Background: LOXL2 inhibits keratinocyte differentiation. This activity was thought to require LOXL2 enzyme activity.
Results: LOXL2 mutants lacking enzyme activity nevertheless inhibit keratinocyte differentiation. The fourth scavenger receptor-cysteine-rich domain of LOXL2 is required for this activity.
Conclusion: LOXL2 induces keratinocyte differentiation independently of its enzyme activity.
Significance: Our results suggest that LOXL2 may affect diverse biological processes independently of its enzyme activity.
Keywords: Calcium, Cell differentiation, Enzyme inactivation, Keratinocytes, Promoters, Skin, Lysyl oxidase like-2
Abstract
Lysyl oxidase-like-2 (LOXL2) induces tumor progression and fibrosis. It also inhibits the differentiation of keratinocytes promoting development of squamous cell carcinomas. Stimulation of HaCaT skin keratinocytes with exogenous LOXL2 or overexpression of LOXL2 in these cells inhibits their differentiation as manifested by inhibition of calcium or vitamin D-induced involucrin expression. The inhibition was abrogated by the LOXL2 function-blocking monoclonal antibody AB0023 as well as by an anti-LOXL2 polyclonal antibody. Surprisingly, a point-mutated form of LOXL2 (LOXL2Y689F) lacking enzymatic activity, as well as a LOXL2 deletion mutant lacking the entire catalytic domain, also inhibited calcium or vitamin D-induced up-regulation of involucrin expression, suggesting that the enzymatic activity of LOXL2 is not required for this activity. This conclusion was supported by experiments that showed that β-aminoproprionitrile, an irreversible competitive inhibitor of the enzymatic activity of all lysyl oxidases, is unable to abolish the LOXL2-induced inhibition of HaCaT cell differentiation. The activity of LOXL2Y689F required the presence of the fourth scavenger receptor-cysteine-rich (SRCR) domain of LOXL2, which is also the binding target of AB0023. Epitope-tagged LOXL2Y689F was internalized at 37 °C by HaCaT cells. The internalization was inhibited by AB0023 and by competition with unlabeled LOXL2, suggesting that these cells may express a LOXL2 receptor. Our results suggest that agents that inhibit the enzymatic activity of LOXL2 may not suffice to inhibit completely the effects of LOXL2 on complex processes that involve altered states of cellular differentiation.
Introduction
Lysyl oxidase-like-2 (LOXL2)2 was identified as an up-regulated gene in Werner's syndrome (1). It belongs to the family of the lysyl oxidases and, like the other four members, catalyzes the deamination of the ϵ-amino groups of lysines of collagen monomers thereby promoting the formation of covalent cross-linkages (2, 3). The catalytic domain of the lysyl oxidases is highly conserved among lysyl oxidases as is the lysyl-tyrosyl-quinone (LTQ) cofactor domain that is absolutely required for their enzymatic activity and is unique to these copper-binding enzymes (2). The enzyme activity of LOXL2 can be inhibited, like the activity of all lysyl oxidases, by the competitive irreversible inhibitor β-aminoproprionitrile (BAPN) (4, 5). LOXL2 is an inducer of lung and liver fibrosis and also promotes fibrosis in LOXL2-expressing tumors, and these activities require the enzymatic activity of LOXL2 (6–8). LOXL2 as well as lysyl oxidase (LOX) also functions as an inducer of tumor cell invasiveness (6, 9, 10). These activities too were found to be dependent on the enzymatic activity of the lysyl oxidases. It was observed that lysyl oxidase enhanced cross-linking of collagen in the tumor microenvironment can by itself promote tumor cells invasiveness (11). Furthermore, hydrogen peroxide produced as a side product of lysyl oxidase activity can also induce tumor cell invasiveness (12). Additional studies revealed that tumor metastasis can be induced as a result of the oxidation of the transcription factor Snail by intracellular LOXL2, which subsequently leads to the induction of epithelial to mesenchymal transition (9, 13, 14). Thus, all the reported effects of the lysyl oxidases have so far been associated with their enzymatic activity.
The progression of squamous cell carcinoma of the skin is associated with enhanced expression of LOXL2 (13). Cells of such tumors are derived from keratinocyte stem cell precursors and are characterized by aberrant differentiation as manifested by their reduced expression of differentiation markers (15). Silencing LOXL2 expression in keratinocytes up-regulates the expression of genes associated with keratinocyte differentiation (16), suggesting that the LOXL2 overexpression observed in squamous cell carcinomas may contribute to tumor progression either through inhibition of the differentiation of keratinocyte-derived tumor cells or through enhancement of their proliferation, which in turn inhibits differentiation (13). Interestingly, the expression of LOX and LOXL2 is inversely regulated in keratinocytes as a function of their state of differentiation even though both enzymes catalyze similar enzymatic reactions and even though both oxidize lysine residues of collagen (2, 7). Thus, LOX expression is induced in differentiating keratinocytes, whereas the expression of LOXL2 is inhibited (16). Furthermore, silencing LOX expression, in contrast to the silencing of LOXL2, impairs keratinocyte differentiation (17).
Enzymes such as heparanase have been recently found to induce biological responses by mechanisms that do not require enzyme activity (18). The N-terminal domain of LOXL2 contains four scavenger receptor-cysteine rich (SRCR) domains of unknown function. These domains are implicated as mediators or protein-protein interactions in a variety of systems (19, 20). These observations suggest that the SRCR domains of LOXL2 may serve in as yet unexplored roles of LOXL2. Here we provide evidence suggesting that the enzymatic activity of LOXL2 is not required for LOXL2-induced inhibition of keratinocyte differentiation and show that this activity of LOXL2 requires the presence of the fourth SRCR domain of LOXL2 (2). Furthermore, we provide preliminary evidence suggesting that keratinocytes express a functional LOXL2 receptor on their cell surface.
