminin in 6- or 12-well plates in the retinal culture medium (15% FBS, 0.6% glucose, penicillin/streptomycin in DMEM/F-12). Then retinal axons were treated with 0.3 μm/ml AP fusion proteins or control AP. The explants were incubated at 37 °C for up to 30 min, fixed, and stained with Alexa Fluor 488 Phalloidin (Invitrogen). In each experiment, at least 30 growth cones were scored for each treatment as collapsed or not collapsed, and three independent experiments were performed.
RESULTS
Expression of Nel Domains Fused with an AP Tag or a myc Tag
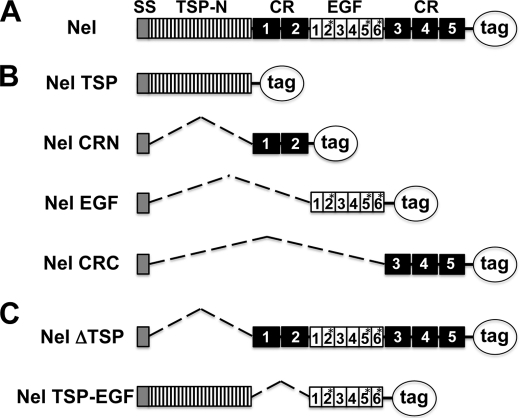
To investigate the functions of different domains of Nel, we have made a series of deletion mutant constructs that are designed to express one or more specific domains of the Nel protein (Fig. 1, A and B). We divided the full-length chicken Nel protein into the following four regions: (i) the TSP-N domain (Nel TSP), (ii) the first and second cysteine-rich domains (Nel CRN), (iii) the six EGF-like domains (Nel EGF), and (iv) the third to fifth cysteine-rich domains (Nel CRC). Those regions of Nel, as well as the full-length Nel and mouse Nell1, were individually fused to either an AP tag (15) or a myc tag.
FIGURE 1.
Schematic representation of Nel domain structure and expression constructs for different Nel domains used in this study. A, the Nel protein consists of a signal sequence (SS), an N-terminal thrombospondin-like domain (TSP-N), five cysteine-rich domains (CR 1–5), and six EGF-like domains (EGF 1–6). Asterisks indicate the three Ca2+ binding type EGF-like domains. B and C, Nel mutant constructs created for this study. B, a series of constructs for expression of different regions of the Nel protein. C, constructs that lack specific domains of Nel. Deleted regions are indicated by dotted lines. The full-length and mutant Nel proteins were fused to either an AP- or myc tag.
Nel Forms Homo-multimer through the TSP-N Domain
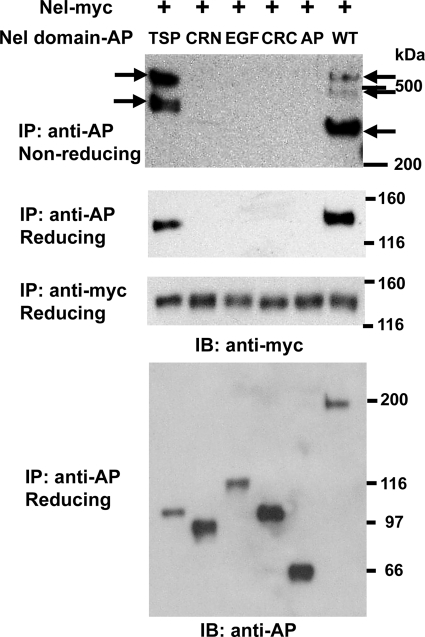
It was previously reported that the secreted Nel protein (130 kDa as a monomer) exists as trimers of ∼400 kDa in solution (4). We first determined which domain is required for homo-multimer formation. To test this, Nel-myc was co-expressed with Nel-AP or a series of AP-fused Nel domains in HEK293T cells, and multimer formation in culture media was examined by immunoprecipitation with anti-AP antibody, followed by immunoblot analysis using anti-myc antibody (Fig. 2). When Nel-myc was co-expressed with Nel-AP and SDS-PAGE was performed under reducing conditions, anti-myc antibody detected a single band of ∼130 kDa, which is the expected mobility for Nel-myc monomers. In the absence of reductants, several high molecular bands were detected, indicating formation of oligomeric complexes of Nel-AP and Nel-myc, which is consistent with the previous description of Nel homo-oligomers in solution (4). When Nel-myc was co-expressed with individual AP-fused domains, Nel TSP-AP was found to form multimers with Nel-myc. In contrast, Nel-myc was not co-immunoprecipitated with either Nel CRN-AP, Nel EGF-AP, Nel CRC-AP, or control AP. These results indicate that the TSP-N domain of Nel is responsible for homo-multimer formation.
FIGURE 2.
Multimer formation of Nel is mediated by the TSP-N domain. A Nel-myc expression vector was transfected into HEK293T cells with an expression construct for AP-fused domains of Nel (TSP, CRN, EGF, and CRC), unconjugated AP (AP), or full-length Nel-AP (WT). Culture media were collected after 4–6 days and subjected to immunoprecipitation (IP) and immunoblot analysis (IB) as indicated. SDS-PAGE was performed under non-reducing or reducing conditions. Nel-myc was co-immunoprecipitated with Nel TSP-AP (TSP) and full-length Nel-AP (WT), but not with Nel CRN-AP (CRN), Nel EGF-AP (EGF), or Nel CRC-AP (CRC). High molecular bands of Nel-myc/Nel-AP oligomers were detected under non-reducing conditions (top, arrows), whereas reducing conditions yielded a single band of Nel-myc monomer (130 kDa).
