Abstract
Investigation of abnormal sexual development in companion animals can allow for the elimination of inherited disorders from breeding populations while contributing to the understanding of the complex process of mammalian sexual development and differentiation. A 1-year-old mixed-breed cat, presented for neutering, was tentatively diagnosed as a male with bilateral cryptorchidism. During surgery, the surgeon identified gonads in an ovarian position and a complete bicornuate uterus. Both testicular and ovarian architecture in the gonads and Mullerian and Wolffian duct derivatives were identified histologically. The karyotype was that of a normal male (38,XY), and no causative mutation was identified in the feline SRY coding sequence amplified from genomic DNA. All features of the case were compatible with a diagnosis of SRY-positive 38,XY sex reversal, true hermaphrodite phenotype. To the authors’ knowledge, this is the first report of this disorder in a domestic cat.
Keywords: feline SRY, ovotestes, XY disorder of sexual development, XY sex reversal, XY true hermaphrodite
In mammals, sexual development is controlled at three sequential steps: the establishment of chromosomal sex, the determination of gonadal sex, and the development of phenotypic sex (reviewed in Meyers-Wallen10). Normal XX and XY embryos develop similarly until the gonad differentiates as testis or ovary. If the gonad is removed at this stage, both XY and XX embryos develop as phenotypic females, indicating that a functional testis is critical to male phenotypic development. The Y chromosome carries the sex-determining region Y gene (SRY), which promotes the testicular pathway in the bipotential gonad. Both testicular and ovarian pathways are thought to be active and compete in the bipotential gonad.4 Although SRY and SOX9 promote testicular induction (reviewed in Sekido and Lovell-Badge14), they suppress beta catenin, a major effector of the ovarian pathway.1 Similarly, RSPO1 regulates the WNT pathway and beta catenin production, which promotes ovarian induction and competes with SOX9 (reviewed in Tevosian and Manuylov15 and Cool and Capel4). In the presence of SRY, SOX9 expression is markedly increased, promoting the testicular pathway and blocking the ovarian pathway. In the absence of SRY and elevated SOX9 expression, beta catenin predominates, promoting the ovarian pathway and blocking the testicular pathway.
Testicular secretions directly or indirectly masculinize the internal ducts and external genitalia. These include Mullerian inhibiting substance (MIS, also known as anti-Mullerian hormone), testosterone, and insulin-like peptide 3 (INSL3). Although Mullerian (paramesonephric) ducts are present in both male and female embryos at the sexually indifferent stage, they regress soon after testicular differentiation under the influence of MIS but persist if MIS is absent during the critical period for Mullerian duct regression. The Wolffian (mesonephric) ducts are stimulated by testosterone to form the epididymis and deferent ducts, but they regress in the absence of testosterone. In precursors of the external genitalia, the enzyme 5 alpha reductase converts testosterone to dihydrotestosterone (DHT). In the presence of DHT, the urogenital sinus, urogenital folds, and genital tubercle form the prostate and male urethra, the scrotum, and the prepuce and penis, respectively; in the absence of DHT, these structures form the caudal vagina, vulva, and the clitoris, respectively. Testicular descent is a complex process that is poorly understood and likely to involve several genes, such as those controlling testosterone and INSL3 secretion in the testis, the androgen receptor, the INSL3 receptor, and other incompletely understood factors in the target organs.
The study of inherited disorders of sexual development in humans and other mammals has increased our understanding of the genetic control of the complex process of sex determination and differentiation. These disorders are of clinical interest as a cause of infertility, but a definitive diagnosis can be important in preventing adverse health outcomes associated with some of these disorders and in eliminating inherited disorders from breeding populations. Diagnosis is based on identification of chromosomal sex, gonadal and reproductive tract histopathology, and description of the internal and external genital morphology (reviewed in Meyers-Wallen10). Inherited disorders of sexual development in cats have rarely been reported.12 Most reports describe 39,XXY or 38,XX/38,XY chimeras that were investigated because the cats appeared to be males with a tortoiseshell or calico coat color.7,8,11 This report describes a case of SRY-positive 38,XY sex reversal with a true hermaphrodite phenotype in a domestic cat, which to our knowledge, is the first such report in this species.
