Abstract
Mutationsin cytosolic isocitrate dehydrogenase 1 (IDH1) or its mitochondrial homolog IDH2 can lead to R(-)-2-hydroxyglutarate (2HG) production. To date, mutations in three active site arginine residues IDH1 R132, IDH2 R172, and IDH2 R140 have been shown to result in the neomorphic production of 2HG. Here we report three additional 2HG-producing IDH1 mutations: IDH1 R100 which is affected in adult glioma, IDH1 G97 which is mutated in colon cancer cell lines and pediatric glioblastoma, and IDH1 Y139. These new mutants all stereospecifically produced 2HG’s (R) enantiomer. In contrast, we find that the IDH1SNPs V71I and V178I, as well as a number of other single sample reports of IDH non-synonymous mutation, did not elevate cellular 2HG levels in cells and retained the wild-type ability for isocitrate-dependent NADPH production. Finally, we report the existence of additional rare but recurring mutations found in lymphoma and thyroid cancer which, while failing to elevate 2HG, nonetheless displayed loss of function, indicating a possible tumorigenic mechanism for a non-2HG producing subset of IDH mutations in some malignancies. These data broaden our understanding of how IDH mutations may contribute to cancer through either neomorphic R(-)-2HG production or reduced wild-type enzymatic activity, and highlight the potential value of metabolite screening to identify IDH-mutated tumors associated with elevated oncometabolite levels.
Keywords: IDH1, IDH2, 2-hydroxyglutarate, oncometabolite, NADPH
INTRODUCTION
Somatic mutations in human cytosolic isocitrate dehydrogenase 1 (IDH1) were initially found to be recurrent in adult glioma and acute myeloid leukemia (AML), where the reported mutations were missense and specific for a single R132 residue( Parsons et al., 2008;Mardis et al., 2009;Yan et al., 2009). Initial experiments demonstrated these IDH1 R132 mutations caused loss of the enzyme’s normalability to catalyze the conversion of isocitrate to α-ketoglutarate. Subsequently, it was discovered that these mutations also conferred an enzymatic gain-of-function: the novel N ADPH-dependent reduction of α-ketoglutarate to the normally trace metabolite R(-)-2-hydroxyglutarate(2HG) (Dang et al., 2009).
Some gliomas lacking cytosolic IDH1 mutations were later observed to have mutations in IDH2, the mitochondrial homolog of IDH1 (Yan et al., 2009). These glioma IDH2 mutations were detected at the R172 residue, analogous to IDH1 R132. Mutations in IDH1 R132 and IDH2 R172 have also been described recently in cartilaginous neoplasms including chondrosarcoma (Amary et al., 2011). While initially studies failed to observe mitochondrial IDH2 mutations in AML, prospective metabolite screening of primary AML samples found that 2HG elevation was much more common than expected from the described IDH1 mutational frequency (Ward et al., 2010). Subsequently it was determined that the majority of 2HG-elevated AML samples harbored mutations in IDH2 rather than IDH1. While some of these were R172 mutations, most were unexpectedly found at a different arginine in IDH2’s active site, R140.
Based on this establishment that multiple residues in the active site of mitochondrial IDH2 can be mutated to produce 2HG, the present study was undertaken to see if additional residues in cytosolic IDH1had the potential to become neomorphic 2HG-producing alleles. We also investigated the enzymatic activity of additional reported IDH1/2 alterations.