Nel Can Form Hetero-complexes with Mouse Nell1 through the TSP-N Domain
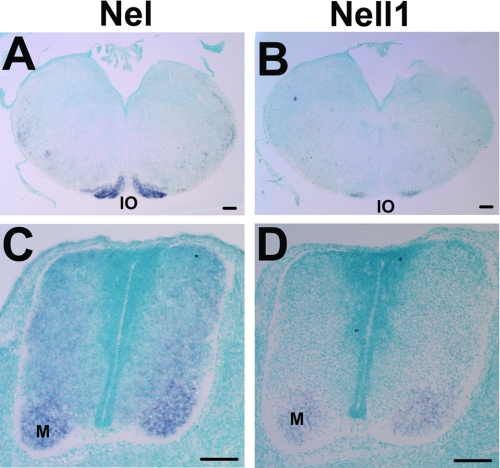
Although previous studies of Nell1 have mainly focused on its expression and functions in non-neural tissues, particularly in skeletal tissues (17–20), Nell1 expression has been detected in the developing and adult nervous system (4). Remarkably, Nel and Nell1 are co-expressed in the rat hippocampus, although expression of Nell1 is weaker than that of Nel (4). In examinations of their expression patterns during mouse development, we also have found that Nel and Nell1 are both strongly expressed in the embryonic inferior olive and spinal cord with partly overlapping patterns (Fig. 3). In both regions, Nel is expressed in broader areas than Nell1. Structurally, Nel and Nell1 share significant sequence similarities, and their overall domain organizations are conserved. In addition, thrombospondin-1, which has structural similarities with the Nel family members, has been shown to form both homo- and hetero-trimers (21). These findings raised the possibility that Nel may be able to form a hetero-complex with Nell1.
FIGURE 3.
Partly overlapping expression of Nel and Nell1 in the developing mouse central nervous system. Coronal sections of the developing mouse central nervous system were hybridized with RNA probes for Nel (A and C) or Nell1 (B and D). Dorsal is at the top. A and B, Nel is strongly expressed in most parts of the E17.5 inferior olive (IO), whereas Nell1 expression is restricted to ventral regions of the nucleus. C and D, Nel and Nell1 are co-expressed in the motor column (M) of the E13.5 spinal cord. Weak expression of Nel extends to dorsal parts of the spinal cord. Scale bars, 100 μm.
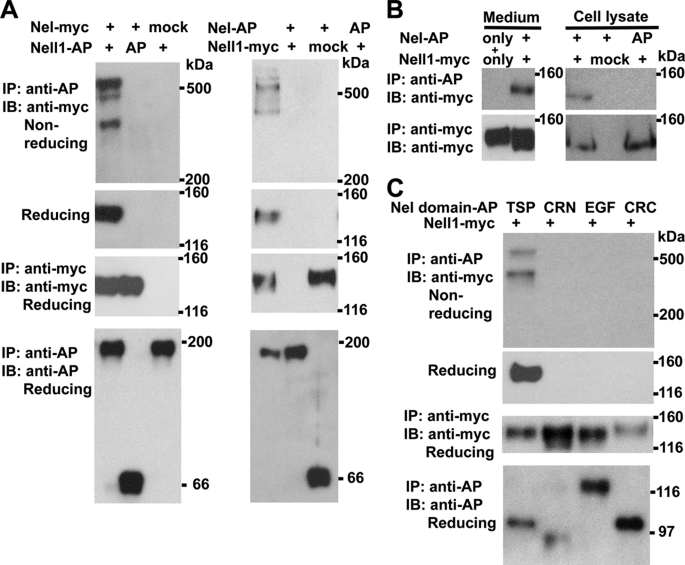
To test this possibility, Nel-myc and Nell1-AP were co-transfected into HEK293T cells, and Nel-Nell1 association was examined by immunoprecipitation with anti-AP antibody and immunoblot analysis with anti-myc antibody. Under reducing conditions, a single band of 130 kDa that corresponds to the size of Nel-myc monomers was detected by anti-myc antibody, whereas several high molecular size bands were detected under the non-reducing conditions, indicating that Nel-Nell1 hetero-complexes are formed in solution (Fig. 4A, left). To confirm the results, HEK293T cells were transfected with Nel-AP and Nell1-myc expression constructs, and culture media were examined similarly. As expected, Nel-AP was co-immunoprecipitated with Nell1-myc, and high molecular bands of hetero-complexes were detected under non-reducing conditions (Fig. 4A, right). These results indicate that Nel and Nell1 can form hetero-oligomer when co-expressed in the same cells.
FIGURE 4.
Hetero-oligomer formation of Nel and Nell1. A, HEK293T cells were transfected with expression constructs of Nel-myc and Nell1-AP (left), or Nel-AP and Nell1-myc (right). Culture media were collected after 4–6 days and subjected to immunoprecipitation (IP) and immunoblot analysis (IB) as indicated. SDS-PAGE was performed under non-reducing or reducing conditions. Co-immunoprecipitation of Nel and Nell1 was detected as high molecular bands of Nel-Nell1 heterocomplexes (non-reducing conditions) or a single band of Nel or Nell1 monomers (reducing conditions). B, conditioned media of HEK293T cells that had been individually transfected with Nel-AP and Nell1-myc expression constructs were mixed and incubated (Medium, Nel-AP only +, and Nell1-myc only), and formation of Nel-Nell1 hetero-complexes was examined by immunoprecipitation and immunoblotting under reducing conditions. No hetero-complex was detected. In contrast, when HEK293T cells were co-transfected with Nel and Nell1 expression vectors, Nel-Nell1 hetero-complex was detected both in medium (Medium, Nel-AP +, and Nell1-myc +) and in cell lysate. An empty vector (mock) and unconjugated AP (AP) were used as negative controls. C, a Nell1-myc expression construct was transfected into HEK293T cells with expression constructs for AP-fused domains of Nel (TSP, CRN, EGF, and CRC), and Nel-Nell1 association was examined as indicated. Nell1-myc was co-immunoprecipitated with Nel TSP-AP (TSP), but not with Nel CRN-AP (CRN), Nel EGF-AP (EGF), or Nel CRC-AP (CRC).