EXPERIMENTAL PROCEDURES
Materials
DMEM, non-essential amino acids, trypsin-EDTA solution, fungizone, and fetal bovine serum were from Biological Industries (Beit Haemek, Israel). Cell culture plates were obtained from Corning Inc. (Corning, NY). A PerfectPure RNA Cultured Cell kit for RNA purification was from 5Prime (Hamburg, Germany). A Verso cDNA synthesis kit and AbsoluteTM Blue QPCR SYBR® Green Rox Mix were from Thermo Scientific (Lafayette, CO). The polyclonal LOXL2 antibody was purified on protein-A-Sepharose as previously described (6). Mouse anti-Myc (sc-40) was from Santa Cruz Biotechnologies (Santa Cruz, CA). Mouse anti-human β-actin (A5316) and mouse anti-human involucrin (I9018) were from Sigma (St. Louis, MO). The AB0023 monoclonal antibody was previously described (5, 8). All trans retinoic acid (ATRA), 9-cis-retinoic acid, troglitazone, and clofibrinic acid were purchased from Sigma-Aldrich, and vitamin D was purchased from Biomol (Hamburg, Germany). Dynabeads and protein G were from Invitrogen. β-Aminopropionitrile fumarate (BAPN) was from Sigma. Anti-Myc antibody-conjugated beads were from Enco Diagnostics (Petach Tikva, Israel). BamHI, KpnI, BglII, and XhoI were from New England Biolabs (Ipswich, MA). Lipofectamine 2000 was from Invitrogen.
Cell Culture
HaCaT cells were kindly provided by Dr. Fusenig (German Cancer Research Center, Heidelberg, Germany). The cells were cultured in MEM-Eagle's medium supplemented with 10% fetal calf serum, 2 mm l-glutamine, 50 μg/ml penicillin/streptomycin (Biological Industries, Beit Haemek, Israel) supplemented with a low (0.03 mm) or high (1.2 mm) concentration of calcium. HEK293 and HEK293T cells were purchased from the American Type Culture Collection (Manassas, VA).
Plasmids and Lentiviral Vector
The NSPI-CMV-Myc lentiviral expression vector was previously described (21). LOXL2 shRNA constructs were previously described (22). Plasmids used for functional analysis of the LOXL2 promoter activity were generated using the pGL2 basic vector (Promega, Madison, WI), which contains a promoterless luciferase reporter gene.
Isolation of the LOXL2 Promoter and Measurements of LOXL2 Promoter Activity
To identify the LOXL2 promoter, we cloned a fragment of ∼3.2 kb of DNA located upstream to the translation start site of LOXL2. The first untranslated intron of LOXL2, which was previously found to contain a functional hypoxia response element (23) was not included in this fragment (Fig. 1C) due to its size (∼35 kb). Thus this fragment contains 300 bp located downstream to intron 1 and 2.9 kb from the DNA region located upstream of intron 1 of LOXL2. This promoter fragment was inserted upstream to the luciferase reporter of the pGL2 plasmid and transfected into HaCaT cells using Lipofectamine 2000 according to the manufacturer's instructions. Measurements of LOXL2 promoter activity using the firefly and Renilla luciferase assay were preformed as described previously (24).
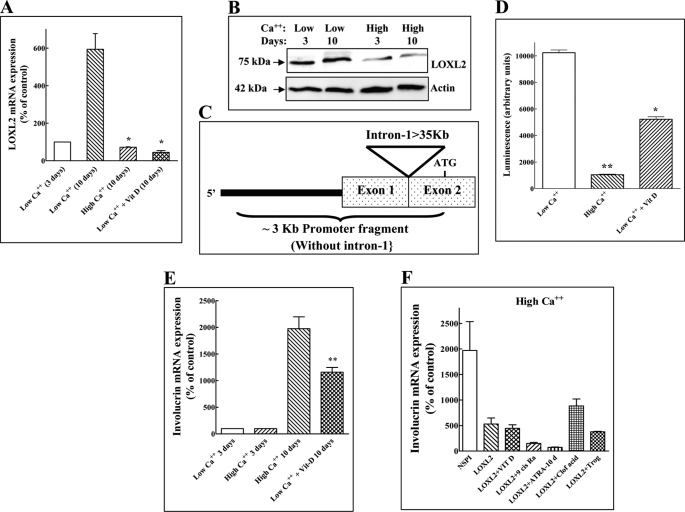
FIGURE 1.
LOXL2 inhibits involucrin expression induced by keratinocyte differentiation inducing factors. A, HaCaT cells were cultured in medium containing low or high calcium concentrations. Some low calcium dishes received, in addition, vitamin D (10−8 m) as indicated. After 10 days endogenous LOXL2 mRNA levels were measured using real-time qPCR. The mRNA levels of cells cultured 10 days in the presence of high calcium concentrations or in the presence of vitamin D were compared with the levels of LOXL2 mRNA expression in cells cultured for 10 days in the presence of low calcium concentrations for 10 days. B, cell lysates were prepared from HaCaT cells cultured in low and high Ca2+ after 3 or 10 days as indicated. Aliquots containing equal protein concentrations were subjected to Western blot analysis using polyclonal anti-LOXL2 antibodies. Blots were then stripped and probed for β-actin. C, picture showing the structure of the LOXL2 promoter fragment used. The large intron containing the hypoxia response element (23) downstream to untranslated exon-1 is not included in the promoter fragment. D, the luciferase activity induced by the LOXL2 promoter in HaCaT cells. In response to high calcium concentrations or in response to 10−8 m vitamin D was measured. The y axis represents luciferase activity normalized to a Renilla control. The data shown represent mean values of two independent experiments preformed in triplicate. Means were compared with the low Ca2+ control. E, the effects of calcium and vitamin D on involucrin mRNA expression were measured as described under A for LOXL2 mRNA expression. Means were compared with the high Ca2+ control. F, HaCaT cells cultured in medium containing high calcium concentrations were infected or not with empty lentivirus expression vector (NSPI) or with lentiviruses directing expression of LOXL2Myc (LOXL2) as indicated. Some dishes containing cells infected with lentiviruses directing expression of LOXL2Myc were supplemented with 10−8 m vitamin D (Vit D), 2 μm ATRA (ATRA), 20 μm troglitazone, 2 μm 9-cis-retinoic acid or 400 μm clofibrinic acids. The differentiation inducers were added every other day. Involucrin mRNA expression was measured after 10 days using real-time qPCR and normalized to β-actin mRNA expression.
