Background: Hypoxia-induced CA IX contributes to pH control in tumor cells, and control of pH is important for cell migration.
Results: CA IX increases migration through catalytic domain and interacts with bicarbonate transporters in lamellipodia.
Conclusion: CA IX is an active component of the molecular machinery that facilitates migration of tumor cells through pH regulation at the leading edge membranes.
Significance: This identifies CA IX as a target to suppress cell migration and reduce tumor aggressiveness.
Keywords: Acidosis, Cancer, Cell Migration, Cell pH, Hypoxia, Bicarbonate Transporters, Carbonic Anhydrase IX
Abstract
Carbonic anhydrase IX (CA IX) is a hypoxia-induced cell surface enzyme expressed in solid tumors, and functionally involved in acidification of extracellular pH and destabilization of intercellular contacts. Since both extracellular acidosis and reduced cell adhesion facilitate invasion and metastasis, we investigated the role of CA IX in cell migration, which promotes the metastatic cascade. As demonstrated here, ectopically expressed CA IX increases scattering, wound healing and transwell migration of MDCK cells, while an inactive CA IX variant lacking the catalytic domain (ΔCA) fails to do so. Correspondingly, hypoxic HeLa cells exhibit diminished migration upon inactivation of the endogenous CA IX either by forced expression of the dominant-negative ΔCA variant or by treatment with CA inhibitor, implying that the catalytic activity is indispensable for the CA IX function. Interestingly, CA IX improves cell migration both in the absence and presence of hepatocyte growth factor (HGF), an established inducer of epithelial-mesenchymal transition. On the other hand, HGF up-regulates CA IX transcription and triggers CA IX protein accumulation at the leading edge of lamellipodia. In these membrane regions CA IX co-localizes with sodium bicarbonate co-transporter (NBCe1) and anion exchanger 2 (AE2) that are both components of the migration apparatus and form bicarbonate transport metabolon with CA IX. Moreover, CA IX physically interacts with AE2 and NBCe1 in situ, as shown here for the first time. Thus, our findings suggest that CA IX actively contributes to cell migration via its ability to facilitate ion transport and pH control at protruding fronts of moving cells.
Introduction
Carbonic anhydrase IX (CA IX)4 is a cancer-associated transmembrane enzyme catalyzing the reversible reaction CO2 ⇆ H+ + HCO3− involved in ion transport and pH balance (1). Its catalytic domain (CA) is exposed at the cell surface and contributes to acidification of the outer microenvironment by producing extracellular protons and accelerating CO2 diffusion (2, 3). At the same time, it facilitates neutralization of intracellular pH by generating bicarbonate ions for their coupled transport across plasma membrane via bicarbonate transporters, including AE2 and NBCe1 (4, 5). Unlike other carbonic anhydrases, CA IX possesses a proteoglycan-like region (PG), which represents an N-terminal extension of the catalytic domain (6). A short intracellular tail at the C terminus of CA IX is regulated by phosphorylation of Thr-443 by protein kinase A and mediates inside-out signaling to extracellular catalytic domain (7, 8).
Expression of CA IX is principally driven by hypoxia through the hypoxia-inducible factor (HIF) pathway, which can also be induced in response to genetic inactivation of the von Hippel Lindau tumor suppressor, or activation of oncogenes in tumor tissues (9, 10). Hypoxia significantly contributes to the acquisition of an aggressive tumor phenotype by HIF-mediated transcriptional reprogramming, consequences of which involve adaptive metabolic changes and a perturbed acid-base balance. This results in activation of pH control mechanisms (including expression and activation of CA IX) that maintain neutral intracellular pH and create extracellular acidosis (11). Acidosis as an inherent sequel of hypoxia further supports tumor progression by reducing cell adhesion, increasing motility and migration, inducing neo-vascularization, activating proteases, and enhancing other hypoxia-induced processes. These changes reinforce the complex remodeling of tumor cells known as epithelial-mesenchymal transition and thereby enhance their metastatic propensity (12).
Reduced cell-to-cell adhesion is the first prerequisite for epithelial cell migration that is usually connected to altered expression, topographic localization and/or functional inactivation of cadherins and other junctional proteins (13). This is facilitated by receptor-mediated signaling induced in response to extracellular growth factors, such as the hepatocyte growth factor (HGF), which binds to the c-Met receptor tyrosine kinase and thereby activates intracellular effector molecules (14). Cell migration itself then depends on the establishment of spatial asymmetry including formation of polarized leading/trailing edges, redistribution of functionally relevant molecules and development of polarized pH gradients (15). Pericellular pH is more acidic at the cell front than at the rear end of migrating cells while the intracellular pH slope is reversed. Such a pH gradient is important for the migratory cycle composed of lamellipodium extension, formation of stable attachments near the leading edge, release of adhesions and retraction at the cell rear end (16). Active pH regulation in the migrating cell is facilitated by ion channels/transporters (including anion exchanger AE2 and Na+/HCO3− co-transporter NBCe1), which are redistributed from their original basolateral position to the leading edge, where they intensify ion transport across the front plasma membrane (17).
Interestingly, CA IX occupies basolateral membranes in intact polarized epithelial cells both in vivo and in vitro. It is also capable of weakening intercellular adhesion and increasing cell dissociation by reducing E-cadherin binding to β-catenin (18). Moreover, it has been shown to cooperate with bicarbonate transporters in pH regulation (4). All these attributes together with induction by hypoxia predispose CA IX to participate in the migration machinery.
This prompted us to study the CA IX role in cell migration. Here we show that ectopically expressed CA IX enhances migration rate and scattering of polarized epithelial MDCK cells and that the CA domain is required for both effects. In contrast, expression of an enzyme-dead mutant as well as treatment with CA inhibitors can reduce migration of HeLa cells expressing wild type CA IX. Furthermore, in A549 and SiHa carcinoma cells stimulated to migration, CA IX is redistributed to leading edges, co-localizes and interacts with bicarbonate transporters NBCe1 and AE2. Thus, our findings support the view that CA IX actively contributes to cell migration and that the catalytic domain is required for its proper function.
EXPERIMENTAL PROCEDURES
Cell Culture
A549, SiHa, HeLa, and MDCK cells and their transfected derivatives were grown in DMEM with 10% fetal calf serum. CA IX expression in the studied cell lines was verified by immunoblotting as described below and shown in supplemental Figs. S1 and S2. Hypoxic experiments were performed in an anaerobic Workstation (Ruskinn Technologies) in a 2% O2, 2% H2, 5% CO2, 91% N2 atmosphere. Parallel normoxic dishes were incubated in ambient atmosphere with 5% CO2. Preparation of cells transfected with wt CA IX and with its deletion mutant (ΔCA) was described previously (16, 2). For migration and co-localization experiments, A549 lung carcinoma cells were incubated in hypoxia for 24 h in medium with 10% FCS and for an additional 24 h in medium with 0.5% FCS. Then a straight scratch was made using a pipette tip, the cells were washed, and stimulated to migrate with HGF. SiHa cells were grown in spheroids preformed in hanging drops for 3 days and then expanded in a non-adherent dish for an additional 11 days. Next, they were transferred to tissue culture dishes and allowed to attach and spread for 24 h. Resulting cell colonies were stimulated with HGF and kept in hypoxia for 24 h.