RESULTS AND DISCUSSION
Mutations at IDH1 R100 and G97 result in 2HG production
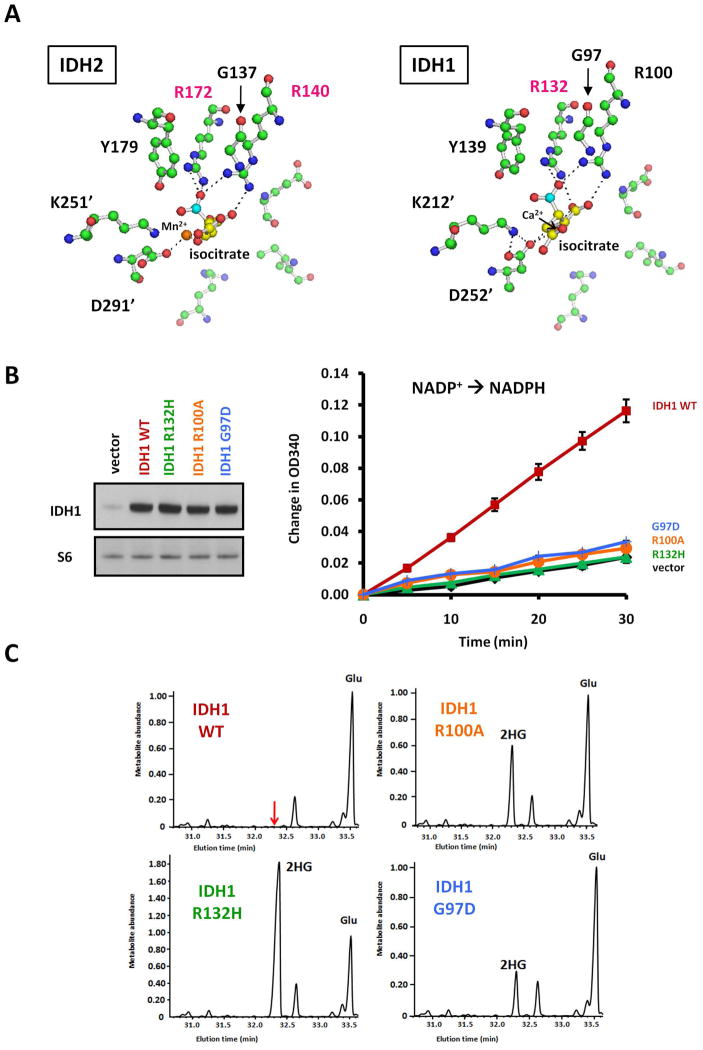
A metabolite screening test of AML samples established that IDH2 mutations at R140 produce 2HG (Ward et al., 2010). Since mutations at two other IDH residues which are analogous to each other, mitochondrial IDH2 R172 and cytosolic IDH1 R132, both lead to 2HG production, we asked whether the same relationship held between known neomorphic mutation s at IDH2 R140 and mutations at the uncharacterized analogous position in IDH1, R100. Like IDH2 R140, IDH1 R100 normally stabilizes isocitrate’s β-carboxyl group through a charge interaction from its guanidinium moiety (Figure 1A). We predicted that removing this stabilization of the β-carboxyl through mutation of R100 would hinder the enzyme’s ability to use isocitrate as a substrate and, like mutation of IDH2 R140, facilitate the non-carboxylating reduction of α-ketoglutarate to 2HG. The effect of IDH1 G97D mutation was also examined, as this alteration has been identified in colon cancer cell lines and pediatric glioblastoma ( Bleeker et al., 2009;Paugh et al., 2010), and we predicted that this mutation to a negatively charged aspartate would disfavor coordination of isocitrate’s negatively charged β-carboxyl.
Figure 1. Mutation of IDH1 R100 or G97 leads to 2HG production.
(A) The active site of human IDH2, modeled based on the highly homologous porcine IDH2 structure (Ceccarelli et al., 2002), and the active site of crystallized human IDH1 with isocitrate are shown. Isocitrate carbons are yellow except the β-carboxyl carbon in cyan. Also shown are oxygens (red), amines (blue), and carbons of amino acids (green). Dashed lines represent hydrogen and ionic bonds < 3.1 Å. The prime ( )designates residues from the other monomer of the IDH dimer. Modeling of human IDH2 was performed as described previously (Ward et al., 2010), and images generated using PyMOL viewer (DeLano 2006).
