Abstract
We previously reported that quinacrine inhibited the formation of an abnormal prion protein (PrPres), a key molecule in the pathogenesis of transmissible spongiform encephalopathy, or prion disease, in scrapie-infected neuroblastoma cells. To elucidate the structural aspects of its inhibiting action, various chemicals with a quinoline ring were screened in the present study. Assays of the scrapie-infected neuroblastoma cells revealed that chemicals with a side chain containing a quinuclidine ring at the 4 position of a quinoline ring (represented by quinine) inhibited the PrPres formation at a 50% inhibitory dose ranging from 10−1 to 101 μM. On the other hand, chemicals with a side chain at the 2 position of a quinoline ring (represented by 2,2′-biquinoline) more effectively inhibited the PrPres formation at a 50% inhibitory dose ranging from 10−3 to 10−1 μM. A metabolic labeling study revealed that the action of quinine or biquinoline was not due to any alteration in the biosynthesis or turnover of normal prion protein, whereas surface plasmon resonance analysis showed a strong binding affinity of biquinoline with a recombinant prion protein. In vivo studies revealed that 4-week intraventricular infusion of quinine or biquinoline was effective in prolonging the incubation period in experimental mouse models of intracerebral infection. The findings suggest that quinoline derivatives with a nitrogen-containing side chain have the potential of both inhibiting PrPres formation in vitro and prolonging the incubation period of infected animals. These chemicals are new candidates for therapeutic drugs for use in the treatment of transmissible spongiform encephalopathies.
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a group of fatal neurodegenerative disorders that include Creutzfeldt-Jakob disease and Gerstmann-Sträussler-Scheinker disease (GSS) in humans and scrapie, bovine spongiform encephalopathy, and chronic wasting disease in animals. These disorders are characterized by the accumulation of an abnormal isoform of prion protein (PrPres), which is high in beta-sheet content and resistant to digestion with proteases (15). Recent outbreaks in younger people of acquired forms of human TSEs, such as variant Creutzfeldt-Jakob disease (19) and iatrogenic Creutzfeldt-Jakob disease with cadaveric growth hormone or dura graft (4), are prompting the development of therapeutic interventions as well as early diagnostics.
One possible therapeutic strategy is to inhibit PrPres formation in the infected host. Doh-ura et al. first reported that cysteine protease inhibitors and lysomotropic agents inhibited PrPres formation in scrapie-infected neuroblastoma (ScNB) cells and that among them, quinacrine was one of the most potent inhibitors (8). Another research group has also reported that quinacrine and its related tricyclic compounds are effective in inhibiting PrPres formation (11). Quinacrine is a synthesized chemical which has a quinoline ring in its structure. It is used as a substitute for quinine in the treatment of malaria. Accordingly, in this study we chose to focus on the quinoline derivatives to examine the structure-activity relationship involved in inhibiting PrPres formation as well as in prolonging the incubation time of infected animals.
MATERIALS AND METHODS
Chemicals and ScNB cells.
Chemicals were purchased from Sigma, Maybridge (Cornwall, United Kingdom), Peakdale (Derbyshire, United Kingdom), Specs (Rijswijk, The Netherlands), and Bionet (Cornwall, United Kingdom) and were dissolved in 100% dimethyl sulfoxide (DMSO) or 96% ethanol just before use. ScNB cells (16) were grown in six-well culture plates in Opti-MEM (Invitrogen) supplemented with 10% fetal bovine serum. Chemicals at various concentrations were added to the medium when 1/20 of the confluent cells were passed. The final concentration of either DMSO or ethanol in the medium was less than 0.2%. The cultures were allowed to grow to confluence for 4 days.
Western blot analysis.
PrPres was analyzed as described previously (5) with slight modification. Briefly, the cells in confluency were rinsed with phosphate-buffered saline (PBS) and lysed with lysis buffer (0.5% sodium deoxycholate, 0.5% Nonidet P-40, PBS). After low-speed centrifugation, the supernatant was treated with 10 μg of proteinase K/ml for 30 min at 37°C. Digestion was stopped with 0.5 mM phenylmethylsulfonyl fluoride, and the supernatant was centrifuged at 100,000 × g for 30 min at 4°C. Pellets were resuspended in 30 μl of the sample buffer by sonication. After being boiled, the sample was separated by electrophoresis on a Tris-glycine-sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-PAGE) and then electroblotted onto a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with PrP-2B, an anti-PrP polyclonal antibody, against a mouse-hamster PrP fragment (amino acids 89 to 103) and then with an alkaline phosphatase-conjugated goat anti-rabbit antibody (Promega). Signals were visualized with CDP-Star detection reagent (Amersham) and were densitometrically analyzed. Either the concentration of a chemical giving 50% inhibition of PrPres formation relative to the control 50% inhibitory concentration (IC50) or the maximal concentration of a chemical that does not affect the rate of cell growth to confluence (TC) was estimated from more than three independent experiments.
