Background: Nitroalkenes are cardioprotective. We investigated the role of ANT1 in this process.
Results: Nitro-linoleate modifies Cys57 on ANT1. Knockdown of ANT1 inhibits cytoprotection by nitro-linoleate.
Conclusion: ANT1 is required for nitro-linoleate cytoprotection and possibly for its cardioprotection.
Significance: This is the first evidence for modification of a specific cysteine in a mitochondrial protein by a physiologically (and clinically) relevant electrophile.
Keywords: Ischemia, Mitochondria, Nitric Oxide, Preconditioning, Signaling, Electrophiles, Nitrated Lipids, siRNA
Abstract
Electrophilic nitrated lipids (nitroalkenes) are emerging as an important class of protective cardiovascular signaling molecules. Although species such as nitro-linoleate (LNO2) and nitro-oleate can confer acute protection against cardiac ischemic injury, their mechanism of action is unclear. Mild uncoupling of mitochondria is known to be cardioprotective, and adenine nucleotide translocase 1 (ANT1) is a key mediator of mitochondrial uncoupling. ANT1 also contains redox-sensitive cysteines that may be targets for modification by nitroalkenes. Therefore, in this study we tested the hypothesis that nitroalkenes directly modify ANT1 and that nitroalkene-mediated cardioprotection requires ANT1. Using biotin-tagged LNO2 infused into intact perfused hearts, we obtained mass spectrometric (MALDI-TOF-TOF) evidence for direct modification (nitroalkylation) of ANT1 on cysteine 57. Furthermore, in a cell model of ischemia-reperfusion injury, siRNA knockdown of ANT1 inhibited the cardioprotective effect of LNO2. Although the molecular mechanism linking ANT1-Cys57 nitroalkylation and uncoupling is not yet known, these data suggest that ANT1-mediated uncoupling may be a mechanism for nitroalkene-induced cardioprotection.
Introduction
Mitochondria play a central role in cardiac ischemia-reperfusion (IR)3 injury (1). IR severely impairs mitochondrial function, causing inhibition of Ox-Phos (2), enhanced ROS generation, and Ca2+ dysregulation (3), which in combination with high inorganic phosphate and low ATP promote opening of the mitochondrial permeability transition (PT) pore (4). The latter is a key factor in IR-induced cell death (5). The composition of the PT pore is still a matter of debate, and whereas a role for cyclophilin D is well defined (6), opposing data exist regarding involvement of the adenine nucleotide translocase (ANT) (7, 8). In addition to its potential role in the PT pore and cell death regulation (7, 9, 10), ANT plays essential roles in mitochondrial bioenergetics (10) and ANT1 (the major cardiac isoform (11)) is known to mediate a basal proton leak in mitochondria (12, 13). Mild increases in mitochondrial proton leak (uncoupling) can confer protection against IR injury (14–16), and this raises the possibility that ANT may play a role in cardioprotection.
Nitroalkenes, such as nitro-linoleic acid (LNO2) and nitro-oleic acid (OANO2), are electrophiles that trigger a variety of intracellular signaling events (17, 18). The mechanisms of endogenous nitroalkene formation are poorly understood, but several factors present in mitochondria are thought to be important (19). This includes a large mitochondrial pool of unsaturated membrane lipids (20), a ready availability of nitrite (21), and elevated levels of reactive oxygen/nitrogen species (22). The conditions encountered during ischemia, including acidosis, may promote endogenous nitroalkene formation (23, 24). However, far from being pathologic in nature, nitroalkenes are thought to play an important role in the endogenous cardioprotective paradigm of ischemic preconditioning (IPC) (15). Formation of LNO2 has been shown in mitochondria isolated from perfused hearts subjected to IPC (15). Furthermore, prior administration of synthetic nitroalkenes prevented IR injury in both in vivo hearts and isolated cardiomyocytes (15, 24).
One mechanism of nitroalkene signaling is the nitroalkylation of nucleophilic protein residues such as cysteines (25). We previously obtained immunoblot data strongly supportive of ANT1 nitroalkylation in isolated mitochondria and cardiomyocytes. This was accompanied by an increase in mitochondrial proton leak and by potent cytoprotection against simulated IR injury (15). ANT1 is an attractive candidate for cardioprotective nitroalkene signaling, as it contains a number of redox active cysteines (26) that have been shown to play critical roles in adenine nucleotide binding (27) and opening of the PT pore (28, 29). Notably, however, the role of these cysteines in regulating proton conductance by ANT1 is unknown. Furthermore, a direct role for ANT1 in the acute cardioprotective effect of nitroalkenes has not been established. Therefore, the goal of this study was to determine the requirement for ANT1 in nitroalkene-mediated cardioprotection.
EXPERIMENTAL PROCEDURES
Animals and Reagents
Full experimental details are in the supplemental material. Two-month-old male C57/BL6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed under a 12 h light/dark cycle with food and water available ad libitum. All chemicals were purchased from Sigma unless otherwise specified.
Nitroalkene Synthesis, Purification, and Quantification
Nitroalkenes (OANO2, LNO2) were synthesized by nitroselynation as previously described (30). Biotin-tagged LNO2 (Bt-LNO2) was synthesized from fresh LNO2 stock using EZ-Link Biotin Hydrazide (Pierce) followed by purification and quantification as previously published (31). All nitroalkenes and native fatty acids (OA and LA) were stored in pure HPLC grade methanol at −80 °C under argon.
Heart Preparation
Isolated heart perfusion was performed as previously described (15, 32). Hearts were divided in several groups: (i) IR alone (n = 8); (ii) LA + IR (n = 7); (iii) LNO2 + IR (n = 8); (iv) Bt-LNO2 + IR (n = 7); (v) OA + IR (n = 7); (vi) OANO2 + IR (n = 7). Nitroalkenes and related agents were infused via a port above the perfusion cannula for 20 min before ischemia at 100 nm. Global ischemia was induced by stopping flow and immersing the heart in deoxygenated buffer at 37 °C for 25 min. Reperfusion (1 h) was initiated by raising flow back to the preischemic rate over a period of 1 min. Parameters measured included heart rate, left ventricular developed pressure (LVDP), rate pressure product (heart rate × LVDP), and infarct size (2,3,5-triphenyltetrazolium chloride staining).