Quantitative RT-PCR
Cells (5 × 104) were seeded in 6-well dishes and grown to confluence. Total RNA was isolated using the 5-Prime kit. cDNA was synthesized from 1 μg of total RNA and random hexamers using the Verso cDNA synthesis kit (Thermo Scientific, Lafayette, CO). PCR amplification of cDNA was carried out using the AbsoluteTM Blue QPCR SYBR® Green Rox Mix (Thermo Scientific) using an ABI Prism 7000 sequence detection system with oligonucleotide pairs specific for human LOXL2 (5′-GCGTCACTGACTGCAAGCAC and 5′-CGAATCCGAATGTCCTCCAC), human involucrin (5′-TGTTCCTCCTCCAGTCAATA and 5′-GCTTTGATGGGACCTCCACT), human keratin-10 (5′-GGAAGAATCAAACTATGAGCTG and 5′-ATTGTCGATCTGAAGCAGG) or β-actin (5′-TTGCCGACAGGATGCAGAAGGA and 5′-AGGTGGACAGCGAGGCCAGGAT). To measure the expression levels of endogenous LOXL2 mRNA we used another pair of primers of which one is derived from the 3′ UTR of the LOXL2 mRNA 5′-GGTTGACATCACTGACGTGC and 5′-GAGTTGACCACGCAGGCTTC. To ensure the specificity of the reaction conditions, at the end of the individual runs, the melting temperature of the amplified products was measured to confirm its homogeneity. The following conditions were used: 50 °C for 2 min, 95 °C for 15 min, 95 °C for 10 s, and 60 °C for 1 min for a total of 40 cycles. Each sample was analyzed in duplicate. Amplified cDNA levels were normalized using the β-actin cDNA as a reference. Products were resolved on a 2% agarose gel to confirm the identity of the amplified cDNA. The target gene expression level was calculated by the 2-ΔΔCt method (25). In experiments in which the expression is shown as percentage of control, the control was always the expression level of the gene of interest in untreated cells cultured for 3 days in the presence of high or low calcium concentrations as indicated.
Western Blot Analysis
Cell lysis and Western blot analysis were performed as described previously (26).
Inhibition of LOXL2 Expression
shRNA-targeting LOXL2 (22) were generated in HaCaT cells using lentiviral vectors as previously described (27).
Production and Purification of Recombinant LOXL2Variants
HEK293 or HaCaT cell lines expressing either LOXL2Myc, LOXL2Y689F, or various deletion mutants of LOXL2Y689F were generated using lentiviral vectors as previously described (27). Some of these recombinant LOXL2 species were purified from serum-free conditioned medium as follows: HEK293 cells expressing LOXL2Myc variants were grown to 70% confluence and incubated for 48 h with serum-free DMEM medium. Conditioned medium (200 ml) was incubated with 1 ml of anti-Myc conjugated beads for 24 h at 4 °C. The beads were then collected and washed with 20 volumes of PBS. LOXL2 was eluted with ammonium hydroxide (0.1 m). Acetic acid (1 m) was used to neutralize the eluant back to pH 7.
Determination of LOXL2 Enzyme Activity
The fluorometric Amplex Red assay was performed essentially as described before (28). Purified LOXL2Myc or LOXL2Y689F (2.5 μg) or a corresponding volume of a similarly purified fraction from control conditioned medium were added to a reaction mix (10 mm K2HPO4, pH 7.2, 10 μm Amplex Red, and 0.1 unit of horseradish peroxidase). The substrate was a lysine-rich PF4-derived peptide LYKKIIKKLLES that we identified as a good LOXL2 substrate. This peptide was added to a final concentration of 20 μm in a final reaction volume of 100 μl. The enzymatic reaction was carried out at 37 °C for 4 h. Fluorescence was measured with excitation at 540 and emission at 580 nm.
Generation of Deletions in the LOXL2 SRCR and Catalytic Domains
The cDNA-encoding LOXL2Y689F was used as a template for the generation of the depicted SRCR and catalytic domain deletions (Fig. 4A). For each deletion the cDNA upstream and downstream of the deletion was amplified using PfuUltra II fusion HS DNA polymerase (Stratagene, Santa Clara, CA) and the primer pairs shown (supplemental Table S1). Amplified fragments upstream and downstream from the deletions were joined using the Expand Long Template PCR system (Roche, Mannheim, Germany), sequenced, and stitched together essentially following a previously described protocol (29). The cDNA containing the deletion was then ligated into the NSPI-CMV-Myc lentiviral expression vector.
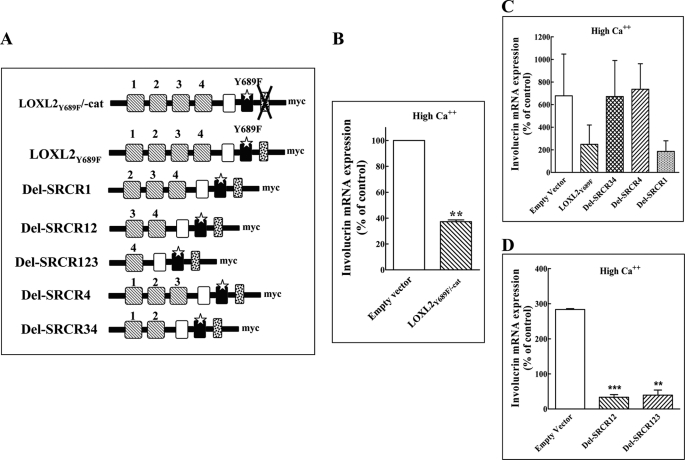
FIGURE 4.
The non-enzymatic effects of LOXL2 on the differentiation of HaCaT cells are mediated by the fourth SRCR domain of LOXL2. A, a schematic presentation of the catalytic domain deletion mutant derived from LOXL2Y689F and of the various SRCR deletion mutants of LOXL2Y689F. B–D, HaCaT cells grown in medium containing high calcium concentrations were infected with empty lentiviruses (Empty vector) and with lentiviruses directing expression of LOXL2Y689F/-cat. B, or lentiviruses directing expression of various LOXL2Y689F deletion mutants lacking various SRCR domains as indicated (C and D). The cells were cultured in 6-well plates for 10 days. The data shown represent mean values of two independent experiments preformed in triplicate. The expression of involucrin mRNA was then determined using real-time qPCR. Means were compared with the empty vector control.