Metabolic labeling study.
Metabolic labeling of prion protein was performed as described previously (5). Briefly, subconfluent ScNB cells in 25-cm2 flasks were rinsed three times with PBS and preincubated at 37°C in 1.5 ml of methionine-free minimal essential medium with 1% dialyzed fetal bovine serum and 1 μM quinine or 2,2′-biquinoline. After 60 min of preincubation, 125 μCi of 35S-labeled methionine (Amersham) was added to each flask and incubated for 60 min. Then 10 ml of chase medium with 1 μM quinine or biquinoline was added, and the incubation was continued for 18 min, 2 h, or 8 h. Cells were rinsed three times with PBS and lysed with lysis buffer. After low-speed centrifugation, an aliquot of the supernatant was electrophoresed for total protein analysis; the remainder was used for immunoprecipitation of total prion protein. For the detection of cell surface phosphatidylinositol-anchored prion protein, cells were incubated for 30 min in the chase medium with 1 μM quinine or biquinoline after pulse labeling, rinsed three times with PBS, and then incubated with 1.33 U of phosphatidylinositol-specific phospholipase C (PIPLC)/ml in PBS at 37°C for 60 min. The soup was used for immunoprecipitation of cell-surface prion protein. Immunoprecipitation was performed with a PrP-2B antibody after whole proteins in the soup were precipitated with methanol and resuspended in detergent-lipid-protein complex solution.
Surface plasmon resonance sensorgram study.
Interaction between prion protein and a chemical was analyzed using a BIAcore X systems. A recombinant murine prion protein fragment (amino acids 121 to 231) (PrP121-231) was immobilized on a sensor chip (CM5) according to the manufacturer's instructions. Each chemical was injected at a 100 μM concentration in running buffer (2.5% DMSO in PBS) for 1 min at a flow rate of 20 μl/min; then running buffer without a chemical was injected for 1 min at the same flow rate. Data were corrected by using a blank sensor chip as a control.
In vivo study.
In vivo evaluation of the effectiveness of a chemical at prolonging the incubation times in infected animals was performed by using a mouse model of Tg7 (14, 17) or Tg20 (10), both of which have substantially shorter incubation periods than wild mice. Briefly, a 20-μl aliquot of 1% 263K pathogen homogenate for Tg7 mice, or the same amount of aliquot of 1% Rocky Mountain Laboratory (RML) pathogen homogenate or Fukuoka-1 pathogen homogenate for Tg20 mice, was inoculated into the right parietal portion of the brain. A 4-week continuous intraventricular infusion of vehicle alone (25% DMSO) or of a chemical dissolved in 25% DMSO was initiated at day 10 or 35 in Tg7 mice or at day 14 or 49 in Tg20 mice by using an Alzet osmotic pump equipped with a brain infusion kit (Durect, Cupertino, Calif.). An intraventricular infusion cannula from the brain infusion kit was fitted into the left frontal portion of the brain.
The infusion initiation date was selected at an early stage of the infection (day 10 or 14), or at a late stage (day 35 or 49), when abnormal PrP deposition in the brain definitely appeared in the 263K-infected Tg7 or RML-infected Tg20 mice. However, day 49 postinoculation in the Fukuoka-1-infected Tg20 mice was not exactly at a late stage of the infection, and no information on when abnormal PrP deposition appeared in this model was available.
In some experiments, intraperitoneal administration of a chemical was provided by a single injection once a day for 5 days per week from day 10 or day 35 postintracerebral inoculation until death. The incubation period during which the animals were observed every day lasted from the time of intracerebral infection until the time of death. Five male mice (each weighing about 30 g) per group were used in the experiments. Animal handling and killing were in accordance with national prescribed guidelines, with ethical approval for the study granted by the Animal Experiment Committee of Kyushu University.
Mice which died within a few days due to operational procedures were excepted from the statistical analysis after pathological confirmation. Doses of less than 8 nmol of quinine/day were examined, because toxicity shortened life span at doses beyond 8 nmol/day. Biquinoline was examined at doses of less than 16 nmol/day, which provided no toxicity yet solubility in 25% DMSO.