Immunoprecipitation, SDS-PAGE, and In-gel Digestion
In separate experiments hearts from groups (i) and (iv) were harvested before ischemia and subjected to two separate immunoprecipitation protocols, described previously (15, 33). Briefly, whole hearts were homogenized in buffer containing 20 mm Tris, 50 mm NaCl, 1 mm EGTA, 0.5% (v/v) Nonidet P-40, 1% (v/v) Triton X-100, 1% (w/v) n-dodecyl-β-d-maltoside supplemented with protease inhibitor mixture tablets (Roche Applied Science), pH 7.4, at 4 °C. ANT1 was pulled down from 2 mg of whole heart homogenate using anti-ANT1 antibodies (1:200). Immunocomplexes were captured using protein G-agarose (EMD, Gibbstown, NJ) suspension (15 μl/μg antibody). After dissociation of immunocomplexes from the beads by boiling in sample buffer, proteins were separated by 12.5% SDS-PAGE gel and immunoblotted using either anti-ANT1 antibodies (MitoSciences, Eugene, OR) or streptavidin-HRP (GE Healthcare).
In reverse experiments, biotinylated proteins (i.e. from hearts treated with biotin-tagged nitroalkenes) were immunoprecipitated from whole heart homogenates using neutravidin-agarose (Pierce) (15). Samples were subjected to 12.5% SDS-PAGE and run in duplicate. Proteins from one gel were transferred to nitrocellulose, and membranes were probed with anti-ANT1 antibodies (1:2000 dilution). Proteins from the second gel were visualized by Coomassie Blue stain, and the prominent bands at >250, 80, 55, and 32 kDa were excised. Bands were destained and subjected to overnight in-gel digestion with sequencing grade trypsin (25 ng/μl in 25 mm ammonium bicarbonate buffer, pH 7.8). Peptides were extracted with 0.1% TFA, 75% acetonitrile and evaporated to near dryness.
MALDI-TOF-TOF Mass Spectrometry
Peptide calibration standards and matrix CHCA were from Applied Biosystems (Carlsbad, CA). All spectra were taken on an ABSciex 5800 MALDI-TOF-TOF mass spectrometer in positive reflector mode (10 kV) with a matrix of CHCA. At least 1000 laser shots were averaged to obtain each spectrum. Masses were calibrated to known peptide standards. 5-μl aliquots of the ANT1 (band at 32 kDa) tryptic digest were cleaned on a C18 ZipTip (Millipore, Bedford MA) as per manufacturer's instructions. Bound peptides were desalted with two 5-μl washes of 0.1% TFA and then eluted with 2.5 μl of aqueous, acidic acetonitrile (75% CH3CN, 0.1% TFA). The eluant was mixed with 2.5 μl of freshly prepared CHCA stock solution (20 mg/ml CHCA in aqueous acetonitrile as above), and 1.5-μl portions of this mixture were spotted onto a MALDI sample plate for air drying. 1.5 μl of crude peptides were additionally mixed with 1.5 μl of CHCA and spotted. MS/MS of the1438.8 m/z peak was performed in positive reflector mode without collision-induced dissociation. MS and MS/MS spectra were analyzed in Protein Pilot 3.0, Mascot Distiller, and PEAKS software packages.
Cell Culture and siRNA Transfection
H9c2 cardiomyocytes were maintained in DMEM with 4 mm glutamine, 1% penicillin/streptomycin, and 10% heat inactivated FBS at 37 °C in a 5% CO2 atmosphere. Cells were used between passages 20 and 40 and plated at 30,000/well on 22-mm 12-well plates (Greiner Bio-One) in antibiotic-free media. After 24 h, half the wells were transfected with 100 nm ANT1 siRNA (Qiagen, Valencia CA; #SI03107496) using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. The remaining wells were transfected with 100 nm concentrations of scrambled (control) siRNA (#1027281). Media were changed after 24 h. Successful knockdown was verified after 72 h using ANT1 immunoblotting.
In Vitro IR Model
72 h post-transfection, growth medium was replaced with 1 ml/well normoxic buffer comprising DMEM, 10 mm glucose, 10 mm HEPES, pH 7.4, at 37 °C. LNO2 (100 nm) was added in half of controls and half of ANT1 siRNA wells and incubated for 30 min at 37 °C. After treatment, medium in each well was replaced with hypoxic buffer (glucose-free DMEM, 10 mm HEPES, pH 6.5, at 37 °C), which was pregassed with N2. The plate was then transferred to a hypoxic chamber (Coy Inc., Grass Lake, MI) pre-equilibrated with N2, thermostatic to 37 °C. Reperfusion comprised removal of the plate from the hypoxic chamber, replacement of media with normoxic buffer, and incubation at 37 °C for 1.5 h. Cell death was then assayed by measuring LDH release using a kit (Cytotoxicity Detection, Roche Applied Science) according to the manufacturer's protocol. LDH release was expressed as a percentage of total LDH, the latter obtained by adding 0.1% Triton X-100 to lyse cells. The model of IR injury was validated by testing a known cardioprotective agent, the volatile anesthetic isoflurane, which yielded a 31 ± 3% reduction in IR-induced cell death (data not shown).
Statistics
Where appropriate, Student's t test and multiple way analysis of variance were applied to the data.