Case History
Upon physical examination of a 1-year-old mixed-breed male cat, presented for neutering, a normal penis and scrotum were identified, but testes were not palpable in the scrotum or inguinal region (Fig. 1). The cat was born and raised on a dairy farm with 5 littermates: 1 male that died as a young kitten and 4 females with apparently normal external genitalia. The parents and siblings of this cat were either already neutered or unavailable for study. Because resting serum testosterone concentration measured by radioimmunoassay (1.06 ng/ml) was within normal range (1–7 ng/ml) for an intact male, the tentative diagnosis was bilateral cryptorchidism.
Figure 1.
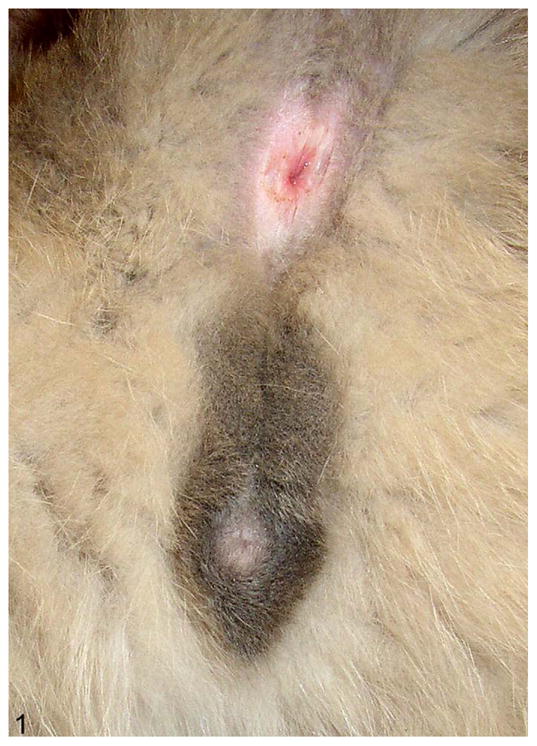
External genitalia; cat. The prepuce and scrotum are located in a normal male position, but the testes are not present in the scrotum.
Laparotomy for orchiectomy was performed. Both gonads resembled testes externally but were found in an ovarian position, caudal to the kidneys, and were attached to the proximal ends of a typical feline bicornuate uterus. The uterus contained a moderate amount of mucus. The gonads and uterus were surgically excised, fixed in 10% buffered formalin, and submitted in their entirety for histologic examination. The cat remained healthy during the 1-year postoperative follow-up period.
Peripheral blood was submitted for karyotypic analysis and sequence analysis of SRY. Chromosome preparations were generated from peripheral blood lymphocyte cultures according to standard techniques.6 The protocol was modified for cat lymphocytes as follows: cell culture medium was RPMI 1640 supplemented with 15% fetal bovine serum, 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA), 0.2% primocin (Invitrogen), 8 U/ml sodium heparin (Becton Dickinson, Franklin Lakes, NJ), and 20 μg/ml Concanavalin A (Sigma, St Louis, MO); the cell culture was performed for 96 hours. Air-dried metaphase spreads were stained by GTG banding following the protocol of Seabright13 using Giemsa stain (Sigma) and analyzed by bright-field microscopy. The entire feline SRY coding region was amplified by polymerase chain reaction (PCR) from genomic DNA extracted from cultured peripheral blood lymphocytes of the affected cat and from peripheral blood of a normal male control cat. To amplify the SRY HMG box region, primers (SRYA1 and SRYA2) and reaction conditions were used as described in Ciani et al.3 The remainder was amplified with PCR primers flanking the coding region of feline SRY, designed in Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). These reactions (25 μl) contained 1.25mM dNTPs, 2.0mM MgCl2, 1× PCR buffer II (Applied Biosystems, Foster City, CA), 0.375 U of AmpliTaq polymerase (Applied Biosystems), 25 ng of genomic DNA, and 1.0 uM of each primer (forward, 5′-ACAACCTTCAACGGTTAGATAA-TTC-3′; reverse, 5′-CCAGGCTGGATCAGGTAGAC-3′). The thermal reaction profile was 3 minutes of incubation at 94°C, followed by 40 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 20 seconds, and extension at 72°C for 45 seconds, followed by a final extension step of 10 minutes at 72°C. All PCR products were isolated and sequenced by automated dideoxy chain termination sequencing as previously described.2 Publicly available feline SRY (NM_001009240, National Center for Biotechnology Information) was aligned to the sequences generated for the affected and normal male control cats, using multiple alignment software (http://www.ebi.ac.uk/Tools/clustalw2).