Antibodies and Reagents
Recombinant human hepatocyte growth factor (HGF, Sigma) was used to stimulate migration and CA IX transcription. Expression of the HGF receptor c-Met was verified by immunoblotting in all studied cell lines (supplemental Figs. S1 and S2). Immunodetection was performed using the following antibodies and reagents: anti-human CA IX mouse monoclonal antibody M75 in undiluted hybridoma medium (19); mouse mAb clone ZK-31, specific for desmosomes, diluted 1:300 (Sigma Aldrich), rabbit anti-E-cadherin polyclonal antibody, diluted 1:500 (Santa Cruz Biotechnology); goat anti-β-actin polyclonal antibody, diluted 1:150 (Santa Cruz Biotechnology); rabbit anti-NBCe1 polyclonal antibody raised against amino acids 338–391 (1:100, AB 3212 Millipore). Rabbit anti-AE2 polyclonal antibody was raised against a peptide mapping a portion of the C-terminal region of human AE2 (amino acids 1228–1241) by GeneScript USA, Inc. Alexa Fluor® 488 donkey anti-mouse IgG, Alexa Fluor® 594 donkey anti-goat IgG, Alexa Fluor® 594 goat anti-rabbit IgG, and Alexa Fluor® 633 donkey anti-mouse IgG secondary antibodies were from Invitrogen. CA inhibitor acetazolamide (Sigma) was used at 1 mm final concentration.
Wound Healing Assay
Transfected MDCK cells and HeLa cells were seeded to confluence at 2,8 × 105/well in 12-well tissue culture plates. The following day, the cells were starved overnight in DMEM with 0.5% FCS. A wound was made with a sterile micropipette tip. Floating cells were removed by washing with PBS. Fresh DMEM + 0.5% FCS was then added, with or without HGF (Sigma). Cells were photographed immediately after wound initiation and then at indicated time points using inverted Zeiss microscope (Axiovert 40 CFL), ×10 objective. Wound healing was quantified using ImageJ software as the percentage of closed wound area (mean ± S.D.), and results were compared by t test.
Scatter Assay
The cell aggregates were preformed from a single-cell suspension seeded in Petri dish with nonadhesive surface (Greiner) in DMEM with 10% FCS and incubated overnight on an orbital rotation shaker (100 rpm). The next day, the aggregates were moved into 6-well tissue culture plates and allowed to attach. After their spread into cell islands (t0) they were either induced with HGF or left untreated. Cell islands were imaged on an inverted microscope Zeiss (Axiovert 40 CFL) with a 5× objective at indicated times. Extent of cell dispersion was analyzed as island area increase at indicated time intervals. Cellular islands were measured at each time point, and results were expressed as mean ± S.D. and compared by t test.
Transwell Migration Assays
Transwell Migration Assays were carried out in BD Falcon FluoroBlok 24-Multiwell Inserts (BD Biosciences). Overnight starved cells (0.5% FCS) were labeled with a lipophilic fluorescent dye DiO (Invitrogen), and then seeded on the surface of a fluorescence-blocking microporous membrane at 1 × 105 cells/insert in a 24-well plate in 0.5% FCS DMEM (phenol red-free). HGF was added into the lower chamber. Fluorescence intensity was measured from the bottom of the plate to detect only the cells that had migrated across the insert membrane at different time points (Synergy HT, Biotek). Fluorescence intensity at the starting point (t0) was considered as baseline.
Cell Dissociation Assay
The assay was performed as described before (2).
Quantitative Reverse Transcriptase PCR
Total RNA was extracted using the Instapure reagent (Eurogentec) from cells that were cultured in hypoxic or normoxic conditions for 17 h. RNA (2 μg) was reverse transcribed with M-MuLV reverse transcriptase (Finnzymes) using random heptameric primers. Quantitative RT PCR analysis of CA9 and β-actin as internal standard were performed on a StepOneTM Real-Time PCR System (Applied Biosystems) using POWER SYBR® Green PCR Master Mix (Applied Biosystems) and the following primers: CA9-S, 5′-CCGAGCGACGCAGCCTTTGA-3′, CA9-A, 5′-GGCTCCAGTCTCGGCTACCT-3′, β-actin-S, 5′-TCCTCCCTGGAGAAGAGCTA-3′, β-actin-A, 5′-ACATCTGCTGGAAGGTGGAC-3′.
Immunofluorescence and Confocal Microscopy
Cells grown on glass coverslips were fixed in methanol at −20 °C for 5 min. Nonspecific binding was blocked with PBS containing 1% BSA for 30 min at 37 °C. The cells were sequentially incubated with primary antibodies (M75 followed by the other antibody) diluted in PBS with 0.5% BSA (PBS-BSA), each for 1 h at 37 °C, washed four times with PBS containing 0.02% Tween 20 for 10 min, incubated with fluorescent secondary antibodies (always added together in one step) diluted in PBS-BSA for 45 min at 37 °C, washed once with PBS, incubated with DAPI (1:36 000) in PBS to stain nuclei, and then washed three times with PBS for 10 min. Finally, the cells were mounted onto slides in Fluorescent Mounting Medium (Calbiochem), analyzed by Zeiss LSM 510 Meta confocal microscope, scanned in multitrack mode and deconvoluted by Huygens software (Scientific Volume Imaging). The acquired images were processed in ImageJ using JACoP plugin and Pearson's correlation coefficient for the leading edge area of cells stimulated into migration was calculated.
Proximity Ligation Assay (PLA)
Proximity ligation assay (PLA) was used for in situ detection of CA IX interaction with AE2 and NBCe1. The assay was performed in a humid chamber at 37 °C according to the manufacturer's instructions (Olink Bioscience). SiHa and A549 cells were prepared as described under “Cell Culture” paragraph above. The cells were fixed with methanol and blocked with blocking solution for 30 min. Then the samples were incubated with a mixture of mouse anti-CA IX monoclonal antibody M75 and rabbit anti-AE2 or anti-NBCe1 polyclonal serum for 1 h, washed three times, and incubated with plus and minus PLA probes. After washing, the ligation mixture containing connector oligos was added for 30 min. The washing step was repeated, and amplification mixture containing fluorescently labeled DNA probe was added for 100 min. Finally, the samples were washed and mounted with DAPI mounting medium. The signal representing interaction was analyzed by Zeiss LSM 510 Meta confocal microscope.
