Abstract
OBJECTIVE
To evaluate the association between body mass index (BMI, kg m−2) and mortality rate among Hispanic adults.
METHODS AND PROCEDURES
Analysis of five data sets (total N = 16 798) identified after searching for publicly available, prospective cohort data sets containing relevant information for at least 500 Hispanic respondents (≥18 years at baseline), at least 5 years of mortality follow-up, and measured height and weight. Data sets included the third National Health and Nutrition Examination Survey, the Puerto Rico Heart Health Program (PRHHP), the Hispanic Established Population for Epidemiologic Studies of the Elderly (HEPESE), the San Antonio Heart Study (SAHS) and the Sacramento Area Latino Study on Aging.
RESULTS
Cox proportional hazards regression models, adjusting for sex and smoking, were fit within three attained-age strata (18 to younger than 60 years, 60 to younger than 70 years, and 70 years and older). We found that underweight was associated with elevated mortality rate for all age groups in the PRHHP (hazard ratios [HRs] = 1.38–1.60) and the SAHS (HRs = 1.88–2.51). Overweight (HRs = 0.38 and 0.84) and obesity grade 2–3 (HRs = 0.75 and 0.60) associated with reduced mortality rate in the HEPESE dataset for those in the 60 to younger than 70 years, and 70 years and older attained-age strata. Weighted estimates combining the HRs across the data sets revealed a similar pattern.
CONCLUSION
Among Hispanic adults, there was no clear evidence that overweight and obesity associate with elevated mortality rate.
Keywords: body mass index, hispanic adults, mortality rate
INTRODUCTION
There are approximately 45 million hispanic persons in the United States comprising 15% of the population, and it is estimated that Hispanic persons will comprise about 30% of the US population by 2050.1,2 Although Hispanics can be of any race, the national identity reflects relatively heterogeneous groups. Mexican Americans comprise about two-thirds of all US-residing hispanics, followed by Puerto Ricans, Cuban Americans and Central/South Americans.2 Recent data from the National Health and Nutrition Examination Surveys (NHANES) indicate that overweight and obesity are more prevalent among Mexican Americans than non-Hispanic persons. Specifically, 77.9% of Mexican-American adults aged 20 years or older are estimated to be overweight or obese compared with 68% among non-Hispanic persons, and from 1999 to 2008 the age-adjusted prevalence of obesity among Mexican-American men and women increased by 7% and 5.4%, respectively.3 Moreover, compared with non-Hispanic children, male MexicanAmerican children have 73% higher odds of being overweight, whereas female MexicanAmerican children have 56% higher odds of being overweight.4
The high prevalence of overweight and obesity is a concern because obesity is a major risk factor for many diseases, and it has been shown to decrease lifespan in white middle-aged cohorts.5,6 Hispanic persons appear to be particularly vulnerable to the metabolic syndrome, diabetes and asthma.6–8 Specifically, they have the highest prevalence of age-adjusted metabolic syndrome of any racial/ethnic group, and Hispanic women have a higher prevalence than Hispanic men.7 Regarding diabetes, compared with non-Hispanic white adults, the risk of being diagnosed with diabetes is 66% higher among Hispanics.8 Finally, national survey data indicate about a two-fold increase in asthma prevalence among obese Hispanic persons compared with obese whites.9,10
To date, however, our knowledge of the association between body mass index (BMI, kg m−2) and mortality rate is based primarily on analyses of European-American and African-American samples.11 A recent analysis among approximately 310 000 healthy, middle-aged (median age of 58) nonsmoking white adults6 indicated that the lowest mortality was for individuals with BMIs between 22.5 and 24.9 kg m−2, and that every 5-BMI unit increase above these BMI values is associated with a 31% increase in mortality, with BMIs below this point exhibiting an inverse association with mortality.
In this study, we estimated the association between BMI and mortality rate among Hispanic adults using data from five large, well-characterized epidemiologic data sets. By using multiple data sets and identical statistical procedures, we (1) evaluated the reproducibility of the results, (2) derived estimates of the magnitude of the association across an array of epidemiologic data sets meeting a consistent set of inclusion criteria, and (3) generated combined estimates that incorporate all the data across data sets.
MATERIALS AND METHODS
Inclusion criteria for data sets
To evaluate the association between BMI and mortality rate, we used prospective epidemiological data sets that met the following requirements: (1) contained measured weight and height to allow for the calculation of BMI; (2) contained at least 500 Hispanic respondents 18 years of age or older at baseline; (3) had available mortality follow-up data for at least 5 years; (4) contained each respondents’ age, sex and smoking status; and (5) were publicly available or obtainable.
Overview of data sets used
We briefly describe here and in Table 1 the characteristics of the five data sets used.
Table 1.