(B) 293T cells were lysed 48 h after transfection with IDH1 WT, mutants, or empty vector, and IDH1 expression was confirmed by Western blot with goat polyclonal antibody (Santa Cruz Biotechnology, sc49996) S6 ribosomal protein levels were measured to assess equal loading with rabbit monoclonal antibody (Cell Signaling, 2217). These lysates were then assayed for isocitrate-dependent NADPH production from 3 μg lysate protein in an assay solution containing 100 mM Tris-HCl buffer (pH 7.5), 1.3 mM MnCl2, 0.33 mM EDTA, 0.1 mM β-NADP+, and 0.1 mM D-(+)-threo-isocitrate as described previously (Ward et al., 2010). Data are depicted as the mean and SD from three independent measurements at the indicated time points.
(C) Parallel-transfected cells were extracted for intracellular metabolites by gently removing culture medium and then rapidly quenching cells in ice-cold 80% methanol. After incubation at −80°C for 20 min, extracts were centrifuged to remove precipitated protein, and aqueous metabolites in the supernatant were then dried under nitrogen gas and redissolved in 1:1 acetonitrile: N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (MTBSTFA; Regis) and heated at 70°C for 75 min to derivatize metabolites. Derivatized samples were then analyzed by GC-MS as described previously (Ward et al., 2010). Representative gas chromatographs are show n for metabolites eluting from 31–33.5 minutes, including glutamate (Glu) and 2HG. Metabolite abundance refers to signal intensity. Data for (B) and (C) are from a representative of three independent experiments.
To test our predictions, we expressed IDH1 R100A and IDH1 G97D mutants in cells, along with IDH1 WT and the established 2HG-producing IDH1 R132Hmutant (Figure 1B). We first found that, unlike overexpression of IDH1 WT, expression of either IDH1 R100A or G97D failed to increase isocitrate-dependent NADPH production in cell lysates above the levels invector-transfected cells. Thus, like the IDH1 R132H mutation as shown previously (Dang et al., 2009;Yan et al., 2009), IDH1 R100Aand G97D mutations are loss-of-function for generating NADPH in the presence of isocitrate. To determine if theR100A and G97D mutants also share with the R132H mutant the gain-of-function to produce 2HG, we extracted metabolites from parallel-transfected cells and measured 2HG elevation by GC-MS. 2HG levels were observed to be elevated in cells expressing either IDH1 R100A or G97D (Figure 1C). As confirmation we found that 2HG was also elevated in the HCT15 colon cancer line containing an endogenous G97D mutation (data not shown). Of note, following the initiation of this study, IDH1mutations at R100 were described in adult glioma( Pusch et al., 2011), further supporting the importance of predicting and then characterizing additional 2HG-producing alleles.
Electrostatics analysis predicts an other 2HG-producing mutation:IDH1 Y139D
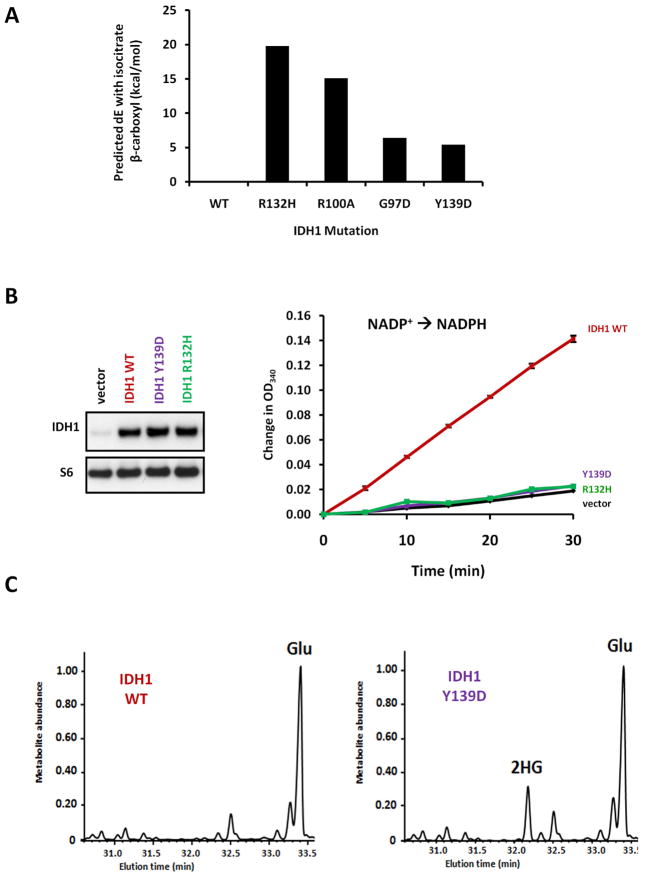