Immunohistochemistry.
An indirect immunoperoxidase method was applied as described previously (9) with slight modification. Briefly, brains were obtained postmortem and fixed in 10% buffered formalin for several weeks. The tissue was immersed in 98% formic acid for 1 h to reduce infectivity and then embedded in paraffin. The samples were cut into 5-μm-thick sections, and then the sections were deparaffinized in xylene and hydrated using an ethanol gradient. The endogenous peroxidase activity was blocked with 0.3% H2O2 in absolute methanol for 30 min at room temperature. After being rinsed with tap water, the sections were treated with a hydrolytic autoclave (1 mM or 1.5 mM HCl, 121°C, 10 min) and washed in 50 mM Tris-HCl, pH 7.6, before being incubated with PrP-C polyclonal antibody (Immuno-Biological Laboratories, Gunma, Japan) (1:200) at 4°C overnight. The sections were then incubated with a horseradish peroxidase-conjugated secondary antibody (Vector Laboratories, Burlingame, Calif.) (1:200). The color reaction product was developed with 3,3′-diaminobenzidine tetrahydrochloride solution, and the sections were then counterstained with hematoxylin.
RESULTS
Screening of chemicals in vitro.
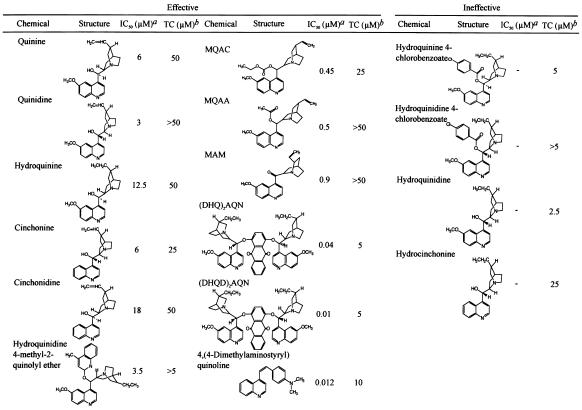
Clinically available drugs with a quinoline ring and their related chemicals were first screened for the inhibition of PrPres formation in ScNB cells. The antimalarial drug quinine and its related chemicals (such as quinidine, hydroquinine, cinchonine, cinchonidine, and hydroquinidine 4-methyl-2-quinolyl ether) were found to be effective (Table 1, left column). The IC50 doses of these chemicals ranged from 3 to 18 μM, and the effective dose range between the IC50 and the TC was relatively narrow. Hydroquinidine 4-methyl-2-quinolyl ether, which has two quinoline rings, was slightly more effective than the chemicals with only one quinoline ring. Quinine-related chemicals with a carbonyl base located between a quinoline ring and a quinuclidine ring, such as MQAC (cinchonan-9-ol, 6′-methoxy-ethylcarbonate), MQAA (cinchonan-9-ol, 6′-methoxy-acetate), and MAM [(6-ethynyl-1-azabicyclo[2.2.2.]oct-2yl) (6-methoxy-4-quinolinyl) methanone], were more effective, and their IC50 dose ranges were 0.45 to 0.9 μM (Table 1, middle column). Chemicals with either the motif of quinine or that of quinidine on each lateral side of anthraquinone, (DHQ)2AQN (hydroquinine anthraquinone-1,4-diyl diether) and (DHQD)2AQN (hydroquinidine anthraquinone-1,4-diyl diether), were much more effective than those with only one motif, and their IC50 doses were 0.04 and 0.01 μM, respectively. A chemical with a 4-dimethylaminostyryl moiety was also very effective; and its IC50 was 0.012 μM. Except for this chemical, all of the effective chemicals shared a common structure composed of a quinoline ring plus a relative large side chain containing a quinuclidine ring at the 4 position of the quinoline ring. The chemicals listed in the right column of Table 1 also had this common structure, but they showed toxicity at a lower dose and were not effective within a nontoxic dose range.
TABLE 1.
Structure-activity relationship of quinine analogues on PrPres inhibition
IC50, approximate concentration of a chemical giving 50% inhibition of PrPres formation relative to the control.
TC, approximate maximal concentration of a chemical that does not affect the rate of cell growth to confluence.
