Background: Induction of the iodide transporter in cancer cells confers targeted cytotoxicity with radioiodide.
Results: Isoforms of p38 MAPK were identified that specifically promote iodide uptake in breast cancer cells.
Conclusion: p38 isoform-specific stimulation may induce iodide uptake sufficient for radioiodide therapy in breast cancer.
Significance: Study of p38 isoform-specific signaling improves understanding of cancer cell differentiation and identifies novel therapeutic targets.
Keywords: Breast Cancer, Gene Expression, p38, Rac1, Thyroid, Retinoic Acid, Sodium Iodide Symporter
Abstract
Activation of p38 MAPK is a key pathway for cell proliferation and differentiation in breast cancer and thyroid cells. The sodium/iodide symporter (NIS) concentrates iodide in the thyroid and lactating breast. All-trans-retinoic acid (tRA) markedly induces NIS activity in some breast cancer cell lines and promotes uptake of β-emitting radioiodide 131I sufficient for targeted cytotoxicity. To identify a signal transduction pathway that selectively stimulates NIS expression, we investigated regulation by the Rac1-p38 signaling pathway in MCF-7 breast cancer cells and compared it with regulation in FRTL-5 rat thyroid cells. Loss of function experiments with pharmacologic inhibitors and small interfering RNA, as well as RT-PCR analysis of p38 isoforms, demonstrated the requirement of Rac1, MAPK kinase 3B, and p38β for the full expression of NIS in MCF-7 cells. In contrast, p38α was critical for NIS expression in FRTL-5 cells. Treatment with tRA or overexpression of Rac1 induced the phosphorylation of p38 isoforms, including p38β. A dominant negative mutant of Rac1 abolished tRA-induced phosphorylation in MCF-7 cells. Overexpression of p38β or Rac1 significantly enhanced (1.9- and 3.9-fold, respectively), the tRA-stimulated NIS expression in MCF-7 cells. This study demonstrates differential regulation of NIS by distinct p38 isoforms in breast cancer cells and thyroid cells. Targeting isoform-selective activation of p38 may enhance NIS induction, resulting in higher efficacy of 131I concentration and treatment of breast cancer.
Introduction
p38 kinase, a member of the MAPK family, is a key regulator of intracellular signal pathways influencing cell proliferation, differentiation, cell survival, and migration. The catalytic activity of p38 requires dual phosphorylation of a conserved motif, Thr-Gly-Tyr. Because p38 is activated in many types of cancer, including breast cancer and thyroid cancer, p38 has been proposed as a therapeutic target to modulate cell growth and differentiation (1). A range of stimuli, including cytokines, growth factors, hormones, and cell stress, stimulate p38 activity through selective MAPK kinases (MKKs), MKK3 and/or MKK6. Activation of p38 by small GTPases, such as Rac1 and Rho, contributes to cell differentiation and transformation.
The sodium iodide symporter (NIS,3 or solute carrier family 5, member 5 (SLC5A5)) is expressed predominantly in the thyroid gland and lactating breast and mediates accumulation of iodide from the blood stream to these tissues (2). In the majority of differentiated thyroid cancer, after stimulation with high levels of thyroid-stimulating hormone (TSH), NIS is induced sufficiently to ablate residual tumor with β-emitting radioiodide-131 (131I). Recent studies of NIS gene therapy have demonstrated that NIS expression sufficient for tumor shrinkage with 131I can be achieved in several types of non-thyroidal cancer (3–6).
Approximately 70% of breast cancer expresses endogenous NIS (7, 8) and has been considered as a potential target of radioiodide therapy for breast cancer (9, 10). The native expression level of NIS, however, is insufficient to deliver an effective dose of 131I. Extensive experience with 131I therapy in thyroid cancer indicates the importance of maximizing the magnitude of iodide uptake specifically in the tumor.
All-trans-retinoic acid (tRA) is the most potent NIS inducer in breast cancer models, including MCF-7 cells (11–15). NIS expression sufficient for iodide uptake and cytotoxicity with 131I has been demonstrated in several in vitro breast cancer models (11, 14). Our study with in vivo breast cancer models has shown that iodide uptake can be achieved but that a large dose of tRA is required. Elucidation of signaling pathways involved in the NIS induction by tRA may lead to more effective induction of NIS in some breast cancer.
The induction of NIS by tRA is primarily mediated by the heterodimer of retinoic acid receptor (RAR)-β and retinoid X receptor (RXR)-α (14, 16). tRA-stimulated RAR-RXR has been shown to up-regulate NIS expression in MCF-7 cells (16, 17). Although RAR-RXR can act as a transcription factor and directly stimulate gene expression, it can also activate signaling pathways, such as phosphoinositide 3-kinase and p38 (16, 18).
NIS gene expression is differentially regulated in thyroid and breast tissues. In thyroid cells, stimulation with TSH, followed by cAMP accumulation, is critical for NIS expression (15, 19). In contrast, cAMP does not influence NIS expression in breast cancer cells (11). tRA, the NIS inducer in breast cancer cells, reduces NIS expression in FRTL-5 rat thyroid cells (11, 20). Stimulation of p38 MAPK activity is required for TSH-induced NIS expression in thyroid cells (21) as well as tRA-induced NIS expression in MCF-7 cells (17). The Rac1-p38 pathway is up-regulated by TSH-cAMP stimulation in thyroid cells (21) and by tRA treatment in MCF-7 breast cancer cells (18). We hypothesized that the Rac1-p38 pathway was a common pathway for NIS induction in breast cancer cells and thyroid cells.
Four isoforms of p38, α, β, γ, and δ, have been reported in mammalian cells. There are isoform differences in tissue distribution and substrate specificity (22–24), which may confer differential activation of downstream signaling pathways. We therefore determined the role of each p38 isoform in the regulation of NIS in MCF-7 breast cancer cells and compared it with the role in FRTL-5 rat thyroid cells. We identified a p38 MAPK cascade that selectively regulates NIS expression in MCF-7 cells. The effect of tRA treatment on the NIS-selective p38 signal transduction pathway was also investigated.
EXPERIMENTAL PROCEDURES
Materials
Signal transduction inhibitors were purchased from EMD Biosciences (La Jolla, CA). tRA and other chemicals were purchased from Sigma, unless otherwise noted. pCMV6-Myc/FLAG-p38β and pCMV6-Entry vector were obtained from Origene (Rockville, MD). Rac1 constructs, pcDNA3.1–3xHA-Rac1 G12V and pcDNA3.1–3xHA-Rac1 T17N, were obtained from Missouri S&T cDNA Resource Center (Rolla, MO). pcDNA3.1+ vector was purchased from Invitrogen. Anti-p38β antibodies (Abs), P38-11A5 and C28C2, were purchased from Zymed Laboratories (San Francisco, CA) and Cell Signaling Technology (Danvers, MA), respectively. Anti-phospho-p38 (rabbit polyclonal Ab and rabbit monoclonal Ab, D3F9), anti-DYKDDDDK (FLAG) tag, anti-HA tag (C29F4), and anti-MKK3B Abs were purchased from Cell Signaling Technology.
Cell Culture
MCF-7 cells (lot F15100 and 205623 from the ATTC, Manassas, VA), MBA-MD-231 cells, and FRTL-5 cells were maintained as described previously (11, 25, 26). BT-474 cells were grown in Dulbecco's modified Eagle's medium (ATCC) with 10% fetal bovine serum (ATCC). When cells were treated with tRA and/or signal transduction inhibitors, they were maintained with 0.1% DMSO vehicle and fed with fresh media with the agents every 24 h.