To better understand the loss of isocitrate utilization and gain of 2HG production from IDH1 R132H, R100A, and G97D mutations, we performed an electrostatics analysis to calculate the destabilizing effect of each mutation on isocitrate’s β-carboxyl (Figure 2A). Consistent with IDH1 R132H, R100A, and G97D mutations all favoring conversion of α-ketoglutarate to 2HG rather than inter conversion of α-ketoglutarate and isocitrate, all three mutations were calculated to be unfavorable for isocitrate β-carboxyl stabilization. While performing this analysis, we also examined if mutations at other IDH1 active site residues could be predicted to disfavor coordination of the isocitrate β-carboxyl. Surprisingly, we found that an additional mutation, Y139D, could be modeled to have an electrostatic effect comparable to that for the 2HG-producing mutant G97D. As Y139 islocated near the substrate in t he IDH1 active site (Figure 1A), w e investigated whether IDH1 Y139D could be yet another 2HG-producing neomorph. We first found thatY139D mutant overexpression failed to increase isocitrate-dependent NADPH production from cell lysates, similar to R132H mutant overexpression and in contrast to cells overexpressing IDH1 WT(Figure 2B). From parallel-transfected cells, we then assessed whether IDH1 Y139D could produce 2HG. Unlike cells expressing IDH1WT, cells expressing the predicted Y139D mutantdisplayed 2HG elevation(Figure 2C).
Figure 2. Predicted Y139D mutation of IDH1 is another 2HG-producing allele.
(A) The changes in electrostatic interaction energy with isocitrate’s β-carboxyl group were calculated for various IDH1 mutations relative to wild-type. A positive change in energy indicates decreased stabilization of the β-carboxylate in both the isocitrate-IDH1 complex and in the transition state of the α-ketoglutarate→isocitrate reverse reaction, thus favoring the production of 2HG product which lacks this carboxylate. Mutations were modeled using the structure PDB ID1T0L of wild type human IDH1 in complex with NAD PH, isocitrate, and calcium as the template( Xu et al., 2004). Details for modeling and electrostatic interaction calculations are described in Supplementary Materials and Methods.
(B) 293T cells were lysed 48 h after transfection with IDH1 WT, Y139D, or R132H, or empty vector. IDH1 protein levels were assessed by Western blot, and 3 μg of lysate protein was assessed for NADPH production with 0.1 mM isocitrate. (C) Parallel-transfected cells were extracted for intracellular metabolites and analyzed by GC-MS. Data for (B) and
(C) are from are presentative of three independent experiments.
While mutation of IDH1 Y139 has yet to be described in any cancer, the residue does lie outside IDH1’s fourth exon, which has been the region of exclusive focus in many sequencing studies. The lack of samples to date with reported mutation of IDH1 Y139 (or the analogous IDH2 Y179)m ay also be structurally explained by the strict requirement for this position to incorporate a negatively charged residue in order to facilitate neomorphic enzyme activity (Supplementary Table 1). In contrast, for the commonly affected residues IDH1 R132, IDH2 R172, and IDH2 R140, the diversity of substitutions observed suggests a less specific structural requirement. Still, specific substitutions of IDH1 R132H/C, IDH2 R172K, and IDH2 R140Q are observed with higher frequency. These can all arise through C→T or G→Atransitions in CpG dinucelotides at these codons, potentially from the frequent methylation-induced deamination of 5-methylcytosine (Cooper and Youssoufian 1988), in contrast to IDH1 Y139D where a T→G transversion must occur at a non-CpG site.