LOXL2 Internalization Assay
HaCaT cells were seeded in 10-cm dishes (16 × 104 cells/dish) and cultured in medium containing a high calcium concentration overnight. The following day the cells were supplemented with 4 μg/ml LOXL2Myc or with LOXL2Myc that was preincubated for 30 min at 4 °C with AB0023. After 30-min incubation at 37 °C or 4 °C, cells were trypsinized, washed, and lysed at 4 °C using Western blot lysis buffer. The lysate was mixed with anti-Myc antibody (200 μg/ml) for 2 h at 4 °C. LOXL2Myc was then precipitated from the lysate using protein-G magnetic beads. The beads were washed three times with PBS, boiled, and subjected to Western blot analysis using anti-Myc antibodies to detect internalized LOXL2Myc.
Proliferation Assay
HaCaT cells were seeded in 24-wells dishes (5 × 103 cells/well) and cultured in medium containing a high calcium concentration. Wells received either no additions, elution buffer (17 μl/ml, the same volume in which LOXL2 was added), LOXL2Myc (5 μg/ml), or LOXL2Y689F (5 μg/ml). Added factors were replenished every other day. Adherent cells were counted every other day using a coulter counter.
Statistical Analysis
The one-tailed, unpaired Student's t test with Welch's correction was used. Error bars represent the ±S.E. Statistical significance is presented in the following manner: *, p < 0.05; **, p < 0.01; and ***, p < 0.001. All the experiments were performed independently three times in triplicate unless otherwise stated in the figure legend. The variation between triplicates in experiments was <10%.
RESULTS
The Expression of LOXL2 in HaCaT Cells Is Regulated by Inducers of Keratinocyte Differentiation, and High Levels of LOXL2 Inhibit the Differentiation of These Cells
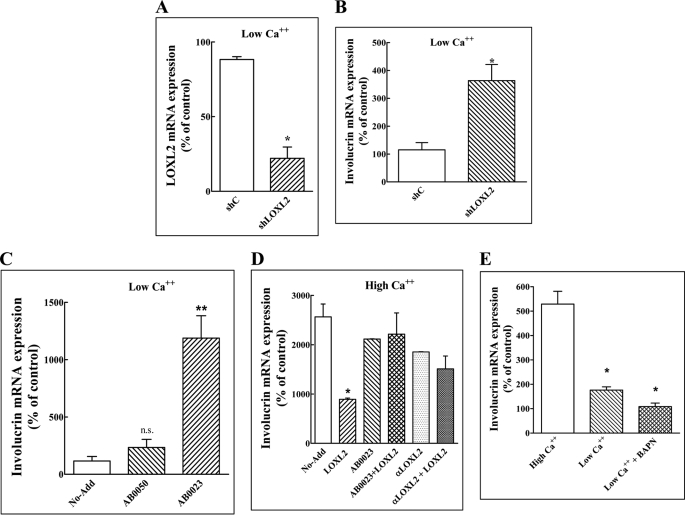
The HaCaT cell line is a spontaneously transformed non-tumorigenic human epithelial cell line derived from adult skin, which maintains full epidermal differentiation capacity. It undergoes differentiation when exposed to calcium or to additional inducers of keratinocyte differentiation such as vitamin D, which is manifested by the up-regulation of the expression of keratinocyte differentiation markers such as involucrin, keratin-10, or filaggrin (30). HaCaT cells expressed the LOXL2 mRNA when cultured in medium containing a low calcium concentration (Fig. 1A). The expression of the LOXL2 mRNA was strongly down-regulated when the cells were cultured in medium containing a high calcium concentration or when stimulated with vitamin D (Fig. 1A). Likewise, the expression of LOXL2 protein was also down-regulated by calcium (Fig. 2B). We have also cloned a 3-kb fragment containing part of the LOXL2 promoter (Fig. 1C). This DNA fragment was able to down-regulate the expression of a luciferase reporter gene fused downstream to it when HaCaT cells transfected with this construct were exposed to high calcium concentrations or when they were stimulated with vitamin D, suggesting that the decreased LOXL2 mRNA concentrations found in differentiating HaCaT cells were due to inhibition of LOXL2 transcription (Fig. 1D). As expected, stimulation of HaCaT cells with high calcium concentrations or with vitamin D up-regulated the expression of the keratinocyte differentiation marker involucrin (Fig. 1E). However, HaCaT cells overexpressing recombinant LOXL2 tagged at the C-terminal with an Myc epitope tag (LOXL2Myc) (7) failed to up-regulate involucrin expression in response to either calcium, vitamin D, or to several other known inducers of keratinocyte differentiation (Fig. 1F) (31). As far as we could tell, the addition of the Myc epitope tag did not alter the biological properties of LOXL2 (6, 22). In contrast, inhibition of endogenous LOXL2 expression in HaCaT cells cultured in the presence of low calcium concentrations by using a specific shRNA (Fig. 2A) (22) induced the expression of involucrin (Fig. 2B), suggesting that endogenously produced LOXL2 functions in keratinocytes as a gatekeeper that inhibits differentiation regardless of the nature of the external stimuli. The induction of differentiation following the expression of this shRNA was not due to up-regulation of LOX or other lysyl oxidases, because the expression levels of other lysyl oxidases did not change significantly in response to this shRNA (supplemental Fig. S1B). Interestingly, the LOXL2 function-blocking antibody AB0023 was also able to induce involucrin mRNA expression in HaCaT cell cultured in medium containing low calcium concentrations, suggesting that the differentiation-inhibiting effect of LOXL2 was induced by secreted extracellular LOXL2 (Fig. 2C). This last conclusion was supported by another experiment in which we observed that addition of purified LOXL2Myc to HaCaT cells cultured in the presence of high calcium concentration also inhibits the up-regulation of involucrin. In this experiment too addition of AB0023 or addition of a polyclonal antibody directed against LOXL2 (6) inhibited the effect of LOXL2Myc on involucrin expression suggesting that the inhibitory effect was transduced by extracellular LOXL2Myc (Fig. 2D).
FIGURE 2.
