Background: C. jejuni modify the flagellar protein FlgG and lipid A with phosphoethanolamine.
Results: The C. jejuni phosphoethanolamine transferase EptC modifies FlgG at position Thr75.
Conclusion: Phosphoethanolamine modification of FlgG is required for motility, whereas modification of lipid A with phosphoethanolamine results in resistance to antimicrobial peptides.
Significance: Understanding how bacterial surface modifications influence and regulate virulence is key for the development of novel therapeutics.
Keywords: Antimicrobial Peptides, Glycerophospholipid, Lipid Metabolism, Membrane, Post-translational Modification, Antimicrobial Peptide, Flagella, Lipid A, Lipopolysaccharide, Outer Membrane
Abstract
Gram-negative bacteria assemble complex surface structures that interface with the surrounding environment and are involved in pathogenesis. Recent work in Campylobacter jejuni identified a gene encoding a novel phosphoethanolamine (pEtN) transferase Cj0256, renamed EptC, that serves a dual role in modifying the flagellar rod protein, FlgG, and the lipid A domain of C. jejuni lipooligosaccharide with a pEtN residue. In this work, we characterize the unique post-translational pEtN modification of FlgG using collision-induced and electron transfer dissociation mass spectrometry, as well as a genetic approach using site-directed mutagenesis to determine the site of modification. Specifically, we show that FlgG is modified with pEtN at a single site (Thr75) by EptC and demonstrate enzyme specificity by showing that EptC is unable to modify other amino acids (e.g. serine and tyrosine). Using Campylobacter strains expressing site-directed FlgG mutants, we also show that defects in motility arise directly from the loss of pEtN modification of FlgG. Interestingly, alignments of FlgG from most epsilon proteobacteria reveal a conserved site of modification. Characterization of EptC and its enzymatic targets expands on the increasingly important field of prokaryotic post-translational modification of bacterial surface structures and the unidentified role they may play in pathogenesis.
Introduction
Campylobacter jejuni is a major cause of bacterial diarrhea worldwide (1). Infection with this pathogen results in significant acute illness as well as serious life-threatening consequences such as Guillain-Barré syndrome (2). Similar to other bacterial pathogens, C. jejuni assembles complex surface structures critical for commensal colonization of avian reservoirs as well as pathogenesis in humans. Two major surface components, the outer membrane glycolipid lipooligosaccharide (LOS)3 and flagella, are often subject to phase variation or targets for modification, making them highly variable, presumably to provide antigenic diversity and a competitive advantage to the bacterium. For example, variation in the surface exposed LOS in C. jejuni results in a form of molecular mimicry between these bacterial glycolipids and peripheral nerve gangliosides, implicating them in the post-infectious neuropathies Guillain-Barré and Miller Fisher syndromes (2, 3). Another example of surface modification is O- and N-linked glycosylation of the flagella filament by the Pse and Pgl family of enzymes found in C. jejuni and many epsilon proteobacteria. These events not only result in antigenic variation but also are required for assembly of the filament (4–6). Considering the importance of surface glycolipids (e.g. LOS) in pathogen-host interactions and a requirement for flagellar locomotion in many pathogens, the roles played by variation and modification of surface structures in C. jejuni in its pathogenesis are now apparent.
Recently, Cj0256 (EptC) was found to catalyze the addition of a phosphoethanolamine (pEtN) residue to two periplasmic targets adding to the repertoire of surface modification and variation found in C. jejuni (Fig. 1) (7). During transport of LOS to the outer surface, EptC was shown to transfer pEtN residues to the phosphate groups of lipid A. Lipid A is unique saccharolipid that serves as the hydrophobic anchor of LOS making up the outer layer of the Gram-negative outer membrane. Decoration of C. jejuni lipid A phosphate groups with pEtN residues provides resistance to cationic antimicrobial peptides (CAMPs) (7). Many Gram-negative bacteria modify their lipid A to provide protection against CAMPs and to avoid detection by the host Toll-like receptor 4/MD2 innate immune receptor (8, 9). Remarkably, EptC was also shown to catalyze the addition of a single pEtN to the flagellar rod protein FlgG (7). EptC-deficient strains showed decreased motility and greatly reduced flagella production, suggesting a functional role for pEtN modification of FlgG. However, the actual site of pEtN modification on FlgG was not determined, and the role pEtN modification plays in bacterial motility could not be clearly examined.
FIGURE 1.
Proposed model for pEtN modifications catalyzed by EptC. The model illustrates the bifunctional nature of EptC in the periplasmic modification of lipid A and the flagellar rod component FlgG of C. jejuni with pEtN. C. jejuni lipid A is shown as a disaccharide of 2,3-diamino-2,3-dideoxy-d-gluopyranose that is hexa-acylated and bis-phosphorylated. Phosphoethanolamine modification of Campylobacter lipid A occurs on the phosphate groups (7) and modification of FlgG occurs on threonine 75. The model indicates that phosphatidylethanolamine serves as the pEtN donor, resulting in the production of diacylglycerol, which can be recycled for phospholipid synthesis.
EptC is member of a large family of proteins (COG 2194) found in a number of pathogenic bacteria, many of which have identified functions in periplasmic decoration of bacterial structures with phosphoryl substituents (Table 1). Most commonly, members of this family of proteins (e.g. EptB, CptA, and Lpt3) have been shown to decorate LOS or LPS of various pathogens with pEtN presumably derived from phosphatidylethanolamine, similar to the lipid A modification catalyzed by EptC (Fig. 1) (10–16). Another noteworthy member of this family PptA (pilin phosphorylcholine transferase A), an enzyme characterized in Neisseria sp., was found to catalyze the transfer of pEtN and phosphocholine to serine residues of PilE (17). These multisite modifications were shown not only to provide structural and antigenic diversity to Neisserial Type IV pili but also to initiate dissemination of N. meningitidis upon cell contact (18, 19), illustrating the importance of such post-translational protein modifications. EptC stands out among members of this large family of mostly uncharacterized proteins in that it catalyzes the addition of pEtN to two periplasmic targets: a membrane lipid and a flagellar protein (7).
TABLE 1.
Orthologous proteins and identified function
| Organism | Protein | I, P, Ga | E valuea | Enzyme target | Role in pathogenesis |
|---|---|---|---|---|---|
| % | |||||
| C. jejuni | EptC | 100, 100, 0 | 0.0 | Lipid A, FlgG (7) | CAMP resistance, motility (7) |
| H. pylori | Hp0022 | 33, 50, 11 | 1e-63 | Lipid A (16) | Unknown |
| N. meningitidis | Lpt3 | 29, 43, 29 | 1.4 | LOS core (3-HepII) (14) | Bactericidal resistance (14) |
| Lpt6 | 29, 43, 29 | 1.4 | LOS core (6-HepII) (15) | Unknown | |
| PptA | 23, 41, 19 | 2e-12 | PilE (17) | Antigenic variation (19), Dissemination of bacteria (18) | |
| E. coli | EptB | 25, 49, 14 | 5e-38 | LPS core (Kdo) (10) | Unknown |
| S. enterica | PmrC | 40, 58, 7 | 1e-101 | Lipid A (12) | CAMP resistance (12) |
| CptA | 25, 42, 9 | 5e-18 | LPS core (11) | CAMP resistance (11) | |
| V. cholera | Vca1102 | 34, 53, 10 | 2e-77 | Unknown | Unknown |
| P. aeruginosa | Pa1972 | 37, 56, 7 | 4e-89 | Unknown | Unknown |
| Pa3310 | 39, 58, 6 | 4e-92 | Unknown | Unknown | |
| Pa4517 | 23, 42, 17 | 7e-24 | Unknown | Unknown | |
| C. coli | Cco0328 | 76, 86, 1 | 0.0 | Unknown | Unknown |
| C. fetus | Cff1723 | 48, 65, 2 | 7e-162 | Unknown | Unknown |
a Alignments scores (I, identities; P, positives; G, gaps) and E value for each protein compared with C. jejuni EptC using NCBI BLASTP2.2.25.
