Abstract
Most heart attacks and strokes are caused by blood clots (thrombi) that block the vasculature. Since arterial thrombosis is a blood platelet-dependent disease, platelet inhibitors such as aspirin and clopidogrel are effective; however, they also impair platelet-dependent hemostasis, and can produce excessive bleeding. Experimental studies show that a reduction in the number of platelets also inhibits thrombosis but these treatments also interfere with platelet function. Because normal hemostasis requires that the platelet concentration remain within a physiological range in the circulation, we evaluated whether lowering platelet count to a value within the normal range by inhibiting their source in the bone marrow reduces acute thrombogenesis in a baboon model. Experimental occlusive thrombogenesis on collagen-coated vascular grafts was inhibited without primary hemostasis impairment when platelet counts were reduced within the normal range using an inhibitor against the megakaryocyte promoting hormone, thrombopoietin, in baboons. This suggests that targeting platelet production without interfering with the hemostatic function of platelets may offer a safe alternative to direct platelet inhibitors for thromboprophylaxis.
Introduction
Platelets participate in arterial thrombosis-associated ischemic stroke and heart attack, as shown by observations that inhibitors of platelet function such as aspirin and clopidogrel reduce thrombosis (1-3). Platelet antagonists can however produce an undesirable increase in bleeding when administered at their most effective antithrombotic doses (2-5). An alternative antithrombotic strategy – reducing the number of circulating platelets – is suggested by clinical observations that lower platelet counts within the normal physiologic range (150,000–450,000/μL) (6, 7) correlate with a significant reduction in adverse cardiovascular events (8-12), even in patients receiving conventional anti-platelet therapy (11, 12). At present, it is not known whether reducing the number of platelets within or below the normal range, without affecting platelet function, has antithrombotic activity. The relative safety of reducing platelet count as an antithrombotic strategy is suggested by the fact that, in the majority of cases, only a relatively small proportion of the normal platelet pool appears to be required for the maintenance of vascular integrity (13-17). Indeed, in many cases it is only when the platelet count falls to approximately 10,000/μL that patients are at markedly increased risk of severe spontaneous internal bleeding (15-17), though mild thrombocytopenia (platelet counts of less than 150,000/μL) has been documented to increase the risk of bleeding in some patients and has been used as a trigger for platelet transfusion (18-20). While normal platelet numbers may be saturating for platelet-dependent hemostasis, higher platelet counts may increase the capacity of platelets to participate in pathological thrombus formation, including thrombotic complications associated with essential thrombocythemia, when platelet counts are above normal (21).
Data from several clinical studies have consistently shown that recurrent thrombosis and mortality correlate with baseline platelet numbers in some cardiovascular diseases (8-12), suggesting that, together with experimental observations over several decades of thrombosis research, platelet numbers are directly related to thrombosis and resulting mortality. However, other than studies performed under thrombocytopenic conditions experimentally induced by anti-platelet antibodies, which may also alter platelet function (22-26), it is not known whether electively lowering platelet counts within the normal range, without affecting their functional integrity, influences thrombus formation, especially in primates. The present study conducted in baboons was designed to answer this question, and to assess the antithrombotic and antihemostatic effects of moderate pharmacological platelet count reduction by selectively inhibiting thrombopoietin (TPO)-dependent platelet production in the bone marrow. Isolated thrombocytopenia has been reported in some patients who received recombinant human TPO (rhTPO) treatment in clinical trials (27-29). The drug-induced thrombocytopenia in some of these patients was shown to be caused by autoantibodies to TPO (megakaryocyte growth and development factor), which is an important glycoprotein hormone essential for platelet production (30, 31). We reasoned that this response to rhTPO could be replicated in baboons. Therefore, to evaluate the efficacy and safety of lowering circulating platelet numbers within the normal range in baboons, we raised neutralizing baboon anti-TPO autoantibodies and tested their effects on platelet function and thrombosis, comparing the results to those obtained following aspirin treatment as well as to historical results with this model.
Results
Platelet count reduction by TPO inhibition in baboons
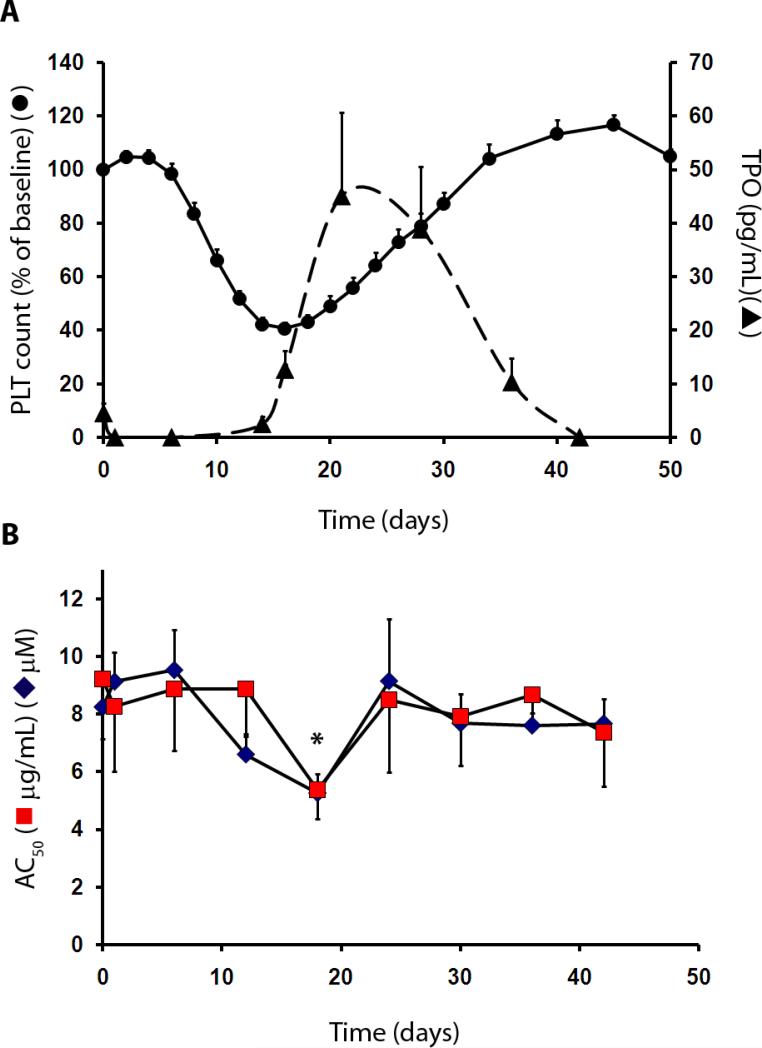