The lysyl oxidase enzyme activity inhibitor BAPN fails to neutralize the inhibitory effect of LOXL2 on involucrin expression. A, HaCaT cells were cultured in low calcium medium and infected with lentiviruses expressing a non-targeting shRNA (Shc) or with lentiviruses expressing a shRNA directed against LOXL2 (shLOXL2) for 10 days. The LOXL2 mRNA concentrations in the cells were then measured by real-time qPCR. Means were compared with the low Ca2+ (10 days) control. B, the expression of involucrin mRNA was measured in HaCaT cells expressing a non-targeting shRNA (Shc) or a LOXL2 targeting shRNA (shLOXL2) as described under A for the LOXL2 mRNA. C, HaCaT cells were cultured in medium containing low calcium concentrations in the absence of any additions (No-Add), in the presence of 5 μg/ml of a control monoclonal antibody that binds to the enzymatic site of LOXL2 but does not inhibit the enzymatic activity (AB0050) or in the presence of 5 μg/ml of an LOXL2-neutralizing antibody (AB0023), which were replenished every other day. The relative concentrations of the involucrin mRNA were measured using real-time qPCR as described. Means were compared with the control. D, HaCaT cells were cultured in medium containing high calcium concentrations in the absence of any additions (No-Add), 5 μg/ml LOXL2Myc (LOXL2), 20 μg/ml AB0023, or 20 μg/ml of a purified anti-LOXL2 polyclonal antibody (αLOXL2). Some of the wells the received LOXL2 also received antibodies as indicated. Added proteins were replenished every other day. After 10 days the expression of involucrin mRNA was determined by real-time qPCR as described. Means were compared with the control. E, HaCaT cells were cultured in medium containing high or low calcium concentrations as indicated for 10 days. Some of the low calcium wells received in addition BAPN (0.2 mm). Medium and BAPN were replenished every other day. After 10 days involucrin mRNA expression levels were determined using real-time qPCR. Means were compared with the high Ca2+ control.
The Enzymatic Activity of LOXL2 Is Not Required for the Inhibition of HaCaT Differentiation
It was previously observed that the LOXL2 function inhibiting antibody AB0023 inhibits the effects of LOXL2 on tumor progression more efficiently than BAPN (8) even though at saturating concentrations BAPN inhibits the enzymatic activity of LOXL2 “in vitro” enzyme assays more efficiently than AB0023 (5), possibly because AB0023 targets the fourth SRCR domain of LOXL2 rather than the catalytic domain (2, 5). These observations suggest that some of the biological activities of LOXL2 may not require its enzymatic activity. This hypothesis is strengthened by the results of an experiment showing that addition of BAPN to HaCaT cells cultured in the presence of low calcium concentrations failed to alleviate the LOXL2-induced repression of involucrin expression, whereas in contrast addition of AB0023 was able to abrogate LOXL2-induced inhibition of involucrin expression (Fig. 2, compare D and E). The failure to restore involucrin expression was not due to cytotoxicity, because the cells proliferated normally in the presence of BAPN (data not shown). This experiment also suggests that the enzymatic activity of LOXL2 may not be required for LOXL2-induced repression of involucrin expression.
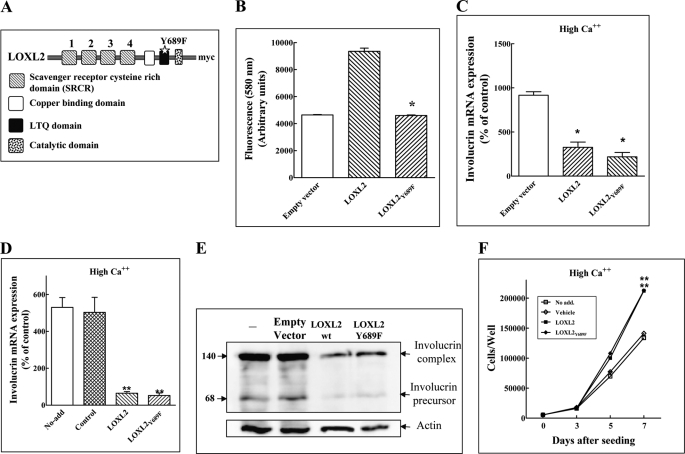
To further examine this hypothesis, we introduced a point mutation into the conserved LTQ domain of LOXL2Myc to generate LOXL2Y689F (Fig. 3A). This mutation was based on previous work showing that mutation of a conserved tyrosine residue required for the formation of the LTQ of LOX results in complete loss of enzymatic activity (32). Indeed, we found that LOXL2Y689F also lacks enzymatic activity (Fig. 3B). The expression of LOXL2Myc or LOXL2Y689F in HaCaT cells did not affect significantly the expression levels of endogenously produced LOXL2 mRNA (supplemental Fig. S1A). However, in HaCaT cells cultured in the presence of high calcium concentrations, the expression of recombinant LOXL2Y689F inhibited the expression of the involucrin mRNA as well as the expression of the cytokeratin-10 mRNA (Fig. 3C and supplemental Fig. S1C). Furthermore, purified LOXL2Y689F that was added to HaCaT cells cultured in the presence of a high calcium concentration was able to inhibit the expression of involucrin mRNA and protein as efficiently as non-mutated LOXL2Myc (Fig. 3, D and E). Addition of LOXL2Myc to the growth medium of HaCaT cells enhanced the number of adherent cells in medium containing high calcium concentrations (Fig. 3F), either because LOXL2 inhibited their differentiation or because LOXL2 promoted their proliferation directly. However, we could not detect LOXL2-induced cyclin-D1 expression in the cells nor was LOXL2 able to induce the phosphorylation of ERK1/2 (data not shown). Because LOXL2 did not appear to stimulate proliferation, we conclude that reduced differentiation in the presence of LOXL2 accounts for the apparent increase in cell numbers, because the shedding of fully differentiated cornified envelopes from the culture well will be reduced. In this experiment too LOXL2Y689F enhanced the accumulation of the cells as efficiently as LOXL2Myc (Fig. 3F).
FIGURE 3.