The Nel/Nell1 hetero-complexes could be formed either within the cells prior to secretion and/or in the extracellular solution after secretion. To distinguish these possibilities, we examined whether Nel and Nell1 exist as hetero-complexes in lysates of HEK293T cells that express both Nel-AP and Nell1-myc. Cell lysates were immunoprecipitated with anti-AP antibody and immunoblotted with anti-myc antibody. As shown in Fig. 4B, formation of Nel/Nell1 hetero-complex could be detected in the cell lysates, indicating that Nel/Nell complexes already exist within the cells. We next tested by pull-down assays whether Nel and Nell1 can assemble into hetero-complexes in solution after secretion. Culture medium of Nel-transfected HEK293T cells was mixed with that of Nell1-transfected cells, incubated overnight at 4 °C, and then analyzed by co-immunoprecipitation and immunoblotting. In contrast to cell lysates, formation of hetero-complexes could not be detected when Nel and Nell1 were mixed in solution (Fig. 4B). These results indicate that formation of Nel-Nell1 hetero-complexes occurs prior to secretion, in the secretion pathway within the cells.
Next we examined which domain of Nel is involved in its association with Nell1. Because the TSP-N domain is responsible for homo-oligomer formation of Nel, we were interested in whether the same domain is also involved in the Nel-Nell1 interaction. To examine this, we expressed AP-tagged domains of Nel with Nell1-myc in HEK293T cells, and complex formation was examined by immunoprecipitation and immunoblotting. As expected, Nell1-myc was co-immunoprecipitated with Nel TSP-AP, but not with Nel CRN-AP, Nel EGF-AP, or Nel CRC-AP (Fig. 4C). Taken together, our results show that the TSP-N domain can mediate both homo- and hetero-multimer formation of Nel.
Thrombospondin-1 Does Not Form Hetero-complexes with Nel or Nell1
Because the TSP-N domain of the Nel family members has structural similarities to the corresponding region of thrombospondin-1, we tested whether Nel and Nell1 can form hetero-complexes with thrombospondin-1. To examine this, we co-transfected an expression construct for AP-tagged thrombospondin-1 (TSP-1-AP) into HEK293T cells, with a construct for myc-tagged thrombospondin-1 (TSP-1-myc), Nel-myc, or Nell1-myc. Then, oligomer formation was examined by immunoprecipitation with anti-AP antibody and immunoblot analysis with anti-myc antibody (Fig. 5). Consistent with previous reports (21, 22), co-immunoprecipitation of TSP-1-AP and TSP-1-myc was observed. In contrast, TSP-1-AP did not appear to form hetero-complex with Nel-myc or Nell1-myc. These finding suggests that there is some specificity in interactions between different TSP-N domains.
FIGURE 5.
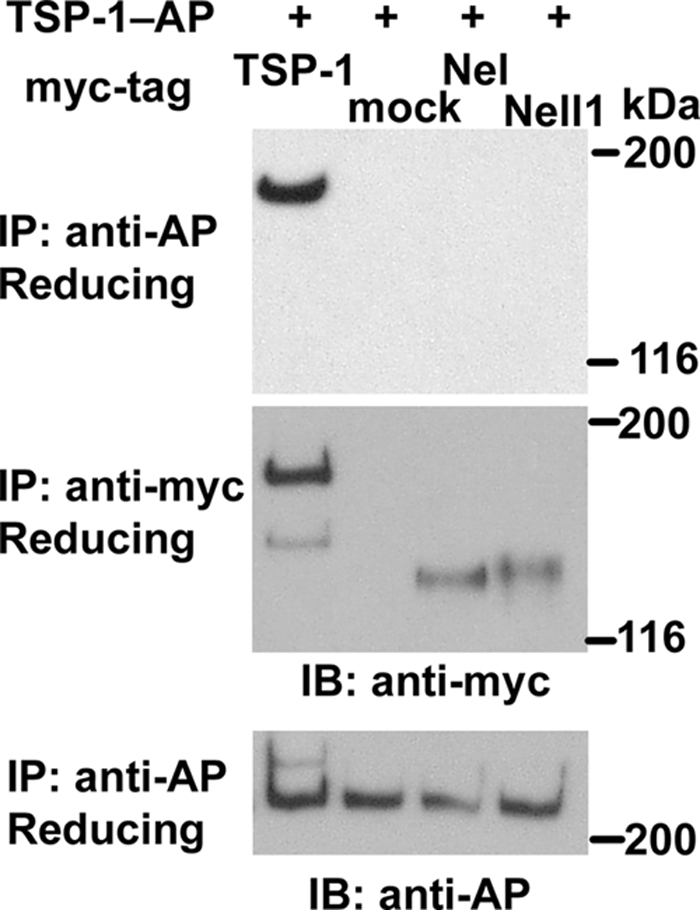
Thrombospondin-1 does not form hetero-complexes with Nel or Nell1. An expression construct for AP-fused thrombospondin-1 (TSP-1-AP) was co-transfected into HEK293T cells with a construct for myc-tagged thrombospondin-1 (TSP-1-myc), Nel-myc, or Nell1-myc. Culture media were collected after 4–6 days and subjected to immunoprecipitation (IP) and immunoblot analysis (IB) as indicated. SDS-PAGE was performed under reducing conditions. TSP-1-AP was co-immunoprecipitated with TSP-1-myc, but not with Nel-myc or Nell1-myc. mock, an empty vector.