Results
Multiple sections of both gonads, tissue adjacent to the gonads, and the uterus and cervix were processed routinely for histopathology. Findings were similar in both gonads, which were identified as ovotestes predominated by the testicular portion in the medulla, with a smaller ovarian portion forming a thin cortical rim (Figs. 2–5). The majority of each gonad consisted of seminiferous tubules containing few germ cells, intervening interstitial cells (Fig. 4, 5), and a thin cortical rim of poorly differentiated ovarian tissue with rare primordial follicles. Oocytes, which were rarely observed, were frequently singular (Fig. 3). A few oocytes were partially mineralized.
Figure 2.
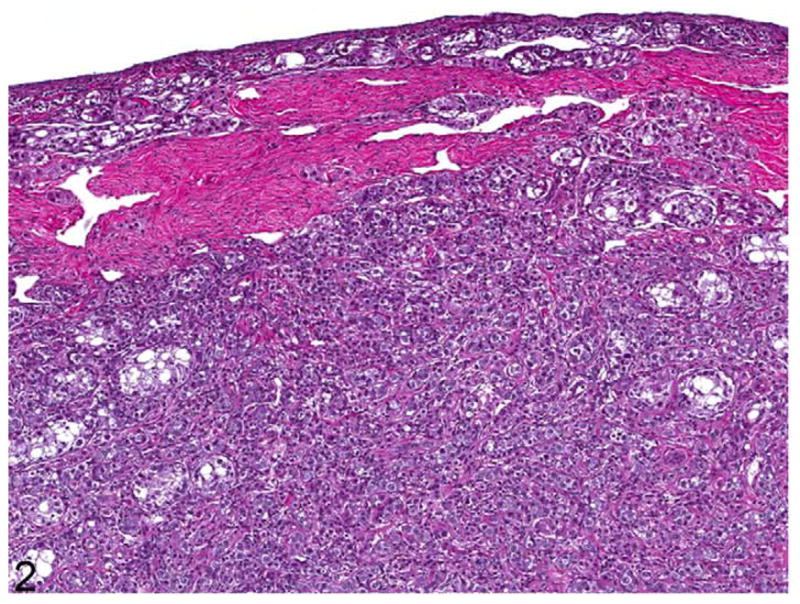
Ovotestis; cat. Central medullary testicular differentiation consists of hypoplastic seminiferous tubules lined by Sertoli cells, interstitial cells, and small irregular sex cords. A band of connective tissue separates the testicular tissue from a thin rim of cortical ovarian tissue. HE.
Figure 5.
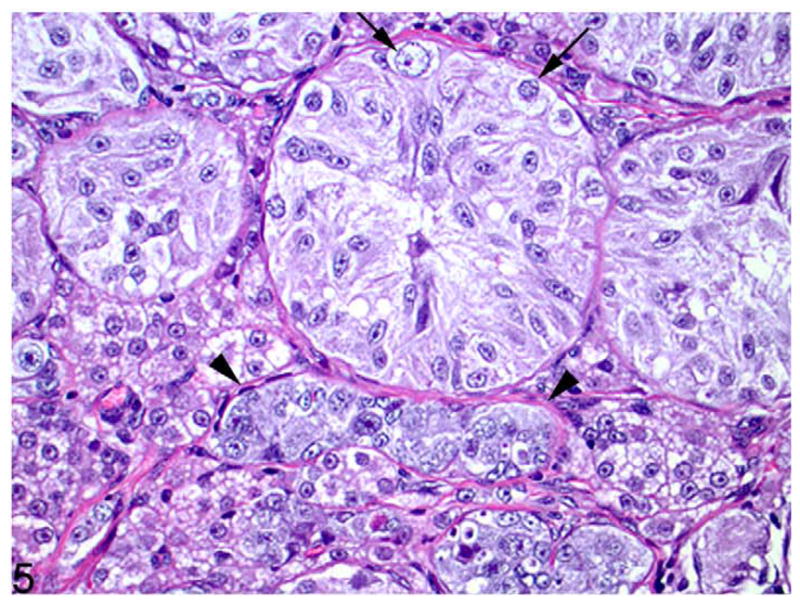
Ovotestis; cat. Poorly differentiated seminiferous tubules vary greatly in diameter. Spermatogonia are present along the basement membrane in some tubules (arrows and arrowheads). Clusters of vacuolated interstitial cells are in the interstitium near each end of the tubule with arrowheads. HE.
Figure 4.