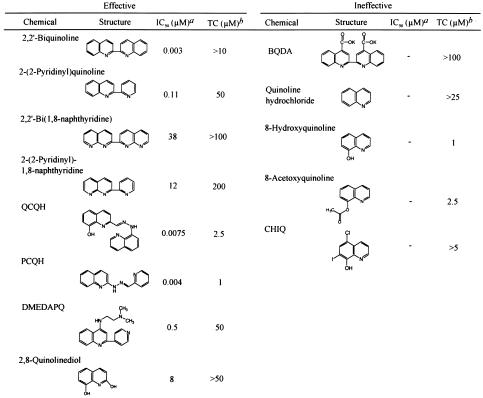
Other quinoline chemicals unrelated to quinine were also screened. Chemicals with a side chain at the 2 position of a quinoline ring, such as 2,2′-biquinoline, inhibited PrPres formation at 0.003 μM, the minimum IC50 dose (Fig. 1A), and this effectiveness was reduced by the replacement of the quinoline ring by a pyridine ring or a naphthyridine ring (Table 2, left column). The addition of a carboxyl moiety to both the 4 position and the 4′ position of the quinoline ring abolished the inhibiting activity of biquinoline (Table 2, right column, top). QCQH (8-hydroxy-8-quinolinylhydrazone-2-quinolinecarboxaldehyde) and PCQH (2-quinolinylhydrazone-2-pyridinecarboxaldehyde) were also very effective in inhibiting PrPres formation at an IC50 dose of 0.0075 and 0.004 μM, respectively. They shared a common structure with biquinoline respecting the arrangement of nitrogen atoms. DMEDAPQ (N,N-dimethyl-N′-[2-{4-pyridinyl}-4-quinolinyl]-1,2-ethanediamine) a chemical with a nitrogen-containing side chain at both the 2 position and the 4 position of a quinoline ring (thereby resembling quinine rather than biquinoline in terms of the arrangement of nitrogen atoms), was less effective than biquinoline, and its IC50 dose was 0.5 μM.
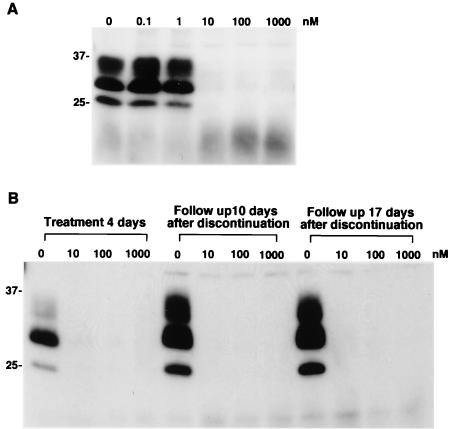
FIG. 1.
Inhibition of PrPres accumulation in ScNB cells grown with 2,2′-biquinoline (A) and lack of restoration of PrPres formation in ScNB cells treated once with biquinoline (B). (A) Biquinoline was added at designated concentrations to the medium when the cells were passed, and the culture was allowed to grow to confluence. Then, PrPres in the cells was analyzed by immunoblotting. (B) ScNB cells were treated with 10, 100, or 1,000 nM biquinoline for 4 days. The medium was replaced by fresh medium, and the cells were left without treatment for an additional 10 or 17 days. Then PrPres levels were assayed. Molecular size markers (in kilodaltons) are indicated.
TABLE 2.
Structure-activity relationship of biquinoline analogues on PrPres inhibition
IC50, approximate concentration of a chemical giving 50% inhibition of PrPres formation relative to the control.
TC, approximate maximal concentration of a chemical that does not affect the rate of cell growth to confluence.
Chemicals containing a quinoline ring without a large side chain were also examined. They included quinoline hydrochloride, 8-hydroxyquinoline, 2,8-quinolinediol, 8-acetoxyquinoline, and CHIQ (5-chloro-7-iodo-8-quinolinol). All of them, with the exception of 2,8-quinolinediol, were ineffective at inhibiting PrPres within a nontoxic dose range (Table 2, right column). Quinolinediol showed an IC50 dose of 8 μM, which was much higher than those of other chemicals with a side chain at the 2 position of a quinoline ring (Table 2, left column, bottom).
Mechanism of inhibition of PrPres formation.
Because quinine and biquinoline represented the effective chemicals found here, we focused on these chemicals and studied the mechanism behind their action. After ScNB cells had been treated with different concentrations of quinine or biquinoline for 4 days and then left without treatment for an additional 10 or 17 days, PrPres signals did not reappear even 17 days after discontinuation of the chemical treatment (Fig. 1B [for biquinoline] and data not shown [for quinine]). Thus, treatment with the chemicals permanently cured the cells of the accumulation of PrPres.