All IDH mutations specifically produce the (R) enantiomer of 2HG
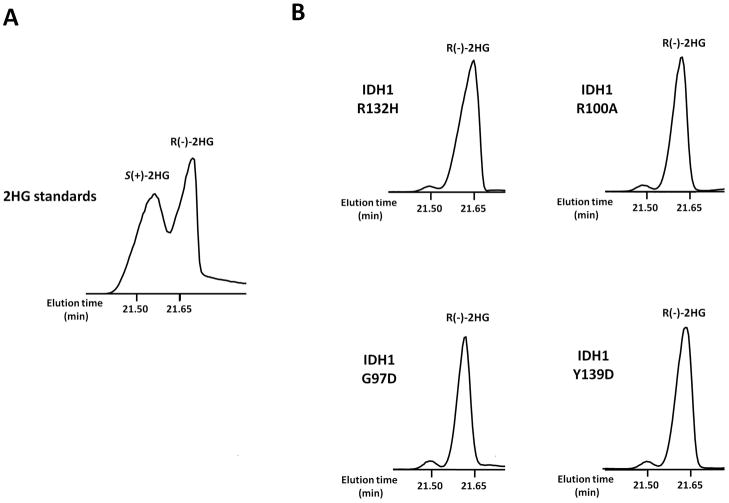
While recent investigations have focused on the differing biological effects of the ( R) and (S) enantiomers of 2HG, IDHR132 mutations were shown to exclusively produce R(-)-2HGboth in vitro and in primary tumor tissue (Dang et al., 2009), and the same stereospecificity was demonstrated for both R172 and R140 mutations in IDH2 (Ward et al,. 2010; Kranendijk et al., 2010). Somatic mutations in tumors resulting in elevation of S(+)-2HG have yet to be found. To determine the chirality of 2HG produced by the additional IDH neomorphs identified in this study, we used a previously described procedure to separate the (S) and (R) enantiomers of 2HG by GC-MS( Kamerling et al., 1981, Ward et al., 2010). We determined that like in cells expressing IDH1 R132H, the 2HG produced in cells expressing IDH1 R100A, G97D, or Y139D specifically corresponds to the (R) enantiomer (Figure 3 ).
Figure 3. All IDH1 mutants specifically generate 2HG’s (R) enantiomer.
(A)S eparation of the two stereoisomers of 2HG on GC-MS as the O-acetylated di(-)-2-butyl esters was demonstrated using standards obtained from Sigma and purified by elution from an AG-1 X8 100–200 anion exchange resin (Bio-Rad). A previously described extended derivatization procedure was then performed (Kamerling et al., 1981, Ward et al., 2010). Eluates were dried under nitrogen, redisssolved in 1 M HCl in R(-)-2-butanol, and heated for 3 h at 95°C. After drying again under nitrogen, samples were redissolved in pyridine and acetic anhydride at a 1:1 ratio, heated for 30 min at 100°C, dried, and redissolved in acetonitrile before analysis of the ion at m/e−173 by GC-MS.
(B) Cells expressing IDH1 R132H, R100A, G97D, or Y139D were analyzed by the same method to determine the specific 2HG enantiomer produced by each mutant. Data are from a representative of three independent experiments.
Rare recurring mutations in lymphoma and thyroid cancer do not produce 2HG, but can result in loss of function
At the time of this article’s submission, over 100 papers had been published that reported patient samples containing tumor-specific IDH mutations. Greater than 99% of these patient samples have an IDH1/2 mutation that has now been confirmed to be R(-)-2HG-producing, either from this or prior studies(Supplementary Table 2). However, several reports have recently identified IDH1/2 variants which have yet to be enzymatically characterized(Table 1). We first examined the effects of reported IDH1 SNPs V71I and V178I, as well as non-arginine variants only reported in a single sample to date( Hemerly et al.,2010 ; Marcucci et al., 2010; Ho et al., 2010; Murugan et al., 2010; Zou et al., 2010; Forbes et al., 2011; Rakheja et al., 2011). All were expressed at comparable levels to IDH1/2 WT in cells (Supplementary Figure 1). None were observed to increase intracellular 2HG, while all retained the ability to increase isocitrate-dependent NADPH production from cell lysates.
Table 1.