The TSP-N Domain of Nel Has Heparin-binding Activity
It has been previously shown that the Nel family proteins have heparin-binding activity, suggesting that secreted Nel proteins interact with heparan sulfate proteoglycans (HSPGs) on the cell surface (4). To determine the domain of Nel that interacts with heparin, HEK293T cells were transfected with expression constructs for different regions of Nel. Culture media were applied to heparin-Sepharose, and bound proteins were eluted with 500 mm NaCl. As shown in Fig. 6, Nel TSP-AP showed significant binding to heparin, whereas no binding activity was detected for Nel CRN-AP, Nel EGF-AP, or Nel CRC-AP, indicating that Nel interacts with heparin through the TSP-N domain.
FIGURE 6.
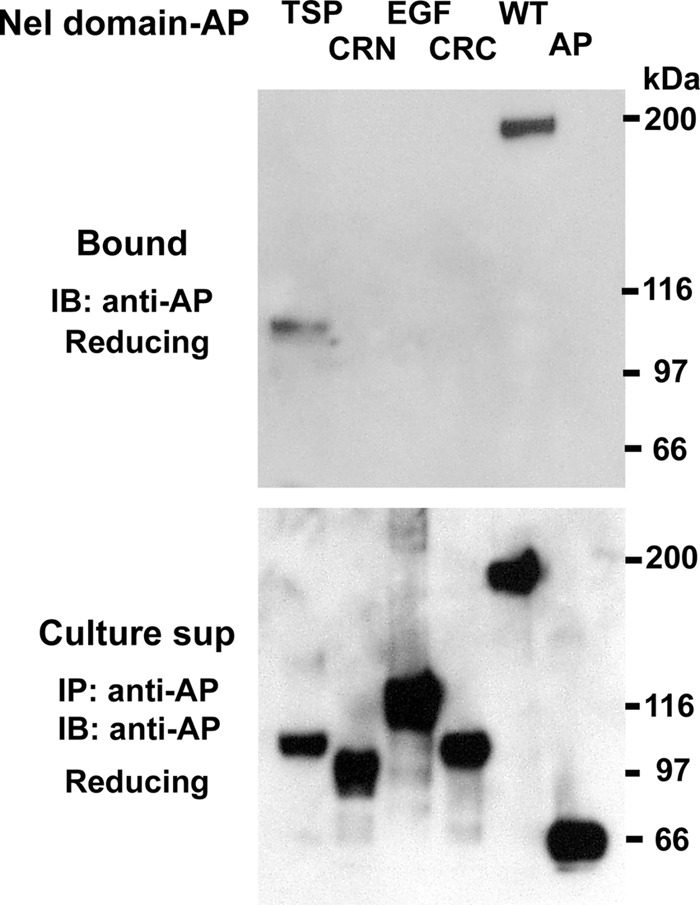
Nel binds to heparin through its TSP-N domain. Culture media of HEK293T cells that express AP-tagged domains of Nel (TSP, CRN, EGF, and CRC), full-length Nel-AP (WT) or unconjugated AP (AP) were incubated with heparin-Sepharose beads. Top, after washing, the bound proteins (Bound) were eluted with 500 mm NaCl and subjected to SDS-PAGE and immunoblot analysis using anti-AP antibody. Nel TSP-AP (TSP) and the full-length Nel-AP bound to heparin-Sepharose, whereas the other domains of Nel (CRN, EGF, and CRC) or unconjugated AP (AP) did not show detectable heparin-binding activity. Bottom, culture supernatants (Culture sup) were immunoprecipitated and immunoblotted with anti-AP antibody. SDS-PAGE was performed under reducing conditions.
Nel Binds to Retinal Axons with Its TSP-N and Cysteine-rich Domains
In our previous studies, we have shown that Nel can bind to retinal axons in vivo and that this interaction induces growth cone collapse in vitro (9). Whereas the molecular nature of Nel receptor(s) has not been identified, our results suggest that Nel binds to a specific receptor expressed on retinal axons and acts as an inhibitory guidance molecule. Therefore, we were interested in determining which domains of Nel interact with the potential receptor expressed in retinal axons in vivo. In addition, we evaluated whether Nell1 has similar retinal axon-binding activities. To this end, we performed affinity probe in situ assays (15) using specific domains of Nel and the full-length Nell1 fused to an AP tag as probes. When individual AP-tagged domains of Nel were expressed in HEK293T cells, however, we found that the yields of Nel TSP-AP were very low, and we were unable to collect sufficient amounts of purified Nel TSP-AP for the assay. The low yields of Nel TSP-AP are consistent with the previous report that an alternative splice variant of rat Nel that contains only the signal peptide and TSP-N domain is secreted at notably lower levels compared with the full-length Nel (23). We therefore tested binding activity of the other three regions of Nel (Nel CRN-AP, Nel EGF-AP, and Nel CRC-AP) as well as the full-length Nell1 (Nell1-AP).
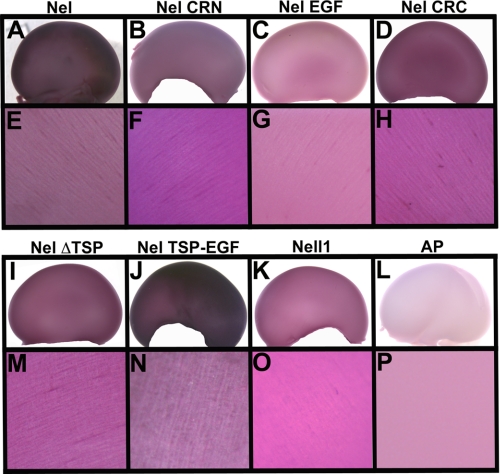
In the chicken visual system, the optic tectum is the primary target of the retinal axons. Because retinal axons enter the tectum through its most superficial layer, stratum opticum, they can be labeled and detected in whole mount preparations of the tectum (e.g. Ref. 24). We dissected out chick tecta at embryonic day (E) 17 or 18, when most of the retinal axons have entered the tectum, and incubated them with either Nel-AP, AP-fused Nel domains, Nell1-AP, or unconjugated AP control. Binding of each fusion protein was then detected by the AP enzyme reaction. Consistent with our previous results, strong binding of the full-length Nel-AP was detected on the entire surface of the tectum (Fig. 7, A and E). Among the AP-tagged domains used in the study, Nel CRN-AP (Fig. 7, B and F) and Nel CRC-AP (Fig. 7, D and H) showed significant binding activity, followed by weaker binding activity of Nel EGF-AP (Fig. 7, C and G). No significant binding was observed for control AP (Fig. 7, L and P). These results suggest that cysteine-rich domains are involved in binding to retinal axons. In contrast, Nell1-AP showed significantly weaker binding to retinal axons on the tectal surface than Nel-AP (Fig. 7, K and O).