In this work we further characterize the unique post-translational pEtN modification of C. jejuni FlgG, identifying a single site of modification at Thr75 employing collision-induced and electron transfer dissociation (CID and ETD) mass spectrometry of tryptic peptides. We also determine substrate specificity using site-directed mutants to show that EptC is unable to modify serine or tyrosine residues. Biological characterization of these mutants confirmed that motility defects previously reported in eptC deficient strains result directly from a loss of FlgG modification.
EXPERIMENTAL PROCEDURES
General Recombinant DNA Techniques
Enzymes, DNA purification kits, and primers were purchased from New England Biolabs, Qiagen, Stratagene, and Invitrogen. All of the DNA-modifying enzymes and kits were used according to the manufacturers' instructions. Restriction sites incorporated into primers used are listed in supplemental Table S2. All of the vectors were confirmed by DNA sequencing.
Bacterial Strains and Growth
A complete list of bacterial strains used in this study can be found in Table 2. Escherichia coli strains were grown routinely at 37 °C in LB broth or on LB agar. C. jejuni strains were grown routinely at 37 °C in a Mueller-Hinton (MH) broth, on MH agar, or tryptic-soy agar supplemented with 5% blood under microaerophilic conditions.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain | Genotype or descriptiona | Reference |
|---|---|---|
| E. coli strains | ||
| XL 1 Blue | General cloning strain, recA1 endA1 gyrA96thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZM15::Tn10], TetR | Stratagene |
| C. jejuni strains | ||
| 81-176 | Serotype HS: 23, 26 | S. A. Thompson |
| 81-176 eptC | 81-176 cj0256, CamR | Ref. 7 |
| 81-176 eptC, eptC+ | 81-176 cj0256, atsA:cj0256+, CamR, KanR | Ref. 7 |
| 81-176 flgGhis6+ | 81-176 flgGhis6+, KanR | Ref. 7 |
| 81-176 eptC, flgGhis6+ | 81-176 cj0256, flgGhis6+, CamR, KanR | Ref. 7 |
| 81-176 flgFG | 81-176 flgFG, CamR | This study |
| 81-176 flgFG, flgFGhis6+ | 81-176 flgFG, atsA:flgFGhis6+, CamR, KanR | This study |
| 81-176 flgFG, flgFGhis6(T75A)+ | 81-176 flgFG, atsA:flgFGhis6 (T75A)+, CamR, KanR | This study |
| 81-176 flgFG, flgFGhis6(T75S)+ | 81-176 flgFG, atsA:flgFGhis6 (T75S)+, CamR, KanR | This study |
| 81-176 flgFG, flgFGhis6(T75Y)+ | 81-176 flgFG, atsA:flgFGhis6 (T75Y)+, CamR, KanR | This study |
| Plasmids | ||
| pBluescript II SK(+) | High-copy Cloning Vector, AmpR | Stratagene |
| pGEMatsAKO:KanR | Complementation Vector created from pGEM-T Easy with atsA interrupted with a kanamycin cassette | Ref. 7 |
| pBflgFGKO:CamR | pBflgFG with flgF and flgG operon interrupted with a chloramphenicol cassette. | This study |
| pAtsAKO:flgFGhis6-KanR | pGEMatsAKO:KanR with flgF and flgGhis6 insertion | This study |
| pAtsAKO:flgFGhis6(T75A)-KanR | pGEMatsAKO:KanR with flgF and flgGhis6(T75A) insertion | This study |
| pAtsAKO:flgFGhis6(T75S)-KanR | pGEMatsAKO:KanR with flgF and flgGhis6(T75S) insertion | This study |
| pAtsAKO:flgFGhis6(T75Y)-KanR | pGEMatsAKO:KanR with flgF and flgGhis6(T75Y) insertion | This study |
a Antibiotics were used at the following concentrations: for E. coli cultures, 100 μg/ml ampicillin (AmpR), 30 μg/ml kanamycin (KanR), 10 μg/ml tetracycline (TetR), and 30 μg/ml chloramphenicol (CamR); and for C. jejuni cultures, 25 μg/ml chloramphenicol (CamR) and 50 μg/ml kanamycin (KanR).
Construction of Select Deletion Mutants in C. jejuni
Deletion mutants of flgF and flgG gene operon (CJJ81176_720 and _721) were constructed as described previously (20). Briefly, an upstream (primers 1 and 3) and downstream (primers 2 and 6) region flanking the flgFG operon (± 1,000 bp) was PCR-amplified from C. jejuni strain 81-176 genomic DNA. Primers 3 and 6 incorporated an overhang complimentary to the chloramphenicol resistance cassette (Cam) from cloning vector pRY111 (21). The Cam cassette was PCR-amplified from pRY111 (primers 4, 5). The three amplicons (upstream, downstream, and Cam cassette) were combined in an assembly PCR and served as templates for primers 1 and 2, which incorporated restriction sites KpnI and SacII. The assembled PCR product was restriction-digested, gel-isolated, and inserted into cloning vector pBluescript SKII (+) to yield the completed knock-out vector pBflgFGKO:CamR. Knock-out vector, pBflgFGKO:CamR, was transformed into C. jejuni by natural transformation, and resistant colonies were selected on blood agar plates containing 25 μg/ml of chloramphenicol. Knock-out of the flgFG operon was confirmed by PCR analysis and named 81-176 flgFG.
Complementation of the 81-176 flgFG mutant was achieved by insertion of the flgFG operon into the arylsulfatase gene atsA (22). Briefly, C. jejuni complementation vector pGEMatsAKO:KanR (7), containing atsA interrupted with a kanamycin resistance cassette (aph3) was digested with AgeI. AgeI cuts the vector in a noncoding region of the Kan cassette, upstream of its promotor. The flgFG operon plus 200 bp of upstream sequence was amplified by PCR (primers 7 and 8) from 81-176 genomic DNA and inserted into the AgeI cut site. Primer 8 was engineered to add an in-frame His6 coding sequence before the stop codon of flgG. The resulting vector, pAtsAKO:flgFGhis6-KanR, was used to transform 81-176 flgFG for complementation studies. For site-directed mutagenesis (SDM), vector pAtsAKO:flgFGhis6-KanR was subject to further manipulation using a QuikChange XL site-Directed mutagenesis kit (Stratagene) (primers 9–14) to create SDM complementation vectors pAtsAKO:flgFGhis6(T75A)-KanR, pAtsAKO:flgFGhis6(T75S)-KanR, and pAtsAKO:flgFGhis6(T75Y)-KanR. SDM complementation vectors were used to transform 81-176 flgFG. C. jejuni was transformed using natural transformation, kanamycin-resistant colonies selected on blood agar plates containing 50 μg/ml of kanamycin and then screened for loss of AstA activity as previously described (22). Complemented strains were named 81-176 flgFG, flgFGhis6+, 81-176 flgFG, flgFGhis6(T75A)+, 81-176 flgFG, flgFGhis6(T75S)+, and 81-176 flgFG, flgFGhis6(T75Y)+, respectively. During mutant construction, allelic replacement was confirmed by PCR of genomic DNA from isolated colonies.