Summary of the effect of IDH mutations on enzyme expression and activity
| Occurs In | Somatic | Expressed in cells | WT activity | Neomorphic | Effect | |
|---|---|---|---|---|---|---|
| IDH1 R132_ | glioma | YES | YES | NO | YES | R(-)-2HG productiona |
| leukemia | ||||||
| chondrosarcoma | ||||||
| colon CA | ||||||
| IDH1 R100_ | glioma | YES | YES | NO | YES | R(-)-2HG production |
| IDH1 G97D | colon CA cell lines | YES | YES | NO | YES | R(-)-2HG production |
| pediatric glioblastoma | ||||||
| IDH1 Y139D | predicted | predicted | YES | NO | YES | R(-)-2HG production |
| IDH2 R172_ | glioma | YES | YES | NO | YES | R(-)-2HG production |
| leukemia | ||||||
| chondrosarcoma | ||||||
| IDH2 R140_ | leukemia | YES | YES | NO | YES | R(-)-2HG production |
| R(-)-2HG aciduria | (germline in 2HG aciduria) | |||||
| IDH1 V71I | SNP | NO | YES | YES | NO | WT activity |
| IDH1 V178I | SNP | NO | YES | YES | NO | WT activity |
| IDH1 I99M | leukemia (one case) | unknown | YES | YES | NO | WT activity |
| IDH1 G123R | thyroid CA (one case) | unknown | YES | YES | NO | WT activity |
| IDH1 I130M | thyroid CA (one case) | YES | YES | YES | NO | WT activity |
| IDH1 H133Q | thyroid CA (one case) | YES | YES | YES | NO | WT activity |
| IDH2 V294Mb | melanoma (one case) | YES | YES | YES | NO | WT activity |
| IDH1 G70D | thyroid CA (six cases) | YES | NO | NO | NO | loss of function |
| IDH1 A134D | thryoid CA (two cases) | YES | YES | NO | NO | loss of function |
| IDH1 R49C | pediatric glioblastoma (one case) | YES | NO | NO | NO | loss of function |
| IDH2 F394_ | T-cell angioimmunoblastic lymphoma (two cases) | YES | NO | NO | NO | loss of function |
See Supplementary Table 2 for information on total reported cases with R(-)-2HG producing IDH1/2 mutations (6629 patients to date).
Two additional single-sample occurrences of IDH2 mutation have recently been reported in melanoma: IDH2 G171D and P158T (Shibata et al., 2011).
In addition to these variants which we found to behave like wild-type IDH enzymes, we also investigated several rare yet recurring somatic mutations. Although previously undescribed in cancer, we have found somatic mutations at IDH2 F394 in two T-cell angioimmunoblastic lymphoma (AILT) samples(one sample with IDH2 F394I, and one with F394V). By transfection we were unable to express these mutants, despite achieving comparable levels of IDH2 mRNA in the transfected cells (Supplementary Figure 2). Transfection of these mutants did not elevate intracellular 2HG, and we confirmed that their transfection did not increase isocitrate-dependent NADPH production.
We then investigated IDH1 G70D and A134D mutations described in six and two thyroid cancer samples, respectively, as well as an IDH1 R49C mutation described in one pediatric glioblastoma (Hemerly et al., 2010, Paugh et al., 2010). All of these mutations have been demonstrated to occur at a single allele in a somatic manner. Only the IDH1 A134D mutant was able to be effectively overexpressed to levels comparable to IDH1 WT or R132H, despite a clear increase in IDH1 mRNA levels for all mutant-transfected cells (Supplementary Figure 3). While not producing 2HG, the expressed A134D mutant also failed to increase isocitrate-dependent NADPH production. Together these data suggest that there is a subset of rare IDH somatic mutations that result in decreased wild-type activity without a concomitant increase in 2HG production.