FIGURE 7.
Binding activity of specific regions of Nel to retinal axons innervating into the tectum in vivo. Tecta of E17–18 chick were incubated with AP fusions of the full-length Nel (A and E), Nel CRN (B and F), Nel EGF (C and G), Nel CRC (D and H), Nel ΔTSP (I and M), Nel TSP-EGF (J and N), Nell1 (K and O), or unconjugated AP control (L and P). E–H and M–P are higher magnification views of A–D and I–L, respectively. Nel-AP showed strong binding activity to retinal axons navigating the tectal surface, whereas no binding was detected for control AP. Among the deletion mutant proteins of Nel, Nel TSP-EGF-AP showed strong binding activity that is comparable to that of Nel-AP. Nel CRN-AP, Nel CRC-AP, and Nel ΔTSP-AP also had significant binding activity, followed by weaker binding of Nel EGF-AP. Nell1-AP appeared to have weaker binding activity than Nel-AP. In higher magnification views, linear patterns of labeled retinal axon bundles can be seen.
To examine whether the TSP-N domain is involved in interaction with retinal axons, we made two additional AP-tagged deletion mutants; one construct lacks the TSP-N domain (Nel ΔTSP-AP), and the other mutant contains the TSP-N domain and EGF-like domains but lacks all the cysteine-rich domains (Nel TSP-EGF-AP) (Fig. 1C). We then tested them for binding to retinal axons. Significant binding activity was detected for Nel ΔTSP-AP (Fig. 7, I and M), which is consistent with the observed binding activity of cysteine-rich domains (Nel CRN-AP and Nel CRC-AP). Interestingly, Nel TSP-EGF-AP showed strong binding activity (Fig. 7, J and N) that is comparable to that of the full-length Nel-AP. Because Nel TSP-EGF-AP consists of the TSP-N and EGF-like domains of Nel, and the latter by themselves did not show strong binding, these results suggest that the TSP-N domain has strong binding activity to retinal axons. Taken together, our results suggest that Nel can interact with retinal axons through two distinct regions, the TSP-N domain and cysteine-rich domains.
Cysteine-rich Domains but Not the TSP-N Domain of Nel Can Induce Growth Cone Collapse in Retinal Axons
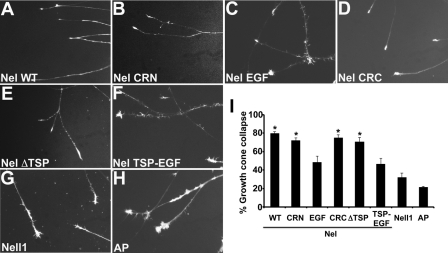
Next we examined whether the binding of the TSP-N and cysteine-rich domains to retinal axons have any functional consequences. Because we have previously shown that Nel can act as an inhibitory guidance cue for retinal axons (9), we tested whether the fusion proteins used for binding assay can cause growth cone collapse. Retinal explants were prepared from E6–7 chick and cultured on a laminin-coated substratum to allow retinal axons to grow out. After 24–36 h culture, the tip of almost all axons had growth cones with well developed lamellipodia and filopodia. AP-tagged domain fragments were then added to the culture, and the morphology of growth cones was observed after 30 min (Fig. 8). As shown previously, treatment with Nel-AP induced growth cone collapse in ∼80% of retinal axons. Consistent with the binding activities of cysteine-rich domains of Nel, Nel CRN-AP, Nel CRC-AP, and Nel ΔTSP-AP exerted remarkable growth cone collapsing activities. Nel EGF-AP was found to have weak collapsing activity. Surprisingly, despite its strong binding activity to retinal axons, Nel TSP-EGF-AP did not induce strong growth cone collapse, but showed only a weak activity that is comparable to that of Nel EGF-AP. Nell1-AP or control AP did not show any obvious effects on the growth cone morphology. These results indicate that cysteine-rich domains play major roles in Nel-induced growth cone collapse of retinal axons.
FIGURE 8.
Growth cone collapse of retinal axons is induced by cysteine-rich domains of Nel. Retinal explants were prepared from E6–7 chick embryos and cultured in vitro for 24–36 h to allow retinal axons to grow out. Then, Nel-AP (Nel WT), AP-fused domains of Nel (Nel CRN, Nel EGF, Nel CRC, Nel ΔTSP, and Nel TSP-EGF), Nell1-AP, or unconjugated AP (AP), was added to the culture, and the growth cone morphology was observed 30 min later. A–H, representative growth cone morphology after the treatment with Nel-AP (A), AP-fused domains of Nel (B–F), Nell1-AP (G), or unconjugated AP (H). Treatment with Nel-AP induced growth cone collapse in most of retinal axons, whereas Nell1-AP or control AP did not cause obvious effects. AP fusion constructs that contain cysteine-rich domains of Nel (Nel CRN-AP, Nel CRC-AP, and Nel ΔTSP-AP) exerted strong growth cone-collapsing activity. Nel EGF-AP and Nel TSP-EGF-AP showed weak growth cone-collapsing activity. I, quantification of the growth cone-collapsing activities. The percentages of collapsed growth cones were plotted as mean ± S.E. The growth cone-collapsing activity of each construct was compared with that of AP. *, p < 0.001.