Transfection of siRNA
Reverse transfection of RNAi duplex was performed with Lipofectamine RNAiMAX (Invitrogen), as recommended. Briefly, MCF-7 cells (2 × 105 cells/well), digested with 2.5 g/liter trypsin (Invitrogen), were incubated with RNAi duplex-Lipofectamine RNAiMAX complexes, containing 20 nmol of SMARTpool siRNA (Dharmacon, Lafayette, CO) or Stealth RNAi (Invitrogen), in 6-well plates for 48 h. Cells were then fed with fresh media and treated with or without tRA for 12 h starting 60 h after the beginning of transfection.
Transfection of Plasmid
In transient expression experiments, 2 μg of plasmid was transfected into ∼106 MCF-7 cells with the Nucleofector system (Lonza, Gaithersburg, MD), as recommended by the manufacturer. For stable expression experiments, the transfected cells were selected in 400 mg/liter G418 (Invitrogen) for 3–4 weeks.
Iodide Uptake
The iodide uptake assay was performed as described previously (27) with minor modifications. Briefly, cells were grown in 12-well dishes, washed with Hanks' balanced salt solution, and incubated for 1 h at 37 °C with 500 μl of Hanks' balanced salt solution containing ∼0.1 μCi of carrier-free Na125I (MP Biomedicals, Solon, OH) and 10 μm NaI. The specific activity under these conditions was 20 mCi/mmol. In some experiments, 30 μm KClO4, the specific inhibitor of NIS, was added to the Hanks' balanced salt solution to measure iodide uptake independent of NIS. After incubation, the cells were washed once with ice-cold PBS and scraped from each well, and radioactivity was measured in a γ-counter. Cell number was determined by counting in a hemocytometer. The radioactivity was normalized to the cell number at the time of the assay. In the signal transduction inhibitor dose-response experiments, iodide uptake was normalized to the amount of cellular protein. Cells were incubated with Na125I and then lysed with the addition of 200 μl of passive lysis buffer (Promega) to each well. The radioactivity in the cell lysate was determined by a γ-counter, and the protein content was determined by the Bio-Rad protein assay (Bio-Rad).
Analysis of mRNA Expression
Two-step quantitative RT-PCR was performed, as described previously (14, 26). Primers for human NIS, GAPDH, and rat Gapdh were designed, as described (14, 26). Quantitative PCR of isoforms of human and rat p38 cDNA was performed with the QuantiTect primer assay (Qiagen). Quantitative RT-PCR of human MKK3A (NM_002756) and MKK3B (NM_145109) was carried out with custom primers (Invitrogen), 5′-GTTCTCAGTTGGCCCGTGTG/5′-CCAAGTCATCAGCCTCCACCTC and 5′-CCACTTGCAGCATGGAGTCG/5′-CCACTTGCAGCATGGAGTCG, respectively. Standard curves representing 6-point serial dilution of the corresponding control group were analyzed in each assay and used for calculation of relative expression values. The sample quantifications were normalized by the internal control GAPDH mRNA.
Western Blot Analysis
Cells grown in 10-cm Petri dishes were rinsed, scraped with PBS containing phosphatase inhibitor mixture 2 (Sigma), spun down by centrifugation, and lysed in cell lysis buffer (Sigma), containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, protease inhibitor mixture (Sigma), and phosphatase inhibitor mixture 2 (Sigma). The cell extracts were clarified by centrifugation, and protein concentrations were determined by using a protein assay reagent (Bio-Rad). In some experiments, cells grown in 6-well plates were directly lysed in a culture dish with SDS sample buffer, containing 62.5 mm, Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mm DTT, 0.01% bromphenol blue, protease inhibitor mixture (Sigma), and phosphatase inhibitor mixture 2 (Sigma). Aliquots of protein were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) by using standard procedures. The membranes were then subjected to Western blotting, and the blots were developed with the advanced ECL Western blot detection kit (GE Healthcare).
Immunoprecipitation
Exogenous FLAG-tagged p38β expressed in MCF-7 cells was immunoprecipitated with the FLAG immunoprecipitation kit (Sigma), as recommended. Briefly, clarified cell lysate in the cell lysis buffer with protease inhibitor mixture (Sigma) and phosphatase inhibitor mixture 2 (Sigma) was incubated with anti-FLAG M2 agarose gel (Sigma) at 4 °C for 16 h. The fraction eluted with SDS sample buffer (Sigma) and the cell lysate before the immunoprecipitation were applied to Western blot analysis.
Statistical Analysis
Unless otherwise noted, statistical significance, at a p value of <0.05, was determined by conducting a paired Student's t test. Mann-Whitney U test was performed for data that were not normally distributed.
RESULTS
Effects of MAPK Cascade Inhibitors on Iodide Uptake in MCF-7 Cells
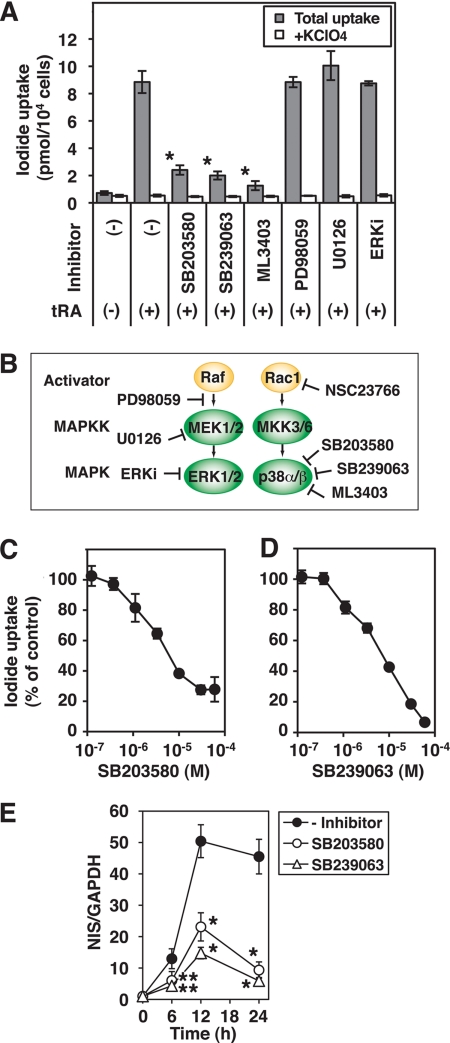
The potent p38 inhibitor, ML3403, significantly reduces tRA-induced NIS mRNA expression in MCF-7 cells (17). We tested the specificity of p38 inhibition on tRA-induced iodide uptake in MCF-7 cells with specific pharmacological inhibitors to determine the pathways involved. The p38 inhibitors, SB203580 (30 μm), SB239063 (10 μm), and ML3403 (30 μm), significantly reduced tRA-stimulated iodide uptake (Fig. 1A). Inhibitors of the MEK/ERK signaling pathway, PD98059 (30 μm), U0126 (30 μm), or ERK inhibitor (30 μm) (Fig. 1B), however, did not significantly influence tRA-stimulated iodide uptake (Fig. 1A). The p38 inhibitors, SB203580 and SB239063, inhibited iodide uptake in a dose-dependent fashion (Fig. 1C), although the IC50 value of these inhibitors (5.32 ± 0.72 and 7.73 ± 0.69 μm, respectively), was higher than the usual reported range in cell culture (<1 μm) (28). The marked tRA induction of NIS mRNA was significantly reduced by the addition of the p38 inhibitors, SB203580 (30 μm) and SB239063 (30 μm) (Fig. 1D), consistent with the effects of these inhibitors on iodide uptake. The p38 inhibitors utilized for these studies target p38 α and β but not γ and δ (29–31). These data indicate that p38 α and/or β play a major role in mediating tRA induction of NIS expression and iodide uptake in MCF-7 cells.