RESULTS
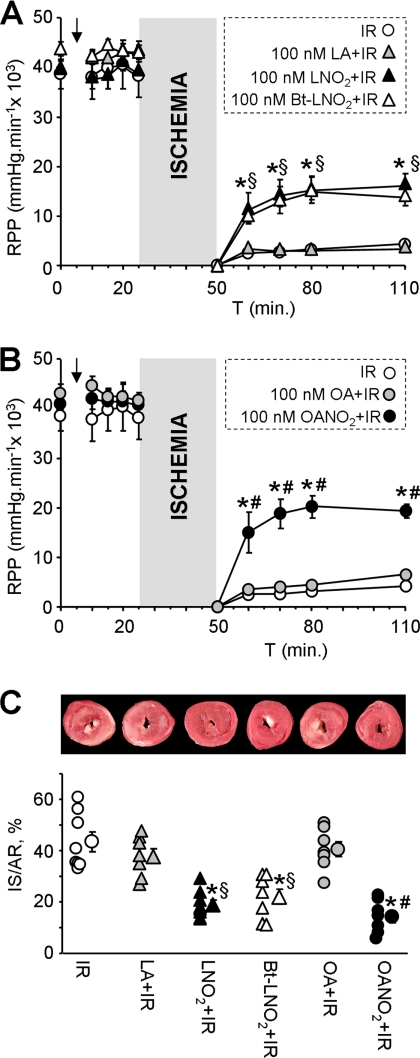
An acute cardioprotective effect of nitroalkenes was demonstrated in adult cardiomyocytes (15). Furthermore, a long term in vivo treatment with nitroalkenes in mice conferred protection against IR injury. However, the ability of nitroalkenes to afford acute protection at the isolated heart level has not been investigated. Fig. 1 demonstrates that nitroalkenes significantly improved cardiac contractile function (rate × pressure product) during reperfusion and drastically decreased infarct size compared with vehicle treated controls. Both LNO2 and OANO2 provided equal magnitudes of protection, and regular non-nitrated LA and OA were not protective, indicating that the small doses used herein (100 nm) were not sufficient to provide any cardioprotective effect of free fatty acids, as has been observed in other studies (34, 35). Together these data support the cardioprotective efficacy of nitroalkenes against IR injury.
FIGURE 1.
Cardioprotective effects of nitroalkenes in perfused mouse hearts. A and B, perfused hearts were subjected to IR injury with or without infusion of agents (see “Experimental Procedures”). Left-ventricular contractile function was monitored, and graphs show rate × pressure product (RPP). For clarity, experimental groups with linoleate-based and oleate-based treatments are shown in two separate panels, although the IR alone group is the same in each panel. Final concentration of nitroalkenes and related reagents was 100 nm. Data are the means ± S.E., n ≥ 7; *, p < 0.05 versus IR alone; §, p < 0.05 versus IR + LA; #, p < 0.05 versus IR + OA (analysis of variance). C, following IR protocols, hearts were stained with 2,3,5-triphenyltetrazolium chloride, and infarct size was measured as the % of the left ventricular area. The upper images show representative 2,3,5-triphenyltetrazolium chloride-stained hearts. In the graph, infarct is quantified, with individual data points for each condition shown on the left and the means ± S.E. on the right (n ≥ 7). *, p < 0.05 versus IR alone; §, p < 0.05 versus IR + LA; #, p < 0.05 versus IR + OA (analysis of variance). IS/AR, infarct/area-at-risk.
The time-frame for this effect (15 min. infusion) indicates that the mechanism of protection was most likely via protein nitroalkylation rather than at the level of transcriptional activity. To investigate protein modifications by LNO2 in the perfused heart, Bt-LNO2 was synthesized. Bt-LNO2 was administered in the same manner as LNO2 and exhibited the same degree of protection against IR injury (Figs. 1, A and C). These data indicate that the addition of the biotin group did not interfere with the bioactivity of LNO2, suggesting that the active portion of the LNO2 molecule is not the biotin-blocked carboxyl group.
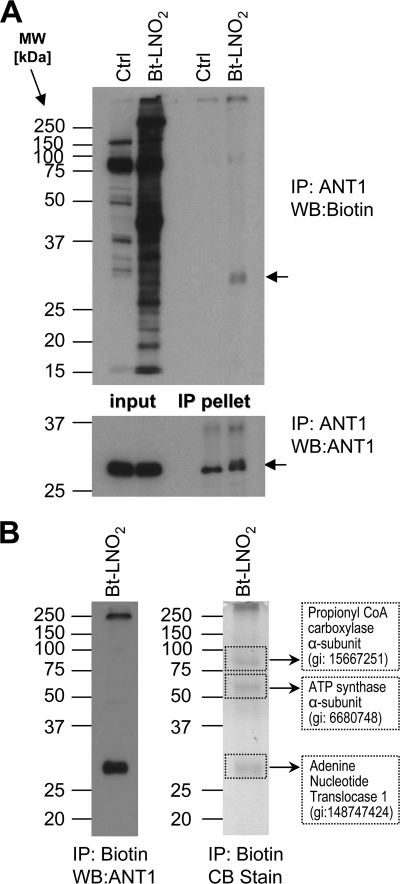
Based on our previous studies (15), we focused next on the nitroalkene modification of ANT1. After Bt-LNO2 delivery to perfused hearts, homogenates were subjected to immunoprecipitation with either anti-ANT1 antibodies or neutravidin to capture ANT1 and other biotin-tagged proteins followed by Western blotting. Fig. 2 shows that Bt-LNO2 adducted several proteins, including ANT1, suggesting that nitroalkenes can cross the vascular and plasma membranes and enter mitochondria. This is in agreement with our previous results (15) where Bt-LNO2 modified ANT1 in both isolated mitochondria and intact adult cardiomyocytes. Although the modification site was not elucidated, we demonstrated that pretreatment with the ANT inhibitor carboxyatractyloside abrogated Bt-LNO2 modification (15). Because carboxyatractyloside is known to block the accessibility of a cysteine residue in ANT (Cys57), we therefore speculated this may be a site of LNO2 modification.
FIGURE 2.
ANT1 modification by Bt-LNO2. A, ANT1 was immunoprecipitated from whole heart homogenates (Ctrl or treated with 100 nm Bt-LNO2) using anti-ANT1 antibodies (see “Experimental Procedures”). The first two lanes are inputs (i.e. intact homogenate), and the next two lanes are immunoprecipitated (IP) pellets. Upper and lower panels correspond to immunoblots (WB) for biotin and ANT1, respectively. At least two independent experiments were performed for each condition. B, biotinylated proteins were immunoprecipitated from whole heart homogenates using neutravidin. The left panel represents immunoblot for ANT1, and the right panel is the corresponding Coomassie Blue (CB)-stained gel. Four bands at >250, 80, 55, and 32 kDa (highlighted by dotted lines) were analyzed by mass spectrometry (MALDI-TOF) and are identified as labeled in the figure. Mass spectrometry of the gel band at 32 kDa corresponding to ANT1 is shown in supplemental Fig. S1B.