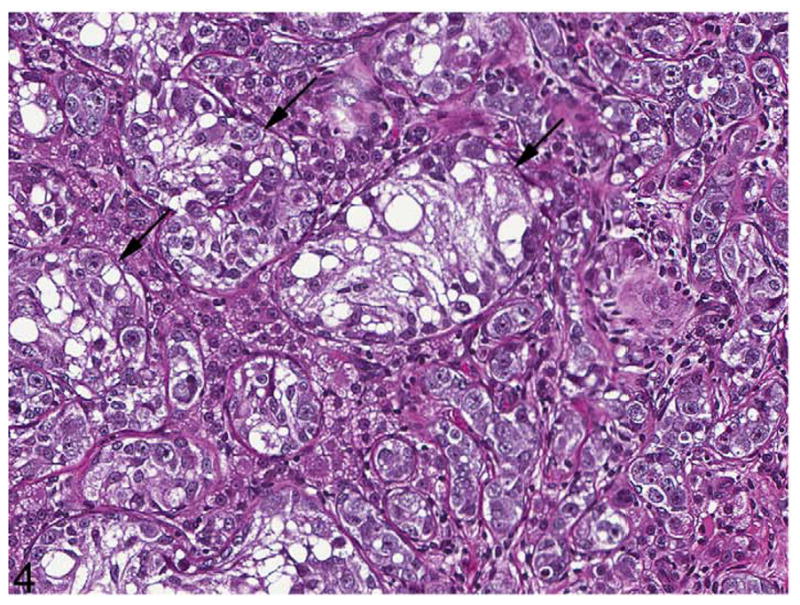
Ovotestis; cat. The medulla contains enlarged poorly differentiated seminiferous tubules lined by Sertoli cells (arrows). Tubules of smaller diameter are also present. HE.
Figure 3.
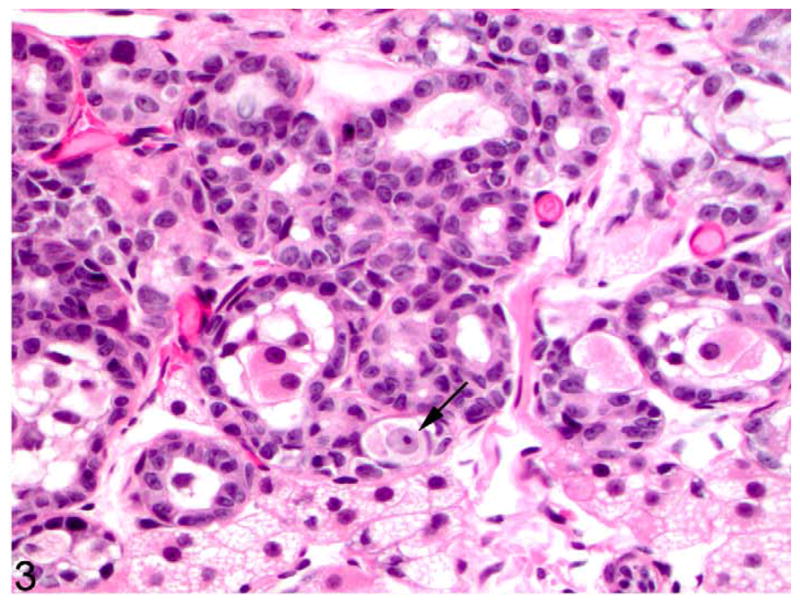
Ovotestis; cat. The cortex contains an oocyte (arrow) and adjacent poorly differentiated sex cords. HE.
Uterine tubes, along with fimbria and epididymides, were identified adjacent to each gonad (Fig. 6). Within the wall of the cranial aspect of the uterine horns, deferent ducts were identified as coiled tubular structures lined by epithelium (Fig. 7). More caudal sections of the uterine horns did not contain deferent duct remnants.
Figure 6.