Motility Assays
Motility was determined using semi-solid motility agar consisting of Mueller-Hinton medium supplemented with 0.4% agar and 10 μg/ml vancomycin. The plates were inoculated using 0.5 μl of bacteria in MH broth at A600 of ∼ 0.05. Inoculated plates were incubated at 37 °C in a microaerophilic environment, and the diameter of the area of motility was measured after 24 h.
Determination of Polymyxin B Minimum Inhibitory Concentration
Minimum inhibitory concentrations (MIC) were determined using Polymyxin B Etest® strips (Biomérieux). Two hundred microliters of culture at an A600 of 0.05 was spread evenly onto a Mueller-Hinton agar plate and allowed to dry completely, followed by the addition of the Etest® strip to the center of the plate. The plates were incubated at 37 °C in a microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) for 24 h before reading. Each experiment was repeated at least three times, the results were averaged, and a standard deviation was calculated.
Purification and Immunoblotting Analysis of FlgGhis6
The indicated strains were grown in 1.0 liter of MH broth and harvested at A600 of ∼ 1.0. The cells were lysed in PBS by French press, and the crude lysate was cleared twice by centrifugation at 10,000 × g for 10 min. The cell-free extract was diluted ∼8-fold in Buffer A (20 mm HEPES, pH 7.5, 6.0 m urea, 0.5 m NaCl, 10 mm imidazole) and applied to a 1.0-ml His-Trap nickel-Sepharose affinity column (GE Healthcare), using the AKTA FPLC purification system. Target protein was eluted with a linear gradient of Buffer B (20 mm HEPES, pH 7.5, 6.0 m urea, 0.5 m NaCl, 500 mm imidazole. Fractions containing purified protein were pooled, concentrated, and dialyzed against 20 mm HEPES, 0.5 m urea, pH 7.5, to a final protein concentration of 0.10–0.44 mg/ml in preparation for ESI, CID, and ETD-MS analysis.
All of the purified protein samples were resolved using standard SDS-PAGE procedures on Invitrogen NuPAGE 4–12% Bis-Tris gels. 15.0 μg of crude lysate or 2.0 μg of purified protein was loaded into each lane. Polyacrylamide gels were run in triplicate for Western blotting. The resolved proteins were transferred to nitrocellulose membranes using an X Cell II blot module (Invitrogen). For anti-polyhistidine, nitrocellulose membranes were blocked using 2.5% BSA and probed with 1:2000 dilution of mouse monoclonal anti-polyhistidine-alkaline phosphatase antibody (Sigma). The blots were then developed using Western Blue Stabilized Substrate (Promega). For anti-FlgG, nitrocellulose membranes were blocked using 2.5% BSA and probed with 1:1,000 dilution of mouse anti-FlgG M69 antibody (gift from Hendrixson, DR) (23). To visualize, the membranes were then blotted with rabbit anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (1:10,000) (GE Healthcare) and developed using the ECL plus Western blotting detection system (GE Healthcare).
ESI-MS Analysis of Purified Protein
An ESI-mass spectrometer (LCQ, Finnigan MAT, San Jose, CA) coupled on-line with a microbore HPLC (Magic 2002; Michrom BioResources, Auburn, CA) was used to acquire spectra of purified proteins. The protein sample was eluted with a 0.5 × 50-mm PLRP-S column (8 μ, 4000 Å; Michrom BioResources, Auburn, CA) with mobile phase A and B (acetonitrile:water:formic acid:trifluoroacetic acid; 2:98:0.1:0.01 for phase A and 90:10:0.1:0.01 for phase B). The gradient used to elute protein was from 5 to 65% B in 20 min at a flow rate of 20 μl/min. Automated acquisition of full scan mass spectra was executed by Finnigan ExcaliburTM software. The full scan range for MS was 450–2000 Da. The acquired convoluted protein spectra from LCQ were deconvoluted by the ProMass for Xcalibur version 2.5.0 software (Novatia LLC, Princeton, NJ) to afford the MH+ m/z value(s) of the protein sample.
CID and ETD MS Analysis of Peptides
Purified proteins were digested with trypsin using a 1:20 enzyme:substrate ratio, a pH of ∼8, and an incubation time of 16 h at 37 °C. All of the MS experiments were undertaken on a Thermo Fisher Scientific LTQ XL mass spectrometer (San Jose, CA) equipped with an ETD unit. Liquid chromatography was performed using a Dionex UltiMate 3000 system (Sunnyvale, CA), and an Agilent ZORBAX 300SB-C18 column (Santa Clara, CA) (150 × 0.3 mm, 5-μm particle size). Eluent A consisted of 0.1% formic acid in water and eluent B 0.1% formic acid in acetonitrile. A linear gradient from 5% eluent B to 40% eluent B over 65 min at 5 μl/min was used. The samples were injected at ∼50 pmol of digested protein. LC-MS/MS was performed in two different ways. For LC-MS/CID runs (i.e. only CID was used for activation), the first event was the full mass scan (m/z range of 400–2000) followed by 10 data-dependent CID events on the 10 most abundant ions from the full mass scan. A q value of 0.25, an activation time of 30 ms, and a normalized collision energy of 35% were used for all CID events. For selected ion LC-MS/ETD of 81-176 WT protein, the first event was the full mass scan (m/z range of 400–2000) followed by ETD on the 2+ and 3+ charge states. Fluoranthene anions were introduced as the electron transfer reagent for ETD experiments with reaction times of 100 ms. For all LC-MS/MS experiments, the maximum injection time for full mass scans and MS/MS events was set to 100 ms, the dynamic exclusion duration was set to 50 s, and the exclusion list size allowed for 500 specified m/z values. A single repeat count was used for LC-MS/CID.
SEQUEST was used for automated LC-MS/CID analysis through the Thermo Fisher Scientific Proteome Discoverer 1.0 software package. A signal:noise ratio of 3, a precursor mass tolerance of 1.5 Da, and a fragment mass tolerance of 0.8 Da were used for processing. Phosphoethanolamine modification of serine, threonine, and tyrosine were set as dynamic side chain modifications. Experimental CID spectra were searched against databases consisting of the sequence of the targeted protein. Peptide hits were filtered based on Xcorr versus charge state (i.e. a minimum Xcorr score for 1+ precursors = 1.5, 2+ precursors = 2.0, 3+ precursors = 2.25, 4+ precursors = 2.50, and 5+ precursors = 2.75), a minimum probability score of 1.1, a max peptide ranking of one, “high” confidence scoring, and manual verification.