Since retention of at least one wild-type IDH allele is important for proliferation in MYC-driven cancer cells and for IDH1/2-mediated shuttling of NADPH from the mitochondria to cytoplasm (Ward et al., 2010; Lemons et al., 2010), we examined if the expressed but loss-of-function A134D mutant IDH1 could dominantly inhibit IDH1 WT activity in cells. For the 2HG-producing mutant IDH1 R132H, it was shown first in Dang et al. (2009), testing a range of physiological isocitrate concentrations up to 100 μM, that R132H mutant expression does not substantially inhibit the isocitrate-dependent NADPH production of IDH1WT, a finding recently confirmed extensively by an independent group (Jin et al., 2011). We repeated this analysis for IDH1 A134D. Co-transfecting IDH1 A134D with IDH1WT at either a 1:1 or 3:1 ratio, we did not observe a decrease in isocitrate-dependent NADPH production from transfection of the A134D mutant (Supplementary Figure 4).
For the IDH1 G70D, IDH1 R49C, and IDH2 F394I/V mutants that were unable to be effectively overexpressed in cells, we performed in vitro transcription coupled translation with [35S] methionine and rabbit reticulocyte lysates (Supplementary Figure 5). We found that in vitro transcription/translation of IDH1 G70D or IDH1 R49Cgenerated a major band corresponding to the predicted IDH1 molecular weight of 47 kD, matching the product observed from IDH1 WT. Similarly, we found that in vitro transcription/translation of IDH2 F394I or F394V generated a major band matching that produced from IDH2 WT. These results further indicate that the inability of these mutants to be effectively overexpressed following transfection reflects an impairment in cellular accumulation.
No IDH mutations producing 2HG have been confirmed to date in thyroid cancer. Conversely, none of the recurring but non-2HG-producingsomatic mutations found in thyroid cancer or AILT have ever been described in leukemia or adult glioma. We further confirmed in this study, in 973 myeloid hematologic malignancy samples, that no recurring somatic mutations were found between IDH1 residues41–438 or IDH2 residues 125–226 except for the previously characterized 2HG-producing IDH1 R132, IDH2 R172K, and IDH2 R140Q alleles. Full-length sequencing of the entire IDH1/2coding regions in 20 samples also failed to detect any additional somatic alterations.
These data support the neomorphic activity converting α-ketoglutarate to R (-)-2HG, rather than solely the loss of normal IDH function, being the common feature selected for in adult leukemia, glioma, and the vast majority of other IDH1/2 mutant cancers. This is further evidenced by recent work demonstrating the clustering of R(-)-2HG-producing IDH mutant cases in a distinct DNA hypermethylation signature in tumor samples from both glioma and AML patients (Figueroa et al., 2010, Noushmehr et al., 2010). While those rare IDH mutations indicative of IDH haploinsufficiency in AILT and thyroid cancer require further investigation and may potentially result in impairment of cytosolic NADPH production and redox control, any of the residues in cytosolic IDH1 or mitochondrial IDH2 that can be altered to produce the R(-)-2HG oncometabolite can be screened for their mutation by a GC-MS metabolite assay.
The data presented here suggest that a screening and diagnostic approach based on elevated oncometabolite levels may not be of the highest utility for thyroid cancer, as IDH mutations found to date in thyroid cancer do not produce 2HG and may function to promote tumorigenesis in an alternative manner. However, based on this study there are at least five reproducible cancer-associated mutations that can result in 2HG production, and these appear to be selected for in several well-characterized tumor types including AML, gliomas, chondrosarcomas, and gastrointestinal cancers. Thus, a screening and diagnostic approach for these malignancies based on elevated 2HG levels may be of substantial value. Firstly, given the rarity and marked developmental consequences of inborn errors of metabolism leading to elevated 2HG, tumors displaying increased levels of 2HG are unlikely to be false positives, in contrast to conventional gene sequencing in which numerous SNPs and uncharacterized sequencing artifacts and/or passenger alterations that have no effect on IDH enzyme activity are detected. Screening for elevated 2HG levels may also be a more sensitive test, as it can allow for the detection of neomorphic mutations at residues like IDH1 R100, G97, and Y139 that are not normally examined by sequencing or mutation-specific antibody approaches focused on the most common alleles. Finally, screening for 2HG can be non-invasive: patient sera/plasma can be assayed in the case of leukemia, while radiologic approaches for 2HG detection can be refined in the case of glioma and other solid tumors, and urinalysis may also be employed.