FIGURE 1.
Effects of MAPK cascade inhibitors on tRA-induced iodide uptake in MCF-7 cells. Cells were treated with or without tRA (1 μm) and the indicated inhibitors for 24 h (A, C, and D) or the duration of time indicated (E), and iodide uptake assay or RT-PCR of NIS was performed. A, effects of p38 kinase inhibitors SB203580 (10 μm), SB239063 (10 μm), and ML3403 (10 μm); MEK inhibitors PD98059 (10 μm) and U0126 (10 μm); and the ERK inhibitor (ERKi) (10 μm) on iodide uptake. B, pharmacological inhibitors of MAPK cascades used in this study. C and D, dose-dependent inhibition of SB203580 (C) and SB239063 (D) on the tRA-induced iodide uptake. E, time course of NIS mRNA induction by tRA with or without SB203580 (10 μm) or SB239063 (10 μm), relative to GAPDH. The samples with only tRA at 12 h were used as standards for quantification in the RT-PCR. Values are the mean ± S.D. (error bars) (n = 3). *, p < 0.01; **, p < 0.05, compared with the group with only tRA (A, C, D, and E) at each time point (E).
Differential Effects of p38 Inhibitors on Iodide Uptake in MCF-7 Cells and FRTL-5 Cells
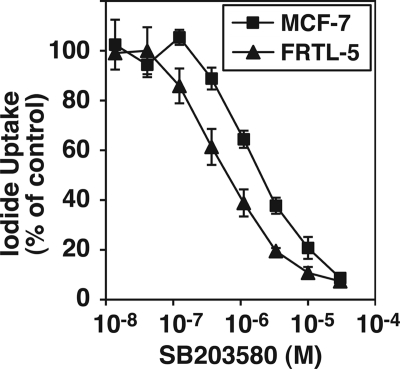
TSH receptor/cAMP signaling, critical for the maintenance of NIS expression in thyroid cells, up-regulates the p38 MAPK cascade (15, 21). Inhibition of p38, by SB203580 and ML3403, significantly reduced NIS expression in FRTL-5 cells (17, 21). Because the IC50 for p38 inhibition of iodide uptake in MCF-7 cells was relatively high, we compared the kinetics of the inhibitory effect of SB203580 in MCF-7 cells with that in FRTL-5 rat thyroid cells. The IC50 in FRTL-5 cells, 0.65 ± 0.01 μm, was significantly lower than that in MCF-7 cells, 5.17 ± 0.12 μm (Fig. 2).
FIGURE 2.
Comparison of dose-dependent effects of the p38 kinase inhibitor SB203580 on iodide uptake in MCF-7 cells with those in FRTL-5 cells. MCF-7 cells were treated with tRA (1 μm) and the indicated concentration of SB203580 for 24 h. FRTL-5 cells, maintained in the growth medium (with 10 μg/ml of bovine insulin and 1 milliunit/ml bovine TSH), were treated with the indicated concentration of SB203580 for 24 h. Iodide uptake was normalized by the cellular protein amount in each well. Data are shown as percentage of control (iodide uptake without inhibitor was set at 100%). Values are the mean ± S.D. (error bars) (n = 3).
The IC50 of SB203580 required to inhibit p38α activity was more than 10 times lower than that reported for p38β inhibition (29). We therefore determined if the difference in IC50 for p38α inhibition between MCF-7 and FRTL-5 cells was due to the differential expression of p38 isoforms. We evaluated the endogenous expression profile of p38 isoforms in these cell lines by quantitative RT-PCR. In MCF-7 cells, all p38 isoforms were abundantly expressed with a relative ranking of α > δ > γ > β (Table 1), consistent with a previous report (24). In FRTL-5 cells, there was abundant expression of p38α and -δ, modest expression of p38γ, and undetectable p38β mRNA (Table 1). The expression of predominantly p38α and -δ isoforms in FRTL5 cells is consistent with the pattern of expression reported in rat thyroid tissue (32). These data indicate that p38α is most likely essential for NIS expression in FRTL-5 cells.
TABLE 1.
mRNA expression of p38 isoforms in MCF-7 cells and FRTL-5 cells
MCF-7 cells were treated with or without tRA (1 μm) for 12 h. FRTL-5 cells were maintained with TSH and insulin. For comparison among isoforms and cell lines, expression of each gene was quantified with standard curves of GAPDH in each cell line and condition. Values shown are arbitrary units (measured p38 isoform relative to GAPDH × 104), indicating the mean ± S.D. (n = 3).
| MCF-7 |
FRTL5 | ||
|---|---|---|---|
| Without tRA | With tRA | ||
| p38α | 57.9 ± 3.36 | 46.9 ± 7.15 | 122 ± 34.5 |
| p38β | 1.31 ± 0.04 | 1.66 ± 0.05 | Undetectable |
| p38γ | 4.98 ± 0.53 | 4.34 ± 0.28 | 0.07 ± 0.01 |
| p38δ | 18.0 ± 0.50 | 8.19 ± 0.37 | 110 ± 4.08 |
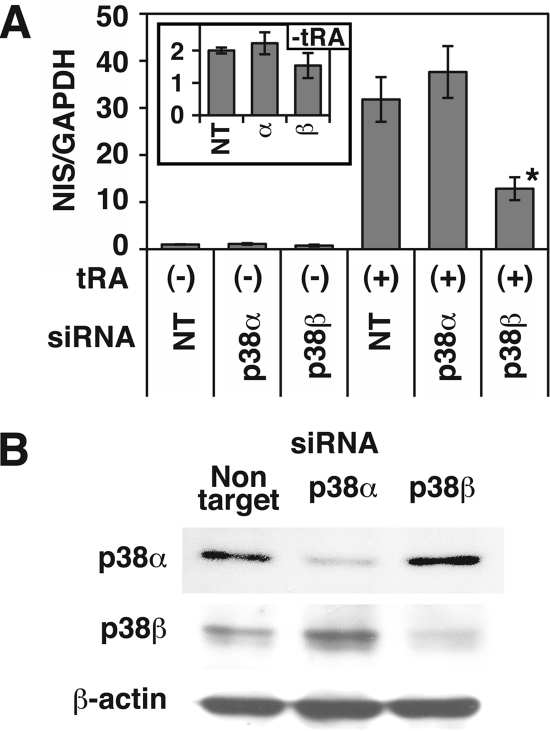
Effects of Selective siRNA Knockdown of p38 Isoforms on the NIS mRNA Expression in MCF-7 Cells
Our analysis with pharmacologic inhibitors indicate the importance of p38α and/or p38β for NIS expression in MCF-7 cells. To determine which isoform of p38 is critical, we utilized siRNA to knock down the isoforms in MCF-7 cells. The transfection of p38β-specific siRNA, but not p38α siRNA, significantly decreased tRA-induced NIS mRNA expression in MCF-7 cells (∼60% reduction compared with non-targeting siRNA; Fig. 3A). The siRNA knockdown of p38β also modestly decreased (∼24% reduction) the basal NIS expression before tRA treatment (Fig. 3A). Although the p38β protein sequence has ∼70% homology with p38α (22), the isoform-selective siRNAs reduced expression of only the targeted isoform (Fig. 3B). These data demonstrate that p38β, but not p38α, is required for full expression of NIS in MCF-7 cells.
FIGURE 3.