Visualization of 32Pi-Labeled Flagellar Components from C. jejuni
Bacterial strains were grown as described above in 200 ml of medium supplemented with 1.5 μCi/ml 32Pi at a starting A600 of ∼0.05. The strains were harvested at an A600 of ∼1.0. The cells were harvested by centrifugation at 10,000 × g for 10 min at 4 °C and washed once with PBS. The cells were lysed in a French press in the presence of a protease inhibitor. The crude cell lysate was cleared twice by centrifugation at 10,000 × g for 10 min, creating a cell-free extract.
C. jejuni cell-free extract samples were further processed as follows because of the low chromosomal expression of FlgG (C-terminal His tag). A ProFound pulldown polyhistidine protein/protein interaction kit (Thermo Scientific) was adapted and used for purification of FlgG (C-terminal His tag). Supplied spin columns were assembled and loaded with 100 μl of 50% immobilized cobalt chelate resin slurry. The loaded column was equilibrated with wash buffer (supplied wash buffer supplemented with 6 m urea and 40 mm imidazole). Next, 8.0 mg of cell-free extract in 6 m urea was loaded into each column and allowed to incubate for 2 h at 4 °C. The column was later washed 10 times with wash buffer to remove nonspecific binding proteins. The target protein was later eluted with 150 μl of elution buffer (supplied wash buffer supplemented with 6 m urea and 290 mm imidazole).
All of the protein samples were resolved using standard SDS-PAGE procedures on Invitrogen NuPAGE 4–12% Bis-Tris gels. 22.5 μl (15%) of the total elution volume was loaded into each lane (∼ 2 μg). Polyacrylamide gels were run in duplicate for Western blotting. The resolved proteins were transferred to nitrocellulose membranes using an X Cell II blot module (Invitrogen). Nitrocellulose membranes were blocked using 2.5% BSA and probed with 1:2000 dilution of monoclonal anti-polyhistidine-alkaline phosphatase antibody produced in a mouse (Sigma). The blots were then developed using Western Blue stabilized substrate (Promega). To visualize 32P-labeled protein, the Western blots were exposed overnight to a PhosphorImager screen for ∼24 h and visualized using a Bio-Rad phosphorimaging equipped with Quantity One software.
RESULTS
Purification and Identification of Site of pEtN Modification of Flagellar Rod Component FlgG
Previous analysis of FlgG-His6 purified from C. jejuni 81-176 wild type background by ESI-MS revealed modification with a single pEtN residue (7). Modification of FlgG-His6 was not seen when purified from eptC-deficient strains (7). However, the actual site of modification was not identified. Considering recent work in Neisseria sp. showing that modification of the Type IV pili with phosphoryl substituents was important for antigenic diversity and involved in dissemination of N. meningitidis upon cell contact (18), we felt it was important to map the actual site of pEtN modification on FlgG. Moreover, identification of the actual site of modification would permit functional studies, separating motility changes from membrane changes (i.e. CAMP resistance) and perhaps identify a conserved residue or motif present in other pathogens that may serve as a target for post-translational modification.
FlgG-His6 was purified as previously described (7) from strains 81-176 flgGhis6+ and 81-176 eptC, flgGhis6+, giving us the ability to analyze pEtN-modified and unmodified FlgG-His6. Fig. 2A illustrates the purity of FlgG-His6 from strain 81-176 flgGhis6+; a similar result was seen for FlgG-His6 purified from 81-176 eptC, flgGhis6+ (data not shown). Additional lower molecular weight proteins seen after purification were identified as FlgG-His6 using C. jejuni-specific anti-FlgG antiserum and assumed to be proteolytic degradation of FlgG-His6 (Fig. 2A).
FIGURE 2.
Purification of FlgG-His6 and immunoblotting analysis. FlgG-His6 (A) and FlgG-His6(T75S) or FlgG-His6(T75Y) (B) purified via affinity chromatography from C. jejuni and Western blotted using anti-His6 and anti-FlgG antibodies to show purity. Crude cell extracts (CCE) and purified FlgG from indicated strains were resolved by SDS-PAGE and stained with Coomassie (top panel), blotted with anti-polyhistidine (middle panel), and anti-FlgG M69 murine antiserum (bottom panel) (23). Purified FlgG is seen along with additional smaller bands assumed to be FlgG degradation products.
Prior to mapping, ESI-MS of whole intact protein was performed using a previously described method to confirm pEtN modification of whole protein (7). Briefly, FlgG-His6 purified from wild type background revealed a mass difference of ∼122.9 Da when comparing observed mass to that of expected mass, the predicted size of a single pEtN residue (Table 3). This mass difference was not present in FlgG-His6 purified from an eptC-deficient background confirming previous findings (7).
TABLE 3.
ESI-MS of FlgG-His6 purified from the indicated background
| Background: C. jejuni 81-176 | Expected massa | Observed massa | ΔMass |
|---|---|---|---|
| Da | Da | Da | |
| flgGhis6+ | 28546.3 | 28669.2 | +122.9 (pEtN) |
| eptC, flgGhis6+ | 28546.3 | 28547.9 | +1.6 (N/A) |
| flgFG, flgFGhis6(T75A)+ | 28516.9 | 28514.4 | −2.5 (N/A) |
| flgFG, flgFGhis6(T75S)+ | 28532.9 | 28528.7 | −4.2 (N/A) |
| flgFG, flgFGhis6(T75Y)+ | 28609.0 | 28612.7 | +3.7 (N/A) |
a Average masses.