Overall, the data presented here demonstrate the complexity inherent in correlating the genetic alterations in the IDH1/2 enzymes found in sequencing studies with altered metabolic activity. Most IDH mutant tumor samples reported to date harbor a mutation which has now been shown to be R(-)-2HG producing, based on the work of this study and others. Yet we also report here the existence of rare subsets of IDH loss-of-function mutations which do not produce 2HG. Taken together, these data highlight the value of metabolite screening approaches to more specifically and sensitively identify those IDH mutant tumors which harbor elevations in the level of the R(-)-2HG oncometabolite.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by grants from the NCI and NIH. R.L. Levine is a HHMI Early Career Award Recipient and Geoffrey Beene Junior Faculty Chair at MSKCC. D.M. Weinstock is supported by a Stand Up To Cancer Innovative Research Grant and American Cancer Society Research Scholar Grant.
Footnotes
Conflict of interest
Dr. Thompson’s work has been funded by the NCI and NIH. He is a co-founder of Agios Pharmaceuticals, is the chair of its scientific advisory board, and has financial interest in the company. The authors declare no other potential conflicts of interest.
References
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- Ceccarelli C, Grodsky NB, Ariyaratne N, Colman RF, Bahnson BJ. Crystal structure of porcine mitochondrial NADP+-dependent isocitrate dehydrogenase complexed with Mn2+ and isocitrate. Insights into the enzyme mechanism. J Biol Chem. 2002;277:43454–43462. doi: 10.1074/jbc.M207306200. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988;78:151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular GraphicsSystem. DeLano Scientific; 2006. [Google Scholar]
- Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly JP, Bastos AU, Cerutti JM. Identification of several novel non-p.R132 IDH1 variants in thyroid carcinomas. Eur J Endocrinol. 2010;163:747–755. doi: 10.1530/EJE-10-0473. [DOI] [PubMed] [Google Scholar]
- Ho PA, Alonzo TA, Kopecky KJ, Miller KL, Kuhn J, Zeng R, et al. Molecular alterations of the IDH1 gene in AML: a Children’s Oncology Group and Southwest Oncology Group study. Leukemia. 2010;24:909–913. doi: 10.1038/leu.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, et al. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One. 2011;6:e16812. doi: 10.1371/journal.pone.0016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerling JP, Duran M, Gerwig GJ, Ketting D, Bruinvis L, Vliegenthart JF, et al. Determination of the absolute configuration of some biologically important urinary 2-hydroxydicarboxylic acids by capillary gas--liquid chromatography. J Chromatogr. 1981;222:276–283. doi: 10.1016/s0378-4347(00)81061-0. [DOI] [PubMed] [Google Scholar]
- Kranendijk M, Struys EA, van Schaftingen E, Gibson KM, Kanhai WA, van der Knaap MS, et al. IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science. 2010;330:336. doi: 10.1126/science.1192632. [DOI] [PubMed] [Google Scholar]
- Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan AK, Bojdani E, Xing M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun. 2010;393:555–559. doi: 10.1016/j.bbrc.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary LJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch S, Sahm F, Meyer J, Mittelbronn M, Hartmann C, von Deimling A. Glioma IDH1 mutation patterns off the beaten track. Neuropathol Appl Neurobiol. 2011;37:428–430. doi: 10.1111/j.1365-2990.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- Rakheja D, Mitui M, Boriack RL, DeBerardinis RJ. Isocitrate dehydrogenase 1/2 mutational analyses and 2-hydroxyglutarate measurements in Wilms tumors. Pediatr Blood Cancer. 2011;56:379–383. doi: 10.1002/pbc.22697. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kokubu A, Miyamoto M, Sasajima Y, Yamazaki N. Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am J Pathol. 2011;178:1395–1402. doi: 10.1016/j.ajpath.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004;279:33946–33957. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zeng Y, Zhang DF, Zou SH, Cheng YF, Yao YG. IDH1 and IDH2 mutations are frequent in Chinese patients with acute myeloid leukemia but rare in other types of hematological disorders. Biochem Biophys Res Commun. 2010;402:378–383. doi: 10.1016/j.bbrc.2010.10.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.