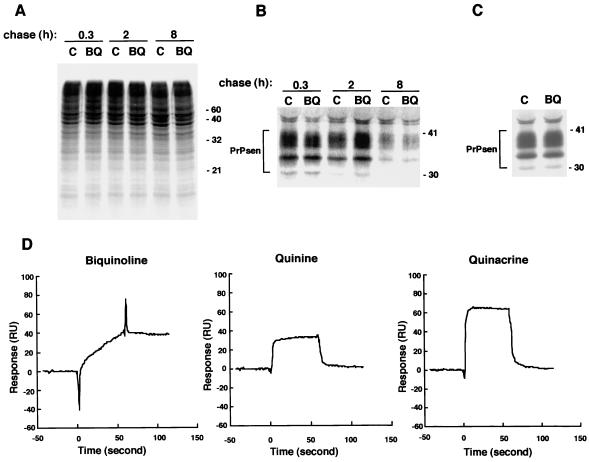
Because phospholipase-sensitive cell surface PrP (PrPsen) is the precursor of PrPres, it is possible that the inhibition of PrPres accumulation by these chemicals was due to an indirect effect on PrPsen metabolism or turnover. However, biquinoline showed no effects on the metabolic labeling of cellular proteins or on the biosynthesis and turnover of PrPsen (Fig. 2A, B, and C).
FIG. 2.
Lack of effect of the presence of biquinoline on the metabolic labeling of total protein (A), total PrPsen (B), and PIPLC-sensitive, cell surface PrPsen (C). (D) Direct interaction of biquinoline with recombinant PrP121-231 analyzed using a surface plasmon resonance sensorgram. (A) Control ScNB cells (lanes C) and biquinoline-treated cells (lanes BQ) were pulse labeled and then incubated in chase medium for the indicated chase time. The total lysate proteins were methanol precipitated from the detergent lysates of the cells and analyzed by SDS-PAGE. Equal flask equivalents were loaded onto all lanes in each panel. Molecular size markers (in kilodaltons) are indicated. (B) PrPsen was isolated from the total lysate proteins by immunoprecipitation and analyzed by SDS-PAGE. (C) PrPsen was immunoprecipitated from the cell soup treated with PIPLC. Biquinoline at 1 μM was included in all media, starting with the preincubation, except in the case of the control cells. (D) Interaction between a PrP121-231 fragment and a chemical was analyzed using a BIAcore system. A recombinant murine PrP121-231 fragment was immobilized on a CM5 sensor chip; biquinoline, quinine, or quinacrine (at 100 μM in buffer solution) was injected for 1 min at a flow rate of 20 μl/min for the association, and then the buffer solution without a chemical was injected at the same flow rate for the dissociation.
Surface plasmon resonance analysis showed that the interaction of biquinoline with recombinant PrP121-231 occurred very slowly and failed to reach saturation even after 1 min. During the dissociation phase, furthermore, complete dissociation did not occur (Fig. 2D). On the other hand, the interaction of quinine or quinacrine occurred very quickly, reaching saturation within several seconds, and dissociation was completely over within seconds.
From observations of the structure of the effective chemicals, it was predicted that they might exert their inhibiting action through some mechanism which involved chelating metals. Thus, quinine and biquinoline were preincubated (before being added to the ScNB culture medium) with an equivalent dose of, a 10-times-higher dose of, or a 100-times-higher dose of various metal ions, including copper, zinc, manganese, iron, cobalt, and aluminum ions. The results showed no change in the inhibiting activities of the chemicals (data not shown).
In vivo study.