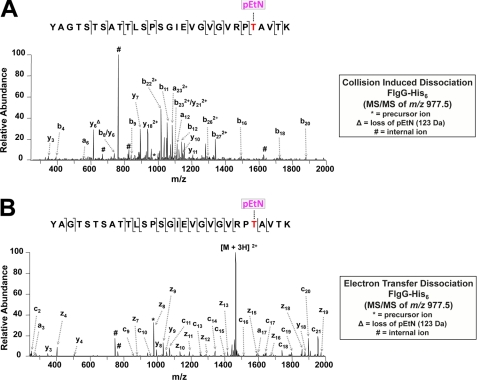
For mapping, FlgG-His6 purified from a wild type background was subjected to trypsin digestion and then analyzed by liquid chromatography MS/MS employing two different fragmentation techniques: CID and ETD. CID has a long history of proteomics applications but often fails to identify post-translational modifications that are labile (24). ETD has proven to be particularly well suited for the identification of post-translational modifications because these labile modifications are often retained on the amino acid side chain during ion dissociation, allowing them to be readily tracked (25, 26). For LC-MS/CID analysis of the tryptic peptides, the first MS event was a full mass scan (m/z range of 400–2000) followed by 10 data-dependent CID events on the most abundant ions from the full mass scan. Phosphoethanolamine modification of serine, threonine, and tyrosine were set as dynamic side chain modifications, and CID spectra were searched against a data base consisting of the sequence of the targeted protein, FlgG from C. jejuni 81-176. Characterization of peptide species at m/z 977.5 [M + 3H]3+ revealed the peptide 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 modified with a single pEtN (Fig. 3A). No other pEtN-modified peptides were seen; however, the site specificity of the pEtN modification could not be confirmed from the CID spectrum alone. As seen in supplemental Table S1, both Thr75 (labeled T25) and Thr78 (labeled T28) were identified by SEQUEST to have a pEtN modification. This ambiguous site determination is likely due to increased spectral complexity upon CID from unique internal ions directed by the pEtN modification, neutral loss of pEtN from product ions (Fig. 3A), and the several possible sites of modification in the identified tryptic peptide (one tyrosine, four serines, and six threonines). Identical analysis of FlgG-His6 purified from 81-176 eptC, flgGhis6+ revealed an unmodified 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 peptide. All of the peptides identified by SEQUEST for both CID analyses are seen in supplemental Table S1. To pinpoint the exact site of pEtN modification, selected ion LC-MS/ETD analysis of tryptic peptides from FlgG-His6 purified from 81-176 flgGhis6+ was performed. The first event was the full mass scan (m/z range of 400–2000) followed by ETD of the [M + 2H]2+ and [M + 3H]3+ precursor ions of the pEtN-modified 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 (identified previously from LC-MS/CID). ETD analysis revealed the exact site of pEtN modification at solely Thr75 (51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79; Fig. 3B). Thus, the combination of CID and ETD proved to be a powerful tool set for identifying and unambiguously assigning the site of the pEtN modification.
FIGURE 3.
A, LC MS/MS of pEtN-modified FlgG-His6 tryptic peptides fragmented by CID. Characterization of the species at m/z 977.5 [M + 3H]2+ from wild type background (strain 81-176 flgGhis6+). The peptide 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 is modified with pEtN at Thr75. No other pEtN-modified peptides were detected. Fragmentation patterns are indicated. The complete SEQUEST output data can be seen in supplemental Table S1. B, LC MS/MS of pEtN-modified FlgG-His6 tryptic peptides fragmented by ETD. Characterization of the species at m/z 977.5 [M+3H]2+ from wild type background (strain 81-176 flgGhis6+). The peptide 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 is modified with pEtN at Thr75. No other pEtN-modified peptides were detected. The fragmentation patterns are indicated.
Generation of Site-directed Mutants in C. jejuni and Mass Spectrometry Analysis of Purified FlgG
Amino acids threonine, serine, and tyrosine are often targets for post-translational modification in bacteria by phosphoryl substituents or O-linked glycans because of a free hydroxyl group (4, 17, 18). For example, many pathogens glycosylate their flagellar filaments contributing to antigenic diversity (6). In C. jejuni, the Pse enzymatic machinery responsible for these modifications, often nonspecifically, modify surface-exposed Thr, Ser, and Tyr residues of the filament proteins FlaA and FlaB (4). An alignment of FlgG proteins from various pathogens revealed a conserved Thr75 or Ser75 among members of the epsilon proteobacteria (e.g. Helicobacter pylori and Wolinella succinogenes) and all Campylobacter species at the identified site of modification Thr75, suggesting that pEtN modification may be conserved among this group of bacteria. An alignment of FlgG from representative organisms can be seen in Fig. 4. The genomes of most members of the epsilon proteobacteria contain an orthologous protein to EptC, and in the case of H. pylori, a lipid A pEtN modification enzyme (Hp0022) has already been identified by our laboratory (16). In light of the Hp0022 role in lipid A decoration and a conserved site of pEtN modification on FlgG (Fig. 4), a similar role in flagellar modification seems likely.
FIGURE 4.
Alignments of partial amino acid sequence (residues 61–119) of C. jejuni FlgG from various bacteria using ClustalW2 (Multiple Sequence Alignment program). The identified site of modification in C. jejuni is aligned with complementary regions of various organisms (blue box). A conserved possible site of modification is seen among epsilon proteobacteria (red). This site is absent in nonepsilon pathogens (black). Alignment of FlgG from all sequenced Campylobacter sp. show a conserved Thr75 (data not shown).
Considering the alignment of FlgG from various epsilon proteobacteria, we wanted to determine whether EptC could modify serine or tyrosine residues. To test this, we first created a deletion mutant of the flgF and flgG gene operon (flgFG) by interruption of the coding sequence with a chloramphenicol resistance cassette (strain 81-176 flgFG). We then chromosomally complemented the flgFG deletion strain by restoring the flgFG operon but with an in-frame polyhistidine coding sequence addition to flgG, giving us the ability to easily purify FlgG-His6 from C. jejuni [strain 81-176 flgFGhis6+]. For site-directed mutants the same complementation strategy was used, replacing Thr75 with Ala, Ser, or Tyr to generate strains 81-176 flgFGhis6(T75A)+, 81-176 flgFGhis6(T75S)+, and 81-176 flgFGhis6(T75Y)+.
For the identification of possible pEtN modification of FlgG, we purified FlgG-His6(T75S) and FlgG-His6(T75Y) using affinity chromatography (Fig. 2B). Purified FlgG mutants were subjected to LC-MS/CID using the same procedure described above. For FlgG-His6(T75S), characterization of the species at m/z 932.3 [M + 3H]2+ revealed a peptide 51YAGTSTSATTLSPSGIEVGVGVRPSAVTK79 devoid of modification at Ser75(Fig. 5A). For FlgG-His6(T75Y), characterization of the species at m/z 957.9 [M + 3H]2+ revealed a peptide 51YAGTSTSATTLSPSGIEVGVGVRPYAVTK79 devoid of modification at Tyr75 (Fig. 5B). All of the peptides identified by SEQUEST for FlgG mutants are listed in supplemental Table S1. To confirm our findings, purified intact protein was also analyzed by ESI-MS. Purified FlgG-His6(T75A), FlgG-His6(T75S), and FlgG-His6(T75Y) all showed an observed mass close to that of the expected mass, hence devoid of pEtN modification (Table 3). These data clearly demonstrate that EptC exhibits single target specificity for the Thr75 residue of FlgG.
FIGURE 5.
LC MS/MS of FlgG-His6(T75S) (A) and FlgG-His6(T75Y) (B) tryptic peptides fragmented by CID. Characterization of the species at m/z 932.3 [M + 3H]2+ from background 81-176flgFG, flgFGhis6(T75S)+ and species at m/z 957.9 [M + 3H]2+ 81-176flgFG, flgFGhis6(T75Y)+, respectively, is shown. In both cases, peptide 51YAGTSTSATTLSPSGIEVGVGVRPTAVTK79 lacks modification with pEtN despite purification from a background with an active copy of EptC, suggesting specificity for Thr75. No other pEtN-modified peptides were detected. Fragmentation patterns are indicated. The complete SEQUEST output data can be seen in supplemental Table S1.