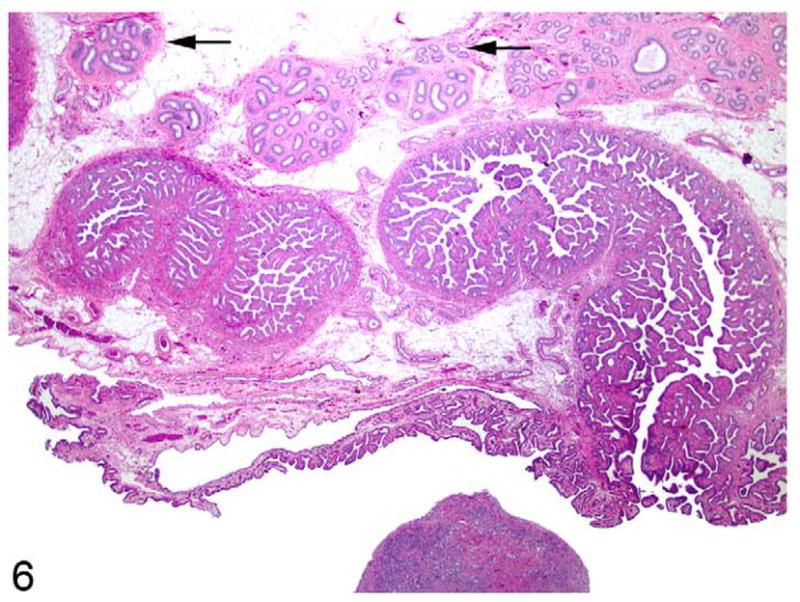
Mesosalpinx near ovotestis; cat. Cross sections of hypoplastic epididymal tubules (arrows) run parallel to longitudinal and oblique profiles of a uterine tube (center). Fimbriae of the uterine tube extend along its lower surface, adjacent to an ovotestis at bottom center. HE.
Figure 7.
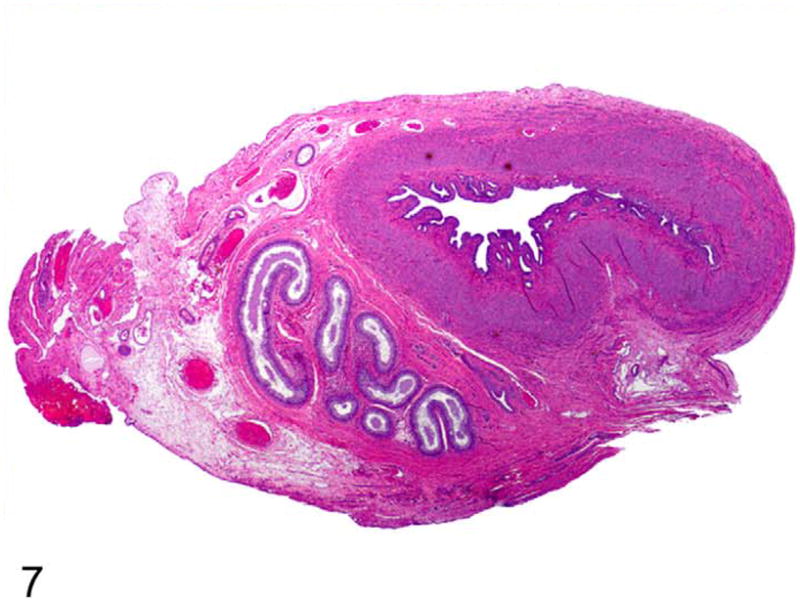
Uterine horn; cat. Cross section of the cranial portion of one uterine horn (paramesonephric duct derivative) contains cross sections of deferent ducts (mesonephric duct derivative) within its mesometrium. HE.
Chromosome number was determined for 40 cells; all cells had 38 chromosomes. Fifteen cells were analyzed by GTG banding. All karyotyped cells had a normal male domestic cat complement of 38,XY (not shown). A single-sequence polymorphism was found at reference position No. 389 (base pairs) in both the control male cat and the affected cat (Table 1). This variant presents in the heterozygous state (G/C) and occurs at the only coding position in SRY where wild cats (Felis silvestris [DQ095187], Felis margarita [DQ095169], and Felis libyca [DQ095168]) differ from domestic cats.
Table 1.