Effects of siRNA targeting to each p38 isoform on the tRA-induced NIS mRNA expression in MCF-7 cells. Cells were transfected with SMARTpool siRNA targeted to p38α or p38β or non-targeting siRNA (NT), grown in 6-well plates, and treated with or without tRA (1 μm) for 12 h. A, results of quantitative RT-PCR of NIS. The samples with both non-targeting siRNA and tRA were used as standards for quantification. The inset graph shows results without tRA treatment. Values are the mean ± S.D. (error bars) (n = 3). *, p < 0.01, compared with the group with non-targeting siRNA and tRA treatment. B, Western blot analysis of whole cell extracts with anti-p38α Ab, anti-p38β Ab (P38-11A5), and anti-β-actin Ab. Cells were harvested 60 h after transfection.
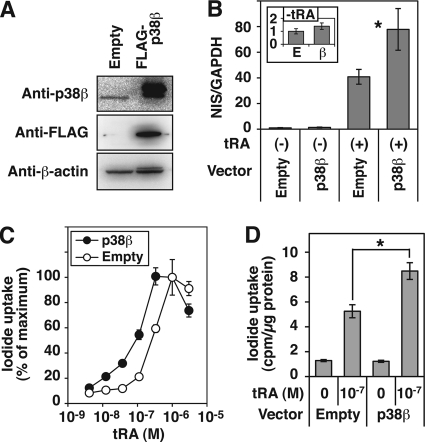
Overexpression of p38β Enhances NIS Expression in MCF-7 Cells
To investigate if a gain of p38β function influences NIS expression, we established a stable transfectant of FLAG-tagged p38β in MCF-7 cells with G418 as a selection marker (Fig. 4A). The constitutive expression of p38β consistently increased the NIS mRNA expression in MCF-7 cells, both with and without tRA treatment (∼1.9- and ∼1.4-fold, respectively), although the induction without tRA was not statistically significant (Fig. 4B). The sensitivity to tRA for the induction of iodide uptake was significantly enhanced by overexpression of p38β; the EC50 of tRA was 9.58 × 10−8 m with pCMV6-p38β, whereas that with empty vector was 4.34 × 10−7 m (Fig. 4C). Iodide uptake in the p38β-overexpressing cells, after treatment with 10−7 m tRA, was significantly higher than that in cells transfected with the empty vector (Fig. 4D).
FIGURE 4.
Effects of constitutive expression of p38β on NIS expression in MCF-7 cells. Cells were transfected with pCMV6-Myc/FLAG-p38β (FLAG-p38β) or pCMV6-Entry (Empty) and selected in G418 for 3 weeks. A, Western blot analysis of whole cell extracts with anti-p38β Ab (C28C2), anti-FLAG tag Ab, and anti-β-actin Ab. B, quantitative RT-PCR of NIS in the cells treated with or without tRA (1 μm) for 24 h. The inset graph shows results without tRA treatment. Values are the mean ± S.D. (error bars) (n = 4). *, p < 0.05, compared with the group with an empty vector. C and D, iodide uptake in the cells treated with the indicated concentration of tRA for 60 h. Radioactivity of intracellular 125I was normalized by cellular protein amount in each well. Data are shown as percentage of control in C (iodide uptake with 10−6 m tRA was set at 100%). In D, iodide uptake in cells treated with 10−7 m tRA is shown. Values are the mean ± S.D. (n = 3). *, p < 0.05.
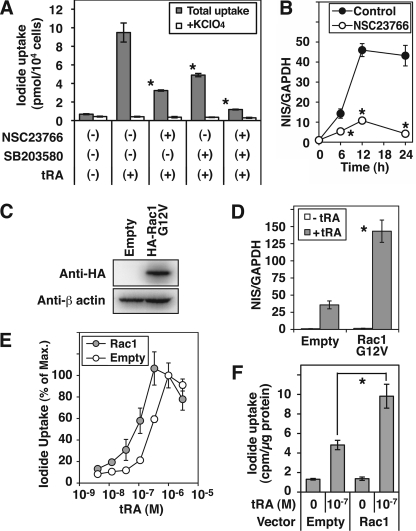
Rac1 Regulates NIS Expression in MCF-7 Cells
The activity of the p38 MAPK cascade is regulated by tRA through Rac1 (18). To investigate if Rac1 plays an important role in NIS expression in MCF-7 cells, we added a pharmacological Rac1 inhibitor, NSC23766. tRA-induced iodide uptake was significantly decreased by NSC23766 (∼66% reduction compared with tRA only), and the inhibitory effect was enhanced by the addition of the p38 inhibitor SB203580 (Fig. 5A). Induction of NIS mRNA by tRA was also significantly decreased by the Rac1 inhibitor NSC23766 (Fig. 5B) at every time point tested (76% reduction after 12 h of treatment with tRA). A constitutively active mutant of Rac1, Rac1 G12V, stably transfected in MCF-7 cells (Fig. 5C), significantly increased the tRA-induced NIS mRNA expression (3.4-fold compared with empty vector; Fig. 5D). The sensitivity of iodide uptake induction to tRA was significantly increased by Rac1 G12V. The EC50 of tRA (3.03 × 10−7 m) was significantly decreased to 9.57 × 10−8 m by the Rac1 activation (Fig. 5E). The iodide uptake in Rac1-overexpressing cells, treated with 10−7 m tRA, was significantly higher than that in cells transfected with the empty vector (Fig. 5F). These data indicate a critical role of Rac1 in the tRA-induced NIS expression in MCF-7 cells.
FIGURE 5.
Regulation of NIS expression by Rac1 in MCF-7 cells. A and B, effects of a Rac1 inhibitor, NSC23766, on NIS expression. A, iodide uptake in cells treated with tRA (1 μm), NSC23766 (50 μm), and/or p38 kinase inhibitor SB203580 (10 μm) for 24 h. *, p < 0.01, compared with the group with only tRA (n = 3). B, quantitative RT-PCR of NIS in tRA-stimulated MCF-7 cells treated with or without NSC23766 (50 μm) for the indicated time. *, p < 0.01, compared with the group without NSC23766 at each time point. C–E, effects of exogenous expression of constitutively active Rac1 on the NIS expression in MCF-7 cells. Cells were transfected with pcDNA3.1–3xHA-Rac1 G12V or pcDNA3.1+ (Empty) and selected in G418 for 3 weeks. C, Western blot analysis of whole cell lysates with anti-HA tag Ab and anti-β-actin Ab. D, quantitative RT-PCR of NIS in the cells treated with or without tRA (1 μm) for 24 h. *, p < 0.01, compared with the group of empty vector. E and F, iodide uptake in the cells treated with the indicated concentration of tRA for 60 h. Radioactivity of intracellular 125I was normalized by the cellular protein amount in each well. Data are shown as percentage of control in E (iodide uptake with 10−6 m tRA was set at 100%). In F, iodide uptake in cells treated with 10−7 m tRA is shown. Values are the mean ± S.D. (error bars) (n = 3). *, p < 0.03.