Collagen Rafts
Collagen type I from rat tail was mixed with normal human fibroblasts suspended in 2xDMEM with 20% FCS and incubated overnight in 24-well plates to form gels. Monolayer of HeLa cells was trypsinized and cells were seeded over collagen gels. Alternatively, HeLa cell spheroids formed in agarose-coated wells of a 96-well microplate for 12 days were transferred on top of the collagen gels. Resulting collagen rafts were transferred onto metal grids and cultured at the air-liquid interface to allow for their growth and/or invasion. The medium was changed every second day until day 12. Then the rafts were fixed in formalin, embedded in paraffin, sectioned, deparaffinized, and stained to detect CA IX.
Immunohistochemistry
Rehydrated 5-μm sections were immunostained with M75 monoclonal antibody using the UltraTech HRP Streptavidin-Biotin Universal Detection System (Immunotech) as described earlier (20).
RESULTS
CA IX Increases Migration of MDCK Cells in an HGF-independent Manner
To evaluate the role of CA IX in cell migration, we utilized stably transfected MDCK-CA IX cells, which display basolateral localization of CA IX, increased CA IX-mediated extracellular acidification in response to hypoxia, and reduced cell-cell adhesion when compared with mock-transfected CA IX-negative MDCK cells (18, 2). We performed three different migration assays, including wound healing, scattering, and transwell migration. In all cases, CA IX expression led to increased migration of MDCK cells (Figs. 1 and 2). Moreover, the extent of cell migration clearly reflected the proportion of CA IX-expressing cells (supplemental Fig. S3). Remarkably, the CA IX effects on migration were similar in the absence or presence of hepatocyte growth factor (HGF), a well-known inducer of epithelial-mesenchymal transition (Fig. 1, C and D). This was clearly due to CA IX, as the expression levels of the HGF receptor c-Met were equal in CA IX-expressing cells and in negative controls (supplemental Fig. S1B). Out of the range of tested concentrations, HGF displayed the strongest migration-inducing effect on CA IX-expressing cells at 5 ng/ml (supplemental Fig. S4). The effect was similar at higher HGF concentrations in the cells containing CA IX, but was still increasing in their CA IX-negative counterparts. This could suggest that the c-Met-related pathways stimulated by HGF might be already partially activated in the presence of CA IX and their full activation could be achieved at lower HGF level.
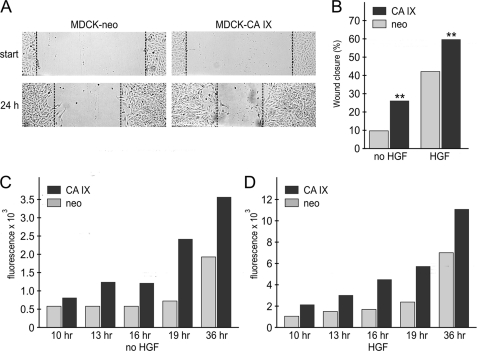
FIGURE 1.
Effect of CA IX expression on wound healing and migration of MDCK cells. A, confluent monolayers of MDCK-CA IX cells and mock-transfected MDCK-neo cells, were starved overnight, wounded, and allowed to migrate as described under “Experimental Procedures.” The wound was photographed at start and after 24 h. The healing capacity of MDCK-CA IX cells was visibly increased compared with the MDCK-neo counterpart. B, same experiment was performed in the absence and presence of 20 ng/ml HGF. Wound width was measured in 16 positions immediately after wounding (t0) and 24 h later (t), and wound closure was calculated. CA IX expression stimulated the healing independently of HGF. C and D, MDCK-CA IX and MDCK-neo cells loaded with DiO dye were seeded in a 24-well plate inside florescence-blocking transwell inserts (in triplicates) and allowed to migrate toward lower chambers containing medium either without (C) or with 20 ng/ml HGF (D). Fluorescence intensity was measured at the bottom of the plate at indicated time points (t) and expressed as a value subtracted by baseline fluorescence measured at the start of migration (t0). HGF showed 3–4× increased migration and CA IX expression stimulated migration about 2× independently of HGF.
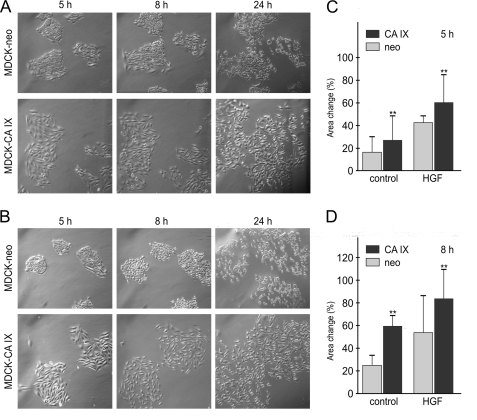
FIGURE 2.
Effect of CA IX on scattering and morphology of MDCK cells. A, islands of MDCK-CA IX and MDCK-neo cells, were generated by plating preformed cell aggregates. Spreading and outward migration were allowed to proceed in the absence of HGF for 24 h. B, same experiment was done in the presence of 20 ng/ml HGF. Pictures of the selected colonies were taken at different time points. Increased migration and mesenchymal-like morphology was clearly apparent in CA IX-expressing cells and was independent of HGF. C, significant increase (**, p < 0.001) in areas of MDCK-CA IX cell islands (n = 20) compared with MDCK-neo islands (n = 20) was evident as early as after 5 h of scattering for both HGF-treated and non-treated cells (D) and was further improved after an additional 3 h.
CA IX also endowed the MDCK cells with a mesenchymal-like morphology, which was mainly apparent in the scatter assay (Fig. 2). Because MDCK-CA IX cells cannot grow in tight colonies when seeded from single cell suspension (unlike the mock-transfected MDCK cells), we modified the assay to pre-form cell aggregates and allow them to attach and spread. Then we observed their morphology and scattering. In accord with above data, MDCK-CA IX islands displayed greater dispersion than MDCK-neo counterparts (both with and without HGF, see Fig. 2, A–D).
CA IX Catalytic Domain/Activity Is Required for Increased Migration
Next we wanted to learn whether the CA domain, which executes the enzymatic activity of CA IX, is involved in the above-observed phenomena. For this purpose, we used MDCK-ΔCA cells transfected with an enzymatically inactive ΔCA mutant of CA IX, which lacks a major part of the catalytic domain (2). These MDCK-ΔCA cells displayed reduced dissociation when compared with MDCK-CA IX cells indicating that the capacity of CA IX to destabilize cell-cell adhesion was perturbed due to the deletion of the catalytic domain (Fig. 3A). In the colony scatter assay, the ability of MDCK-ΔCA cells to migrate was significantly lower than that of MDCK cells expressing intact CA IX (Fig. 3B), suggesting that the CA domain is necessary for a full demonstration of the CA IX pro-migratory properties.