Affinity-purified polyclonal IgG from immunized baboon serum, but not from control serum, dose-dependently inhibited cell proliferation of a TPO-dependent human hematopoietic cell line (HU-03)(32), with an IC50 of 0.76 μg/ml. A single intravenous injection (30 to 35 mL) of the anti-TPO serum into 9 baboons (9-13 kg body weight) decreased circulating platelet numbers that reached a nadir at day 16, followed by a rebound increase in platelet counts until day 40 (Fig. 1A). The concentrations of red blood cells (RBC) and leukocytes were unaffected (pre-treatment counts of 5.1 ± 0.2 × 109/μL and 6,500 ± 600/μL for RBC and leukocytes, vs. 4.8 ± 0.2 × 109/μL and 6,900 ± 1000/μL at day 16, respectively; n=9 each, P > 0.05 for both). Single intravenous injections of anti-TPO antiserum caused mean platelet counts to decrease from 302,000 ± 21,000/μL at baseline to 116,000 ± 7,000/μL by day 16 (Fig. 1A). Concurrently, TPO concentrations (Fig. 1A) decreased from 4.6 ± 1.8 pg/mL at baseline to below the assay limit of detection (<2.8 pg/mL) 24 hours after infusion, then increased to 45.1 ± 15.6 pg/mL by day 21 before returning to pre-treatment values. For collagen- and adenosine diphosphate (ADP)-induced platelet aggregation (Fig. 1B), baseline AC50 values, defined as the agonist concentration that produces half-maximal platelet aggregation, which is an index of platelet reactivity, were 9.2 ± 2.1 μg/mL and 8.2 ± 0.9 μM, respectively. There was a modest increase in ex vivo platelet reactivity (decreased AC50) to these two agents by day 18 ( AC50 values of 5.4 ± 1.0 μg/mL and 5.3 ± 0.7 μM at day 18, respectively; n=5 each, P < 0.05 for ADP), while mean AC50 values returned to baseline by day 40. These data show that although platelet count decreased with administration of antiserum to TPO, platelet aggregation was not impaired for the duration of the thrombosis studies, though there was some indication that the platelets were more prone to aggregate at lower agonist concentrations when circulating TPO levels were high. Control serum containing normal baboon IgG was also injected into 2 additional baboons to assess possible effects on platelet aggregation and on thrombosis. Platelet counts remained normal by day 16 compared with baseline counts (mean of 303,000/μL at baseline vs. 370,000/μL at day 16). The small increase in platelet count observed may have resulted from repeated blood collections during the study. The sensitivity of platelets to aggregate in response to ADP and collagen was also normal 18 days after control serum injection (mean pre-treatment AC50 values of 5.2 μM and 9.6 μg/mL for ADP and collagen, vs. 6.7 μM and 6.7 μg/mL at day 18, respectively; n=2 each). Previously, a single high dose (>20 mg/kg) of aspirin has been shown to inhibit platelet aggregation in baboons (33, 34). Thus, measurements of aggregation impairment due to aspirin administration were not repeated in this study.
Fig 1. Platelet count reduction by inhibition of TPO in baboons.
(A) Platelet count and TPO blood concentrations in response to treatment with antiserum to TPO in 9 baboons. PLT, platelet count, solid line; circulating TPO, dashed line. (B) Ex vivo platelet responsivity to aggregation agonists adenosine diphosphate (ADP) and collagen after in vivo treatment with antiserum to TPO that was given on day 0. Platelet aggregation was measured during the time-course of platelet count depletion (n=5 each). Aggregation tests were performed in platelet-rich plasma, with the platelet count adjusted to 150,000/μL. Results were expressed as the concentration of each agonist (◆ ADP, μM; ■ collagen, μg/mL) that induced half-maximal aggregation (AC50). Platelet aggregation was sensitized to ADP 18 days after TPO antiserum treatment (*P < 0.05), which corresponded to the peak circulating TPO concentrations. All values are mean ±1 SEM.
Correlation between platelet count and thrombus formation
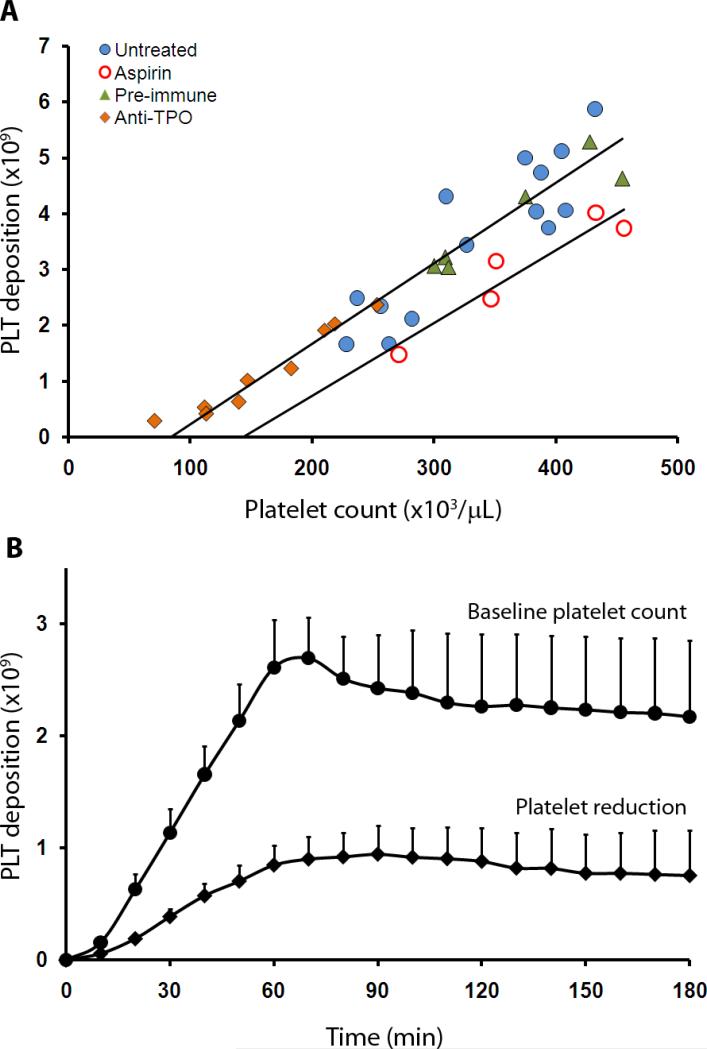
To test the anti-thrombotic potential of platelet count lowering we used an established baboon vascular graft thrombosis model (34, 35). For these experiments, a pro-thrombotic 4 mm (id) collagen-coated synthetic vascular graft segment, which mimics a damaged or stenosed blood vessel, was interposed between the femoral artery and vein, and blood was allowed to flow through the device while imaging in real-time the deposition of autologous radiolabeled platelets. In untreated animals, platelet deposition onto the grafts after 60 min of blood exposure showed a positive linear correlation with platelet count (Fig. 2A) (R2 = 0.91, P < 0.0001), consistent with the premise that platelet concentrations within the normal range do not saturate arterial-type thrombogenesis in large vessels, and with earlier observations that normal platelet count variations predict arterial-type thrombus propagation rate in primates (36). In animals given control pre-immune serum, thrombosis experiments also showed a correlation between platelet count and deposition (Fig. 2A), similar to that seen in untreated animal. In aspirin-treated animals, a similar relationship was observed within the platelet count range tested (R2 = 0.90, P = 0.01; Fig. 2A), consistent with observations that platelet count may be an independent predictor of thrombotic events in coronary artery disease patients receiving anti-platelet therapy (11, 12). Fibrin accumulation on the grafts after 60 min averaged 1.2 ± 0.1 mg in untreated animals, and 1.0 ± 0.1 mg aspirin-treated animals.