Visualization of 32P-labeled FlgG-His6
ESI, CID, and ETD MS techniques work well for the identification of post-translational modifications, and the use of all three techniques in our characterization of wild type FlgG revealed pEtN modification. However, some MS techniques can prove inefficient in the detection of post-translational modifications if they are in low abundance or labile (26). To rule out the possibility that we missed pEtN modification of FlgG-His6 mutants because of inefficient transfer of pEtN to Ser or Tyr, we employed a previously published technique that allows for visualization of radiolabeled pEtN-modified FlgG-His6 (7). Briefly, cultures were labeled with 32Pi and FlgG-His6 purified via affinity chromatography followed by SDS/PAGE. FlgG-His6 was easily purified and detectable by Western blotting followed by phosphorimaging analysis (Fig. 6). As expected, FlgG-His6 purified from a background with an active EptC revealed 32P-modified protein, whereas 32P labeling was absent on FlgG-His6 purified from eptC-deficient strains. Furthermore, FlgG-His6(T75A), FlgG-His6(T75S), and FlgG-His6(T75Y) purified from an active EptC background revealed no 32P labeling, thus confirming the lack of pEtN modification.
FIGURE 6.
32P labeling of FlgG-His6 purified from C. jejuni strains. 32P-Labeled FlgGs were purified via affinity chromatography from C. jejuni, Western blotted using anti-His antibody, and visualized by phosphorimaging. FlgG-His6 purified from wild type background showed pEtN modification, whereas FlgG-His6 purified from an eptC-deficient background was unmodified. FlgG-His6(T75A, T75S, or T75Y) purified from wild type background were also unmodified, demonstrating the specificity of EptC for threonine.
Characterization of FlgG Site-directed Mutants
In C. jejuni, the flagellum or flagellar motility is required for effective adherence and invasion of intestinal epithelial cells (27–29). Moreover, it was demonstrated in several pathogens that decoration of LOS/LPS with pEtN is critical for resistance to polymyxin B (PMB), a positively charged lipopeptide that binds to the negatively charged surface of Gram-negative bacteria killing in a manner similar to CAMPs of the innate immune system (7, 12, 16). Because we were previously unable to completely separate the two phenotypic changes observed in eptC-deficient strains (i.e. decreased motility and PMB resistance), it was assumed that the loss of PMB resistance and decreased motility resulted from loss of pEtN modification of lipid A and FlgG, respectively. However, there remained the possibility that pEtN modification of FlgG contributed to PMB resistance or that modification of lipid A with pEtN was required for efficient flagella production and motility. For example, E. coli mutants unable to produce phosphatidylethanolamine are also nonmotile, linking phospholipid composition and flagellar motility (30). The possibility remained that changes in membrane lipid composition in C. jejuni had a similar effect on motility. Fortuitously, phenotypic characterization of our site-directed mutants afforded us an opportunity to distinguish between these two changes and conclusively demonstrate the contribution of each modification.
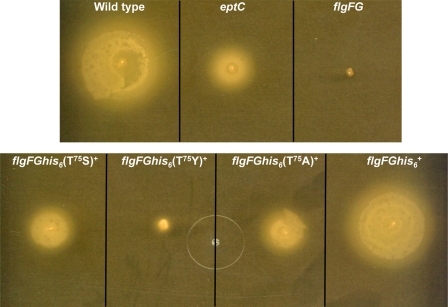
Strains 81-176 flgFGhis6(T75A)+, 81-176 flgFGhis6(T75S)+, and 81-176 flgFGhis6(T75Y)+ were analyzed for motility and PMB resistance. Swimming motility was measure using a soft agar assay. As expected, eptC-deficient strains of C. jejuni showed reduced motility, whereas flgFG-deficient strains were completely nonmotile (Fig. 7). Complementation of eptC-deficient strains restored wild type motility (Table 4), as previously reported (7). Complementation of flgFG-deficient C. jejuni strains with flgFGhis6 demonstrated wild type motility, whereas those complemented with site-directed mutations of flgFGhis6 showed motility defects similar to that of eptC-deficient strains (Fig. 7), with T75Y SDM strains showing the greatest defect. These results suggest that the observed motility phenotypes are not the result of changes in the outer membrane but rather directly related to modification of FlgG with pEtN. Motility of select C. jejuni strains is demonstrated in Fig. 7, whereas quantitative measure of motility is reported as a percentage of motility compared with wild type in Table 4.
FIGURE 7.
Comparison of swimming motility in various strains of C. jejuni. Motility was determined using semi-solid MH agar. Inoculated plates were incubated for 24 h at 37 °C in a microaerophilic environment. As expected, eptC-deficient strains show decreased motility when compared with wild type, and flgFG deletion mutants are nonmotile. Site-directed mutants (T75S, T75Y, and T75A) show motility similar to that of the eptC mutant, suggesting that modification of Thr75 with pEtN is required for wild type motility. C. jejuni strain 81-176 flgFG, flgFGhis6+, shows wild type motility.
TABLE 4.
Characterization of select C. jejuni strains
| Background: C. jejuni 81-176 | Polymyxin B MICa | pEtN modification (lipid A)b | pEtN modification (flagella) | Motilityc |
|---|---|---|---|---|
| μg/ml | % | |||
| flgG | 17.3 ± 3.3 | + | + | 100 |
| eptC | 0.8 ± 0.2 | − | − | 55 |
| eptC, eptC+ | 17.6 ± 3.6 | + | + | 105 |
| flgFG | 17.0 ± 3.0 | + | − | 0 |
| flgFG, flgFGhis6(T75A)+ | 18.6 ± 4.6 | + | − | 55 |
| flgFG, flgFGhis6(T75S)+ | 16.0 ± 0.0 | + | − | 55 |
| flgFG, flgFGhis6(T75Y)+ | 18.6 ± 4.6 | + | − | 15 |
| flgFG, flgFGhis6+ | 17.0 ± 3.0 | + | + | 100 |
a Minimum inhibitory concentrations were determined using polymyxin B Etest® strips (Biomérieux).
b Modification of lipid A by pEtN previously determined for strains wild type, eptC, and eptC, eptC+. Modification of lipid A by pEtN is assumed for other strains because of resistance to polymyxin B.
c Motility of indicated strains compared with that of wild type.
MIC of the strains were determined using Polymyxin B Etest strips (Biomérieux) as previously described. As expected, the eptC-deficient C. jejuni strain showed a dramatic decrease in resistance to PMB (MIC of 0.8 ± 0.2 μg/ml) when compared with wild type (MIC of 17.3 ± 3.3 μg/ml), indicating a loss of pEtN modification of the lipid A backbone (Table 4). All other C. jejuni strains revealed a PMB MIC ranging from 16.0 ± 0.0 μg/ml to 18.6 ± 4.6 μg/ml, similar to that of wild type (Table 4). These data suggest that in C. jejuni, pEtN modification of lipid A provides resistance to CAMPs, whereas modification of FlgG with pEtN plays no role in CAMP resistance.
DISCUSSION
For survival, all of the bacteria must adapt to an ever-changing environment. The bacterial cell surface and appendages protruding from it interface with the surrounding environment bringing them into contact with host cells and immunological defenses, making bacterial surface structures important for evasion of host defenses. To circumvent detection and survive in inhospitable environments bacteria have evolved elaborate systems and surface structures providing antimicrobial resistance and antigenic diversity (6, 9, 19). One of the more common methods of immune deception involves modification of LOS/LPS found in the outer leaflet of the outer membrane in nearly all medically important Gram-negative pathogens (9). C. jejuni EptC modifies the lipid A anchor of LOS with pEtN (Fig. 1), providing resistance to antimicrobial peptides (Table 4) by changing the electrostatic nature of lipid A (7), most likely constituting an advantage for this pathogen.