DISCUSSION
Nel has recently emerged as an important regulator of neural development and functions. The Nel protein has a multimodular structure and contains conserved domains that play important roles in molecular interactions and cell-cell communications in other extracellular molecules. To elucidate the mechanisms by which Nel exerts its diverse functions, it is important to understand the roles of its individual domains. In this study, we have performed structure-function analysis of Nel by using a series of expression constructs for specific Nel domains. Our studies have determined the domains that are responsible for homo- and hetero-multimer formation, heparin-binding activity, binding to potential receptors on retinal axons, and growth cone-collapsing activity.
Homo- and Hetero-multimer Formation Mediated by the TSP-N Domain
The results of co-immunoprecipitation experiments have shown that homo-multimer formation of Nel is mediated by the TSP-N domain (Fig. 2). In addition, when co-expressed in the same cells, Nel and Nell1 can form hetero-complexes through the TSP-N domain (Fig. 4, A and C). Nel/Nell1 complexes are also detected in lysates of cells expressing both proteins (Fig. 4B), indicating that hetero-oligomer formation occurs within the cells prior to secretion. Previous studies have shown that thrombospondin-1, which has structural similarities with Nel, can form both a homo-trimer and hetero-trimers via two conserved cysteine residues in their N-terminal region (21, 22). The TSP-N domain of Nel contains cysteine residues that are conserved in Nell1, and inter-chain disulfide linkages between some of those residues may be involved in Nel/Nell1 hetero-complex formation. Despite their structural similarities, neither Nel nor Nell1 has appeared to form hetero-complexes with thrombospondin-1 (Fig. 5), indicating the specificity in multimer formation through the TSP-N domain of Nel family proteins.
Nel and Nell1 have been found, by using RNA in situ hybridization, to be co-expressed in several regions of the developing nervous system, such as the inferior olive and spinal cord (Fig. 3), suggesting the possibility that Nel/Nell1 hetero-complexes exist and function in vivo. Depending on the relative expression levels of Nel and Nell1 in those areas, there is probably a dynamic balance between the homo- and hetero-oligomeric forms of the molecules. In neuronal cells that express both Nel and Nell1, the spectrum of homo- and hetero-complexes can be simply altered by modulating the synthesis of either or both molecules. If expressions of Nel and Nell1 are regulated by different extracellular factors, signals at the cell surface may preferentially alter the synthesis of Nel or Nell1 and, thereby, influence the homo-/hetero-multimer equilibrium. In our affinity probe in situ and growth cone collapse assays, Nel and Nell1 have shown different binding and growth cone collapsing activities (Figs. 7 and 8). Differential binding patterns between Nel and Nell1 have also been found in other tissues, such as kidney,4 suggesting that they recognize different receptors and/or bind to the same set of receptors with different affinities. Therefore, production of hetero-multimers may regulate the function of Nel and Nell1 by modulating their receptor repertoires and binding affinities.
Heparin-binding Activity of the TSP-N Domain
The affinity chromatography experiments have shown that the TSP-N domain is responsible for heparin-binding activity of Nel (Fig. 6). The N-terminal region of thrombospondin-1 also has heparin-binding activity (25), indicating that this property is conserved between the two proteins. Heparin-binding activity of the TSP-N domain may be important for regulation of the distribution and axon guidance functions of Nel in vivo. We have previously shown that Nel mRNA is expressed in specific layers of the developing chick tectum. Although Nel is a secreted protein, immunohistochemical analysis has revealed that most of the Nel protein remains in the layers of its origin (9). This limited diffusion may be because the secreted Nel protein is trapped in situ by HSPGs, which are abundant in the developing tectum (26). Because Nel exerts inhibitory effects on retinal axons in vitro and retinal axons do not normally invade the tectal layers that express Nel in vivo, we have proposed that Nel may act as an inhibitory guidance cue in the layer-specific retinotectal projection (9). Topographically specific distribution is an essential feature of axon guidance cues, and therefore heparin-binding activity of the TSP-N domain may play a crucial role in Nel-mediated regulation of retinal axon projection.
Binding to Retinal Axons and Induction of Growth Cone Collapse by Cysteine-rich Domains
To date, no specific receptors have been identified for Nel, and its intracellular signaling pathway is still unknown. Affinity probe in situ assays in this study have revealed that the TSP-N domain and cysteine-rich domains can independently bind to retinal axons (Fig. 7). Interestingly, cysteine-rich domains, but not the TSP-N domain, have exerted strong growth cone-collapsing functions (Fig. 8), suggesting that cysteine-rich domains of Nel can interact with a cognate receptor on retinal axons and transduce signals that lead to alterations of growth cone morphology. Because cysteine-rich domains are not involved in interaction between Nel monomers, our results also indicate that oligomerization is not necessary for receptor binding and activation, although the results do not rule out the possibility that valency can influence binding affinities of Nel to the receptor and its functions.
We have observed that, although the TSP-N domain of Nel has strong binding activity to retinal axons, it does not appear to have strong growth cone-collapsing activity. Because the TSP-N domain has heparin-binding activity, its strong binding to retinal axons may be attributed, at least in part, to interaction with cell surface HSPGs (4). Similar interactions have been reported for thrombospondin-1 (27, 28). The binding of the TSP-N domain to cell surface HSPGs may modulate the interaction between Nel cysteine-rich domains and their signal transducing receptors, as is the case for other heparin-binding factors, such as fibroblast growth factors (29). Alternatively, the TSP-N domain may bind to its specific receptor that is different from the receptor for cysteine-rich domains and exert distinct functions. Further studies, particularly identification of the cognate receptors, will be required for understanding molecular mechanisms by which Nel exerts its diverse functions.