Fig 2. Effect of platelet count on platelet deposition on vascular grafts in baboons.
(A) Platelet deposition onto collagen-coated (4 mm id × 2 cm) vascular grafts as a function of platelet count in naïve, pre-immune serum-treated, TPO antiserum-treated, and aspirin-treated baboons. Platelets deposited after 60 min of blood flow through the graft (n=29, combined data from anti-TPO treated, pre-immune, and naïve animals) were correlated with the circulating platelet count (R2 = 0.91, P < 0.001). Pretreatment with oral aspirin (32 mg/kg) in 5 previously naïve animals reduced platelet deposition when compared to untreated controls at similar platelet counts. In aspirin-treated animals total platelet accumulation was correlated with circulating platelet count (R2 = 0.90, P = 0.01). (B) Platelet deposition onto collagen-coated thrombogenic grafts over 3 hours in naïve and anti-TPO-treated baboons. Baseline platelet count was 266,000 ± 32,000/μL, n=3 and lowered platelet count was 134,000 ± 33,000/μL, n=3. All values are mean ±1 SEM.
In longer duration studies, we measured platelet deposition in 4 mm (id) grafts to determine whether platelet count-dependent effects persisted after thrombus stabilization. As reported (37), platelet accumulation in this model reached its maximum between 60 and 90 min, both before and after platelet count-lowering treatments. The number of platelets deposited in the thrombus progressively decreased thereafter in both groups (Fig. 2B). At all time points, animals with lowered platelet counts maintained an advantage, having fewer platelets in the graft than the corresponding controls with higher platelet counts. There appeared to be no trend to suggest that platelet deposition at lowered platelet count would eventually increase and reach the platelet deposition values seen in the pre-treatment group. Fibrin deposition at 3 hours was similar for both groups (1.2 ± 0.4 mg for platelet counts of 134,000 ± 33,000/μL and 1.2 ± 0.1 mg for counts of 266,000 ± 32,000/μL; n=3 for each, P < 0.05 for platelet count change).
Prevention of vascular graft thrombo-occlusion by platelet count reduction
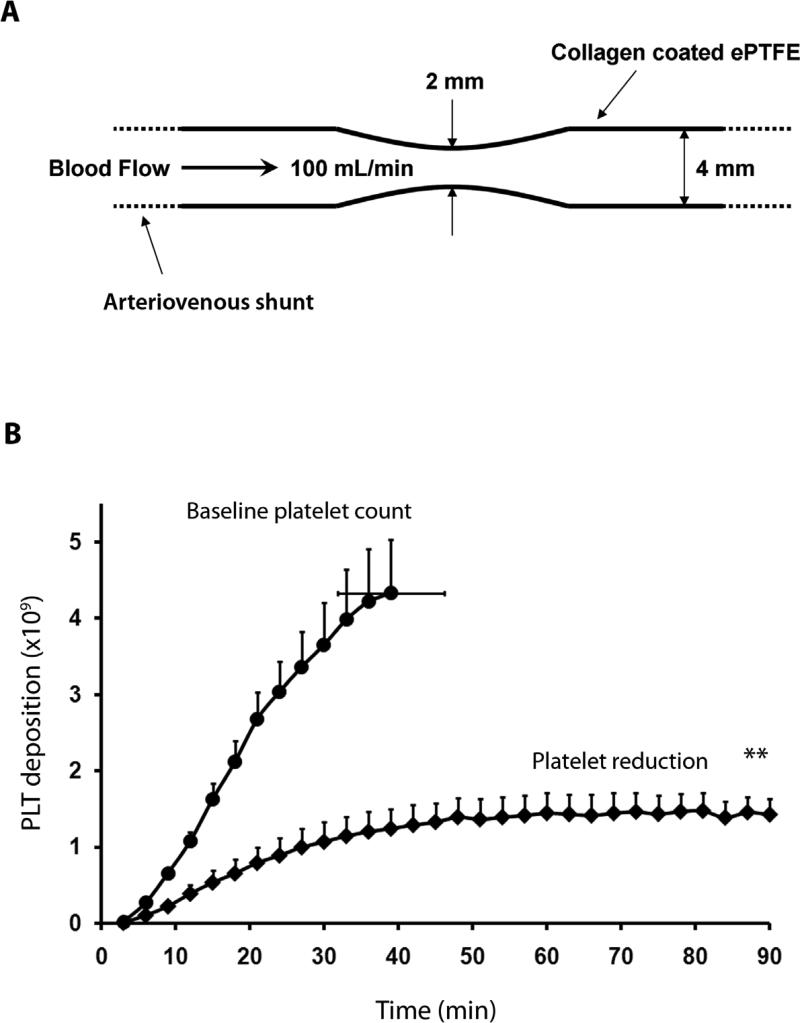
To determine the effects of platelet count lowering on occlusive thrombus formation, we performed a series of experiments using a collagen-coated vascular graft that had a 75% central constriction in cross-sectional area (38). While blood flow through the straight grafts was unchanged over 1 to 3 hours of observation (Fig. 2), the stenotic grafts progressively occluded due to thrombus formation (Fig. 3). In this model, the graft typically occludes by about 40 min at 100 mL/min flow rate; aspirin significantly delays the time to occlusion in a similar model (34). When these stenotic grafts were placed in shunts of untreated animals with platelet counts near the high end of the normal range (372,000 ± 14,000/μL; n=5), platelet deposition increased rapidly and all devices occluded by 40 ± 6 min (range = 21-60 min) (Fig. 2B). Treatment with antiserum to TPO in the same baboons lowered their platelet counts to 194,000 ± 38,000/μL (n=4, P < 0.01) and prevented the occlusion of stenosed grafts for the entire 90 min study interval (P < 0.01). These data suggest that moderate platelet count reduction had a profound effect on the dynamics of acute arterial-type occlusive thrombus formation.
Fig 3. Prevention of vascular graft occlusion by moderate platelet count reduction in baboons.