An enzyme dead LOXL2 point mutant inhibits the differentiation of HaCaT cells. A, a schematic presentation showing the location of the Y689F point mutation introduced into the LOXL2 LTQ domain. B, the enzymatic activity of affinity-purified LOXL2Myc, LOXL2Y689F, or of purified conditioned medium derived from control cells infected with empty lentiviruses and purified identically (Empty vector) were determined using the Amplex Red assay as described under “Experimental Procedures.” The data shown represent mean values of two independent experiments preformed in duplicate. Means were compared with the wild-type LOXL2 Control. C, HaCaT cells were cultured in medium containing high calcium and infected with empty lentiviruses (Empty vector) or lentiviruses directing expression of LOXL2Myc or LOXL2Y689F as indicated. After 10 days involucrin mRNA levels were determined using real-time qPCR. Means were compared with the empty vector control. D, HaCaT cells were grown in medium containing high calcium concentration without additions (No-add), or in medium supplemented with elution buffer (control), purified LOXL2Myc (LOXL2), or LOXL2Y689F (LOXL2Y689F). Added factors were replenished every other day. After 10 days involucrin mRNA levels were determined using real-time qPCR. Means were compared with the control. E, cell lysates were prepared from cells treated as described in D. The presence of involucrin complex and involucrin precursor in the cell lysates was determined by Western blot analysis. F, HaCaT cells were seeded in 24-well plates (5 × 103 cells/well) in the presence of high calcium concentration. Wells received either no additions (No-add), elution buffer (Vehicle), LOXL2Myc (5 μg/ml), or LOXL2Y689F (5 μg/ml). Added factors were replenished every other day. Adherent cells were counted every other day using a Coulter counter. Means were compared with the low LOXL2 and LOXL2Y689F controls, respectively, on day 7.
Even though the enzymatic activity of LOXL2Y689F seemed to be completely inhibited (Fig. 3B), we thought it possible that maybe some residual activity still remained and that this residual activity may be sufficient to inhibit calcium-induced expression of involucrin in HaCaT cells. To exclude this possibility we produced a deletion mutant of LOXL2Y689F that completely lacks the catalytic domain (LOXL2Y689F/-cat) (Fig. 4A). Despite the lack of a catalytic domain, expression of recombinant LOXL2/-cat in HaCaT cells cultured in the presence of high calcium concentrations was able to efficiently inhibit the expression of involucrin (Fig. 4B).
Inhibition of Involucrin Expression by LOXL2 Requires the Presence of the Fourth SRCR Domain of LOXL2
The experiments described above suggested that the enzymatic activity of LOXL2 is not required for LOXL2-induced inhibition of keratinocyte differentiation. These results suggested that other LOXL2 domains such as one of the SRCR domains may be important for that activity. To examine this hypothesis, we produced deletion mutants of LOXL2Y689F lacking one or several SRCR domains as depicted (Fig. 4A). We then expressed the deletion mutants in HaCaT cells and determined which of these mutated LOXL2 variants are able to inhibit calcium-induced induction of involucrin in HaCaT cells. These experiments indicated that inhibition of involucrin expression in HaCaT depended on the retention of the fourth SRCR domain. Loss of this fourth SRCR domain resulted in the loss of the ability to inhibit involucrin expression, whereas the loss of any of the other SRCR domains did not affect that ability (Fig. 4, C and D). Interestingly, AB0023, which is also able to inhibit LOXL2Myc-induced inhibition of involucrin expression (Fig. 2D), also binds to the fourth SRCR domain of LOXL2 (5). Thus, the finding that AB0023 also inhibits LOXL2Myc-induced inhibition of involucrin expression independently supports the identification of that domain as the domain that mediates LOXL2-induced inhibition of keratinocyte differentiation.
LOXL2Myc Is Internalized by HaCaT Cells, and the Internalization Is Inhibited by an Excess of Unlabeled LOXL2
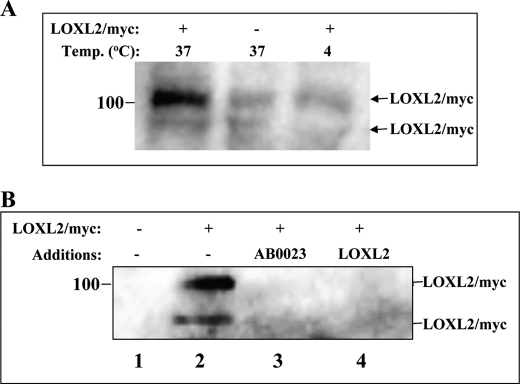
The realization that inhibition of keratinocyte differentiation can be inhibited by the addition of exogenous, enzyme-dead LOXL2 suggested that LOXL2 may bind to a cell membrane-anchored LOXL2 receptor that transduces its inhibitory signals. We hypothesized that, if such a receptor exists, it may also internalize LOXL2 in analogy with other receptors (33, 34). To examine this possibility we incubated HaCaT cells at 37 °C or at 4 °C with LOXL2Myc. After 30 min we treated the cells with a mixture of proteases so as to digest any LOXL2Myc exposed on the cell surface and lysed the cells in the presence of protease inhibitors following washing to remove excess proteases. This experiment revealed that LOXL2Myc is internalized by the cells and that the internalization occurs only at 37 °C (Fig. 5A). Interestingly the internalization could be inhibited by AB0023 as well as by incubation with conditioned medium containing unlabeled LOXL2, suggesting that the internalization of LOXL2Myc may be mediated by a saturable receptor (Fig. 5B). Further experiments will be required in the future to identify this putative LOXL2 receptor.
FIGURE 5.
Evidence for a putative LOXL2 receptor of HaCaT cells. A, HaCaT cells were cultured in medium containing high calcium concentration to confluence in 10-cm dishes. They were then incubated with or without purified LOXL2Myc as indicated for 0.5 h at 4 °C or at 37 °C. At the end of the incubation the cells were washed and trypsinized to remove cell surface-associated LOXL2Myc. The cells were then lysed, and lysate aliquots containing equal amounts of protein were analyzed by Western blot using an anti-Myc antibody to detect LOXL2Myc. B, HaCaT cells were cultured as described above. Upon reaching confluence the medium was exchanged with conditioned medium (high calcium) derived from HEK293 cells infected with empty lentiviruses (lane 1), with fresh HaCaT growth medium containing high calcium (lanes 2 and 3), or conditioned medium from HEK293 cells producing recombinant untagged LOXL2 (lane 4). LOXL2Myc (2 μg/ml) or AB0023 antibody (20 μg/ml) were added as indicated. After a 30-min incubation at 37 °C the cells were trypsinized and lysed, and LOXL2Myc in cell lysates were visualized as described under A.