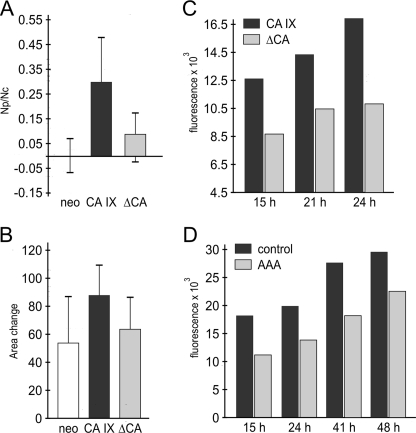
FIGURE 3.
The role of CA domain/activity in cell migration. A, MDCK-CA IX cells, MDCK-ΔCA cells (expressing catalytically inactive deletion variant) and MDCK-neo cells were subjected to dissociation assay. MDCK-ΔCA cells showed a significantly decreased ability to disrupt intercellular contacts compared with MDCK-CA IX cells, but a slightly higher dissociation than the MDCK-neo cells, as evident from the ratio between the number of particles (Np) obtained by monolayer dissociation and total cell number (Nc). B, scatter assay including HGF stimulation was performed with all three MDCK cell types and evaluated as in Fig. 2C. Deletion of the CA domain was associated with reduced scattering. C, transwell migration was performed with hypoxic HeLa cells naturally expressing CA IX versus hypoxic HeLa-ΔCA cells, in which the dominant negative ΔCA variant abolishes the enzymatic activity of wild type CA IX. HeLa-ΔCA cells clearly exhibited reduced migration. D, decreased migration was also observed in hypoxic HeLa cells in the presence of 1 mm carbonic anhydrase inhibitor acetazolamide (AAA).
To examine the significance of the catalytic domain in the context of natural expression of CA IX, we performed the transwell migration experiment with HeLa cells expressing the hypoxia-induced endogenous CA IX protein in comparison to HeLa cells stably transfected with the enzyme-dead ΔCA mutant (see the supplemental Fig. S2C). As described earlier, ΔCA behaves in a dominant-negative manner, since it is capable of diminishing extracellular acidification driven by wild-type CA IX in HeLa cells exposed to hypoxia (2). Indeed, HeLa-ΔCA cells incubated in hypoxia showed a reduced migration speed when compared with the mock-transfected HeLa cells containing only the wild type protein (Fig. 3C), indicating the need for an intact catalytic domain in CA IX-related migration. This assumption was corroborated by pharmacologic inhibition of CA IX activity. Because HeLa cells do not express additional CA isoforms implicated in tumor pH regulation, namely cytosolic CA II and transmembrane CA XII (data not shown), we used acetazolamide, a general inhibitor of carbonic anhydrase activity. In accord with the above experiment, hypoxic HeLa cells treated with acetazolamide showed decreased migration compared with non-treated controls (Fig. 3D). These data clearly suggest that the enzymatic activity represents an important attribute of CA IX function in cell migration and point to the involvement of CA IX in the pH-regulating apparatus of migrating cells.
CA IX Relocalizes to Lamellipodia of the Migrating Cells
Because acquisition of the migratory phenotype is associated with redistribution of relevant regulatory molecules, we analyzed localization of CA IX in MDCK cells stimulated to migration with HGF. In both wound healing and scatter assays, CA IX was repositioned from the basolateral membranes to lamellipodia (Fig. 4, A and B). At the same time, cell-cell adhesion protein E-cadherin and desmosomes were to a large extent depleted from the plasma membrane (Fig. 4C), in accord with the known fact that migration is accompanied by internalization of the junctional proteins (21).
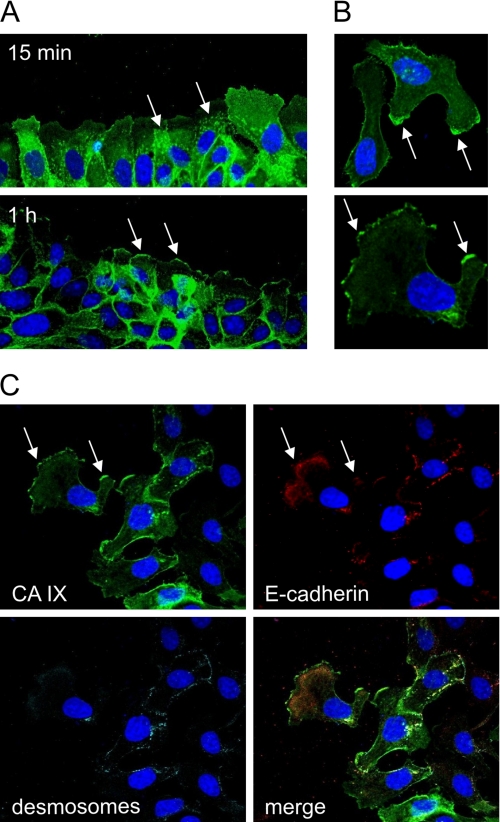
FIGURE 4.
HGF-induced relocalization of CA IX to lamellipodia of migrating MDCK cells. A, confluent MDCK-CA IX cells were wounded and stimulated to migration by HGF. After 15 min and 1 h, the cells were fixed with methanol, and CA IX was detected by immunofluorescence. The upper figure shows intracellular vesicles transporting CA IX toward wound-facing membranes of the cells, whereas the lower figure shows CA IX at the cell fronts. B, CA IX staining signal was confined to lamellipodia of migrating cells. C, border area of scattered cell island contains migrating cells, in which CA IX was accumulated in the protruding cell membranes, whereas E-cadherin and desmosomes were internalized to submembrane areas. In this triple staining, E-cadherin and desmosomes were first stained with the sequentially added primary antibodies, then with the secondary antibodies added together and in the last step with the directly labeled M75 mAb conjugated with Alexa Fluor® 488.
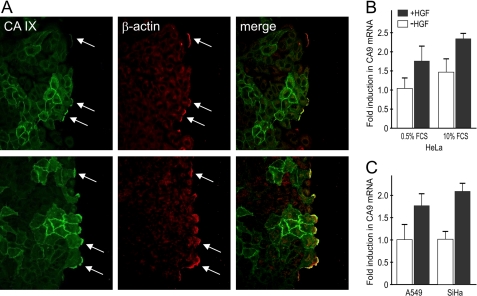
The CA IX protein is widely expressed in hypoxic tumors and therefore, we were interested in its localization in migrating hypoxic tumor cells. For this purpose, lung carcinoma A549 cells were incubated in 2% O2 for 48 h to induce the synthesis of CA IX. Hypoxic monolayer was scratched and stimulated with HGF. As early as 15 min after wounding, CA IX was redistributed into the lamellipodia, where it strongly co-localized with β-actin, an established driving force for lamellipodial extension (Fig. 5A). Extensive formation of lamellipodia and membrane ruffling was clearly visible 1 h after induction of migration and occurred predominantly in CA IX-positive cells.