(A) Schematic of the thrombogenic vascular graft. Thrombosis was initiated by placing collagen-coated expanded polytetrafluoroethylene (ePTFE) vascular graft segments (4 mm id × 2.5 cm) with a symmetrical 75% constriction in cross-sectional area into chronic arteriovenous shunts. Blood flow was restricted by distal clamping of the arteriovenous shunt to maintain 100 mL/min or until flow dropped to ≤20 mL/min, signaling imminent occlusion. Grafts were removed from the shunt at occlusion, or at 90 min, whichever came first. (B) Effects of platelet count lowering on platelet accumulation and occlusive thrombus formation in stenotic collagen-coated vascular grafts. At platelet counts averaging 372,000 ± 14,000/μL (n=5), all devices occluded within 40 ± 6 min. After platelet count reduction to 194,000 ± 38,000/μL, platelet accumulation was reduced and thrombo-occlusion was prevented for 90 min (n=4; **P < 0.01, with significance calculated using the log-rank test and non-occluding devices being censored). All values are mean ±1 SEM.
Fibrin deposition in the stenotic grafts at the time of graft occlusion was similar in both antibody-treated baboons (0.8 ± 0.1 mg) and untreated controls (0.9 ± 0.1 mg at 90 min, P > 0.05). Since the stenotic grafts were exposed to blood flow 2.3-times longer in anti-TPO treated baboons, possible relationships between platelet count and fibrin deposition in this model remain unclear.
Normal bleeding times after platelet count reduction
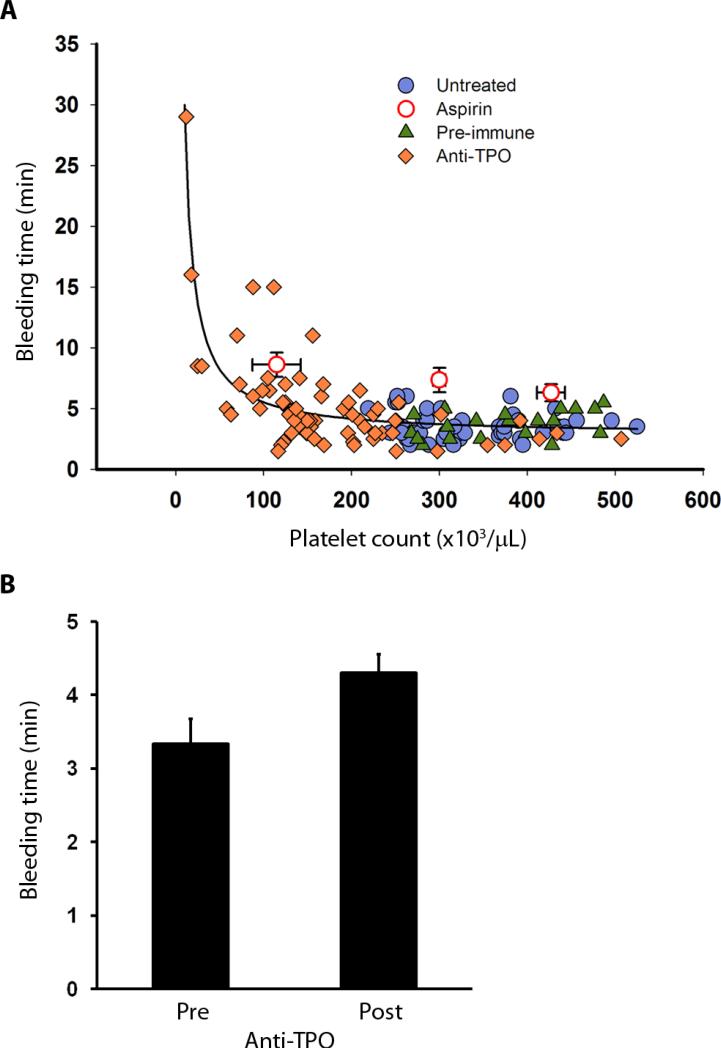
Bleeding times (BT) were measured using the Surgicutt® device, which creates a uniform 5-mm long 1-mm deep incision on the volar surface of the forearm, and the time to bleeding cessation was recorded. After treatment with antibody to TPO, BT remained in the normal range and did not increase until platelet counts dropped below ~100,000/μL (Fig. 4A). The correlation between BT and platelet count in the normal range from all animals was not statistically significant (n=87, R2 = 0.03, P > 0.05). In contrast, BT showed a stronger negative correlation with the platelet count in the sub-normal platelet count range (below 150,000/μL, n=28, R2 = 0.42, P < 0.001) similar to a published clinical report (39). Aspirin treatment significantly prolonged the BT by an average of 3 min to 6.7 ± 0.5 min (n=22) versus pre-treatment values of 3.4 ± 0.2 min (n=16) taken in the same animals (P < 0.001) at similar platelet counts (367,000 ± 19,000/μL vs. 383,000 ± 20,000/μL). Aspirin did not induce a disproportionate prolongation of BT when the platelet count was reduced to below 100,000/μL (maximum BT = 12.5 min at platelet count of 58,000/μL). Thus, although aspirin modestly prolongs the normal BT, and may aggravate the risk of spontaneous bleeding in thrombocytopenia (40), our data do not suggest that moderate platelet count reduction within the normal range enhances the risk of bleeding with aspirin ingestion. Comparison of BT in the same animals immediately before anti-TPO treatment (BT = 3.3 ± 0.3 min, n=9, platelet count = 324,000 ± 19,000/μL) and up to five weeks after anti-TPO treatment (BT = 4.3 ± 0.3 min, n=9, platelet count = 227,000 ± 14,000/μL), showed no significant differences (Fig. 4B, P = 0.08 for bleeding time, P < 0.001 for platelet count, paired t-test).
Fig 4. Independence of bleeding time and platelet count within the normal range.
(A) Bleeding times and platelet counts measured in naïve, aspirin-treated, pre-immune serum treated, and TPO antiserum treated baboons. Aspirin data (open circles) was grouped into tertiles of platelet count: lower tertile (115,000 ± 27,000/μL, n=7), middle tertile (300,000 ± 7,000/μL, n=7), and upper tertile (427,000 ± 16,000/μL, n=13). (B) Bleeding times in the same baboons immediately before and up to five weeks after anti-TPO treatment while platelet counts were within the normal range (150,000/μL to 450,000/μL). Platelet count reduction from 324,000 ± 19,000/μL to 227,000 ± 14,000/μL (P < 0.001) had no significant effect on the average bleeding time (pre: 3.3 ± 0.3 min, versus post: 4.3 ± 0.3 min, n=9 each; P = 0.08, calculated with the paired t-test). Values are mean ± 1 SEM.