Sequencing of the SRY Coding Region From the Feline Genome (NM_001009240), the Affected 38,XY Hermaphrodite Cat, and a Control Male Cata
| Name | Nucleotide Sequence | Position |
|---|---|---|
| NM_001009240 | ATGTTGAGAGTATTGAGCAGCGATGAGCA | 29 |
| Affected cat | ACCCCCCTTTTTTAACGCTGCAATTTTATGCTTCTGGTATGTTGAGAGTATTGAGCAGCGATGAGCA | 67 |
| Control male | ACCCCCCTTTTTTAACGCTGCAATTTTATGCTTCTGGTATGTTGAGAGTATTGAGCAGCGATGAGCA | 67 |
| NM_001009240 | CCGTGAGGCGGTACAGCAACAAAATATCCTCGCTGTGGAGGGAACCTCTTGCGAACTTTGCACGGAG | 96 |
| Affected cat | CCGTGAGGCGGTACAGCAACAAAATATCCTCGCTGTGGAGGGAACCTCTTGCGAACTTTGCACGGAG | 134 |
| Control male | CCGTGAGGCGGTACAGCAACAAAATATCCTCGCTGTGGAGGGAACCTCTTGCGAACTTTGCACGGAG | 134 |
| NM_001009240 | AGTCCTACCTCCAATTACCGGTGTGAAACCAGAGGAAAGGGTAGAGACCGCGGTCAGGACCGCGTCA | 163 |
| Affected cat | AGTCCTACCTCCAATTACCGGTGTGAAACCAGAGGAAAGGGTAGAGACCGCGGTCAGGACCGCGTCA | 201 |
| Control male | AGTCCTACCTCCAATTACCGGTGTGAAACCAGAGGAAAGGGTAGAGACCGCGGTCAGGACCGCGTCA | 201 |
| NM_001009240 | AACGACCCATGAACGCATTCATGGTGTGGTCTCGAGACCAAAGGCGCAAGGTGGCTCTAGAGAATCC | 230 |
| Affected cat | AACGACCCATGAACGCATTCATGGTGTGGTCTCGAGACCAAAGGCGCAAGGTGGCTCTAGAGAATCC | 268 |
| Control male | AACGACCCATGAACGCATTCATGGTGTGGTCTCGAGACCAAAGGCGCAAGGTGGCTCTAGAGAATCC | 268 |
| NM_001009240 | CCAAACGCAAAACTCAGAGATCAGCAAGCAGCTGGGGTACCAGTGGAAAATGCTTACACAAGCCGAA | 297 |
| Affected cat | CCAAACGCAAAACTCAGAGATCAGCAAGCAGCTGGGGTACCAGTGGAAAATGCTTACACAAGCCGAA | 335 |
| Control male | CCAAACGCAAAACTCAGAGATCAGCAAGCAGCTGGGGTACCAGTGGAAAATGCTTACACAAGCCGAA | 335 |
| NM_001009240 | AAATGGCCGTTCTTCGAGGAGGCACAGAGACTACAGGCGCTGCACCGAGAGAAATACCCGGGCTATA | 364 |
| Affected cat | AAATGGCCGTTCTTCGAGGAGGCACAGAGACTACAGGCGCTGCACCGAGAGAAATACCCGGGCTATA | 402 |
| Control male | AAATGGCCGTTCTTCGAGGAGGCACAGAGACTACAGGCGCTGCACCGAGAGAAATACCCGGGCTATA | 402 |
| NM_001009240 | GATACCGACCTCGTCGGAAGGCCAGGCCAGAGAAAAGTGACAAATTGCCCCCCGCAGACGCCTCGTC | 431 |
| Affected cat | GATACCGACCTCGTCGGAAGGCCANGCCAGAGAAAAGTGACAAATTGCCCCCCGCAGACGCCTCGTC | 469 |
| Control male | GATACCGACCTCGTCGGAAGGCCANGCCAGAGAAAAGTGACAAATTGCCCCCCGCAGACGCCTCGTC | 469 |
| NM_001009240 | CACACTCTGTAGTCAGCTGCACGCGGGAGAGAGGTTGTACGCCTTCCCCTACAAGGACGGCTGTACG | 498 |
| Affected cat | CACACTCTGTAGTCAGCTGCACGCGGGAGAGAGGTTGTACGCCTTCCCCTACAAGGACGGCTGTACG | 536 |
| Control male | CACACTCTGTAGTCAGCTGCACGCGGGAGAGAGGTTGTACGCCTTCCCCTACAAGGACGGCTGTACG | 536 |
| NM_001009240 | AAGGCTGCACACTCAGGAATGAAGGACCAGTTATACTCCTCCTCGGAACCCATGAGCATAACCAGCT | 565 |
| Affected cat | AAGGCTGCACACTCAGGAATGAAGGACCAGTTATACTCCTCCTCGGAACCCATGAGCATAACCAGCT | 603 |
| Control male | AAGGCTGCACACTCAGGAATGAAGGACCAGTTATACTCCTCCTCGGAACCCATGAGCATAACCAGCT | 603 |
| NM_001009240 | CGCTGCTGGAACCGGGACCTCACAGGACCTCCACAACCCTCCAAGACAGTCCTGAAGACTTGGCTAT | 632 |
| Affected cat | CGCTGCTGGAACCGGGACCTCACAGGACCTCCACAACCCTCCAAGACAGTCCTGAAGACTTGGCTAT | 670 |
| Control male | CGCTGCTGGAACCGGGACCTCACAGGACCTCCACAACCCTCCAAGACAGTCCTGAAGACTTGGCTAT | 670 |
| NM_001009240 | GCAGCTCTCCGCGGATGCTCCCCTTTATCGTACCTTAGAGCTGGGAGTTTCTGAGGCTTACTTTGCA | 699 |
| Affected cat | GCAGCTCTCCGCGGATGCTCCCCTTTATCGTACCTTAGAGCTGGGAGTTTCTGAGGCTTACTTTGCA | 737 |
| Control male | GCAGCTCTCCGCGGATGCTCCCCTTTATCGTACCTTAGAGCTGGGAGTTTCTGAGGCTTACTTTGCA | 737 |
| NM_001009240 | TGGTGA | 705 |
| Affected cat | TGGTGATTTCCTTAGTGTCGCTAACCCAAGGCCTTATTCATCTTAACTGTACTATTATTTCCTCTGT | 804 |
| Control male | TGGTGATTTCCTTAGTGTCGCTAACCCAAGGCCTTATTCATCTTAACTGTACTATTATTTCCTCTGT | 804 |
| NM_001009240 | ||
| Affected cat | CACTTAATTTCAAAATAAAGCATGTACAC | 833 |
| Control male | CACTTAATTTCAAAATAAAGCATGTACAC | 833 |