FIGURE 5.
Response of CA IX to HGF stimulation of tumor cells. A, hypoxic monolayer of lung carcinoma A549 cells was wounded and stimulated with HGF for 15 min and 1 h. The cells were then fixed, double-stained for CA IX and β-actin, and analyzed by confocal microscopy. Both proteins occupied the same position at lamellipodia (see arrows) that were formed predominantly by CA IX-expressing cells. B, RNA was isolated from HeLa cells grown in medium supplemented with low (0.5%) and high (10%) FCS and incubated in hypoxia for 17 h in the absence or presence of 30 ng/ml HGF. Quantitative RT PCR analysis with gene-specific primers for CA IX, and β-actin was performed as described under “Experimental Procedures.” Data related to CA IX were normalized to β-actin and expressed as fold induction in mRNA level compared with non-treated low serum control. CA IX expression was induced by HGF independently of serum level. C, similar effect of HGF was evident in an analogous experiment with hypoxic A549 and normoxic SiHa cells maintained in 10% FCS.
Interestingly, HGF induced not only relocalization of CA IX, but also its transcriptional levels in tumor cell lines including HeLa, A549, and SiHa (Fig. 5B,C). HGF-mediated effect on CA IX transcription was evident in hypoxic HeLa and A549 cells. However, SiHa cells responded to HGF better in normoxia, possibly because hypoxia alone strongly induces CA IX to its threshold level that does not allow for further increase.5
CA IX Colocalizes with Bicarbonate Transporters
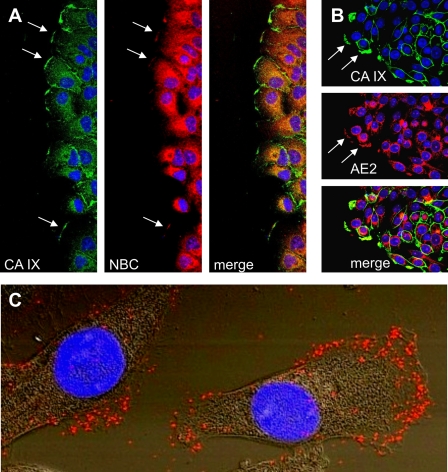
The CA IX position in the leading edge membranes of migrating cells and the requirement for its catalytic domain/activity indicate its spatial and functional association with ion transporters that regulate local pH gradient facilitating migration. Previous studies have demonstrated functional cooperation of CA IX with bicarbonate transporters, namely sodium bicarbonate co-transporters (represented by NBCe-1, encoded by the SLC4A4 gene) and anion exchangers (AE2, the SLC4A2 gene product), within a protein complex called the bicarbonate transport metabolon (22, 4). However, this functional cooperation would require positional coordination, so that the bicarbonate generated by CA IX could be directly delivered to the transporter. In accord with this proposal, hypoxia-induced CA IX showed a high degree of co-localization with NBCe1 in the leading edge of wounded A549 cells, with Pearson's coefficient of 0.7519 ± 0.0639, n = 25 (Fig. 6A). In addition, we could demonstrate that CA IX significantly co-localized with AE2 in the leading edge regions of SiHa cells migrating from attached spheroids incubated under hypoxia, with Pearson's coefficient of 0.628 ± 0.0762, n = 16 (Fig. 6B).
FIGURE 6.
Co-localization of CA IX with bicarbonate transporters in migrating tumor cells. A, hypoxic monolayer of A549 cells was wounded, stimulated with HGF, double-stained for CA IX and NBC, and analyzed by confocal microscopy. Clear overlap of the two staining signals was evident at the leading edge membranes of the cells facing the wound (see arrows). B, spheroids formed from SiHa cells were attached, spread under hypoxia, stimulated to migration with HGF, and double-stained for CA IX and AE2 and subjected to confocal microscopic analysis. Cells migrating out of the spheroid periphery displayed co-localization of CA IX with AE2 suggesting spatial cooperation of these proteins. C, SiHa cells were plated and treated as described above in part B. Then they were fixed with methanol and subjected to PLA analysis with CA IX-specific mAb M75 and AE2-specific polyclonal antibody. The red PLA signal was clearly visible in the lamellipodia of migrating SiHa cells, indicating the interaction of CA IX with AE2 in the protruding cellular fronts. The image represents an overlay of Nomarski view with fluorescence signal.
CA IX Interacts with AE2 and NBCe1
To obtain evidence for a direct cross-talk between CA IX and bicarbonate transporters, we performed proximity ligation assay (PLA), which allows the detection of stable as well as transient protein-protein interactions in situ. This in-cell co-immunoprecipitation is a much better alternative to conventional co-immunoprecipitation of extracted proteins as it preserves the natural context of the analyzed interactors (23). Using this approach, we were able to show that CA IX interacts with AE2 as well as with NBCe1 in the lamellipodia of migrating cells (Fig. 6C, supplemental Figs. S5 and S6). In both cases, the PLA signal was predominantly localized in the leading edge compartments that drive cell migration.
Indeed, the evidence for the physical interaction of CA IX with bicarbonate transporters provides the first proof-of-concept in support of the proposal that CA IX is a functional component of the cellular apparatus involved in generation of a pericellular pH gradient that is important for migration and invasion.