Effects of platelet count on thrombus development in human blood
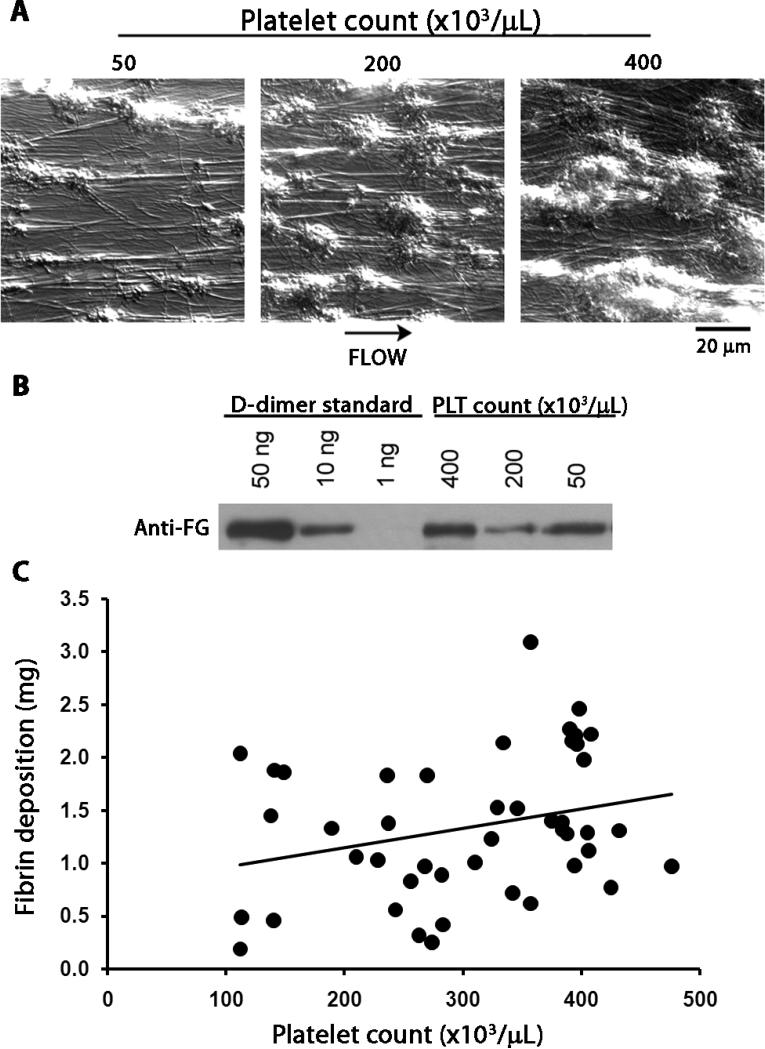
To test the effects of platelet count on thrombus formation in human blood, we also employed a flow chamber thrombosis model (34). The platelet count in the reconstituted blood perfused through the thrombus chamber was adjusted to 0/μL, 50,000/μL, 200,000/μL, or 400,000/μL. On microscopic assessment, the size of platelet-rich thrombi that formed on the collagen-coated surface of the capillary tubes after 10 min of flow was larger at higher platelet counts in each experiment (Fig. 5A). In contrast, similar to the results obtained in the vascular grafts in baboons, the amount of deposited fibrin, assessed as the strength of bands on Western blots for D-dimer content of the digested thrombi, did not correlate well with the platelet counts from the same experiment (Fig. 5B). When there were no platelets added back to plasma, there was minimal visible fibrin deposited in the capillary tubes within 10 min, suggesting that the collagen-coated surface alone did not trigger robust coagulation activation.
Fig 5. Platelet count dependence of platelet deposition on immobilized collagen, ex vivo.
(A) Representative micrographs of thrombus formation in collagen-coated capillary flow chambers. Reconstituted blood with a platelet concentration of 50,000, 200,000 or 400,000/μL was co-perfused with CaCl 2(7.5 mM) and MgCl2 (3.75 mM) over immobilized collagen at 265 s-1 for 10 min. Images were visually evaluated for the number and size of platelet-rich thrombi and for fibrin strands. (B) Fibrin generation in the flow chamber. After blood perfusion, capillary tubes were washed with modified Tyrodes buffer, treated with lysis buffer and then plasmin (1 μM). Capillary eluates were collected, run on 6% SDS-polyacrylamide gel and immunoblotted for the ~200 kDa fibrin degradation product D-dimer. Images were analyzed visually and are representative of 4 experiments. (C) Combined historic and current baboon thrombosis data obtained in the same baboon thrombosis model showing no linear correlation (R2 = 0.08) between platelet count and fibrin deposition in the graft, in vivo. This result is consistent with the ex vivo data (B), suggesting that fibrin deposition in the vascular graft segment may also be regulated independently of platelet count.
When we plotted the fibrin data as a function of platelet count from the current baboon studies, along with 22 historic data points from previous thrombosis experiments with the 4 mm (id) collagen-coated graft in other untreated baboons, we also saw no significant linear correlation between thrombus fibrin and platelet count (Fig. 5C; R2 = 0.08, P = 0.07). Indeed, most data generated in the flow chamber model were consistent with the graft thrombosis data obtained in the baboons, suggesting that although platelet count is a strong predictor of the platelet content of arterial-type thrombi, fibrin formation is regulated by additional factors that are not associated with circulating platelet count in the normal range.
Discussion
Although the contribution of platelets to occlusive arterial thrombus formation has been well documented, the role of circulating platelet numbers in vascular thrombo-occlusion has been less clear. The present study in baboons demonstrates that modestly reducing platelet counts to the lower portion of the normal range, without directly targeting platelets or inhibiting specific cellular platelet functions, can markedly reduce the size of arterial-type platelet-rich thrombi in small diameter conduits, and thereby reduce the frequency of thrombotic occlusion. Thrombi grew significantly slower after platelet count reduction in baboons than they did at the higher baseline platelet counts. Since intraluminal thrombus propagation, evidenced as a positive platelet deposition rate, stopped after about one hour in animals having both baseline and lowered platelet counts, there remained a sustained and apparently steady difference between the platelet contents of thrombi even after 3 hours of observation.
Since baboons are hemostatically similar to humans with respect to concentrations and functions of clotting factors and platelets (41, 42), this model has been useful for studying therapeutic interventions (33-36) and mechanisms of thrombosis (35, 38) under conditions of blood flow and geometry that are clinically relevant. In contrast to our results, Reyers et al. found in a rat model of thrombocytopenia that decreasing platelet count with antibodies directed against platelets delayed, but did not prevent, occlusion of injured small arteries (23). The numerous differences between the two approaches and the models may account for the discrepancy between those findings and the present study.
Although thrombus propagation was directly correlated with platelet count, primary hemostasis, assessed as prolongation of the BT after platelet count reducing anti-TPO treatment, was impaired only when platelet counts dropped below the normal range, a result consistent with clinical observations in diseases that affect the hemostatic system such as thrombocytopenic purpura (14, 17, 43, 44). These data are consistent with the view that platelet counts within the normal range fully support the hemostatic function of platelets, and that lowering platelet count within this range is unlikely to have a negative effect on hemostasis. A single BT value, ~2.5 to 9 min in the clinic (45, 46), is not a particularly useful predictor of hemostasis, but a change of BT from an established baseline can be both predictive and diagnostic in clinical practice (47). In our current and previous baboon studies, intermediate doses of aspirin or heparin were antithrombotic (34, 48), and both drugs markedly prolonged the BT relative to pre-treatment values. These observations support BT prolongation as a reliable predictor of drug-induced hemostasis impairment, because both aspirin and heparin are associated with clinically relevant bleeding (49, 50).