Diversity in Molecular Interactions of Nel
In view of the structural similarities between Nel and thrombospondin-1, it seems likely that Nel interacts with a diverse range of extracellular and cell surface molecules by using different domains (30). The TSP-N domain of thrombospondin-1 interacts with integrins, calreticulin, low density lipoprotein receptor-related protein, sulfatides, and cell surface HSPGs and chondroitin sulfate proteoglycans (31). Because the TSP-N domain of Nel also has heparin-binding activity, some binding partners may be shared by the TSP-N domains of Nel and thrombospondin-1.
The cysteine-rich domains of the Nel family of proteins have structural similarities to those of chordin, which acts as an antagonist for bone morphogenetic proteins (BMPs) in the extracellular space by directly binding to BMPs and thereby preventing the activation of BMP receptors (32, 33). The structural similarities are reflected in the spacing of cysteines and in the presence of the CXXCXC and CCXXC motifs and conserved glycine and tryptophan residues, although the degree of similarity varies between different cysteine-rich domains of Nel. Interestingly, chordin-like cysteine-rich domains are present in a number of extracellular proteins, and some of those proteins have appeared to regulate the BMP and other transforming growth factor (TGF) β signaling positively or negatively (13). Although it remains to be determined whether Nel family proteins bind TGFβ family members, the structural feature of Nel cysteine-rich domain raises the intriguing possibility that Nel may modulate growth factor signaling via binding and sequestration of ligands, as well as via binding to its one or more cognate receptors.
EGF-like domains have been found in a number of eukaryotic proteins that are involved in cell proliferation, growth inhibition, and differentiation (14). For examples, EGF-like domains of thrombospondin-1 have been recently shown to indirectly activate the EGF receptor and downstream phospholipase Cγ and stimulate cell migration (34). Although no functions have been identified in this study for the six EGF-like domains of Nel, it is likely that they are involved in interactions with a variety of extracellular molecules. Interestingly, it has been previously indicated that EGF-like domains of Nel can interact with protein kinase C (4, 35). Because Nel has an alternative splicing form that encodes cytoplasmic protein, Nel may regulate intracellular signaling through its EGF-like repeats.
In summary, the present study has determined the one or more domains of Nel that are responsible for interactions with other extracellular molecules. Taken together with the results of previous reports, these findings strongly suggest that Nel plays a diverse range of functions by using its different domains.
Acknowledgments
We thank Iain McEwan for useful comments on the manuscript and Paul Bornstein for the pcDNA3 mTSP1 vector deposited at Addgene.
This work was supported by grants from the Royal Society (UK) and the Biotechnology and Biological Sciences Research Council (UK) (to M. N.).
R. Nakamura and M. Nakamoto, unpublished observation.
- Nel
- neural EGF-like molecule
- AP
- alkaline phosphatase
- BMP
- bone morphogenetic proteins
- CRC
- C-terminal cysteine-rich domain
- CRN
- N-terminal cysteine-rich domain
- HSPG
- heparan sulfate proteoglycan
- Nell1/2
- Nel-like1/2
- TSP-N
- N-terminal thrombospondin domain.
REFERENCES
- 1. Matsuhashi S., Noji S., Koyama E., Myokai F., Ohuchi H., Taniguchi S., Hori K. (1995) New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev. Dyn. 203, 212–222 [DOI] [PubMed] [Google Scholar]
- 2. Matsuhashi S., Noji S., Koyama E., Myokai F., Ohuchi H., Taniguchi S., Hori K. (1996) New gene, nel, encoding a Mr 91 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev. Dyn. 207, 233–234 [DOI] [PubMed] [Google Scholar]
- 3. Watanabe T. K., Katagiri T., Suzuki M., Shimizu F., Fujiwara T., Kanemoto N., Nakamura Y., Hirai Y., Maekawa H., Takahashi E. (1996) Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics 38, 273–276 [DOI] [PubMed] [Google Scholar]
- 4. Kuroda S., Oyasu M., Kawakami M., Kanayama N., Tanizawa K., Saito N., Abe T., Matsuhashi S., Ting K. (1999) Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem. Biophys. Res. Commun. 265, 79–86 [DOI] [PubMed] [Google Scholar]
- 5. Jeong J. K., Kim H. R., Hwang S. M., Park J. W., Lee B. J. (2008) Region- and neuronal phenotype-specific expression of NELL2 in the adult rat brain. Mol. Cells 26, 186–192 [PubMed] [Google Scholar]
- 6. Nelson B. R., Matsuhashi S., Lefcort F. (2002) Restricted neural epidermal growth factor-like like 2 (NELL2) expression during muscle and neuronal differentiation. Gene Expr. Patterns 2, 7–15 [DOI] [PubMed] [Google Scholar]
- 7. Nelson B. R., Claes K., Todd V., Chaverra M., Lefcort F. (2004) NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev. Biol. 270, 322–335 [DOI] [PubMed] [Google Scholar]
- 8. Aihara K., Kuroda S., Kanayama N., Matsuyama S., Tanizawa K., Horie M. (2003) A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res. Mol. Brain Res. 116, 86–93 [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y., Obama H., Kuan S. L., Nakamura R., Nakamoto C., Ouyang Z., Nakamoto M. (2009) In vitro guidance of retinal axons by a tectal lamina-specific glycoprotein Nel. Mol. Cell. Neurosci. 41, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuyama S., Aihara K., Nishino N., Takeda S., Tanizawa K., Kuroda S., Horie M. (2004) Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport 15, 417–420 [DOI] [PubMed] [Google Scholar]