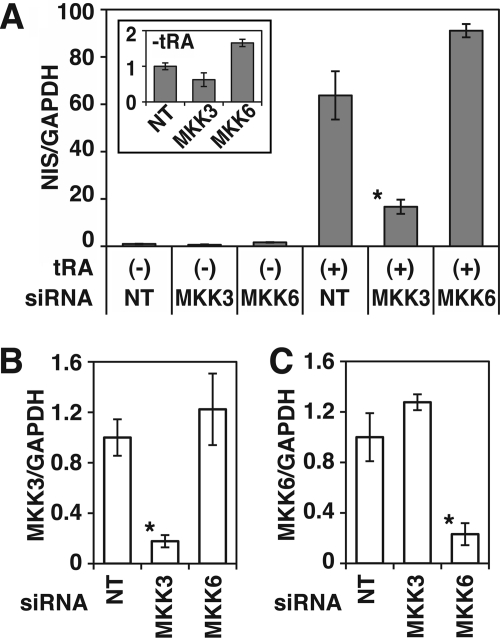
MKK3B Is Important for NIS Expression in MCF-7 Cells
p38 is a substrate of MKK3 and MKK6. To investigate which MKK contributes to NIS expression, we evaluated the effects of selective siRNA knockdown of MKKs on NIS mRNA expression in MCF-7 cells. The knockdown of MKK3, but not MKK6, significantly decreased tRA-induced NIS mRNA expression (∼74% reduction; Fig. 6A). NIS mRNA expression in MCF-7 cells without tRA treatment was also reduced by MKK3 siRNA (∼38% reduction), but the change was not significant, consistent with the p38β siRNA (Fig. 3).
FIGURE 6.
Effects of siRNA knockdown of MKK3 and MKK6 mRNA on NIS expression in MCF-7 cells. Cells were transfected with Stealth RNAi to MKK3 or MKK6 or non-targeting siRNA (NT), grown in 6-well plates, and treated with or without tRA (1 μm) for 12 h. A, results of quantitative RT-PCR of NIS. The samples with both tRA and non-targeting siRNA were used as standards for quantification. The inset graph shows results without tRA treatment. *, p < 0.01, compared with the group with both non-targeting siRNA and tRA treatment (n = 3). B and C, quantitative RT-PCR of MKK3 (B) and MKK6 (C) in the cells 60 h after the beginning of transfection. *, p < 0.01, compared with the group of non-targeting siRNA (n = 3).
Two variants of MKK3, MKK3A and -B, have been reported (33). MKK3B is an alternatively spliced form of MKK3A with an additional 29 amino acids fused to the N-terminal end of MKK3A. MKK3A selectively activates p38α, whereas MKK3B activates both p38α and -β (33). The siRNA used in our knockdown studies targeted both MKK3A and -B. Our quantitative RT-PCR indicated abundant expression of MKK3B as well as modest expression of MKK3A in MCF-7 cells, both with and without tRA treatment (Table 2). These data indicate that among variants of MKK3 and MKK6, the MKK3B is most important for NIS expression in MCF-7 cells.
TABLE 2.
mRNA expression of MKK3 variants in MCF-7 cells
Cells were treated with or without tRA (1 μm) for 12 h, and quantitative RT-PCR was carried out. Expression of MKK3 variants was quantified with standard curves of GAPDH in each condition. Values shown are arbitrary units (measured MKK3 variants relative to GAPDH × 105), indicating the mean ± S.D. (n = 3).
| Without tRA | With tRA | |
|---|---|---|
| MKK3A | 0.03 ± 0.01 | 0.06 ± 0.01 |
| MKK3B | 43.7 ± 2.90 | 71.9 ± 5.81 |
tRA Induces Phosphorylation of p38β in MCF-7 Cells
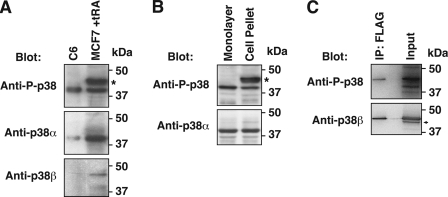
tRA induces the phosphorylation of p38 in MCF-7 cells (18). The commercially available anti-phospho-p38 Abs recognize the common activation site motif contained in all four p38 isoforms. Previous studies of phosphorylation of p38, therefore, have not identified specific isoforms. We determined that Western blot of cell extracts prepared from cell pellet identified phosphorylated p38α as well as other isoforms. Only phospho-p38α was detected when the lysis buffer was directly added to monolayer cells (Fig. 7). Our study demonstrated that tRA treatment (1 μm) stimulated phospho-p38α at 0.5 h, whereas phosphorylation of other p38 isoforms was not induced by tRA until 12 h or later (Fig. 8A).
FIGURE 7.
Detection of phosphorylated p38β in Western blot analysis. The anti-phospho-p38 Abs (rabbit polyclonal Ab and rabbit monoclonal D3F9 Ab) recognizes the sequence around phosphorylation sites of Thr-180/Tyr-182, the common activation site motif among four p38 isoforms. Expected molecular masses of these isoforms are as follows: α, 40 kDa; β and δ, 43 kDa; γ, 46 kDa. A, detection of phosphorylated p38α and other isoforms in MCF-7 cells. Whole cell extracts from MCF-7 cells treated with tRA (1 μm) for 24 h as well as control cell extracts of anisomycin-treated C6 cells (Cell Signaling Technology) were applied to Western blot with the indicated Abs. C6 cells express abundant p38α, but not p38β. In tRA-treated MCF-7 cells, a broad band around 43–46 kDa (denoted by the asterisk), corresponding to p38β, -γ, and -δ, and a narrow band at 40 kDa, corresponding to p38α, were observed with the anti-phospho-p38 polyclonal Ab. Blotting with anti-p38β Ab (P38-11A5) indicated the major band at 43 kDa, corresponding to the upper broad band of phosphorylated p38. B, differences in the Western blot analysis with anti-phospho-p38 polyclonal Ab is dependent on the methods of cell extract preparation. Monolayer MCF-7 cells were directly lysed in a culture dish with SDS sample buffer containing 2% SDS, as described under “Experimental Procedures.” The harvested cell pellet was lysed with the lysis buffer containing 1% Triton X-100, as described under “Experimental Procedures.” The broad band of 43–46 kDa (denoted by the asterisk), probably including phospho-p38β, was not visualized with cell extracts directly prepared from monolayer cells. C, Western blot analysis of FLAG-tagged p38β expressed in MCF-7 cells. Cells were transfected with Myc/FLAG-tagged p38β-expressing pCMV6 vector and treated with tRA (1 μm) for 24 h. Proteins immunoprecipitated by anti-FLAG tag Ab (IP), or whole cell extracts before the immunoprecipitation (input), were applied to Western blot analysis with the indicated Abs. Phospho-p38β with the Myc/FLAG tag migrated around 46 kDa (top), whereas endogenous p38β migrated around 43 kDa as a minor band (indicated by the arrow) in the whole cell extracts (bottom). The predicted molecular mass of the Myc/FLAG tag is 3,620 Da. The result with anti-phospho-p38 Ab (D3F9), shown here, was reproduced with the anti-phospho-p38 polyclonal Ab.
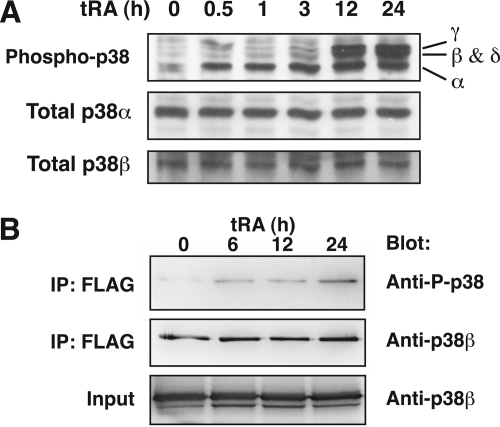
FIGURE 8.