From an evolutionary perspective, higher platelet counts may have been a survival factor in early populations where the risk of trauma was high and lifespans short. Conversely, lower platelet counts may reduce thrombotic risk in modern aging populations where the likelihood of physical trauma is lower; however documenting this assertion would require evidence that is not readily available. In addition, the lack of correlation between BT and platelet counts in the normal range suggests that a reduction of platelet numbers from high normal to the low normal range would carry a relatively small, if any, increased risk of bleeding complications. Our data in primates suggest that even moderate pharmacological reduction in platelet counts, resulting from decreased platelet production, can significantly decrease the rate of occlusive thrombogenesis without appreciably disabling hemostasis.
Because fibrin is an essential structural component of arterial-type thrombi that form in vascular grafts in the baboon model, we also measured the final fibrin content of the thrombi as an indicator of total thrombus size and coagulation tendency at the different platelet counts (35). A number of factors that affect fibrin deposition, including fibrinogen concentrations, the concentrations of coagulation factors, fibrinolytic enzymes, and inhibitors, have been successfully exploited for antithrombotic therapy. In this study, the fibrin content of the experimental thrombi showed no significant correlation with either the circulating platelet count or the number of platelets deposited in vascular grafts in baboons, or with human platelet accumulation in an ex vivo thrombus model. These observations suggested that fibrin deposition may be largely independent of acute platelet deposition in these models. Minimal fibrin thrombus in the absence of platelets in the ex vivo model suggests, however, that platelets are an important component for robust thrombus fibrin generation under arterial flow conditions.
Possible long-term effects of anti-TPO antiserum administration were found to be minimal in this model. Platelet counts rebounded to approximately 120% of baseline values at day 40, returning to normal by day 50 (Fig. 1A). Concurrently, TPO levels were at near baseline levels and platelet aggregation responses were normal by this time (Fig. 1B). Thus in the recovery phase following anti-TPO administration only a minor, transient increase in platelet count was observed. These findings may have implications for platelet reduction in humans as well, although further research will be needed to establish the efficacy of this approach under conditions and in disease states where thrombopoiesis may be regulated by factors other than TPO (51), and during chronic thrombocytopenia that may be associated with hemostatic alterations not reproduced in either baboon or human ex vivo models of acute platelet count reduction. Unlike systemic platelet function inhibitors that reduce the hemostatic activity of all platelets in the circulation, development of pharmacological inhibitors of platelet production that specifically target thrombopoiesis, as shown here, may allow optimization of the prohemostatic versus prothrombotic balance of the circulating platelet pool, and therefore provide a safer alternative to existing strategies for reducing the incidence of arterial thrombo-occlusive events such as heart attack and ischemic stroke.
Currently, two drugs, hydroxyurea and anagrelide, are useful in the treatment of the high platelet counts associated with essential thrombocythemia (52). However, concerns regarding a lack of megakaryocyte specificity and adverse side-effects may limit their use in cardiovascular patients. Alternatively, safer formulations of these drugs or more selective TPO-inhibitors, megakaryocyte-specific TPO receptor (c-Mpl) antagonists, or other megakaryocyte growth and maturation inhibitors may eventually become available, and thereby allow evaluation of the platelet lowering concept described in this study. Our data suggest that targeting platelet-producing megakaryocytes is a strategy worth considering for reducing occlusive thrombotic vascular events.
Materials and methods
Platelet lowering in the baboon
All experiments involving baboons were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. The humane care of all animals was overseen by the veterinary staff of the Oregon National Primate Research Center in adherence to the NIH Guide for the Care and Use of Laboratory Animals. A juvenile male baboon (Papio anubis) was treated with repeated subcutaneous injections of recombinant human TPO (rhTPO, Genentech). After 11 rhTPO injections (5 μg/kg), given over 35 days, the baboon developed transient thrombocythemia (platelet count > 400,000/μL), followed by progressive and sustained thrombocytopenia (platelet count < 150,000/μL), a result of the development of autoantibodies to TPO, a response similar to that observed in human clinical trials. During severe thrombocytopenia (platelet count < 20,000/μL) that lasted for over 6 months, blood was drawn under aseptic conditions weekly and the immune serum was frozen at -80° C. The polyclonal IgG was affinity purified from the serum with an rhTPO sepharose column, and the anti-TPO activity of the autoantibodies was measured with a TPO-dependent (HU-03) cell proliferation assay (32, 53).
Arteriovenous shunt thrombosis model
The effect of reduced platelet count on vascular graft thrombogenesis was evaluated in 49 non-terminal experiments using 15 male baboons (34, 54). Autologous platelets and fibrinogen were radiolabeled with 111Indium (1 mCi) and 125Iodine (4 μCi), respectively, and reinjected into the animals before thrombosis experiments as described (35). Thrombus formation was induced by temporary deployment of collagen-coated, expanded polytetrafluoroethylene (ePTFE) vascular graft segments of constant diameter (4.0 mm internal diameter [id] × 2 cm long) (34), or similar grafts that also contained a symmetrical 75% reduction in cross-sectional area (2 mm id at the stenosis throat), into 3-mm diameter chronic femoral arteriovenous shunts as described (38). Initial blood flow rates were maintained at 100 mL/min, controlled by distal clamping of the shunt and continually measured with a Doppler flow probe, producing initial wall shear rates of 265 sec-1 (straight grafts) or 4,700 sec-1 (stenotic grafts) (34, 38, 54). The platelet content of the grafts was determined by quantitative analysis of the graft images collected using a GE 400T gamma scintillation camera, with a 2-cm wide and 3.5-cm long region of interest centrally located over the thrombogenic vascular graft. Deposition of fibrin in the graft was measured at the study endpoint, after removal of the temporary grafts from the permanent shunt (34, 54). The 4 mm id graft segments were removed from the shunt at 60 min or 3 hours. The stenotic grafts were removed from the shunt either at 90 min or when graft occlusion was imminent, defined as a blood flow rate of ≤ 20 mL/min.
Platelet functionality testing
For platelet aggregation assays, baboon blood was collected into 3.2% sodium citrate anticoagulant (9:1, vol/vol). Platelet rich plasma (PRP) was prepared as described (55). The platelet count was adjusted to 150,000/μL and aggregation assays performed with a Chrono-Log aggregometer with ADP (Sigma) and collagen (Nycomed) as aggregation stimuli. Results were expressed as the concentration that induced half-maximal aggregation (AC50) (55). Other blood samples were collected into 3.8% citrate (9:1, vol/vol) and processed to generate platelet poor plasma, or clotted for serum, and frozen at -80°C. Samples were later assayed with a cross-reacting ELISA for TPO concentrations (Quantikine TPO, R&D Systems). The antihemostatic effects of platelet count reduction and high dose aspirin administration (32 mg/kg orally, 2 hours before graft deployment) were assessed using the template bleeding time (BT) test, where a uniform 5-mm long 1-mm deep incision is created on the volar surface of the forearm and time to bleeding cessation is recorded (54). This test, using the FDA approved Surgicutt® bleeding time device (ITC Medical)(56), is used clinically to screen for platelet defects.