To check if any of the four known cysteines in ANT were modified in perfused heart after Bt-LNO2 treatment, cardiac homogenates were immunoprecipitated using neutravidin beads. The immunoprecipitate pellet was separated on duplicate SDS-PAGE gels. One gel was transferred and probed for ANT1 to verify successful nitroalkylation and pulldown of the Bt-LNO2-ANT1 complex (Fig. 2B, left panel). The second gel was stained (Fig. 2B, right panel), and the prominent bands at >250, 80, 55, and 32 kDa were excised and subjected to mass spectrometric identification. The >250-kDa band could not be identified and is likely a nonspecific protein aggregate. The band at 80 kDa was the α-subunit of propionyl CoA-carboxylase, a biotin-containing enzyme that often contaminates biotin detection systems. The 55-kDa band was the α-subunit of ATP synthase (see “Discussion”).
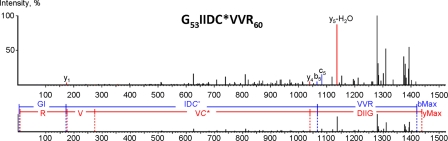
The 32-kDa band was identified as ANT1, with 80% sequence coverage, including all four cysteine residues (supplemental Fig. S1). The confidence of identification was above >98 for at least 5 peptides. Further MS/MS analysis (Fig. 3) revealed that a peptide at m/z 1438.8 containing Cys57 was modified by Bt-LNO2, as indicated by a mass shift of 564 Da corresponding to the molecular mass of Bt-LNO2 (supplemental Fig. S2). These data are the first identification of a nitroalkylation site in ANT1.
FIGURE 3.
Detection of Bt-LNO2-Cys adduct site in ANT1. The MS/MS spectrum of 1438.8 m/z ion was obtained in positive reflector mode fitted with peptide 53GIIDC*VVR60 from a ANT1 mouse sequence (gi:148747424) modified at Cys57 by Bt-LNO2. Colored numbers represent masses found in MS/MS with mass error less than 0.3 ppm. Collision of biotinylated nitro-lipid-modified peptides during MS/MS produces multiple products of nitrolipid side-chain breakage, which PEAKS software cannot take into account. We performed manual assignment of the four major peaks on the MS/MS; 1136.931 corresponds to the y5-H2O ion, 1393.184 corresponds to decarboxylated metastable ion, 1279.124 corresponds to metastable ion with water and biotin ring loss, 1311.005 corresponds to y7 ion with C5H10 neutral loss from nitro-lipid side chain. Supplemental Table S1 shows the full list of b and y ions.
Although supraphysiological doses of electrophiles have previously been shown to modify mitochondrial proteins (36, 37) and modification of ANT cysteines has been shown using various thiol reagents (38), to the best of our knowledge this is the first ever in situ identification of a cysteine modification in ANT by a low dose (100 nm) of a physiologically relevant (15, 39) electrophile, delivered to an intact tissue. Notably, the levels of LNO2 found in mitochondria in preconditioned hearts are in the micromolar range, suggesting they may be capable of nitro-alkylating ANT in situ, although preliminary studies in this area4 have so far failed to detect nitroalkylation of ANT in hearts exposed to IPC.
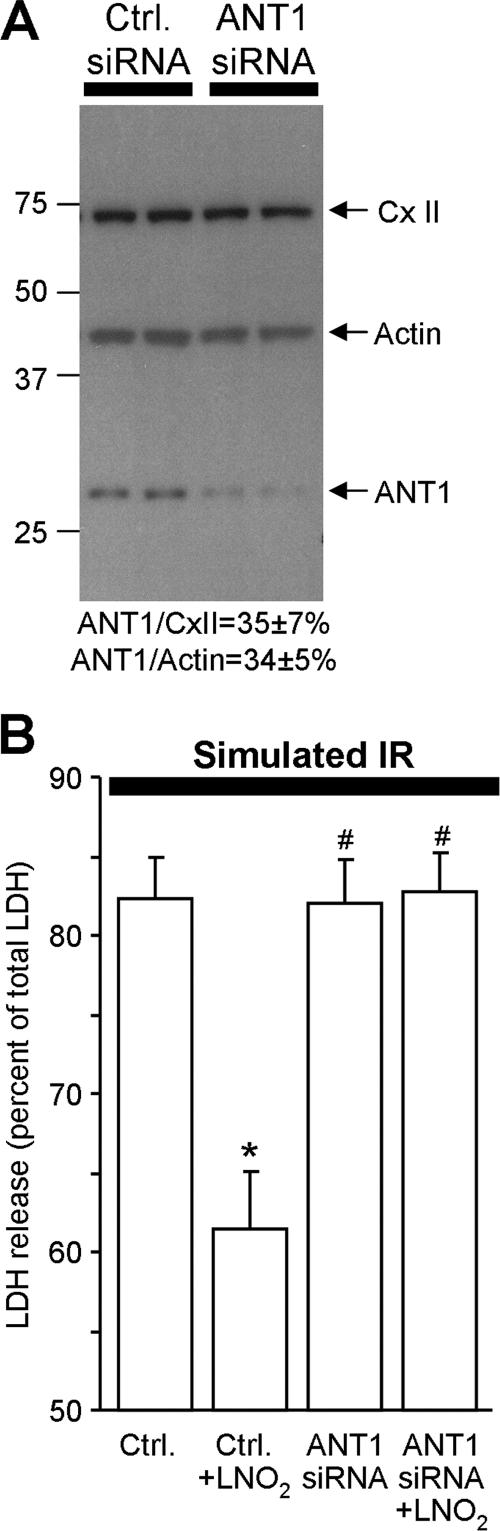
To investigate if ANT1 was required for LNO2-mediated protection, we employed an in vitro model of IR injury. ANT1 was knocked down by siRNA in the H9c2 cardiomyocyte cell line. Fig. 4A demonstrates that 72 h post-transfection, ANT1 levels were 35% of control. The levels of another mitochondrial protein (70-kDa subunit of respiratory complex II) did not change, and a scrambled siRNA control was without effect.
FIGURE 4.