DISCUSSION
The studies in which the mechanisms by which lysyl oxidases induce fibrosis, tumor invasion, and tumor metastasis were examined have found that for all of these activities the enzyme activity of the lysyl oxidases was strictly required (9, 11–13). In agreement, it was also reported that oxidation of the transcription factor snail by intracellular LOXL2 is part of the mechanism by which LOXL2 inhibits the differentiation of keratinocytes and promotes the progression of dermal malignancies such as squamous cell carcinoma of the skin (13, 16).
A few observations nevertheless suggest that LOXL2 may exhibit non-enzymatic activities. The AB0023 monoclonal antibody targeting the fourth SRCR domain of LOXL2 was found to be a better inhibitor of tumor development than the irreversible general lysyl oxidase enzyme inhibitor BAPN. Because this antibody also functions as a partial inhibitor of the enzyme activity, it was thought that allosteric inhibition in the in vivo environment may be more efficient than direct competition for the substrate binding site by BAPN (8). However, the possibility that the fourth SRCR domain may participate directly in the induction of such LOXL2-induced functions was not investigated.
Mutation of a critical tyrosine residue in the LTQ domain of lysyl oxidase results in complete loss of lysyl oxidase activity (32). To determine if LOXL2 has non-enzymatic functions, we introduced a similar point mutation into LOXL2 to generate LOXL2Y689F, resulting apparently in a complete loss of enzymatic activity. Nevertheless, the inhibitory effect that LOXL2 exerts on the differentiation of HaCaT keratinocytes as measured by the inhibition of the calcium-induced expression of the keratinocyte differentiation marker involucrin remained unaffected by the mutation. To circumvent the possibility that the mutation may not have completely inhibited the enzyme activity we have also produced an LOXL2Y689F variant that in addition to the mutation lacks the entire catalytic domain. However, this twice “dead” LOXL2 mutant was also able to inhibit calcium-induced induction of involucrin expression by HaCaT cells further suggesting that LOXL2 inhibits involucrin expression in HaCaT cells independently of its enzymatic activity.
AB0023 inhibited the effect of LOXL2Y689F on involucrin expression, suggesting that inhibition of involucrin expression by LOXL2Y689F was mediated by the fourth SCRC domain of LOXL2, which is the LOXL2 domain targeted by AB0023 (5). Indeed, the only LOXL2Y689F deletion mutants that lost their ability to inhibit involucrin expression were the ones that lacked the fourth SRCR domain, strongly suggesting that inhibition of involucrin expression by LOXL2 in HaCaT cells depends on the presence of this domain. It is intriguing that similar domains are also found in LOXL3 and LOXL4, suggesting that these lysyl oxidases too may exhibit non-enzymatic activities, and this possibility will need to be further examined.
LOXL2Y689F was also able to inhibit involucrin expression when it was added to the growth medium of the HaCaT cells, suggesting the existence of a mechanism able to transduce LOXL2 signals from the extracellular space into the cells. Indeed, our experiments indicate that HaCaT cells may express a signal-transducing LOXL2 receptor on their cell surface. We found that LOXL2Y689F is internalized by HaCaT cells and that the internalization of an epitope-tagged LOXL2 can be inhibited by an excess of unlabeled LOXL2. This observation indicates that the internalization is mediated by a specific high affinity LOXL2 receptor. This possibility is also supported by experiments, which showed that AB0023 can inhibit the internalization of LOXL2, lending further support for this hypothesis. The identity of this putative receptor as well as the signaling cascades that it may trigger in response to LOXL2 binding will have to be examined in greater detail in the future.
Supplementary Material
Acknowledgment
We thank Ronit Leiba, the statistician of the RAMBAM medical center, Haifa, Israel, for her help in statistical analysis.
This work was supported by a research grant from Arresto Biosciences Inc., by a grant from the Israel Cancer Research Fund (to G. N.), and by a grant from the Rappaport Family Institute for Research in the Medical Sciences of Technion. Gilead Sciences is clinically testing a drug targeting LOXL2, which is based upon my patents (G.N.). However, Gilead Sciences does not fund research in my laboratory, nor am I or any of the other authors on their payroll except for V. S., who is a Gilead Sciences employee.

This article contains supplemental Fig. S1 and Table S1.
- LOXL2
- lysyl oxidase-like-2
- LTQ
- lysyl-tyrosyl-quinone
- BAPN
- β-aminoproprionitrile
- LOX
- lysyl oxidase
- SRCR
- scavenger receptor-cysteine-rich
- qPCR
- quantitative PCR.