A single base-pair polymorphism was found in the hermaphrodite and control male cats (N = G/C at position No. 389).
Discussion
Mammals with mutations that interrupt sexual development are frequently presented for abnormal genital development and/or infertility. The mammalian Y chromosome carries the gene SRY, which encodes a critical protein in the pathway that promotes testis differentiation in the bipotential gonad; therefore, mammals with an XY karyotype usually develop testes. However, XY individuals with mutations in SRY, or other important genes in the testis pathway, do not develop normal testes. Such individuals either fail to develop a gonad (gonadal dysgenesis) or develop a testis with abnormal architecture and function (gonadal dysplasia) or develop both testicular and ovarian architecture (ovotestes). The degree of phenotypic masculinization in such individuals depends on the amount of functional testis that develops, and it varies on the basis of the quantity and timing of testicular secretions that are necessary to masculinize the internal and external genitalia. For example, if MIS secretion is delayed or insufficient in quantity during the critical period for Mullerian duct regression, all or part of the Mullerian (paramesonephric) duct system persists (uterus, uterine tubes, cervix, cranial vagina). Similarly, if testicular testosterone is insufficient in quantity or timing during the critical period for androgen-dependent masculinization of the Wolffian (mesonephric) ducts, urogenital sinus, genital swellings, or genital tubercle, the internal and external genitalia fail to masculinize in whole or in part.
All features of this case can be attributed to abnormal gonadal sex determination compatible with the diagnosis of 38,XY true hermaphrodite, which is a type of XY sex reversal or XY disorder of sexual development. The karyotype is that of a normal male cat (38,XY), yet testicular development is abnormal. Apparently MIS was absent during the critical period for Mullerian duct regression, so Mullerian ducts persisted and developed into the uterus and uterine tubes. Because MIS is the first hormone secreted by the fetal testis, this finding suggests that testicular induction or differentiation in this cat was delayed, thereby resulting in delayed MIS secretion. Although testosterone production was evidently sufficient to stimulate the cranial mesonephric ducts to form epididymides and deferent ducts, the level was apparently insufficient to induce deferent ducts caudally at their critical period for masculinization. Sufficient testosterone was apparently available for conversion to DHT in the external genitalia, leading to unambiguously male development of the prepuce and scrotum. The ovotestes failed to descend, which could be due to a combination of factors, such as insufficient production of testosterone and/or INSL3 from the testis, abnormal timing of their secretion at critical periods in testis descent, or physical impedance of descent owing to gonadal attachment of the uterine horns.
Testicular tumors are associated with cryptorchidism in other species, but a clear association has not been demonstrated in cats. However, the standard recommendation to remove both gonads was followed. Furthermore, surgical removal of the reproductive tract is generally necessary for definitive diagnosis in most cases of ambiguous sexual development. In this case, no evidence of associated urinary tract abnormalities was identified, and the cat had no postoperative complications.