CA IX Is Expressed in Invasive Cancer Cells
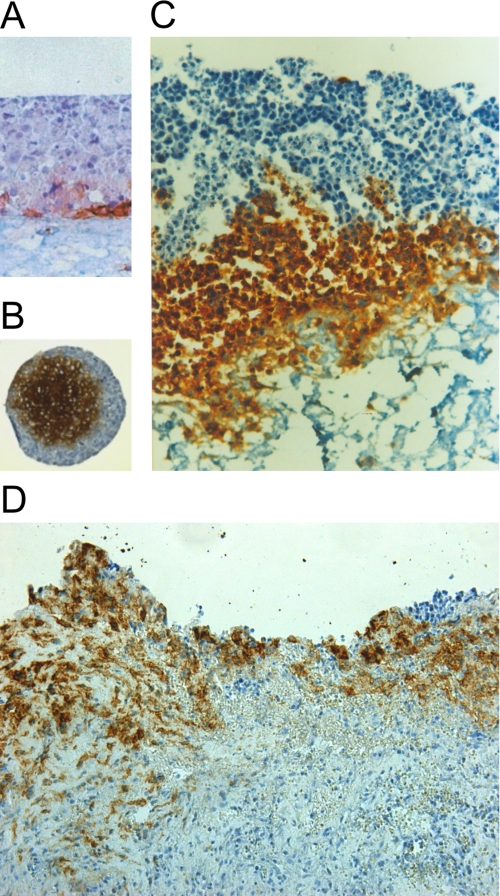
We then examined CA IX expression in relationship to invasive cell behavior using organotypic cultures, in which epithelial cells are cultured on top of a dermal equivalent formed from collagen and normal human fibroblasts. HeLa cells grown in a monolayer before seeding on collagen-embedded fibroblasts, generated multilayer epithelium that did not invade the dermal support and showed CA IX expression only in few cells of the deepest epithelial layer (Fig. 7A). In contrast, multicellular three-dimensional HeLa spheroids formed a hypoxic core with strong CA IX expression (Fig. 7B). Interestingly, these spheroids showed the capacity to invade the fibroblasts-containing collagen lattice via the CA IX-expressing cells (Fig. 7C). A similar invasion pattern involving CA IX-positive tumor cells was found in colon adenocarcinoma tissue specimens (Fig. 7D), in line with data from other tumor types described in literature. These results imply that CA IX is a component of the invasive tumor cell phenotype.
FIGURE 7.
CA IX expression in invasive tumor cells. A, three-dimensional tumor cell growth was reconstructed by plating HeLa cells on a dermal equivalent composed of collagen mixed with fibroblasts. The cells obtained by dissociation of HeLa monolayer were able to form a multilayer collagen raft with weak CA IX expression in basal-like cells. No invasion into the collagen occurred in this model. B, HeLa cells were grown in three-dimensional spheroids. The hypoxic core cells showed strong CA IX staining signal. C, HeLa spheroid plated on the dermal equivalent invaded the collagen via CA IX-positive cells from the hypoxic core, but no invasion of the peripheral CA IX-negative cells could be seen. D, tissue section of metastatic colon adenocarcinoma displays strong CA IX expression with invasive CA IX-positive cells exhibiting mesenchymal morphology. All specimens represent paraffin sections subjected to immunohistochemical staining for CA IX using the M75 monoclonal antibody.
DISCUSSION
The rationale behind the functional involvement of CA IX in cell migration and invasion was based on several merging facts: (a) CA IX expression/function is induced by hypoxia and hypoxia stimulates migration/invasion and drives epithelial-mesenchymal transition, (b) CA IX interferes with E-cadherin-mediated cell adhesion, and down-regulation or disabling of E-cadherin is the prerequisite for migration/invasion, (c) CA IX contributes to pH regulation across plasma membrane and cell migration depends on the proper pH regulation at the protruding cell membrane, (d) CA IX cooperates with bicarbonate transporters and these are known to actively contribute to cell migration, (e) CA IX is expressed in aggressive, treatment refractory tumors and tumor aggressiveness is strongly connected to the migratory/invasive phenotype and epithelial-mesenchymal transition.
The present study adds new components to this complex picture including evidence for the role of CA IX in migration and acquisition of mesenchymal morphology, its recruitment to leading edge membranes and co-localization with bicarbonate transporters. In addition, it brings the first direct evidence for the in situ physical interaction between CA IX and AE2/NBCe1. These data together with the reduced cell migration in the absence of CA IX catalytic activity fit within the migrating cell machinery model recently proposed by Stock and Schwab (15) and suggest that CA IX participates in the pH regulating apparatus of moving cells, particularly under hypoxic conditions. Such view is compatible with our observation that CA IX expression is confined to the hypoxic cores of three-dimensional spheroids composed of cells that are the first to invade collagen and with the data on the CA domain-dependent interaction between CA IX and bicarbonate transporters (4). Interestingly, deletion of the catalytic domain leads not only to reduced migration, but also to loss of cell survival in hypoxia, since HeLa cells expressing the dominant negative ΔCA variant of CA IX are unable to form hypoxic cores of spheroids and display retarded tumor growth in vivo.6 Thus, CA IX appears to affect diverse aspects of the adaptive response of tumor cells to hypoxia.
Although an intact CA domain is clearly needed for CA IX-related effects, contribution of other protein regions cannot be excluded, particularly taking into account the finding that CA deletion causes significant but not complete inhibition of CA IX functioning (see Fig. 3). Indeed, data available so far indicate that the C-terminal intracellular tail of CA IX can transmit signals to the extracellular enzyme domain and that its PKA-mediated phosphorylation at Thr-443 can activate the catalytic performance of CA IX in hypoxia and thereby influence the cell migration propensity of CA IX-expressing cells (7, 8). On the other hand, the PG domain can affect cell-substrate adhesion and enhance enzymatic activity (24, 25) so its contribution to migration is plausible. Nevertheless, at least some effects of both N-terminal and C-terminal regions of CA IX seem to converge on modulating the enzyme activity, which is clearly carried out by the catalytic domain.
Very recently, Kim and co-workers (26) described the effect of constitutive CA IX overexpression on the migration capacity of C33a cervical carcinoma cells. They showed that CA IX increases cell migration and invasion in vitro via transcriptional activation and functional modulation of proteins that regulate and/or execute cytoskeletal reorganization and epithelial-mesenchymal transition. They suggested that CA IX interacts with secreted protein DKK1 and interferes with the Rho/ROCK signaling pathway leading to the activation of paxillin, which promotes dynamic adhesion turnover and migration. Interestingly, Rho/ROCK pathway is pH-sensitive (27, 28) and thus our recent results can provide an explanation for its relationship to overexpression of an intact, catalytically active CA IX.
Additional links between CA IX and migration/invasion phenomena can be forseen based on its enzymatic activity, induction by hypoxia and known signal tranduction capabilities, including activation of the PI3K/Akt pathway through a phosphotyrosine in its intracellular tail (29). Acidic pericellular pH generated by the ion transport apparatus and aided by CA IX-catalyzed proton production is known to influence disassembly of junctional proteins, integrin-ligand interactions, dynamics of focal adhesion contacts, digestion of extracellular matrix etc (30). Hypoxia activates many components of these processes, including several pH regulators and in addition, stimulates canonical HGF/c-Met axis that provides direct signaling to these downstream effectors (31). Finally, activation of the PI3K/Akt pathway by CA IX may further amplify the above-mentioned signals, activate relevant molecular players and lead to enhanced cell migration (32). In this context it is understandable that CA IX can induce cell migration even in the absence of externally added HGF. However, tumor cells can also use intrinsic signals to stimulate expression of CA IX at the transcriptional level by migration-promoting mechanism, including HGF, SLUG and Notch pathways, both under normoxic and hypoxic conditions (this study and Refs. 33, 34). Such convergence of transcriptional and functional activation of CA IX with its downstream effects (illustrated in Fig. 8) clearly suggests that CA IX is really needed for full expression of the pro-migratory phenotype in many tumor types.