Post-translational modifications (PTM) of proteins are well documented in eukaryotic systems and are shown to be involved in a multitude of cellular processes including membrane trafficking, enzymatic activity, and cell signaling (31). However, in prokaryotes, until recently, PTM of proteins were thought to be relatively rare when compared with eukaryotes (6, 31). It is now evident that pathogenic bacteria use PTM of surface-exposed proteins for the purpose of providing/generating antigenic diversity and evading host recognition. Enzymes characterized in Neisseria sp. (i.e. PptA and PglC) were found to catalyze the transfer of pEtN, phosphocholine, and glycans to serine residues of PilE, structural subunits of the Type IV pili (17, 19, 32). These multisite modifications provide structural and antigenic diversity and are also involved in dissemination of N. meningitidis upon cell contact (18). Another example is O-linked protein glycosylation of the flagellar filaments by the Pse family of enzymes found in C. jejuni, also resulting in antigenic diversity of bacterial surface structures (4). Furthermore, C. jejuni is capable of N-linked protein glycosylation (6), the most abundant PTM of secretory and membrane proteins in eukaryotes (31, 33). However, in prokaryotes the purpose of N-linked protein glycosylation remains a mystery.
Using several techniques, we have begun to biochemically characterize another unique bacterial PTM and have conclusively identified the site of pEtN modification of C. jejuni FlgG (Figs. 3 and 4). Replacement of residue Thr75 with serine or tyrosine resulted in unmodified protein (Fig. 5), suggesting specificity for substrate residues. These results were surprising considering the nonspecific nature of PTM machinery involved in flagellin O-linked glycosylation (4), allowing for modification of serine or threonine. Moreover, our analysis revealed a single site of modification in contrast to the multisite modifications seen in other bacterial PTM systems (4, 17), suggesting a defined and specific role in flagella assembly or motility. An alignment of FlgG from epsilon proteobacteria revealed a conserved Thr or Ser at residue 75 with Campylobacter sp. all showing Thr (Fig. 5). Given that BLASTp analysis reveals an EptC ortholog in many epsilon proteobacteria (Table 1), it is likely that FlgG modification occurs in these organisms. Indeed, initial work from our laboratory suggests that deletion of the lipid A phosphoethanolamine transferase of H. pylori results in a loss of motility.4 Alignment of FlgGs also revealed an apparent GXGXRPTA motif possibly serving as a recognition site for pEtN decoration; however, considering the conserved nature of flagellar structural proteins, this may prove to be unrelated.
C. jejuni eptC-deficient stains reveal a decrease in resistance to CAMPs, as well as a reduction in motility and flagella production (Table 4), one enzyme influencing two phenotypes (7). Logically, the decrease in CAMP resistance was attributed to a loss of pEtN modification on the lipid A disaccharide backbone, documented in other pathogens (12), whereas the loss of motility and flagella production was attributed to unmodified FlgG. However, there remained the possibility that changes in membrane composition (i.e. loss of pEtN-modified lipid A) resulted in a motility defect or that unmodified FlgG was the cause of increased CAMP sensitivity. The current work rules out this possibility, yet the role that pEtN-modified FlgG plays in motility is still a mystery. The three-dimensional crystal structure of FlgG has not been solved, and attempts to model FlgG based on the partial crystal structure of FlgE (34), a flagellar hook protein with 38% identical residues, were only successful at partially modeling FlgG (residues 91–223), excluding the N-terminal domain modified by pEtN (34). This limits our discussion of the structural role played by pEtN modification of FlgG, currently under investigation by our laboratory. A recent publication using cryo-electron microscopy for a tomographic study of various flagellar structural components revealed several unidentified and unknown structural components found only in epsilon proteobacteria (35); considering our finding and the possibility that pEtN modification is conserved in these pathogens, a link between pEtN-modified FlgG and these unknown structures is suggested.
In this work, we further characterize the unique PTM of FlgG with a single pEtN at residue Thr75, by the enzyme EptC. EptC is a member of a large family of proteins (COG2194) found in a number of pathogenic bacteria, many of which have not been characterized (Table 1). Those of known function appear to be involved in modification of LOS/LPS (7, 12). However, several members of this family stand out (i.e. EptC and PptA) because of their enzymatic targets (7, 19). Many pathogens harbor multiple orthologs belonging to this family of proteins suggesting a variety of targets (Table 1). C. jejuni only contains a single member of this family of enzymes, EptC, and may explain why it evolved multiple targets. An LOS core sugar of C. jejuni is modified by the addition of pEtN (36) similar to that of S. typhimurium (11). Considering the promiscuous pEtN transferase activity of EptC, a third role in modification of core LOS is possible and currently under investigation in our laboratory. Also of interest is a recent publication showing that EptC itself is subject to PTM by N-linked glycosylation (37). Perhaps N-linked glycosylation of EptC plays a role in its promiscuous nature by regulating substrate specificity of this enzyme. In light of our current findings and the unexpected increase in identified PTM in prokaryotes, more research is needed to identify the functional and regulatory roles that these enzymes may play in bacterial pathogens.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants AI064184 and AI76322 (to M. S. T.).

This article contains supplemental Tables S1 and S2.