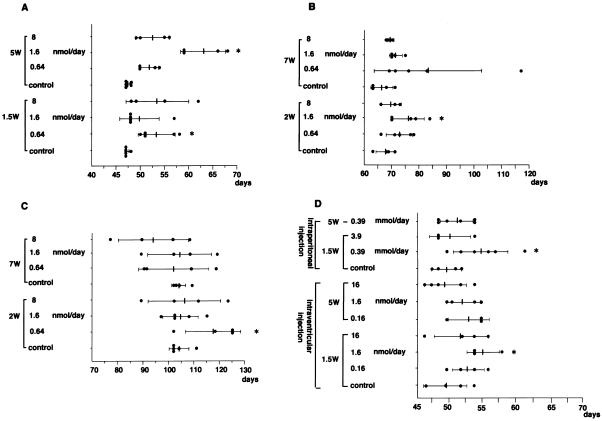
To examine whether these chemicals could be effective in improving the prognosis in vivo, quinine or biquinoline was continuously administered intraventricularly in animal models which had been intracerebrally infected with three different TSE pathogen strains, comprising 263K scrapie agent, RML scrapie agent, and Fukuoka-1 GSS agent. Quinine administration from an early stage of infection prolonged the incubation period by 13.6% (days 47 to 53.4) at 0.64 nmol/day in 263K-infected mice (Fig. 3A), by 10.8% (days 68.6 to 76) at 1.6 nmol/day in RML-infected mice (Fig. 3B), and by 12.8% (days 104.2 to 117.5) at 0.64 nmol/day in Fukuoka-1-infected mice (Fig. 3C). The effect of quinine administration from a late stage of infection was clearly demonstrated in 263K-infected mice, resulting in 36% (days 47 to 63) prolongation of the incubation period at 1.6 nmol/day (Fig. 3A), with some of the RML-infected mice displaying a tendency to survive much longer than the control at 0.64 nmol/day (Fig. 3B). On the other hand, the effect of biquinoline administration was examined only in 263K-infected mice; it demonstrated 10.8% (days 49 to 54.3) prolongation of the incubation period in the group receiving 1.6 nmol/day at an early stage of infection, but no significant effects were observed in the groups which received it at a late stage (Fig. 3D). Intraperitoneal administration of biquinoline was also performed in 263K-infected mice, and this resulted in 7.7% (days 49 to 52.8) prolongation of the incubation period in the group receiving 0.39 mmol/day from an early stage of infection.
FIG. 3.
Prolongation of incubation times in intracerebrally TSE-infected mice treated with quinine or biquinoline. (A) Tg7 mice infected with 263K agent strain and intraventricularly treated with quinine; (B) Tg20 mice infected with RML agent strain and intraventricularly treated with quinine; (C) Tg20 mice infected with Fukuoka-1 agent strain and intraventricularly treated with quinine; (D) Tg7 mice infected with 263K agent strain and intraperitoneally or intraventricularly treated with biquinoline. A 4-week continuous intraventricular infusion of a chemical was initiated by using an osmotic pump at day 10 (1.5W) or day 35 (5W) post-intracerebral inoculation in Tg7 mice or at day 14 (2W) or day 49 (7W) in Tg20 mice. For intraperitoneal treatment, injection of a chemical in Tg7 mice was performed intraperitoneally once a day for 5 days per week from day 10 (1.5W) or day 35 (5W) until the death of the mouse. Each closed circle represents the incubation time of an individual mouse. Each solid line and bar represent the average and standard deviation of the incubation times of each group. The star indicates groups with results with P < 0.05 compared to the results seen with the vehicle control. Each of the experiments was performed independently using different lots of the pathogen homogenate; thus, there was some variation in the data shown in panels A and D even for the same vehicle control.
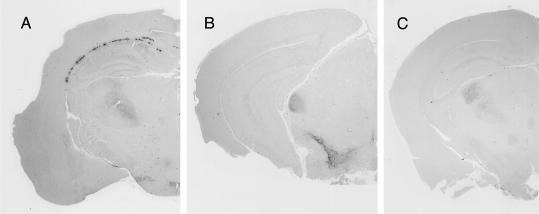
Postmortem histopathological examination of the brains treated with quinine or biquinoline was performed to see whether there was any modification in abnormal PrP deposition patterns following treatment. Those mice with prolonged incubation periods had a tendency to show less PrP deposition in the white matter between the cerebral cortex and the hippocampus of the brain hemisphere implanted with an intraventricular cannula, although they showed no apparent alteration in PrP deposition patterns in the bilateral thalamus or hypothalamus (Fig. 4).
FIG. 4.
Effects of intraventricular treatment with quinine or biquinoline on abnormal PrP deposition in the brain of intracerebrally 263K-infected Tg7 mice. The results for brain treated from day 10 postinfection for 4 weeks with vehicle (25% DMSO) alone (A), 0.64 nmol of quinine/day (B), or 1.6 nmol of biquinoline/day (C) are shown. Immunohistochemistry for abnormal PrP deposition was performed in the brains obtained postmortem from the longest-surviving members in each group, and representative examples of the brain hemisphere at the chemical injection side are shown.