FIGURE 8.
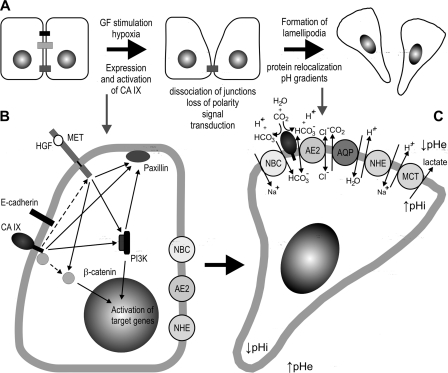
Schematic model illustrating the mechanisms exploited by CA IX in the initiation and maintenance of cell migration, as proposed on the basis of published experimental data. A, upper panel summarizes the sequence of events contributing to acquisition of the migratory phenotype. Hypoxia-induced CA IX appears to participate in both dissociation of intercellular contacts and establishment of a reverse pH gradient at the leading edges of migrating cells. B, in the early phase, the basolaterally localized CA IX could act via its ability to bind β-catenin and displace E-cadherin from adhesion contacts. In addition, CA IX can mediate signal transduction to PI3 kinase (PI3K) and Paxillin as described earlier (18, 26, 29). CA IX might also potentially signal to the c-Met receptor (although this has not been proven so far). Thus, CA IX signaling can feed multiple pathways induced by HGF-activated c-Met (providing an explanation for the HGF-independent pro-migratory effects of CA IX observed in this study). Interestingly, HGF effects include trans-activation of the gene encoding CA IX. C, these signal transduction pathways can contribute to the formation of lamellipodia (characterized by intense glycolytic metabolism) and to re-localization of a range of basolateral proteins, such as CA IX, sodium bicarbonate co-transporter NBCe1 (NBC), anion exchanger 2 (AE2), aquaporins (AQP), sodium-hydrogen exchanger NHE1 (NHE), and monocarboxylate transporter (MCT4) as described elsewhere (15). All these proteins build-up a pH-regulating machinery that generates reverse pH gradient at the cell front, with acidic extracellular pH (↓pHe) and neutral-alkaline intracellular pH (↑pHi). The exchangers and co-transporters extrude lactate and protons and import bicarbonate ions generated by CA IX. To ensure efficient pH regulation via coupled bicarbonate production and transport, CA IX is spatially coordinated and interacts with NBCe1 and AE2. AE2 seems to participate also in the swelling of lamellipodia (by import of chloride ions), however, it is pH-sensitive and thus can potentially import bicarbonate in response to changes in pH gradient (15). CA IX signaling and pH regulatory functions can overlap, as many components of the pathways shown in part B are also pH-sensitive (15).
This suggestion is in line with in vivo effects of CA IX elimination either by an RNA interference approach or by inhibition of CA IX activity with a selective inhibitor, which include delayed tumor growth in mice and enhanced in vivo therapeutic effect of tumor irradiation (35–37). Moreover, many clinical studies of human tumor specimens show significant association of CA IX with invasive phenotype, deeper invasion depth, metastasis, poor prognosis and worse response to therapy (38–40). Although we still do not understand all functional aspects of CA IX behind these observations, our present work brought important evidence for the direct, catalytic domain/activity-dependent role of CA IX in cell migration and created a basis for future investigations of its position in a signal transduction network activated in tumor cells.
Supplementary Material
This work was supported by Grants VEGA 2/0130/11, 2/0129/11 from the Slovak Scientific Grant Agency, from the 7th Framework program of EU (Collaborative Project METOXIA), from the Research and Development Support Agency (DO7RP-0017-09), from the 6th Framework program of EU (Marie Curie RTN project CELLCHECK), and from the Research & Development Operational Program funded by the ERDF (Project TRANSMED 2, ITMS 26240120030).

This article contains supplemental Figs. S1–S6.
S. Pastorekova, unpublished observations.
M. Barathova, A. Gibadulinova, M. Zatovicova, E. Svastova, J. Kopacek, J. Pastorek, and S. Pastorekova, unpublished results.
- CA IX
- carbonic anhydrase IX
- AE2
- anion exchanger 2
- HGF
- hepatocyte growth factor
- HIF
- hypoxia-inducible factor
- NBC
- natrium-bicarbonate co-transporter
- PG
- proteoglycan-like domain.
REFERENCES
- 1. Supuran C. T. (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 [DOI] [PubMed] [Google Scholar]
- 2. Svastová E., Hulíková A., Rafajová M., Zat'ovicová M., Gibadulinová A., Casini A., Cecchi A., Scozzafava A., Supuran C. T., Pastorek J., Pastoreková S. (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 577, 439–445 [DOI] [PubMed] [Google Scholar]
- 3. Swietach P., Hulikova A., Vaughan-Jones R. D., Harris A. L. (2010) New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 16, 6509–6521 [DOI] [PubMed] [Google Scholar]
- 4. Morgan P. E., Pastoreková S., Stuart-Tilley A. K., Alper S. L., Casey J. R. (2007) Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am. J. Physiol. Cell Physiol. 293, C738–C748 [DOI] [PubMed] [Google Scholar]
- 5. Swietach P., Patiar S., Supuran C. T., Harris A. L., Vaughan-Jones R. D. (2009) The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J. Biol. Chem. 284, 20299–20310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Opavský R., Pastoreková S., Zelník V., Gibadulinová A., Stanbridge E. J., Závada J., Kettmann R., Pastorek J. (1996) Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics 33, 480–487 [DOI] [PubMed] [Google Scholar]
- 7. Hulikova A., Zatovicova M., Svastova E., Ditte P., Brasseur R., Kettmann R., Supuran C. T., Kopacek J., Pastorek J., Pastorekova S. (2009) Intact intracellular tail is critical for proper functioning of the tumor-associated, hypoxia-regulated carbonic anhydrase IX. FEBS Letters 583, 3563–3568 [DOI] [PubMed] [Google Scholar]
- 8. Ditte P., Dequiedt F., Svastova E., Hulikova A., Ohradanova-Repic A., Zatovicova M., Csaderova L., Kopacek J., Supuran C. T., Pastorekova S., Pastorek J. (2011) Cancer Res. 71, 7558–7567 [DOI] [PubMed] [Google Scholar]
- 9. Wykoff C. C., Beasley N. J., Watson P. H., Turner K. J., Pastorek J., Sibtain A., Wilson G. D., Turley H., Talks K. L., Maxwell P. H., Pugh C. W., Ratcliffe P. J., Harris A. L. (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60, 7075–7083 [PubMed] [Google Scholar]