Phosphorylation of p38β in tRA-treated MCF-7 cells. Anti-phosphorylated p38 (P-p38) polyclonal Ab recognizes the common phosphorylation site motif among four p38 isoforms; anti-p38α Ab and anti-p38β Ab (P38–11A5) were used for Western blotting. A, Western blot analysis of whole cell lysates from cells treated with tRA (1 μm) for the indicated time. The upper broad band, at 12 and 24 h, consists of at least two closely migrating bands corresponding to p38γ and p38β/δ. B, immunoprecipitation assay of phosphorylated p38β in MCF-7 cells. Cells were transfected with pCMV-Myc/FLAG-p38β and treated with tRA (1 μm) for the indicated time. Immunoprecipitated proteins with anti-FLAG Ab (IP) as well as whole cell extracts (Input) were applied to Western blot analysis with the indicated Abs.
p38β and -δ migrate at ∼43 kDa, so we could not detect the phosphorylation of each isoform individually in the Western blot analysis using the pan-phospho-p38 Ab. In our preliminary experiments, the expression level of endogenous p38β in MCF-7 cells was not sufficient to immunoprecipitate with anti-p38β Ab (data not shown). We therefore transfected the expression vector Myc/FLAG-tagged p38β into MCF-7 cells and isolated the exogenous p38β by immunoprecipitation with anti-FLAG tag Ab after tRA treatment. The phosphorylation of exogenous p38β in MCF-7 cells was induced 6 h or longer after treatment with tRA (1 μm) (Fig. 8B), consistent with the Western blot analysis with the pan-phospho-p38 Ab (Fig. 8A). These data indicate induction of phosphorylated p38β by tRA, although longer stimulation is required for the induction compared with phosphorylated p38α.
Rac1 Regulates p38β in MCF-7 Cells
Our gain and loss of function studies demonstrated critical roles for p38β, MKK3B, and Rac1 in the induction of NIS expression in MCF-7 cells. Although tRA stimulates phosphorylation of p38 through Rac1 in MCF-7 cells (18), selective regulation of p38β by Rac1 has not been reported. We therefore studied if the Rac1 signaling regulates p38β activity in MCF-7 cells.
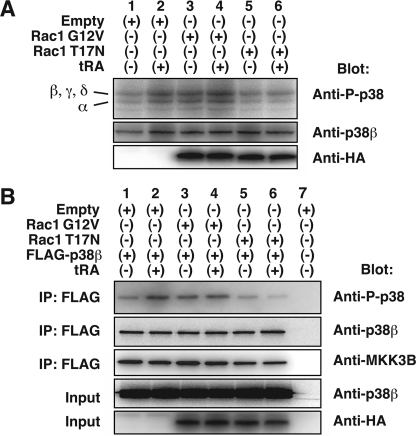
Exogenous Rac1 G12V expression enhanced the expression of phosphorylated p38 isoforms, probably including p38β, in the absence of tRA (Fig. 9A, lanes 1 and 3). The tRA-induced phosphorylation of p38 isoforms was also enhanced (Fig. 9, lanes 2 and 4). A dominant negative mutant of Rac1, Rac1 T17N, abolished the induction of phosphorylated p38 isoforms by tRA (Fig. 9A, lanes 2 and 6). The inhibitory effects of Rac1 T17N were confirmed with the exogenous FLAG-tagged p38β (Fig. 9B, lanes 5 and 6 compared with lanes 1 and 2, respectively). The stimulatory effect of Rac1 G12V on the FLAG-tagged p38β phosphorylation was observed without tRA treatment (Fig. 9B, lanes 1 and 3) whereas the enhancement by Rac1 G12V of tRA-stimulated p38β phosphorylation was not observed (Fig. 9B, lanes 2 and 4).
FIGURE 9.
Regulation of p38β by Rac1 in MCF-7 cells. A, Western blot analysis of whole cell extracts with anti-phosphorylated p38 Ab (D3F9), anti-p38β Ab (C28C2), and anti-HA tag Ab. Cells were transfected with pcDNA3.1–3xHA-Rac1 G12V, pcDNA3.1–3xHA-Rac1 T17N, or pcDNA3.1+ (Empty) and treated with or without tRA (1 μm) for 12 h. B, immunoprecipitation assay of phosphorylated p38β in MCF-7 cells expressing exogenous Rac1 mutants. Cells were transfected with 2 μg/dish of pCMV-Myc/FLAG-p38β or pCMV6-Entry in combination with 4 μg/dish of pcDNA3.1–3xHA-Rac1 G12V, pcDNA3.1–3xHA-Rac1 T17N, or pcDNA3.1+ (Empty) and treated with tRA (1 μm) for 12 h. Proteins immunoprecipitated with anti-FLAG Ab (IP), as well as whole cell extracts (Input), were applied to Western blot analysis with the indicated Abs.
Our loss of function studies identified a requirement of MKK3B for NIS gene regulation. Although activation of p38β by MKK3B has been reported, physical interaction between these kinases has not been studied in MCF-7 cells. We therefore investigated if MKK3B was co-immunoprecipitated with p38β. Endogenous MKK3B was co-immunoprecipitated by anti-FLAG Ab with FLAG-tagged p38β expressed in MCF-7 cells (Fig. 9B). The constitutively active Rac1 G12V or tRA treatment, however, did not clearly increase the amount of co-immunoprecipitated MKK3B, whereas the inhibitory effects by Rac1T17N were still reproduced (Fig. 9B, lanes 5 and 6). These data indicate that MKK3B physically interacts with p38β in MCF-7 cells. The amount of co-immunoprecipitated MKK3B, however, did not change with tRA treatment, Rac1 activator, or Rac1 inhibitor.
DISCUSSION
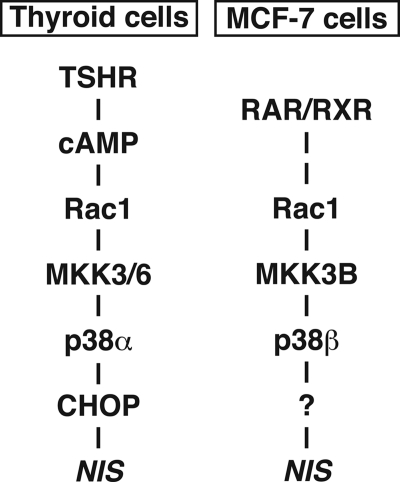
In this study, we demonstrated the importance of Rac1, MKK3B, and p38β for NIS expression in MCF-7 breast cancer cells. tRA up-regulated p38β phosphorylation through Rac1 and MKK3B. The induction of NIS by tRA was, at least partially, through the Rac1-MKK3B-p38β pathway. NIS in thyroid cells was also regulated by p38 signaling; however, p38α, and not p38β, was critical for the expression of thyroid NIS.
In thyroid cells, several signaling pathways downstream of cAMP, such as PKA-CREB, Ref1-PAX8, and Rac1-p38, contribute to maintenance of differentiated function of thyroid, such as iodide uptake by NIS (15). cAMP signaling activates p38 (21) and its downstream effector, CCAAT/enhancer-binding protein-homologous protein (CHOP) (34), through Rac1, MKK3, and/or MKK6 (21). Overexpression of CHOP transcriptionally enhances NIS expression in FRTL-5 cells (34). Our pharmacologic and gene expression analysis demonstrated the importance of p38α for expression of NIS in thyroid. The signaling pathway of cAMP-Rac1-MKK3/6-p38α-CHOP, therefore, contributes to NIS expression in thyroid cells (Fig. 10).
FIGURE 10.
Proposed p38 signal transduction pathways in thyroid cells and MCF-7 breast cancer cells based on data from the present study and previous reports (21, 34).