ANT1 knock down abolishes effects of LNO2 in H9c2 cells. A, knockdown of ANT1 in H9c2 cells is shown. 72 h post-transfection cells (Ctrl and ANT1 siRNA) were harvested for immunoblotting analysis using anti-ANT1, anti-70 kDa complex II, and anti-actin antibodies. A blot image representative of four independent experiments is shown. Densitometry of ANT1, 70-kDa complex II (CX II), and actin bands was performed using Scion Image™ software. Relative ANT1 expression (in siRNA cells versus controls) is shown below the blot (means ± S.E., n = 4). B, is shown LDH release upon simulated IR injury. Transfected cells were subjected to simulated IR (see the “Experimental Procedures”). LDH release (cell death) is expressed as a percentage of total LDH (upon lysis with Triton X-100). Data are the means ± S.E., n = 7. *, p < 0.05 versus Ctrl siRNA; #, p < 0.05 versus Ctrl siRNA + LNO2.
To verify the metabolic effects of ANT1 knockdown in cells, we employed extracellular flux analysis (40) and TMRE spectrofluorimetry (15). ANT1 knockdown caused an ∼30% decrease in basal respiration rate (124 ± 20 pmol/min in ANT1 siRNA versus 177 ± 26 pmol/min in Ctrl siRNA, n = 7, p = 0.001), likely due to the known bioenergetic role of ANT1. To assess mitochondrial uncoupling (proton leak), respiration in the presence of oligomycin (enforced state 4) was used as a surrogate marker, as previously described (15, 41, 42). ANT1 knockdown caused a significant decrease in state 4 respiration (37 ± 5 pmol/min in ANT1 siRNA versus 51 ± 6 pmol/min in Ctrl siRNA, n = 7, p = 0.001), consistent with a role for ANT1 in basal proton leak (12). Furthermore, although the addition of LNO2 caused a small but significant increase in state 4 respiration in control cells (51 ± 6 pmol/min → 61 ± 9 pmol/min, n = 7, p = 0.02), LNO2 was without effect in ANT1 siRNA cells (37 ± 5 pmol/min → 41 ± 6 pmol/min, n = 7, p = 0.185) (supplemental Fig. S3). To further characterize proton leak, mitochondrial membrane potential was also measured in H9c2 cells using TMRE, normalizing the signal to MitoTracker Green. Consistent with the respiration data, in oligomycin-enforced state 4, LNO2 addition caused a significant decrease in Δψ in control cells (TMRE/MitoTracker Green signal 1439 ± 124 → 900 ± 80, p = 0.021) but not in ANT1 knock-down cells (TMRE/MitoTracker Green signal 1456 ± 371 → 1017 ± 216, p = 0.365). Together, these data indicate that the ability of LNO2 to induce mitochondrial proton leak is partly dependent on ANT1.
Finally, because a mild induction of mitochondrial proton leak can elicit cardioprotection (14, 43) and we previously suggested this may be a mechanism of LNO2-induced protection (15), we sought to investigate whether knocking down ANT1 would also block LNO2 protection. Fig. 4B shows that pretreatment with LNO2 resulted in a 26 ± 3% decrease in IR-induced cell death, consistent with our previous finding in adult cardiomyocytes (15) and consistent with the effects of another cardioprotective agent, isoflurane (see “Experimental Procedures”). Most notably, administration of LNO2 to ANT1 knockdown cells did not afford protection. This suggests that ANT1 is required for LNO2-mediated acute protection against IR injury.
DISCUSSION
The present study establishes a role for ANT1 in LNO2-mediated acute protection against IR injury. At least two possible mechanisms of protection have been revealed; (i) modification of Cys57 on ANT1 and (ii) ANT1-mediated mild uncoupling. Currently, the mechanistic details linking these two phenomena are not known. There are 4 cysteines in ANT1 at positions 57, 129, 159, and 256 in the mouse protein. Cys57 is localized on the matrix side of ANT1 in the hydrophobic pocket Gly53-Val59 (44, 45) and is accessible for modification by thiol reactive agents (46). Furthermore, this thiol faces the central pore of ANT1 (supplemental Fig. S4), and the electronegative nitro-group of nitroalkenes is retained upon covalent adduction of the lipid to cysteine. As such, a nitroalkene on Cys57 would be expected to introduce an electronegative moiety into the central pore of ANT, and this may play a role in the mechanism of proton leak.
Modification of Cys57 by Bt-LNO2 is in agreement with this cysteine being inaccessible in the c-conformation of ANT, which is enforced by the inhibitor carboxyatractyloside. We previously showed that carboxyatractyloside prevented ANT adduction by Bt-LNO2, whereas the alternative inhibitor bongkrekate, which enforces the m-conformation in which Cys57 is still accessible, did not (15).
There could be several mechanisms by which modification of ANT1 at Cys57 could protect the heart from IR injury. This includes effects on the PT pore, ADP/ATP transport, and proton leak. From the perspective of PT pore opening, oxidative modification of ANT1 Cys57 has been implicated in PT pore formation (46), potentially via regulation of cyclophilin D binding to ANT1 (29, 47, 48). Although we did show that high concentrations of LNO2 can induce PT pore opening (similar to other electrophiles such as 4-HNE and 15d-PGJ2 (49, 50)), low levels of LNO2 were without effect on the PT pore (15). Given the known importance of ROS in regulating PT pore opening in the post-ischemic heart (5), it is possible that nitroalkylation of Cys57 may serve to prevent further oxidative modification of this residue and thus prevent PT pore opening. Notably, such a “cap and protect” phenomenon has been proposed as one mechanism by which S-nitrosation of thiols can protect them against oxidative damage (51). Clearly, further work is needed to clarify the interplay between nitroalkylation versus oxidative modification at Cys57.
Regarding bioenergetic effects, it is known that irreversible modification of Cys57 drastically reduces ADP/ATP transport via ANT (45) and may impair intracellular ATP levels upon reperfusion regardless of PT pore opening. Thus, reversible nitroalkylation of Cys57 before reperfusion may prevent irreversible oxidative modification of this critical residue and thus permit rescue of ADP/ATP transport in the post-ischemic state.
With respect to proton leak, it is known that LNO2 stimulates mild mitochondrial uncoupling and that mild uncoupling itself is protective against IR injury (14, 16, 33). The mechanism linking mild uncoupling to protection may include inhibition of ROS generation and/or Ca2+ overload (for review, see Ref. 52). Furthermore, it has recently been shown that mild uncoupling of mitochondria can stimulate autophagy (53), and there is substantial evidence for a protective role of autophagy against IR injury (54, 55). It is not yet known if nitroalkenes can stimulate autophagy, but the recent finding that autophagy is required for IPC (56) coupled with our previous discovery of endogenous nitroalkene formation in IPC (15) suggests that nitroalkenes may be an upstream signal-regulating autophagy.