REFERENCES
- 1. Saito H., Papaconstantinou J., Sato H., Goldstein S. (1997) Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J. Biol. Chem. 272, 8157–8160 [DOI] [PubMed] [Google Scholar]
- 2. Lucero H. A., Kagan H. M. (2006) Lysyl oxidase. An oxidative enzyme and effector of cell function. Cell Mol. Life Sci. 63, 2304–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mäki J. M. (2009) Lysyl oxidases in mammalian development and certain pathological conditions. Histol. Histopathol. 24, 651–660 [DOI] [PubMed] [Google Scholar]
- 4. Tang S. S., Trackman P. C., Kagan H. M. (1983) Reaction of aortic lysyl oxidase with β-aminopropionitrile. J. Biol. Chem. 258, 4331–4338 [PubMed] [Google Scholar]
- 5. Rodriguez H. M., Vaysberg M., Mikels A., McCauley S., Velayo A. C., Garcia C., Smith V. (2010) Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J. Biol. Chem. 285, 20964–20974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akiri G., Sabo E., Dafni H., Vadasz Z., Kartvelishvily Y., Gan N., Kessler O., Cohen T., Resnick M., Neeman M., Neufeld G. (2003) Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 63, 1657–1666 [PubMed] [Google Scholar]
- 7. Vadasz Z., Kessler O., Akiri G., Gengrinovitch S., Kagan H. M., Baruch Y., Izhak O. B., Neufeld G. (2005) Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J. Hepatol. 43, 499–507 [DOI] [PubMed] [Google Scholar]
- 8. Barry-Hamilton V., Spangler R., Marshall D., McCauley S., Rodriguez H. M., Oyasu M., Mikels A., Vaysberg M., Ghermazien H., Wai C., Garcia C. A., Velayo A. C., Jorgensen B., Biermann D., Tsai D., Green J., Zaffryar-Eilot S., Holzer A., Ogg S., Thai D., Neufeld G., Van Vlasselaer P., Smith V. (2010) Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 9. Peinado H., Del Carmen Iglesias-de la Cruz M., Olmeda D., Csiszar K., Fong K. S., Vega S., Nieto M. A., Cano A., Portillo F. (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 24, 3446–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erler J. T., Bennewith K. L., Nicolau M., Dornhöfer N., Kong C., Le Q. T., Chi J. T., Jeffrey S. S., Giaccia A. J. (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 [DOI] [PubMed] [Google Scholar]
- 11. Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Payne S. L., Fogelgren B., Hess A. R., Seftor E. A., Wiley E. L., Fong S. F., Csiszar K., Hendrix M. J., Kirschmann D. A. (2005) Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 65, 11429–11436 [DOI] [PubMed] [Google Scholar]
- 13. Peinado H., Moreno-Bueno G., Hardisson D., Pérez-Gomez E., Santos V., Mendiola M., de Diego J. I., Nistal M., Quintanilla M., Portillo F., Cano A. (2008) Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 68, 4541–4550 [DOI] [PubMed] [Google Scholar]
- 14. Moreno-Bueno G., Salvador F., Martín A., Floristán A., Cuevas E. P., Santos V., Montes A., Morales S., Castilla M. A., Rojo-Sebastián A., Martínez A., Hardisson D., Csiszar K., Portillo F., Peinado H., Palacios J., Cano A. (2011) Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol. Med. 3, 528–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slaga T. J., Budunova I. V., Gimenez-Conti I. B., Aldaz C. M. (1996) The mouse skin carcinogenesis model. J. Investig. Dermatol. Symp. Proc. 1, 151–156 [PubMed] [Google Scholar]
- 16. Fujimoto E., Tajima S. (2009) Reciprocal regulation of LOX and LOXL2 expression during cell adhesion and terminal differentiation in epidermal keratinocytes. J. Dermatol. Sci. 55, 91–98 [DOI] [PubMed] [Google Scholar]
- 17. Le Provost G. S., Debret R., Cenizo V., Aimond G., Pez F., Kaniewski B., André V., Sommer P. (2010) Lysyl oxidase silencing impairs keratinocyte differentiation in a reconstructed-epidermis model. Exp. Dermatol. 19, 1080–1087 [DOI] [PubMed] [Google Scholar]
- 18. Fux L., Feibish N., Cohen-Kaplan V., Gingis-Velitski S., Feld S., Geffen C., Vlodavsky I., Ilan N. (2009) Structure-function approach identifies a COOH-terminal domain that mediates heparanase signaling. Cancer Res. 69, 1758–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martínez V. G. V, Moestrup S. K., Holmskov U., Mollenhauer J., Lozano F. (2011) The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol. Rev. 63, 967–1000 [DOI] [PubMed] [Google Scholar]
- 20. Bowen M. A., Bajorath J., Siadak A. W., Modrell B., Malacko A. R., Marquardt H., Nadler S. G., Aruffo A. (1996) The amino-terminal immunoglobulin-like domain of activated leukocyte cell adhesion molecule binds specifically to the membrane-proximal scavenger receptor cysteine-rich domain of CD6 with a 1:1 stoichiometry. J. Biol. Chem. 271, 17390–17396 [DOI] [PubMed] [Google Scholar]
- 21. Akiri G., Cherian M. M., Vijayakumar S., Liu G., Bafico A., Aaronson S. A. (2009) Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene 28, 2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brekhman V., Neufeld G. (2009) A novel asymmetric 3D in vitro assay for the study of tumor cell invasion. BMC Cancer 9, 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schietke R., Warnecke C., Wacker I., Schödel J., Mole D. R., Campean V., Amann K., Goppelt-Struebe M., Behrens J., Eckardt K. U., Wiesener M. S. (2010) The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia. Insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 285, 6658–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohman T., Parish G., Jackson R. M. (1999) Hypoxic modulation of manganese superoxide dismutase promoter activity and gene expression in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 21, 119–127 [DOI] [PubMed] [Google Scholar]
- 25. Huggett J., Dheda K., Bustin S., Zumla A. (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes. Immun. 6, 279–284 [DOI] [PubMed] [Google Scholar]
- 26. Varshavsky A., Kessler O., Abramovitch S., Kigel B., Zaffryar S., Akiri G., Neufeld G. (2008) Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 68, 6922–6931 [DOI] [PubMed] [Google Scholar]
- 27. Kigel B., Varshavsky A., Kessler O., Neufeld G. (2008) Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE 3, e3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palamakumbura A. H., Trackman P. C. (2002) A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal. Biochem. 300, 245–251 [DOI] [PubMed] [Google Scholar]
- 29. Horton R. M., Cai Z. L., Ho S. N., Pease L. R. (1990) Gene splicing by overlap extension. Tailor-made genes using the polymerase chain reaction. BioTechniques 8, 528–535 [PubMed] [Google Scholar]
- 30. Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang Y. J., Lu B., Kim P., Paragh G., Schmitz G., Elias P. M., Feingold K. R. (2008) PPAR and LXR activators regulate ABCA12 expression in human keratinocytes. J. Invest. Dermatol. 128, 104–109 [DOI] [PubMed] [Google Scholar]
- 32. Wang S. X., Mure M., Medzihradszky K. F., Burlingame A. L., Brown D. E., Dooley D. M., Smith A. J., Kagan H. M., Klinman J. P. (1996) A crosslinked cofactor in lysyl oxidase. Redox function for amino acid side chains. Science 273, 1078–1084 [DOI] [PubMed] [Google Scholar]
- 33. Kikuchi A., Yamamoto H. (2007) Regulation of Wnt signalling by receptor-mediated endocytosis. J. Biochem. 141, 443–451 [DOI] [PubMed] [Google Scholar]
- 34. Hussain M. M. (2001) Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. Biosci. 6, D417-D428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.