Ex-vivo flow chamber studies
For flow chamber studies, human venous blood was drawn by venipuncture from 4 healthy volunteers into 3.8% sodium citrate (9:1, vol/vol) and acid/citrate/dextrose (8.5 mM sodium citrate, 7.1 mM citric acid, 11.1 mM glucose, final concentration) (57). The protocol was approved by the Institutional Review Board, Oregon Health & Science University. Washed platelets and red blood cells (RBCs) were prepared as described (58). Human plasma was collected and pooled from 3 healthy individuals, with blood drawn into 3.2% sodium citrate anticoagulant (9:1, vol/vol), and washed RBCs were combined with dilutions of washed platelets to yield a hematocrit of 40% and platelet count ranging from 50,000 to 400,000/μL. The reconstituted blood was co-perfused with a 20% volume of recalcification buffer (37.5 mM CaCl2, 18.8 mM MgCl2) into collagen coated capillary tubes (100 μg/mL HORM collagen) (Chrono-log Corporation) at a shear rate of 265 s-1 for 10 min. Images were obtained by differential interference contrast microscopy after perfusion with modified Tyrodes buffer (129 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2; pH 7.3) to wash out unbound blood components. To evaluate fibrin formation, capillaries were treated with lysis buffer (20 mM Tris, 300 mM NaCl, 2 mM EGTA, 2% NP-40, 2 mM PMSF) for 5 min, followed by treatment with plasmin (1 μM; Enzyme Research Laboratories) for 1 hour. Eluate from capillaries was collected, separated by 6% SDS-PAGE, and Western-immunoblotted for the 200 kD fibrin degradation product D-dimer with fibrinogen antiserum (MP Biomedicals). Protein concentrations were compared with a standard curve generated from serial dilutions of purified D-dimer (1 ng - 50 ng) (Cell Sciences).
Statistical methods and analysis
For statistical comparisons, the two-tailed Student's t-test was used. Values are given ± 1 SEM. Occlusion results were compared with the log-rank test. Correlations were calculated with Pearson's correlation in MATLAB (Mathworks). P < 0.05 was considered statistically significant.
Summary.
Inhibition of platelet production safely reduces thrombogenesis without bleeding side effects in baboons.
References and Notes
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ, Investigators, C. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, Investigators, T.-T. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 5.Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, Cohen EA, Bertrand M, Neumann FJ, Stone GW, DiBattiste PM, Demopoulos L, Trial T. I. D. T. a. R. G. S. E. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344:1888. doi: 10.1056/NEJM200106213442502. [DOI] [PubMed] [Google Scholar]
- 6.Giannini E, Borro P, Botta F, Fumagalli A, Malfatti F, Podesta E, Romagnoli P, Testa E, Chiarbonello B, Polegato S, Mamone M, Testa R. Serum thrombopoietin levels are linked to liver function in untreated patients with hepatitis C virus-related chronic hepatitis. J Hepatol. 2002;37:572. doi: 10.1016/s0168-8278(02)00274-x. [DOI] [PubMed] [Google Scholar]
- 7.Buckley MF, James JW, Brown DE, Whyte GS, Dean MG, Chesterman CN, Donald JA. A novel approach to the assessment of variations in the human platelet count. Thromb Haemost. 2000;83:480. [PubMed] [Google Scholar]
- 8.Ly HQ, Kirtane AJ, Murphy SA, Buros J, Cannon CP, Braunwald E, Gibson CM, Group, T. S. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials) Am J Cardiol. 2006;98:1. doi: 10.1016/j.amjcard.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84:613. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 10.Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schomig A, Kastrati A. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98:852. [PubMed] [Google Scholar]
- 11.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, Mehran R, Lansky AJ, Na Y, Stone GW. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, Braunwald E, Gibson CM, Thrombolysis in Myocardial Infarction Study, G. Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol. 2004;94:1406. doi: 10.1016/j.amjcard.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105. [PubMed] [Google Scholar]
- 14.George JN. Platelets. Lancet. 2000;355:1531. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 15.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 16.McMillan R. Therapy for adults with refractory chronic immune thrombocytopenic purpura. Ann Intern Med. 1997;126:307. doi: 10.7326/0003-4819-126-4-199702150-00007. [DOI] [PubMed] [Google Scholar]
- 17.George JN, el-Harake MA, Raskob GE. Chronic idiopathic thrombocytopenic purpura. N Engl J Med. 1994;331:1207. doi: 10.1056/NEJM199411033311807. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT, Johnston MF, Ramsey G, Sayers MH, Eisenstadt RS, Anderson KC, Rutman RC, Silberstein LE. Guidelines for transfusion support in patients undergoing coronary artery bypass grafting. Transfusion Practices Committee of the American Association of Blood Banks. Ann Thorac Surg. 1990;50:675. doi: 10.1016/0003-4975(90)90221-q. [DOI] [PubMed] [Google Scholar]
- 19.Spiess BD, Gillies BS, Chandler W, Verrier E. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth. 1995;9:168. doi: 10.1016/S1053-0770(05)80189-2. [DOI] [PubMed] [Google Scholar]
- 20.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, Barbui T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332:1132. doi: 10.1056/NEJM199504273321704. [DOI] [PubMed] [Google Scholar]
- 22.Moore S, Friedman RJ, Singal DP, Gauldie J, Blajchman MA, Roberts RS. Inhibition of injury induced thromboatherosclerotic lesions by anti-platelet serum in rabbits. Thromb Haemost. 1976;35:70. [PubMed] [Google Scholar]
- 23.Reyers I, Mussoni L, Donati MB, de Gaetano G. Severe thrombocytopenia delays but does not prevent the occlusion of an arterial prosthesis in rats. Thromb Haemost. 1981;46:558. [PubMed] [Google Scholar]
- 24.Lockyer S, Kambayashi J. Demonstration of flow and platelet dependency in a ferric chloride-induced model of thrombosis. J Cardiovasc Pharmacol. 1999;33:718. doi: 10.1097/00005344-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Herbert JM, Bernat A, Maffrand JP. Importance of platelets in experimental venous thrombosis in the rat. Blood. 1992;80:2281. [PubMed] [Google Scholar]