DISCUSSION
In the studies reported here, we were able to identify quinoline derivatives that inhibited PrPres accumulation in ScNB cells. The commonly shared structure in these chemicals was a quinoline ring bound at its 2 or 4 position with a side chain containing a nitrogen atom, which was located at a particular distance from a nitrogen atom in the ring. Chemicals with a side chain at the 2 position of a quinoline ring were more effective than those with a side chain at the 4 position. Replacement of a quinoline ring with a pyridine ring or a naphthyridine ring resulted in a weaker inhibiting activity, while modification of biquinoline by a moiety that caused less flexibility in the hinge portion between the quinoline rings completely suppressed the inhibiting activity. These findings suggest that a certain proper alignment of two nitrogens, one in a quinoline ring and the other in a side chain, might be important with regard to inhibiting activity.
As for the inhibiting mechanism of these chemicals, the representative chemicals, quinine and biquinoline, demonstrated no alteration either in the protein biosynthesis in general or in the metabolic labeling and turnover of PrPsen in particular. However, biquinoline showed a very strong binding affinity with recombinant PrP121-231 in the Biacore study. Thus, some of the chemicals, including biquinoline, may inhibit the conversion of PrPsen to PrPres through direct interaction with PrPsen molecules. Since biquinoline (IC50 dose, 0.003 μM) was much more effective than quinine (3 μM) or quinacrine (at a concentration of 0.4 μM [8] or 0.3 μM [11]) in ScNB cells, the binding affinity of the PrP fragment (which was much stronger with biquinoline than with quinine or quinacrine) would appear to be clearly correlated with the inhibiting activity of PrPres formation in vitro. The potential binding site(s) of these chemicals in PrPsen molecules remains to be determined.
On the other hand, the involvement of chelating metal(s) in their inhibiting activity (as determined on the basis of the structure of the chemicals which were found to be effective in this study) was predicted. PrPsen is known to bind copper at its N-terminal octameric repeat region (3, 13, 18), and it is suggested that interaction between PrPres and copper stabilizes PrPres conformation (12). Manganese also binds PrP molecules instead of copper and increases proteinase K resistance and beta-sheet content (2). However, our observation suggests that the chelating mechanism seems unlikely to be involved in the inhibiting action of the chemicals found here.
Among the chemicals tested here, CHIQ is an antibiotic (called clioquinol) and a Cu/Zn-selective chelator known to be effective in decreasing beta-amyloid deposits in Alzheimer's disease (6). However, in this study, CHIQ and its related compounds, quinoline hydrochloride, 8-hydroxyquinoline, and 8-acetoxyquinoline, did not inhibit PrPres formation in ScNB cells. These findings also suggest that chelating drugs which are effective in inhibiting beta-amyloid formation are not necessarily effective at inhibiting PrPres formation.
The in vivo study revealed that the chemicals with a quinoline ring were effective not only in inhibiting PrPres formation in vitro but also in prolonging incubation times of intracerebrally infected animals. The greatest effectiveness was obtained by intraventricular administration of quinine at 1.6 nmol/day, which prolonged the incubation time by 36% in 263K-infected mice (compared to the results seen with the control) when initiated at a late stage of infection. Quinine was also effective in prolonging incubation times of the mice inoculated with different pathogen strains such as RML scrapie agent and Fukuoka-1 GSS agent. These findings indicate that application of quinine, an antimalarial drug, to humans infected with other TSE agents could be judicious.
Recently two research groups have reported that quinacrine is not effective in prolonging incubation times of intracerebrally infected TSE animals (1, 7). Our findings regarding quinine, which is a quinacrine-related chemical, appear to be inconsistent with their findings about quinacrine. However, differences in the structures of the chemicals and in the administration routes, doses, and durations as well as experimental models might have caused this gap but it remains to be elucidated.
Biquinoline was 1,000 times more effective than quinine in inhibiting PrPres formation in vitro with respect to the IC50 value, but when initiated from an early stage with intraventricular injections of 1.6 nmol/day or intraperitoneal injections of 0.39 mmol/day, its effectiveness in prolonging incubation times in vivo was clear, albeit marginal. The stability of chemicals and accessibility to targets in vivo might be different between these chemicals, and the reason for the gap between inhibiting activity in vitro and therapeutic activity in vivo remains to be found.
In investigations of the immunohistochemistry of the postmortem materials, abnormal PrP deposition in the white matter adjacent to the ventricle (where a chemical was injected continuously) was less evident in the mice treated with quinine or biquinoline from a early stage than in the control, although abnormal PrP deposition in the thalamus and hypothalamus was demonstrated in a fashion similar to that seen in the control. This would seem to imply that following treatment with a chemical, prolongation of incubation times in mice treated with the chemical might be associated with a reduction in abnormal PrP deposition in the brain.