Although we were able to detect significant changes in mitochondrial proton leak by combining measurements of both membrane potential (TMRE) and respiration (extracellular flux (XF)-24), these methods are not high resolution. Likewise, it was previously shown that a cardioprotective concentration of FCCP (100 nm) stimulated oxygen consumption in cardiomyocytes but did not cause a detectable drop in membrane potential (57). Furthermore, the requirement to measure proton leak in state 4 typically necessitates the use of oligomycin, which depletes cellular ATP and induces mitochondrial fragmentation (58). Thus, development of more reliable methods for measuring proton leak in intact cells is desirable.
An important discrepancy between our cell and intact heart data is that LNO2 reduced infarct size in the heart by 56% but only reduced death in the cell model of IR by 26%. One reason for this may be that LNO2 modifies different proteins in hearts versus cells. Indeed, in addition to ANT1, several other proteins were modified by Bt-LNO2 in intact hearts. One such protein identified herein was the α-subunit of ATP synthase (Fig. 2B). This protein contains a redox-sensitive cysteine (Cys294) that was recently shown to be modified by reactive nitrogen species in the context of heart failure (59). Thus, it is possible that nitroalkylation of Cys294 may modulate ATP synthase activity, with important implications for cardiac dysfunction.
It is also possible that other mitochondrial redox-sensitive proteins not detected in this study may react with nitroalkenes to bring about cardioprotection. Two examples are UCP-2 and p66shc. Previously we obtained evidence suggestive of UCP-2 modification by LNO2 (15), and studies are ongoing to confirm this at the molecular level. Regarding p66shc, it is known to regulate energy metabolism (60) and H2O2 generation during cardiac reperfusion (61). The redox active cysteine (Cys59) in p66shc plays an important role in its activation (62) and may be a target for nitroalkylation. Clearly, as further nitroalkene target proteins continue to be identified, their role in cardioprotection by nitroalkenes can be determined.
Regarding the physiological relevance of the ANT nitroalkylation event in IPC, we have so far failed to detect nitroalkylation of ANT by endogenously generated LNO2 in hearts exposed to IPC. This is likely due to uncertainty regarding the exact molecular nature of the nitroalkenes generated in IPC, thus precluding the ability to use single-reaction monitoring to detect ANT modifications by mass spectrometry. Furthermore, because ANT is such a large part of the mitochondrial protein complement, immuno-precipitating all of the ANT (modified and unmodified) from a sample is technically challenging. In addition, the stability of endogenous nitroalkylation adducts during mitochondrial isolation from tissue is unclear.
Finally, although it is unlikely that the well documented ability of nitroalkenes to regulate transcription factors (24, 63, 64) contributes to the acute timescale protection observed in this study, such long term gene regulation effects might nevertheless be important for nitroalkene-mediated cardioprotection in a larger disease context. It is notable that nitroalkenes are currently being pursued in the context of type 2 diabetes (Complexa, Inc.), raising the possibility that nitroalkene administration in humans may be beneficial both in short term and long term therapeutic windows.
In summary, herein we show that acute protection from IR injury by LNO2 requires ANT1 and that LNO2 modifies a specific cysteine (Cys57) on the protein. The mechanistic links between this post-translational modification event and cardioprotection remain to be explored.
Supplementary Material
Acknowledgments
We thank Andrew Wojtovich (University of Rochester Medical Center) for technical assistance with the isoflurane cytoprotection studies and Keith Nehrke (University of Rochester Medical Center) for assistance with fluorescence microscopy measurements (TMRE).
This work was supported, in whole or in part, by National Institutes of Health Grants HL071158 (to P. S. B.) and HL101902 and HL067841 (to S. M. B.).

This article contains supplemental Table S1 and Figs. S1—S4.
S. M. Nadtochiy and P. S. Brookes, unpublished information.
- IR
- ischemia-reperfusion
- LA
- linoleic acid
- OA
- oleic acid
- LNO2
- nitro-linoleate
- Bt-LNO2
- biotinylated LNO2
- OANO2
- nitro-oleate
- ANT1
- adenine nucleotide translocase 1
- IPC
- ischemic preconditioning
- PT pore
- permeability transition pore
- TMRE
- tetramethylrhodamine ethyl
- Ctrl
- control
- CHCA
- α-cyano-4-hydroxycinnaminic acid.