We demonstrated that NIS expression in MCF-7 breast cancer cells was dependent on p38β but not p38α. MKK6 activates the NIS-inducible CHOP in thyroid cells (34), whereas MKK3B, but not MKK6, was critical for NIS expression in MCF-7. Moreover, our preliminary study showed that overexpression of CHOP did not significantly increase NIS expression in MCF-7 cells.4 Although the Rac1-p38 pathway stimulates NIS expression in both thyroid cells (21, 34) and MCF-7 breast cancer cells, the Rac1 signals diverge into at least two pathways through distinct isoforms of p38, probably resulting in differential regulation of NIS in these cell types (Fig. 10).
Our previous pharmacological study has demonstrated various regulatory mechanisms for NIS expression involving several signaling pathways in MCF-7 cells (17). A phosphoinositide 3-kinase inhibitor, LY294002, significantly reduces tRA-induced NIS expression, but not basal expression, in MCF-7 cells (17). Retinoic acid stimulation of NIS involved direct interaction of an RAR-RXR heterodimer with phosphoinositide 3-kinase (16). In contrast, inhibition of p38 reduces NIS at base line and in tRA-stimulated MCF-7 cells (17). Loss-of-function experiments of p38β, as well as MKK3B, demonstrate a modest reduction of basal NIS expression in MCF-7 cells but very significant reduction of tRA-induced NIS expression. Overexpression of p38β, as well as Rac1, significantly enhanced tRA induction of NIS expression, consistent with the activation of Rac1 (18) and p38β by tRA. These results indicate that p38β probably regulates NIS expression through both retinoic acid signaling-dependent and -independent mechanisms.
Four isoforms of p38 are endogenously expressed in MCF-7 cells (24), confirmed by our RT-PCR. The size of p38α, ∼40 kDa, is smaller than those of other isoforms, 43–46 kDa. Because activation of p38 is dependent on dual phosphorylation of the Thr-Gly-Tyr motif in the T-loop (35), anti-phospho-p38 Abs have been developed to recognize the Thr-Gly-Tyr dual phosphorylation site, common among all p38 isoforms. A previous study using such a pan-phospho-p38 Ab, however, showed a single band for the phosphorylated p38 in MCF-7 cells (18), probably corresponding to p38α. Our Western blot analysis, performed with MCF-7 cell extracts prepared from the cell pellet, showed multiple bands, corresponding to each isoform, with a polyclonal pan-phospho-p38 Ab, as well as a monoclonal pan-phospho-p38 Ab. In contrast, experiments with cell extracts prepared directly from monolayer cells produced a single band, corresponding to p38α, even with the same polyclonal phospho-p38 Ab. To analyze the phosphorylated p38 β-δ in MCF-7 cells by Western blotting, cells need to be pelleted before lysing. Caution is required in analysis of p38 phosphorylation with anti-phospho-p38 Ab, especially if the cells express multiple p38 isoforms.
The treatment of MCF-7 cells with tRA followed by β-emitting 131I results in significant cytotoxicity in vitro (11, 14, 36). Our study with mouse breast cancer models, however, showed that the dose of tRA for the maximum induction of NIS in the tumors is probably higher than would be tolerated for human treatment (13). Retinoic acid signaling regulates a wide variety of physiological events by stimulating signal transduction pathways as well as transcription of a large number of target genes. Indeed, tRA activated not only the NIS-inducing p38β but also other isoforms of p38, which were not required for the full induction of NIS. Targeted stimulation of signaling pathways for NIS expression should promote more efficient induction of radioiodide uptake, with less toxicity, in some breast cancer tissues. The Rac1-MKK3B-p38β signaling is one such pathway, as suggested by our overexpression experiments of Rac1 and p38β in MCF-7 cells.
Most studies on p38 signaling have focused on the p38α isoform, especially its responses to stress and inflammation. p38β is thought to be a minor pathway in immune system or stress signaling (37). Deficiency of p38β in mice actually causes no apparent phenotype, probably due to redundant functions among the isoforms (37). Increasing evidence points to an association of p38α with cell proliferation, malignant transformation, and tumor invasion in cancer cells, whereas the role of p38β in breast cancer is not established (1). A number of in vitro studies, however, have demonstrated that each isoform of p38 activates different downstream effectors (22–24). We demonstrate here that a retinoic acid-regulated p38β signal transduction pathway stimulates NIS expression, a promising target for breast cancer therapy. Confirmation of this p38β isoform specificity in additional in vitro and in vivo breast cancer models will be important. Dissection of isoform-specific signaling pathways is a potential novel strategy for breast cancer treatment.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 CA089364 (to G. A. B.). This work was also supported by Veterans Affairs Merit Review Funds.
T. Kogai and G. A. Brent, unpublished observation.
- NIS
- sodium iodide symporter
- Ab
- antibody
- CHOP
- CCAAT/enhancer-binding protein-homologous protein
- CREB
- cAMP-response element-binding protein
- MKK
- MAPK kinase
- RAR
- retinoic acid receptor
- RXR
- retinoid X receptor
- TSH
- thyroid-stimulating hormone
- tRA
- all-trans-retinoic acid.
REFERENCES
- 1. Wagner E. F., Nebreda A. R. (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 [DOI] [PubMed] [Google Scholar]
- 2. Dohán O., De la Vieja A., Paroder V., Riedel C., Artani M., Reed M., Ginter C. S., Carrasco N. (2003) The sodium/iodide symporter (NIS). Characterization, regulation, and medical significance. Endocr. Rev. 24, 48–77 [DOI] [PubMed] [Google Scholar]
- 3. Trujillo M. A., Oneal M. J., McDonough S., Qin R., Morris J. C. (2010) A probasin promoter, conditionally replicating adenovirus that expresses the sodium iodide symporter (NIS) for radiovirotherapy of prostate cancer. Gene Ther. 17, 1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penheiter A. R., Wegman T. R., Classic K. L., Dingli D., Bender C. E., Russell S. J., Carlson S. K. (2010) Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am. J. Roentgenol. 195, 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riesco-Eizaguirre G., De la Vieja A., Rodríguez I., Miranda S., Martín-Duque P., Vassaux G., Santisteban P. (2011) Telomerase-driven expression of the sodium iodide symporter (NIS) for in vivo radioiodide treatment of cancer. A new broad-spectrum NIS-mediated antitumor approach. J. Clin. Endocrinol. Metab. 96, E1435–E1443 [DOI] [PubMed] [Google Scholar]
- 6. Klutz K., Willhauck M. J., Wunderlich N., Zach C., Anton M., Senekowitsch-Schmidtke R., Göke B., Spitzweg C. (2011) Sodium Iodide Symporter (NIS)-Mediated Radionuclide (131I, 188Re) Therapy of Liver Cancer After Transcriptionally Targeted Intratumoral in Vivo NIS Gene Delivery. Hum. Gene Ther. 22, 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tazebay U. H., Wapnir I. L., Levy O., Dohan O., Zuckier L. S., Zhao Q. H., Deng H. F., Amenta P. S., Fineberg S., Pestell R. G., Carrasco N. (2000) The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 6, 871–878 [DOI] [PubMed] [Google Scholar]