In conclusion, we have demonstrated that quinoline derivatives with a relatively large side chain with a nitrogen are able to inhibit PrPres accumulation in ScNB cells and can prolong the incubation periods of infected mice. The inhibition was not caused by interference in the biosynthesis or turnover of PrPsen or by the chelation of metals. Some of the chemicals, including quinine, are already in clinical use and are known to pass the blood-brain barrier. Thus, these drugs might be immediately available for clinical trials in investigations of the treatment of human TSEs.
Acknowledgments
This study was supported by grants to K.D. from the Ministry of Health, Labor and Welfare (H13-kokoro-025) and from the Ministry of Education, Culture, Sports, Science and Technology (13557118, 14021085), Japan.
We thank B. Chesebro and R. Race in the Rocky Mountain Laboratories, NIAID for providing Tg7 mice, C. Weissmann in the Imperial College School of Medicine at St. Mary's, United Kingdom for Tg20 mice, and S. Katamine and S. Sakaguchi in Nagasaki University, Nagasaki, Japan, for PrP121-231. We also thank N. Suzuki in Daiichi Pharmaceuticals, Tokyo, Japan, for screening the chemical database.
REFERENCES
- 1.Barret, A., F. Tagliavini, G. Forloni, C. Bate, M. Salmona, L. Colombo, A. De Luigi, L. Limido, S. Suardi, G. Rossi, F. Auvre, K. T. Adjou, N. Sales, A. Williams, C. Lasmezas, and J. P. Deslys. 2003. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77:8462-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, D. R., F. Hafiz, L. L. Glasssmith, B. S. Wong, I. M. Jones, C. Clive, and S. J. Haswell. 2000. Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J. 19:1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, D. R., K. Qin, J. W. Herms, A. Madlung, J. Manson, R. Strome, P. E. Fraser, T. Kruck, A. von Bohlen, W. Schulz-Schaeffer, A. Giese, D. Westaway, and H. Kretzschmar. 1997. The cellular prion protein binds copper in vivo. Nature 390:684-687. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P., M. Preece, J. P. Brandel, T. Sato, L. McShane, I. Zerr, A. Fletcher, R. G. Will, M. Pocchiari, N. R. Cashman, J. H. d'Aignaux, L. Cervenakova, J. Fradkin, L. B. Schonberger, and S. J. Collins. 2000. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 55:1075-1081. [DOI] [PubMed] [Google Scholar]
- 5.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherny, R. A., C. S. Atwood, M. E. Xilinas, D. N. Gray, W. D. Jones, C. A. McLean, K. J. Barnham, I. Volitakis, F. W. Fraser, Y. Kim, X. Huang, L. E. Goldstein, R. D. Moir, J. T. Lim, K. Beyreuther, H. Zheng, R. E. Tanzi, C. L. Masters, and A. I. Bush. 2001. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30:665-676. [DOI] [PubMed] [Google Scholar]
- 7.Collins, S. J., V. Lewis, M. Brazier, A. F. Hill, A. Fletcher, and C. L. Masters. 2002. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann. Neurol. 52:503-506. [DOI] [PubMed] [Google Scholar]
- 8.Doh-ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doh-ura, K., E. Mekada, K. Ogomori, and T. Iwaki. 2000. Enhanced CD9 expression in the mouse and human brains infected with transmissible spongiform encephalopathies. J. Neuropathol. Exp. Neurol. 59:774-785. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 11.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie, D., J. Bartz, J. Mirwald, D. Olander, R. Marsh, and J. Aiken. 1998. Reversibility of scrapie inactivation is enhanced by copper. J. Biol. Chem. 273:25545-25547. [DOI] [PubMed] [Google Scholar]
- 13.Miura, T., A. Hori-i, H. Mototani, and H. Takeuchi. 1999. Raman spectroscopic study on the copper(II) binding mode of prion octapeptide and its pH dependence. Biochemistry 38:11560-11569. [DOI] [PubMed] [Google Scholar]
- 14.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 15.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 16.Race, R. E., B. Caughey, K. Graham, D. Ernst, and B. Chesebro. 1988. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J. Virol. 62:2845-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Race, R. E., S. A. Priola, R. A. Bessen, D. Ernst, J. Dockter, G. F. Rall, L. Mucke, B. Chesebro, and M. B. Oldstone. 1995. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron 15:1183-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viles, J. H., F. E. Cohen, S. B. Prusiner, D. B. Goodin, P. E. Wright, and H. J. Dyson. 1999. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc. Natl. Acad. Sci. USA 96:2042-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]