REFERENCES
- 1. Halestrap A. P. (2009) Mitochondria and reperfusion injury of the heart-a holey death but not beyond salvation. J. Bioenerg. Biomembr. 41, 113–121 [DOI] [PubMed] [Google Scholar]
- 2. Chen Q., Moghaddas S., Hoppel C. L., Lesnefsky E. J. (2008) Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am. J. Physiol. Cell Physiol. 294, C460–C466 [DOI] [PubMed] [Google Scholar]
- 3. Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., Sheu S. S. (2004) Calcium, ATP, and ROS. A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287, C817–C833 [DOI] [PubMed] [Google Scholar]
- 4. Di Lisa F., Bernardi P. (2009) A CaPful of mechanisms regulating the mitochondrial permeability transition. J. Mol. Cell. Cardiol. 46, 775–780 [DOI] [PubMed] [Google Scholar]
- 5. Halestrap A. P. (2010) A pore way to die. The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 38, 841–860 [DOI] [PubMed] [Google Scholar]
- 6. Baines C. P., Kaiser R. A., Purcell N. H., Blair N. S., Osinska H., Hambleton M. A., Brunskill E. W., Sayen M. R., Gottlieb R. A., Dorn G. W., Robbins J., Molkentin J. D. (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 [DOI] [PubMed] [Google Scholar]
- 7. Brustovetsky N., Klingenberg M. (1996) Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 35, 8483–8488 [DOI] [PubMed] [Google Scholar]
- 8. Kokoszka J. E., Waymire K. G., Levy S. E., Sligh J. E., Cai J., Jones D. P., MacGregor G. R., Wallace D. C. (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baines C. P., Molkentin J. D. (2009) Adenine nucleotide translocase-1 induces cardiomyocyte death through up-regulation of the pro-apoptotic protein Bax. J. Mol. Cell. Cardiol. 46, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klingenberg M. (2008) The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 1778, 1978–2021 [DOI] [PubMed] [Google Scholar]
- 11. Levy S. E., Chen Y. S., Graham B. H., Wallace D. C. (2000) Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene 254, 57–66 [DOI] [PubMed] [Google Scholar]
- 12. Brand M. D., Pakay J. L., Ocloo A., Kokoszka J., Wallace D. C., Brookes P. S., Cornwall E. J. (2005) The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 392, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cadenas S., Buckingham J. A., St-Pierre J., Dickinson K., Jones R. B., Brand M. D. (2000) AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem. J. 351, 307–311 [PMC free article] [PubMed] [Google Scholar]
- 14. Brennan J. P., Southworth R., Medina R. A., Davidson S. M., Duchen M. R., Shattock M. J. (2006) Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 72, 313–321 [DOI] [PubMed] [Google Scholar]
- 15. Nadtochiy S. M., Baker P. R., Freeman B. A., Brookes P. S. (2009) Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc. Res. 82, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quarrie R., Cramer B. M., Lee D. S., Steinbaugh G. E., Erdahl W., Pfeiffer D. R., Zweier J. L., Crestanello J. A. (2011) Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the post-ischemic heart. J. Surg. Res. 165, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groeger A. L., Freeman B. A. (2010) Signaling actions of electrophiles. Anti-inflammatory therapeutic candidates. Mol. Interv. 10, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker P. R., Schopfer F. J., O'Donnell V. B., Freeman B. A. (2009) Convergence of nitric oxide and lipid signaling. Anti-inflammatory nitro-fatty acids. Free Radic. Biol. Med. 46, 989–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koenitzer J. R., Freeman B. A. (2010) Redox signaling in inflammation. Interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann. N.Y. Acad. Sci. 1203, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daum G. (1985) Lipids of mitochondria. Biochim. Biophys. Acta 822, 1–42 [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez J., Maloney R. E., Rassaf T., Bryan N. S., Feelisch M. (2003) Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc. Natl. Acad. Sci. U.S.A. 100, 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brookes P. S., Levonen A. L., Shiva S., Sarti P., Darley-Usmar V. M. (2002) Mitochondria. Regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 33, 755–764 [DOI] [PubMed] [Google Scholar]
- 23. O'Donnell V. B., Eiserich J. P., Chumley P. H., Jablonsky M. J., Krishna N. R., Kirk M., Barnes S., Darley-Usmar V. M., Freeman B. A. (1999) Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem. Res. Toxicol. 12, 83–92 [DOI] [PubMed] [Google Scholar]
- 24. Rudolph V., Rudolph T. K., Schopfer F. J., Bonacci G., Woodcock S. R., Cole M. P., Baker P. R., Ramani R., Freeman B. A. (2010) Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischemia and reperfusion. Cardiovasc. Res. 85, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batthyany C., Schopfer F. J., Baker P. R., Durán R., Baker L. M., Huang Y., Cerveñansky C., Branchaud B. P., Freeman B. A. (2006) Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 281, 20450–20463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin T. K., Hughes G., Muratovska A., Blaikie F. H., Brookes P. S., Darley-Usmar V., Smith R. A., Murphy M. P. (2002) Specific modification of mitochondrial protein thiols in response to oxidative stress. A proteomics approach. J. Biol. Chem. 277, 17048–17056 [DOI] [PubMed] [Google Scholar]
- 27. Halestrap A. P., Woodfield K. Y., Connern C. P. (1997) Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 272, 3346–3354 [DOI] [PubMed] [Google Scholar]
- 28. McStay G. P., Clarke S. J., Halestrap A. P. (2002) Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 367, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vyssokikh M. Y., Katz A., Rueck A., Wuensch C., Dörner A., Zorov D. B., Brdiczka D. (2001) Adenine nucleotide translocator isoforms 1 and 2 are differently distributed in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem. J. 358, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim D. G., Sweeney S., Bloodsworth A., White C. R., Chumley P. H., Krishna N. R., Schopfer F., O'Donnell V. B., Eiserich J. P., Freeman B. A. (2002) Nitrolinoleate, a nitric oxide-derived mediator of cell function. Synthesis, characterization, and vasomotor activity. Proc. Natl. Acad. Sci. U.S.A. 99, 15941–15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui T., Schopfer F. J., Zhang J., Chen K., Ichikawa T., Baker P. R., Batthyany C., Chacko B. K., Feng X., Patel R. P., Agarwal A., Freeman B. A., Chen Y. E. (2006) Nitrated fatty acids. Endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 281, 35686–35698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nadtochiy S. M., Redman E., Rahman I., Brookes P. S. (2011) Lysine deacetylation in ischaemic preconditioning. The role of SIRT1. Cardiovasc. Res. 89, 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nadtochiy S. M., Tompkins A. J., Brookes P. S. (2006) Different mechanisms of mitochondrial proton leak in ischemia/reperfusion injury and preconditioning. Implications for pathology and cardioprotection. Biochem. J. 395, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Khalifa A., Maddaford T. G., Chahine M. N., Austria J. A., Edel A. L., Richard M. N., Ander B. P., Gavel N., Kopilas M., Ganguly R., Ganguly P. K., Pierce G. N. (2007) Effect of dietary hempseed intake on cardiac ischemia-reperfusion injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1198–R1203 [DOI] [PubMed] [Google Scholar]