- 8. Wapnir I. L., van de Rijn M., Nowels K., Amenta P. S., Walton K., Montgomery K., Greco R. S., Dohán O., Carrasco N. (2003) Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J. Clin. Endocrinol. Metab. 88, 1880–1888 [DOI] [PubMed] [Google Scholar]
- 9. Welcsh P. L., Mankoff D. A. (2000) Taking up iodide in breast tissue. Nature 406, 688–689 [DOI] [PubMed] [Google Scholar]
- 10. Boelaert K., Franklyn J. A. (2003) Sodium iodide symporter. A novel strategy to target breast, prostate, and other cancers? Lancet 361, 796–797 [DOI] [PubMed] [Google Scholar]
- 11. Kogai T., Schultz J. J., Johnson L. S., Huang M., Brent G. A. (2000) Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc. Natl. Acad. Sci. U.S.A. 97, 8519–8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanosaki S., Ikezoe T., Heaney A., Said J. W., Dan K., Akashi M., Koeffler H. P. (2003) Effect of ligands of nuclear hormone receptors on sodium/iodide symporter expression and activity in breast cancer cells. Breast Cancer Res. Treat. 79, 335–345 [DOI] [PubMed] [Google Scholar]
- 13. Kogai T., Kanamoto Y., Che L. H., Taki K., Moatamed F., Schultz J. J., Brent G. A. (2004) Systemic retinoic acid treatment induces sodium/iodide symporter expression and radioiodide uptake in mouse breast cancer models. Cancer Res. 64, 415–422 [DOI] [PubMed] [Google Scholar]
- 14. Kogai T., Kanamoto Y., Li A. I., Che L. H., Ohashi E., Taki K., Chandraratna R. A., Saito T., Brent G. A. (2005) Differential regulation of sodium/iodide symporter gene expression by nuclear receptor ligands in MCF-7 breast cancer cells. Endocrinology 146, 3059–3069 [DOI] [PubMed] [Google Scholar]
- 15. Kogai T., Taki K., Brent G. A. (2006) Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr. Relat. Cancer 13, 797–826 [DOI] [PubMed] [Google Scholar]
- 16. Ohashi E., Kogai T., Kagechika H., Brent G. A. (2009) Activation of the PI3 kinase pathway by retinoic acid mediates sodium/iodide symporter induction and iodide transport in MCF-7 breast cancer cells. Cancer Res. 69, 3443–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kogai T., Ohashi E., Jacobs M. S., Sajid-Crockett S., Fisher M. L., Kanamoto Y., Brent G. A. (2008) Retinoic acid stimulation of the sodium/iodide symporter in MCF-7 breast cancer cells is mediated by the insulin growth factor-I/phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase signaling pathways. J. Clin. Endocrinol. Metab. 93, 1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsayed Y., Uddin S., Mahmud N., Lekmine F., Kalvakolanu D. V., Minucci S., Bokoch G., Platanias L. C. (2001) Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J. Biol. Chem. 276, 4012–4019 [DOI] [PubMed] [Google Scholar]
- 19. Kogai T., Endo T., Saito T., Miyazaki A., Kawaguchi A., Onaya T. (1997) Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology 138, 2227–2232 [DOI] [PubMed] [Google Scholar]
- 20. Schmutzler C., Winzer R., Meissner-Weigl J., Köhrle J. (1997) Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem. Biophys. Res. Commun. 240, 832–838 [DOI] [PubMed] [Google Scholar]
- 21. Pomerance M., Abdullah H. B., Kamerji S., Correze C., Blondeau J. P. (2000) Thyroid-stimulating hormone and cyclic AMP activate p38 mitogen-activated protein kinase cascade. Involvement of protein kinase A, rac1, and reactive oxygen species. J. Biol. Chem. 275, 40539–40546 [DOI] [PubMed] [Google Scholar]
- 22. Jiang Y., Chen C., Li Z., Guo W., Gegner J. A., Lin S., Han J. (1996) Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271, 17920–17926 [DOI] [PubMed] [Google Scholar]
- 23. Conrad P. W., Rust R. T., Han J., Millhorn D. E., Beitner-Johnson D. (1999) Selective activation of p38α and p38γ by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J. Biol. Chem. 274, 23570–23576 [DOI] [PubMed] [Google Scholar]
- 24. Pramanik R., Qi X., Borowicz S., Choubey D., Schultz R. M., Han J., Chen G. (2003) p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J. Biol. Chem. 278, 4831–4839 [DOI] [PubMed] [Google Scholar]
- 25. Kogai T., Kanamoto Y., Brent G. A. (2003) The modified firefly luciferase reporter gene (luc+) but not Renilla luciferase is induced by all-trans-retinoic acid in MCF-7 breast cancer cells. Breast Cancer Res. Treat. 78, 119–126 [DOI] [PubMed] [Google Scholar]
- 26. Kogai T., Sajid-Crockett S., Newmarch L. S., Liu Y. Y., Brent G. A. (2008) Phosphoinositide 3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J. Endocrinol. 199, 243–252 [DOI] [PubMed] [Google Scholar]
- 27. Weiss S. J., Philp N. J., Grollman E. F. (1984) Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology 114, 1090–1098 [DOI] [PubMed] [Google Scholar]
- 28. Barros L. F., Young M., Saklatvala J., Baldwin S. A. (1997) Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin. p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J. Physiol. 504, 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eyers P. A., Craxton M., Morrice N., Cohen P., Goedert M. (1998) Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino acid substitution. Chem. Biol. 5, 321–328 [DOI] [PubMed] [Google Scholar]
- 30. Ju H., Nerurkar S., Sauermelch C. F., Olzinski A. R., Mirabile R., Zimmerman D., Lee J. C., Adams J., Sisko J., Berova M., Willette R. N. (2002) Sustained activation of p38 mitogen-activated protein kinase contributes to the vascular response to injury. J. Pharmacol. Exp. Ther. 301, 15–20 [DOI] [PubMed] [Google Scholar]
- 31. Munoz L., Ramsay E. E., Manetsch M., Ge Q., Peifer C., Laufer S., Ammit A. J. (2010) Novel p38 MAPK inhibitor ML3403 has potent anti-inflammatory activity in airway smooth muscle. Eur. J. Pharmacol. 635, 212–218 [DOI] [PubMed] [Google Scholar]
- 32. Wang X. S., Diener K., Manthey C. L., Wang S., Rosenzweig B., Bray J., Delaney J., Cole C. N., Chan-Hui P. Y., Mantlo N., Lichenstein H. S., Zukowski M., Yao Z. (1997) Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J. Biol. Chem. 272, 23668–23674 [DOI] [PubMed] [Google Scholar]
- 33. Enslen H., Brancho D. M., Davis R. J. (2000) Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 19, 1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pomerance M., Carapau D., Chantoux F., Mockey M., Correze C., Francon J., Blondeau J. P. (2003) CCAAT/enhancer-binding protein-homologous protein expression and transcriptional activity are regulated by 3′,5′-cyclic adenosine monophosphate in thyroid cells. Mol. Endocrinol. 17, 2283–2294 [DOI] [PubMed] [Google Scholar]
- 35. Raingeaud J., Gupta S., Rogers J. S., Dickens M., Han J., Ulevitch R. J., Davis R. J. (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270, 7420–7426 [DOI] [PubMed] [Google Scholar]
- 36. Unterholzner S., Willhauck M. J., Cengic N., Schütz M., Göke B., Morris J. C., Spitzweg C. (2006) Dexamethasone stimulation of retinoic acid-induced sodium iodide symporter expression and cytotoxicity of 131I in breast cancer cells. J. Clin. Endocrinol. Metab. 91, 69–78 [DOI] [PubMed] [Google Scholar]
- 37. Beardmore V. A., Hinton H. J., Eftychi C., Apostolaki M., Armaka M., Darragh J., McIlrath J., Carr J. M., Armit L. J., Clacher C., Malone L., Kollias G., Arthur J. S. (2005) Generation and characterization of p38β (MAPK11) gene-targeted mice. Mol. Cell Biol. 25, 10454–10464 [DOI] [PMC free article] [PubMed] [Google Scholar]