- 26.Merhi Y, Lam JY, Lacoste LL, Latour JG, Guidoin R, Waters D. Effects of thrombocytopenia and shear rate on neutrophil and platelet deposition on endothelial and medial arterial surfaces. Arterioscler Thromb. 1993;13:951. doi: 10.1161/01.atv.13.7.951. [DOI] [PubMed] [Google Scholar]
- 27.Basser RL, O'Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM, Cohen B, Begley CG. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599. doi: 10.1182/blood.v99.7.2599. [DOI] [PubMed] [Google Scholar]
- 28.Bromberg ME. Immune thrombocytopenic purpura--the changing therapeutic landscape. N Engl J Med. 2006;355:1643. doi: 10.1056/NEJMp068169. [DOI] [PubMed] [Google Scholar]
- 29.Shiozaki H, Miyawaki S, Kuwaki T, Hagiwara T, Kato T, Miyazaki H. Autoantibodies neutralizing thrombopoietin in a patient with amegakaryocytic thrombocytopenic purpura. Blood. 2000;95:2187. [PubMed] [Google Scholar]
- 30.Patel SR, Hartwig JH, Italiano JE., Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce KH, Jr., Potts BJ, Presta LG, Bald LN, Fendly BM, Wells JA. Mutational analysis of thrombopoietin for identification of receptor and neutralizing antibody sites. J Biol Chem. 1997;272:20595. doi: 10.1074/jbc.272.33.20595. [DOI] [PubMed] [Google Scholar]
- 33.Hanson SR, Harker LA, Bjornsson TD. Effects of platelet-modifying drugs on arterial thromboembolism in baboons. Aspirin potentiates the antithrombotic actions of dipyridamole and sulfinpyrazone by mechanism(s) independent of platelet cyclooxygenase inhibition. J Clin Invest. 1985;75:1591. doi: 10.1172/JCI111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson SR, Kotze HF, Savage B, Harker LA. Platelet interactions with Dacron vascular grafts. A model of acute thrombosis in baboons. Arteriosclerosis. 1985;5:595. doi: 10.1161/01.atv.5.6.595. [DOI] [PubMed] [Google Scholar]
- 36.Hanson SR, Harker LA. Studies of suloctidil in experimental thrombosis in baboons. Thromb Haemost. 1985;53:423. [PubMed] [Google Scholar]
- 37.Lumsden AB, Kelly AB, Schneider PA, Krupski WC, Dodson T, Hanson SR, Harker LA. Lasting safe interruption of endarterectomy thrombosis by transiently infused antithrombin peptide D-Phe-Pro-ArgCH2Cl in baboons. Blood. 1993;81:1762. [PubMed] [Google Scholar]
- 38.Wootton DM, Markou CP, Hanson SR, Ku DN. A mechanistic model of acute platelet accumulation in thrombogenic stenoses. Ann Biomed Eng. 2001;29:321. doi: 10.1114/1.1359449. [DOI] [PubMed] [Google Scholar]
- 39.Ramanathan J, Sibai BM, Vu T, Chauhan D. Correlation between bleeding times and platelet counts in women with preeclampsia undergoing cesarean section. Anesthesiology. 1989;71:188. doi: 10.1097/00000542-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Barbui T, Buelli M, Cortelazzo S, Viero P, De Gaetano G. Aspirin and risk of bleeding in patients with thrombocythemia. Am J Med. 1987;83:265. doi: 10.1016/0002-9343(87)90696-6. [DOI] [PubMed] [Google Scholar]
- 41.Hampton JW, Matthews C. Similarities between baboon and human blood clotting. J Appl Physiol. 1966;21:1713. doi: 10.1152/jappl.1966.21.6.1713. [DOI] [PubMed] [Google Scholar]
- 42.Todd ME, McDevitt E, Goldsmith EI. Blood-clotting mechanisms of nonhuman primates. Choice of the baboon model to simulate man. J Med Primatol. 1972;1:132. doi: 10.1159/000460376. [DOI] [PubMed] [Google Scholar]
- 43.Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. J Pediatr. 1987;110:457. doi: 10.1016/s0022-3476(87)80517-6. [DOI] [PubMed] [Google Scholar]
- 44.Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- 45.Mielke CH, Jr., Kaneshiro MM, Maher IA, Weiner JM, Rapaport SI. The standardized normal Ivy bleeding time and its prolongation by aspirin. Blood. 1969;34:204. [PubMed] [Google Scholar]
- 46.Samra SK, Harrison RL, Bee DE, Valero V. A study of aspirin induced changes in bleeding time, platelet aggregation, and Sonoclot coagulation analysis in humans. Ann Clin Lab Sci. 1991;21:315. [PubMed] [Google Scholar]
- 47.Gimple LW, Gold HK, Leinbach RC, Coller BS, Werner W, Yasuda T, Johns JA, Ziskind AA, Finkelstein D, Collen D. Correlation between template bleeding times and spontaneous bleeding during treatment of acute myocardial infarction with recombinant tissue-type plasminogen activator. Circulation. 1989;80:581. doi: 10.1161/01.cir.80.3.581. [DOI] [PubMed] [Google Scholar]
- 48.Gruber A, Marzec UM, Bush L, Di Cera E, Fernandez JA, Berny MA, Tucker EI, McCarty OJ, Griffin JH, Hanson SR. Relative antithrombotic and antihemostatic effects of protein C activator versus low-molecular-weight heparin in primates. Blood. 2007;109:3733. doi: 10.1182/blood-2006-07-035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:161. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 50.Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:287S. doi: 10.1378/chest.126.3_suppl.287S. [DOI] [PubMed] [Google Scholar]
- 51.Ceresa IF, Noris P, Ambaglio C, Pecci A, Balduini CL. Thrombopoietin is not uniquely responsible for thrombocytosis in inflammatory disorders. Platelets. 2007;18:579. doi: 10.1080/09537100701593601. [DOI] [PubMed] [Google Scholar]
- 52.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, van der Walt JD, Reilly JT, Grigg AP, Revell P, Woodcock BE, Green AR, United Kingdom Medical Research Council Primary Thrombocythemia, S. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 53.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC, Henzel WJ, Wong SC, Kuang WJ, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 54.Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest. 1993;92:2003. doi: 10.1172/JCI116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harker LA, Hunt P, Marzec UM, Kelly AB, Tomer A, Hanson SR, Stead RB. Regulation of platelet production and function by megakaryocyte growth and development factor in nonhuman primates. Blood. 1996;87:1833. [PubMed] [Google Scholar]
- 56.Schwartz L, Brister SJ, Bourassa MG, Peniston C, Buchanan MR. Interobserver Reproducibility and Biological Variability of the Surgicutt II Bleeding Time. J Thromb Thrombolysis. 1998;6:155. doi: 10.1023/A:1008861924107. [DOI] [PubMed] [Google Scholar]
- 57.White-Adams TC, Berny MA, Tucker EI, Gertz JM, Gailani D, Urbanus RT, de Groot PG, Gruber A, McCarty OJ. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2) Arterioscler Thromb Vasc Biol. 2009;29:1602. doi: 10.1161/ATVBAHA.109.187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]