In 70% of human patients with similar XY disorders of sex determination, the mutation is unknown.9 Several human patients with XY gonadal dysplasia or XY sex reversal have had mutations in SRY,1 but a mutation was not identified in the SRY coding region of this cat. The heterozygous polymorphism at reference position No. 389 probably resulted from a sequence-specific anomaly. The lack of available electrophoretograms for the reference sequence and wild cats prevents direct comparisons. Regardless, this variant does not cause XY sex reversal, because neither domestic nor wild cats routinely demonstrate this phenotype. Mutations in other testis pathway genes have been identified as the cause of naturally occurring XY gonadal dysplasia in humans (reviewed in Hughes et al5 and Matzuk and Lamb9) and in transgenic mice (reviewed in Sekido and Lovell-Badge14). These include heterozygous mutations in SOX9, in which only one mutant allele (haploinsufficiency) causes XY gonadal dysgenesis and skeletal malformations that are generally fatal (OMIM 114290). However, mutations, such as duplications affecting RSPO1 and WNT4, could lead to abundant beta catenin production, interfere with testis induction, and promote ovarian induction, leading to formation of ovotestes. Thus, the XY true hermaphroditism in this cat could have resulted from a mutation in the testis or ovarian pathway, but the exact molecular cause remains to be determined.
Acknowledgments
Serum testosterone radioimmunoassay was performed as a clinical service by the Endocrinology Laboratory, Animal Health Diagnostic Center, Cornell University, Ithaca, New York. We thank Anita Hesser for manuscript preparation and Jennifer Patterson for assisting with images.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure/Funding
This research was supported in part by NIH-NCRR R24 RR0016094 (L.A.L.).
References
- 1.Bernard P, Sim H, Knower K, Vilain E, Harley V. Human SRY inhibits b-catenin-mediated transcription. Int J Biochem Cell Biol. 2008;40:2889–2900. doi: 10.1016/j.biocel.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bighignoli B, Niini T, Grahn RA, Pedersen NC, Millon LV, Polli M, Longeri M, Lyons LA. Cytidine monophospho-N-acetylneuraminic acid hydrosylase (CMAH) mutations associated with the domestic cat AB blood group. Biomed Central. 2007;8:1471–2156. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciani F, Cocchia N, Rizzo M, Ponzio P, Tortora G, Avallone L, Lorizio R. Sex determining of cat embryo and some feline species. Zygote. 2008;16:169–177. doi: 10.1017/S0967199408004681. [DOI] [PubMed] [Google Scholar]
- 4.Cool J, Capel B. Mixed signals: development of the testis. Semin Reprod Med. 2009;27:5–13. doi: 10.1055/s-0028-1108005. [DOI] [PubMed] [Google Scholar]
- 5.Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91:554–563. doi: 10.1136/adc.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hungerford DA. Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCl. Stain Technol. 1965;40:333–338. doi: 10.3109/10520296509116440. [DOI] [PubMed] [Google Scholar]
- 7.Leaman T, Rowland R, Long SE. Male tortoiseshell cats in the United Kingdom. Vet Rec. 1999;144:9–12. doi: 10.1136/vr.144.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Long SE. 38,XX/38,XY chromosome chimaerism in three feline siblings. Vet Rec. 1999;145:404–405. doi: 10.1136/vr.145.14.404. [DOI] [PubMed] [Google Scholar]
- 9.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyers-Wallen VN. CVT update: inherited disorders of the reproductive tract in dogs and cats. In: Bonagura JD, Twedt DC, editors. Kirk’s Current Veterinary Therapy XIV. WB Saunders Co; Philadelphia, PA: 2008. pp. 1034–1040. [Google Scholar]
- 11.Meyers-Wallen VN, Patterson DF. Disorders of sexual development in dogs and cats. In: Kirk RW, Bonagura JD, editors. Current Veterinary Therapy. 10. WB Saunders Co; Philadelphia, PA: 1989. pp. 1261–1269. [Google Scholar]
- 12.Meyers-Wallen VN, Wilson JD, Griffin JE, Fisher S, Moorhead PH, Goldschmidt MH, Haskins ME, Patterson DF. Testicular feminization in a cat. J Am Vet Med Assoc. 1989;195:631–634. [PubMed] [Google Scholar]
- 13.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;298:971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 14.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Tevosian SG, Manuylov NL. To beta or not to beta: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]