- 35. Leprán I., Szekeres L. (1992) Effect of dietary sunflower seed oil on the severity of reperfusion-induced arrhythmias in anesthetized rats. J. Cardiovasc. Pharmacol. 19, 40–44 [DOI] [PubMed] [Google Scholar]
- 36. Chavez J. D., Wu J., Bisson W., Maier C. S. (2011) Site-specific proteomic analysis of lipoxidation adducts in cardiac mitochondria reveals chemical diversity of 2-alkenal adduction. J. Proteomics 74, 2417–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong H. L., Liebler D. C. (2008) Mitochondrial protein targets of thiol-reactive electrophiles. Chem. Res. Toxicol. 21, 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Girón-Calle J., Zwizinski C. W., Schmid H. H. (1994) Peroxidative damage to cardiac mitochondria. II. Immunological analysis of modified adenine nucleotide translocase. Arch. Biochem. Biophys. 315, 1–7 [DOI] [PubMed] [Google Scholar]
- 39. Baker P. R., Schopfer F. J., Sweeney S., Freeman B. A. (2004) Red cell membrane and plasma linoleic acid nitration products. Synthesis, clinical identification, and quantitation. Proc. Natl. Acad. Sci. U.S.A. 101, 11577–11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gerencser A. A., Neilson A., Choi S. W., Edman U., Yadava N., Oh R. J., Ferrick D. A., Nicholls D. G., Brand M. D. (2009) Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. 81, 6868–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bosetti F., Baracca A., Lenaz G., Solaini G. (2004) Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett. 563, 161–164 [DOI] [PubMed] [Google Scholar]
- 42. Muscari C., Bonafè F., Gamberini C., Giordano E., Lenaz G., Caldarera C. M. (2006) Ischemic preconditioning preserves proton leakage from mitochondrial membranes but not oxidative phosphorylation during heart reperfusion. Cell Biochem. Funct. 24, 511–518 [DOI] [PubMed] [Google Scholar]
- 43. Teshima Y., Akao M., Jones S. P., Marbán E. (2003) Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 93, 192–200 [DOI] [PubMed] [Google Scholar]
- 44. Boulay F., Vignais P. V. (1984) Localization of the N-ethylmaleimide reactive cysteine in the beef heart mitochondrial ADP/ATP carrier protein. Biochemistry 23, 4807–4812 [DOI] [PubMed] [Google Scholar]
- 45. Majima E., Koike H., Hong Y. M., Shinohara Y., Terada H. (1993) Characterization of cysteine residues of mitochondrial ADP/ATP carrier with the SH reagents eosin 5-maleimide and N-ethylmaleimide. J. Biol. Chem. 268, 22181–22187 [PubMed] [Google Scholar]
- 46. Costantini P., Belzacq A. S., Vieira H. L., Larochette N., de Pablo M. A., Zamzami N., Susin S. A., Brenner C., Kroemer G. (2000) Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene 19, 307–314 [DOI] [PubMed] [Google Scholar]
- 47. Halestrap A. P., McStay G. P., Clarke S. J. (2002) The permeability transition pore complex. Another view. Biochimie 84, 153–166 [DOI] [PubMed] [Google Scholar]
- 48. Woodfield K., Rück A., Brdiczka D., Halestrap A. P. (1998) Direct demonstration of a specific interaction between cyclophilin D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 336, 287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kristal B. S., Park B. K., Yu B. P. (1996) 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J. Biol. Chem. 271, 6033–6038 [DOI] [PubMed] [Google Scholar]
- 50. Landar A., Shiva S., Levonen A. L., Oh J. Y., Zaragoza C., Johnson M. S., Darley-Usmar V. M. (2006) Induction of the permeability transition and cytochrome c release by 15-deoxy-Δ12,14-prostaglandin J2 in mitochondria. Biochem. J. 394, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kohr M. J., Sun J., Aponte A., Wang G., Gucek M., Murphy E., Steenbergen C. (2011) Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ. Res. 108, 418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brookes P. S. (2005) Mitochondrial H+ leak and ROS generation. An odd couple. Free Radic. Biol. Med. 38, 12–23 [DOI] [PubMed] [Google Scholar]
- 53. Huang C., Andres A. M., Ratliff E. P., Hernandez G., Lee P., Gottlieb R. A. (2011) Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS. One 6, e20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dong Y., Undyala V. V., Gottlieb R. A., Mentzer R. M., Jr., Przyklenk K. (2010) Autophagy. Definition, molecular machinery, and potential role in myocardial ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 15, 220–230 [DOI] [PubMed] [Google Scholar]
- 55. Petrovski G., Das S., Juhasz B., Kertesz A., Tosaki A., Das D. K. (2011) Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid. Redox Signal. 14, 2191–2200 [DOI] [PubMed] [Google Scholar]
- 56. Huang C., Yitzhaki S., Perry C. N., Liu W., Giricz Z., Mentzer R. M., Jr., Gottlieb R. A. (2010) Autophagy induced by ischemic preconditioning is essential for cardioprotection. J. Cardiovasc. Transl. Res. 3, 365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brennan J. P., Berry R. G., Baghai M., Duchen M. R., Shattock M. J. (2006) FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarization. Cardiovasc. Res. 72, 322–330 [DOI] [PubMed] [Google Scholar]
- 58. Legros F., Lombès A., Frachon P., Rojo M. (2002) Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell 13, 4343–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang S. B., Foster D. B., Rucker J., O'Rourke B., Kass D. A., Van Eyk J. E. (2011) Redox regulation of mitochondrial ATP synthase. Implications for cardiac resynchronization therapy. Circ. Res. 109, 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nemoto S., Combs C. A., French S., Ahn B. H., Fergusson M. M., Balaban R. S., Finkel T. (2006) The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J. Biol. Chem. 281, 10555–10560 [DOI] [PubMed] [Google Scholar]
- 61. Carpi A., Menabò R., Kaludercic N., Pelicci P., Di Lisa F., Giorgio M. (2009) The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim. Biophys. Acta 1787, 774–780 [DOI] [PubMed] [Google Scholar]
- 62. Gertz M., Fischer F., Wolters D., Steegborn C. (2008) Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc. Natl. Acad. Sci. U.S.A. 105, 5705–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baker P. R., Lin Y., Schopfer F. J., Woodcock S. R., Groeger A. L., Batthyany C., Sweeney S., Long M. H., Iles K. E., Baker L. M., Branchaud B. P., Chen Y. E., Freeman B. A. (2005) Fatty acid transduction of nitric oxide signaling. Multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 280, 42464–42475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schopfer F. J., Lin Y., Baker P. R., Cui T., Garcia-Barrio M., Zhang J., Chen K., Chen Y. E., Freeman B. A. (2005) Nitrolinoleic acid. An endogenous peroxisome proliferator-activated receptor γ ligand. Proc. Natl. Acad. Sci. U.S.A. 102, 2340–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.