- 11. Matsuyama S., Doe N., Kurihara N., Tanizawa K., Kuroda S., Iso H., Horie M. (2005) Spatial learning of mice lacking a neuron-specific epidermal growth factor family protein, NELL2. J. Pharmacol. Sci. 98, 239–243 [DOI] [PubMed] [Google Scholar]
- 12. Beckmann G., Hanke J., Bork P., Reich J. G. (1998) Merging extracellular domains. Fold prediction for laminin G-like and amino-terminal thrombospondin-like modules based on homology to pentraxins. J. Mol. Biol. 275, 725–730 [DOI] [PubMed] [Google Scholar]
- 13. Garcia Abreu J., Coffinier C., Larraín J., Oelgeschläger M., De Robertis E. M. (2002) Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene 287, 39–47 [DOI] [PubMed] [Google Scholar]
- 14. Davis C. G. (1990) The many faces of epidermal growth factor repeats. New Biol. 2, 410–419 [PubMed] [Google Scholar]
- 15. Flanagan J. G., Cheng H. J., Feldheim D. A., Hattori M., Lu Q., Vanderhaeghen P. (2000) Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol. 327, 19–35 [DOI] [PubMed] [Google Scholar]
- 16. Goshima Y., Nakamura F., Strittmatter P., Strittmatter S. M. (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 [DOI] [PubMed] [Google Scholar]
- 17. Zhang X., Kuroda S., Carpenter D., Nishimura I., Soo C., Moats R., Iida K., Wisner E., Hu F. Y., Miao S., Beanes S., Dang C., Vastardis H., Longaker M., Tanizawa K., Kanayama N., Saito N., Ting K. (2002) Craniosynostosis in transgenic mice overexpressing Nell-1. J. Clin. Invest. 110, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ting K., Vastardis H., Mulliken J. B., Soo C., Tieu A., Do H., Kwong E., Bertolami C. N., Kawamoto H., Kuroda S., Longaker M. T. (1999) Human NELL-1 expressed in unilateral coronal synostosis. J. Bone Miner. Res. 14, 80–89 [DOI] [PubMed] [Google Scholar]
- 19. Desai J., Shannon M. E., Johnson M. D., Ruff D. W., Hughes L. A., Kerley M. K., Carpenter D. A., Johnson D. K., Rinchik E. M., Culiat C. T. (2006) Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum. Mol. Genet. 15, 1329–1341 [DOI] [PubMed] [Google Scholar]
- 20. Bokui N., Otani T., Igarashi K., Kaku J., Oda M., Nagaoka T., Seno M., Tatematsu K., Okajima T., Matsuzaki T., Ting K., Tanizawa K., Kuroda S. (2008) Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 582, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Rourke K. M., Laherty C. D., Dixit V. M. (1992) Thrombospondin 1 and thrombospondin 2 are expressed as both homo- and heterotrimers. J. Biol. Chem. 267, 24921–24924 [PubMed] [Google Scholar]
- 22. Lawler J. W., Slayter H. S., Coligan J. E. (1978) Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J. Biol. Chem. 253, 8609–8616 [PubMed] [Google Scholar]
- 23. Kim D. G., Hwang E. M., Yoo J. C., Kim E., Park N., Rhee S., Ha C. M., Hong S. G., Park J. Y. (2010) Identification and characterization of a truncated isoform of NELL2. Biochem. Biophys. Res. Commun. 391, 529–534 [DOI] [PubMed] [Google Scholar]
- 24. Nakamoto M., Cheng H. J., Friedman G. C., McLaughlin T., Hansen M. J., Yoon C. H., O'Leary D. D., Flanagan J. G. (1996) Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell 86, 755–766 [DOI] [PubMed] [Google Scholar]
- 25. Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. (1984) Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J. Biol. Chem. 259, 10100–10105 [PubMed] [Google Scholar]
- 26. Halfter W. (1993) A heparan sulfate proteoglycan in developing avian axonal tracts. J. Neurosci. 13, 2863–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H., Sottile J., Strickland D. K., Mosher D. F. (1996) Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein. Localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem. J. 318, 959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikhailenko I., Krylov D., Argraves K. M., Roberts D. D., Liau G., Strickland D. K. (1997) Cellular internalization and degradation of thrombospondin-1 is mediated by the amino-terminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J. Biol. Chem. 272, 6784–6791 [DOI] [PubMed] [Google Scholar]
- 29. Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. (1992) Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol. Cell. Biol. 12, 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H., Herndon M. E., Lawler J. (2000) The cell biology of thrombospondin-1. Matrix Biol. 19, 597–614 [DOI] [PubMed] [Google Scholar]
- 31. Elzie C. A., Murphy-Ullrich J. E. (2004) The N terminus of thrombospondin. The domain stands apart. Int. J. Biochem. Cell Biol. 36, 1090–1101 [DOI] [PubMed] [Google Scholar]
- 32. Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. (1995) Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333–336 [DOI] [PubMed] [Google Scholar]
- 33. Piccolo S., Agius E., Lu B., Goodman S., Dale L., De Robertis E. M. (1997) Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91, 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu A., Garg P., Yang S., Gong P., Pallero M. A., Annis D. S., Liu Y., Passaniti A., Mann D., Mosher D. F., Murphy-Ullrich J. E., Goldblum S. E. (2009) Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cγ and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J. Biol. Chem. 284, 6389–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang E. M., Kim D. G., Lee B. J., Choi J., Kim E., Park N., Kang D., Han J., Choi W. S., Hong S. G., Park J. Y. (2007) Alternative splicing generates a novel non-secretable cytosolic isoform of NELL2. Biochem. Biophys. Res. Commun. 353, 805–811 [DOI] [PubMed] [Google Scholar]