D. K. Giles and M. S. Trent, unpublished results.
- LOS
- lipooligosaccharide
- pEtN
- phosphoethanolamine
- CID
- collision-induced dissociation
- ETD
- electron transfer dissociation
- CAMP
- cationic antimicrobial peptides
- MIC
- minimum inhibitory concentration
- PTM
- post-translational modification
- PMB
- polymyxin B
- MH
- Mueller-Hinton
- SDM
- site-directed mutagenesis
- ESI
- electrospray ionization
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Samuel M. C., Vugia D. J., Shallow S., Marcus R., Segler S., McGivern T., Kassenborg H., Reilly K., Kennedy M., Angulo F., Tauxe R. V. (2004) Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin. Infect. Dis. 38, S165–S174 [DOI] [PubMed] [Google Scholar]
- 2. Ang C. W., Laman J. D., Willison H. J., Wagner E. R., Endtz H. P., De Klerk M. A., Tio-Gillen A. P., Van den Braak N., Jacobs B. C., Van Doorn P. A. (2002) Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect. Immun. 70, 1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szymanski C. M., Michael F. S., Jarrell H. C., Li J., Gilbert M., Larocque S., Vinogradov E., Brisson J. R. (2003) Detection of conserved N-linked glycans and phase-variable lipooligosaccharides and capsules from campylobacter cells by mass spectrometry and high resolution magic angle spinning NMR spectroscopy. J. Biol. Chem. 278, 24509–24520 [DOI] [PubMed] [Google Scholar]
- 4. Ewing C. P., Andreishcheva E., Guerry P. (2009) Functional characterization of flagellin glycosylation in Campylobacter jejuni 81-176. J. Bacteriol. 191, 7086–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerry P., Ewing C. P., Schirm M., Lorenzo M., Kelly J., Pattarini D., Majam G., Thibault P., Logan S. (2006) Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 60, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szymanski C. M., Logan S. M., Linton D., Wren B. W. (2003) Campylobacter. A tale of two protein glycosylation systems. Trends. Microbiol. 11, 233–238 [DOI] [PubMed] [Google Scholar]
- 7. Cullen T. W., Trent M. S. (2010) A link between the assembly of flagella and lipooligosaccharide of the Gram-negative bacterium Campylobacter jejuni. Proc. Natl. Acad. Sci. U.S.A. 107, 5160–5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trent M. S., Stead C. M., Tran A. X., Hankins J. V. (2006) Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin. Res. 12, 205–223 [DOI] [PubMed] [Google Scholar]
- 9. Raetz C. R., Reynolds C. M., Trent M. S., Bishop R. E. (2007) Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reynolds C. M., Kalb S. R., Cotter R. J., Raetz C. R. (2005) A phosphoethanolamine transferase specific for the outer 3-deoxy-d-manno-octulosonic acid residue of Escherichia coli lipopolysaccharide. Identification of the eptB gene and Ca2+ hypersensitivity of an eptB deletion mutant. J. Biol. Chem. 280, 21202–21211 [DOI] [PubMed] [Google Scholar]
- 11. Tamayo R., Choudhury B., Septer A., Merighi M., Carlson R., Gunn J. S. (2005) Identification of cptA, a PmrA-regulated locus required for phosphoethanolamine modification of the Salmonella enterica serovar typhimurium lipopolysaccharide core. J. Bacteriol. 187, 3391–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H., Hsu F. F., Turk J., Groisman E. A. (2004) The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 186, 4124–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox A. D., Wright J. C., Li J., Hood D. W., Moxon E. R., Richards J. C. (2003) Phosphorylation of the lipid A region of meningococcal lipopolysaccharide. Identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 185, 3270–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackinnon F. G., Cox A. D., Plested J. S., Tang C. M., Makepeace K., Coull P. A., Wright J. C., Chalmers R., Hood D. W., Richards J. C., Moxon E. R. (2002) Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43, 931–943 [DOI] [PubMed] [Google Scholar]
- 15. Wright J. C., Hood D. W., Randle G. A., Makepeace K., Cox A. D., Li J., Chalmers R., Richards J. C., Moxon E. R. (2004) lpt6, a gene required for addition of phosphoethanolamine to inner-core lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae. J. Bacteriol. 186, 6970–6982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran A. X., Karbarz M. J., Wang X., Raetz C. R., McGrath S. C., Cotter R. J., Trent M. S. (2004) Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J. Biol. Chem. 279, 55780–55791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aas F. E., Egge-Jacobsen W., Winther-Larsen H. C., Løvold C., Hitchen P. G., Dell A., Koomey M. (2006) Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281, 27712–27723 [DOI] [PubMed] [Google Scholar]
- 18. Chamot-Rooke J., Mikaty G., Malosse C., Soyer M., Dumont A., Gault J., Imhaus A. F., Martin P., Trellet M., Clary G., Chafey P., Camoin L., Nilges M., Nassif X., Duménil G. (2011) Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science 331, 778–782 [DOI] [PubMed] [Google Scholar]
- 19. Hegge F. T., Hitchen P. G., Aas F. E., Kristiansen H., Løvold C., Egge-Jacobsen W., Panico M., Leong W. Y., Bull V., Virji M., Morris H. R., Dell A., Koomey M. (2004) Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc. Natl. Acad. Sci. U.S.A. 101, 10798–10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chalker A. F., Minehart H. W., Hughes N. J., Koretke K. K., Lonetto M. A., Brinkman K. K., Warren P. V., Lupas A., Stanhope M. J., Brown J. R., Hoffman P. S. (2001) Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183, 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao R., Alm R. A., Trust T. J., Guerry P. (1993) Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130, 127–130 [DOI] [PubMed] [Google Scholar]
- 22. Yao R., Guerry P. (1996) Molecular cloning and site-specific mutagenesis of a gene involved in arylsulfatase production in Campylobacter jejuni. J. Bacteriol. 178, 3335–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balaban M., Joslin S. N., Hendrixson D. R. (2009) FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J. Bacteriol. 191, 6602–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLuckey S. A., Mentinova M. (2011) Ion/neutral, ion/electron, ion/photon, and ion/ion interactions in tandem mass spectrometry. Do we need them all? Are they enough? J. Am. Soc. Mass Spectrom. 22, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coon J. J. (2009) Collisions or electrons? Protein sequence analysis in the 21st century. Anal. Chem. 81, 3208–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wassenaar T. M., van der Zeijst B. A., Ayling R., Newell D. G. (1993) Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139, 1171–1175 [DOI] [PubMed] [Google Scholar]
- 28. Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J. (1988) Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157, 472–479 [DOI] [PubMed] [Google Scholar]
- 29. Hendrixson D. R., DiRita V. J. (2004) Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 52, 471–484 [DOI] [PubMed] [Google Scholar]
- 30. Shi W., Bogdanov M., Dowhan W., Zusman D. R. (1993) The pss and psd genes are required for motility and chemotaxis in Escherichia coli. J. Bacteriol. 175, 7711–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walsh C. T., Garneau-Tsodikova S., Gatto G. J., Jr. (2005) Protein posttranslational modifications. The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 44, 7342–7372 [DOI] [PubMed] [Google Scholar]
- 32. Naessan C. L., Egge-Jacobsen W., Heiniger R. W., Wolfgang M. C., Aas F. E., Røhr A., Winther-Larsen H. C., Koomey M. (2008) Genetic and functional analyses of PptA, a phospho-form transferase targeting type IV pili in Neisseria gonorrhoeae. J. Bacteriol. 190, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kowarik M., Numao S., Feldman M. F., Schulz B. L., Callewaert N., Kiermaier E., Catrein I., Aebi M. (2006) N-Linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science 314, 1148–1150 [DOI] [PubMed] [Google Scholar]
- 34. Chevance F. F., Takahashi N., Karlinsey J. E., Gnerer J., Hirano T., Samudrala R., Aizawa S., Hughes K. T. (2007) The mechanism of outer membrane penetration by the eubacterial flagellum and implications for spirochete evolution. Genes Dev. 21, 2326–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen S., Beeby M., Murphy G. E., Leadbetter J. R., Hendrixson D. R., Briegel A., Li Z., Shi J., Tocheva E. I., Müller A., Dobro M. J., Jensen G. J. (2011) Structural diversity of bacterial flagellar motors. EMBO J. 30, 2972–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aspinall G. O., Lynch C. M., Pang H., Shaver R. T., Moran A. P. (1995) Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231, 570–578 [PubMed] [Google Scholar]
- 37. Scott N. E., Parker B. L., Connolly A. M., Paulech J., Edwards A. V., Crossett B., Falconer L., Kolarich D., Djordjevic S. P., Hojrup P., Packer N. H., Larsen M. R., Cordwell S. J. (April 1, 2011) Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni. Mol. Cell. Proteomics 10.1074/mcp.M000031-MCP000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.