- 10. Semenza G. L. (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pouysségur J., Dayan F., Mazure N. M. (2006) Hypoxia signaling in cancer and approaches to enforce tumor regression. Nature 441, 437–443 [DOI] [PubMed] [Google Scholar]
- 12. Brahimi-Horn M. C., Bellot G., Pouysségur J. (2011) Hypoxia and energetic tumor metabolism. Curr. Opin. Genet. Dev. 21, 67–72 [DOI] [PubMed] [Google Scholar]
- 13. Yap A. S., Crampton M. S., Hardin J. (2007) Making and breaking contacts: the cellular biology of cadherin regulation. Curr. Opin. Cell Biol. 19, 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 15. Stock C., Schwab A. (2009) Protons make tumor cells move like clockwork. Pflugers Arch. 458, 981–992 [DOI] [PubMed] [Google Scholar]
- 16. Webb D. J., Parsons J. T., Horwitz A. F. (2002) Adhesion assembly, disassembly and turnover in migrating cells, over and over and over again. Nat. Cell Biol. 4, E97–100 [DOI] [PubMed] [Google Scholar]
- 17. Klein M., Seeger P., Schuricht B., Alper S. L., Schwab A. (2000) Polarization of Na(+)/H(+) and Cl(-)/HCO (3)(-) exchangers in migrating renal epithelial cells. J. Gen. Physiol. 115, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svastová E., Zilka N., Zat'ovicová M., Gibadulinová A., Ciampor F., Pastorek J., Pastoreková S. (2003) Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp. Cell Res. 290, 332–345 [DOI] [PubMed] [Google Scholar]
- 19. Pastoreková S., Závadová Z., Kostál M., Babusíková O., Závada J. (1992) A novel quasi-viral agent, MaTu, is a two-component system. Virology 187, 620–626 [DOI] [PubMed] [Google Scholar]
- 20. Zat'ovicová M., Tarábková K., Svastová E., Gibadulinová A., Mucha V., Jakubicková L., Biesová Z., Rafajová M., Ortova Gut M., Parkkila S., Parkkila A. K., Waheed A., Sly W. S., Horak I., Pastorek J., Pastoreková S. (2003) Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumor-associated hypoxia-induced carbonic anhydrase IX. J. Immunol. Methods 282, 117–134 [DOI] [PubMed] [Google Scholar]
- 21. Troyanovsky S. M. (2009) Regulation of cadherin-based epithelial cell adhesion by endocytosis. Front. Biosci. 1, 61–67 [DOI] [PubMed] [Google Scholar]
- 22. Morgan P. E., Pastorekova S., Casey J. R. (2005) Functional and physical interaction between bicarbonate transport proteins and the transmembrane carbonic anhydrase, CA IX. XXV. International Congress of Physiological Sciences March 31-April 5, San Diego, CA, Abstract No 366.5 [Google Scholar]
- 23. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 24. Závada J., Závadová Z., Pastorek J., Biesová Z., Jezek J., Velek J. (2000) Human tumor-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br. J. Cancer. 82, 1808–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Innocenti A., Pastorekova S., Pastorek J., Scozzafava A., De Simone G., Supuran C. T. (2009) The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg Med. Chem. Lett. 19, 5825–5828 [DOI] [PubMed] [Google Scholar]
- 26. Shin H. J., Rho S. B., Jung D. C., Han I. O., Oh E. S., Kim J. Y. (2011) Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J. Cell Sci. 124, 1077–1087 [DOI] [PubMed] [Google Scholar]
- 27. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 28. Worthylake R. A., Burridge K. (2003) RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278, 13578–13584 [DOI] [PubMed] [Google Scholar]
- 29. Dorai T., Sawczuk I. S., Pastorek J., Wiernik P. H., Dutcher J. P. (2005) The role of carbonic anhydrase IX overexpression in kidney cancer. Eur. J. Cancer 41, 2935–2947 [DOI] [PubMed] [Google Scholar]
- 30. Cristini V., Frieboes H. B., Gatenby R., Caserta S., Ferrari M., Sinek J. (2005) Morphologic instability and cancer invasion. Clin. Cancer Res. 11, 6772–6779 [DOI] [PubMed] [Google Scholar]
- 31. Pennacchietti S., Michieli P., Galluzzo M., Mazzone M., Giordano S., Comoglio P. M. (2003) Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3, 347–361 [DOI] [PubMed] [Google Scholar]
- 32. Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L., Arteaga C. L. (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 [DOI] [PubMed] [Google Scholar]
- 33. Studebaker A. W., Storci G., Werbeck J. L., Sansone P., Sasser A. K., Tavolari S., Huang T., Chan M. W., Marini F. C., Rosol T. J., Bonafé M., Hall B. M. (2008) Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087–9095 [DOI] [PubMed] [Google Scholar]
- 34. Sansone P., Piazzi G., Paterini P., Strillacci A., Ceccarelli C., Minni F., Biasco G., Chieco P., Bonafè M. (2009) Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J. Cell Mol. Med. 13, 3876–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiche J., Ilc K., Laferrière J., Trottíer E., Dayan F., Mazure N. M., Brahimi-Horn M. C., Pouysségur J. (2009) Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 [DOI] [PubMed] [Google Scholar]
- 36. Lou Y., McDonald P. C., Oloumi A., Chia S., Ostlund C., Ahmadi A., Kyle A., Auf dem Keller U., Leung S., Huntsman D., Clarke B., Sutherland B. W., Waterhouse D., Bally M., Roskelley C., Overall C. M., Minchinton A., Pacchiano F., Carta F., Scozzafava A., Touisni N., Winum J. Y., Supuran C. T., Dedhar S. (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 [DOI] [PubMed] [Google Scholar]
- 37. Dubois L., Peeters S., Lieuwes N. G., Geusens N., Thiry A., Wigfield S., Carta F., McIntyre A., Scozzafava A., Dogné J. M., Supuran C. T., Harris A. L., Masereel B., Lambin P. (2011) Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol. 99, 424–431 [DOI] [PubMed] [Google Scholar]
- 38. Chen J., Röcken C., Hoffmann J., Krüger S., Lendeckel U., Rocco A., Pastorekova S., Malfertheiner P., Ebert M. P. (2005) Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut. 54, 920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woelber L., Kress K., Kersten J. F., Choschzick M., Kilic E., Herwig U., Lindner C., Schwarz J., Jaenicke F., Mahner S., Milde-Langosch K., Mueller V., Ihnen M. (2011) Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer. BMC Cancer 11, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pastorekova S., Pastorek J. (2010) In: The Tumor Microenvironment. Series: Cancer Drug Discovery and Development (Bagley R. G., ed) The Tumor Microenvironment, Cancer Drug Discovery and Development, pp. 59–90, Springer, New York [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.