Overview of the five data sets used
| Characteristic | NHANES III | PRHHP | HEPESE | SAHS | SALSA |
|---|---|---|---|---|---|
| Dates of study | 1988–1994 | 1965–1980 | 1995–1996 | 1979–1999 | 1998–2006 |
| Age (years) | 42.1±17.9 | 54.4±6.7a | 73.7±6.2 | 43.5±11.1 | 70.4±6.9 |
| Female (%) | 50.8 | 0 | 58.2 | 56.9 | 58.3 |
| Sample size for analysis | 5182 | 5187 | 1882 | 2758 | 1625 |
| Mortality follow-up (years) | 12–18 | 12 | 10 | 14 | 9 |
| Deaths, na | 840 | 2619 | 385 | 923 | 419 |
| Prevalence of BMI level (%) | |||||
| <18.5 | 1.5 | 11.4 | 1.6 | 0.9 | 0.4 |
| 18.5 – <25 | 34.4 | 41.4 | 29.0 | 27.9 | 18.7 |
| 25 – <30 | 37.8 | 35.3 | 39.0 | 39.9 | 38.6 |
| 30 – <35 | 18.0 | 11.0 | 22.0 | 19.6 | 27.2 |
| ≥35 | 8.3 | 0.9 | 8.7 | 11.7 | 15.1 |
Abbreviations: HEPESE, Hispanic Established Population for Epidemiologic Studies of the Elderly; NHANES III, Third National Health and Nutrition Examination Survey; PRHHP, Puerto Rico Heart Health Program; SAHS, San Antonio Heart Study; SALSA, the Sacramento Area Latino Study on Aging.
PRHHP reported age at study entry in contiguous 4-year interval categories from 35 to 79 years. For analysis, participants were assigned an age corresponding to the average of the left and right end points of their age interval.
The third NHANES (NHANES III), 1988–1994, was conducted on a nationwide probability sample of approximately 33 994 persons for 2 months and over.12 NHANES-III contains 5213 Mexican American persons aged ≥18 at baseline with mortality follow-up through 31 December 2006. (NHANES I and II did not meet our inclusion criteria, as they do not contain sufficient numbers of Hispanic respondents across the range of BMI). Mortality information was based on the public-use mortality files, which are linked to the National Death Index.13
The Puerto Rico Heart Health Program (PRHHP), initiated in 1965, investigates morbidity and mortality in Puerto Rican men who were free from coronary heart disease at the time of the first examination.14–17 The men were sampled from three urban areas and four rural areas in the northeast part of Puerto Rico. The original cohort consisted of men who were 45–64 years of age at baseline. Other participants aged 35–44 years (n = 348) and 65–79 years (n = 678), who were not part of the original sampling frame, were also included, making the total number of participants 9824 men between the ages of 35 and 79 years. Precise assessments of age at study entry were not publicly available for this cohort. However, approximate age was available in categories by contiguous 4-year intervals from 35 to 79 years. For analysis, an age was assigned to each participant as the average of the upper and lower bound of the age interval indicated in the database (e.g., age assigned would be 42 for participants in the 40–44-year age category). Follow-up examinations were conducted approximately 3 years apart, and morbidity and mortality follow-up was concluded in 1980.
The Hispanic Established Population for Epidemiologic Studies of the Elderly (HEPESE) was initiated in 1993 to collect data on 3050 community-dwelling Mexican American elderly aged ≥65 years residing in the five southwestern states of Arizona, California, Colorado, New Mexico and Texas.18,19 Respondents completed five waves of follow-up, including mortality follow-up through 2005.
The San Antonio Heart Study (SAHS) is a prospective observational study of diabetes and cardiovascular disease (CVD) among 3301 Mexican Americans, aged 25–64 years, from randomly selected neighborhoods in San Antonio, TX.20 A 7- to 8-year follow-up examination began in 1987 and was completed in 1996. Mortality ascertainment was carried through until 1 January 2000.
The Sacramento Area Latino Study on Aging (SALSA) is a cohort of 1789 community-dwelling Mexican Americans residing in California’s Sacramento Valley, who were aged 60 or older at baseline from 1998 to 1999. Details of the sampling frame and recruitment have been published.21 Clinical data were collected about participants in home visits every 12–15 months for a total of seven follow-up visits. Mortality ascertainment is ongoing.
Study variables
Predictor variable
BMI (kg m−2) was the predictor variable of primary interest, and was calculated from measured weight and height.
Outcome variable
The outcome variable was age at death (attained age) from any cause.
Covariates
Data on sex and smoking status were included as covariates in the analysis. With the exception of HEPESE, where smoking was only available as yes or no, smoking for the remaining data sets was coded as the following: current, former or never smoker.
Missing data
Missing data were handled using a listwise deletion approach. Observations that lacked information concerning baseline age, follow-up for mortality, BMI or any pertinent covariate were removed from the analysis. NHANES III had 31 respondents with missing covariate information among the Hispanic subpopulation of 5213 people (un-weighted sample size) with mortality follow-up information. The PRHHP had 18 observations with missing smoking or BMI information. HEPESE had 329 individuals with missing BMI values; SAHS had no missing covariate information, whereas the SALSA had two individuals with missing data on sex.
Statistical analysis
We calculated hazard ratios (HRs) for the BMI-mortality rate association among Hispanic respondents using Cox proportional hazards (PH) regression models, using age as the time scale.22,23 Thus, in what follows, age refers to attained age, which sums baseline age and the available length of follow-up. Consistent with the approach used by Flegal and colleagues,24,25 we divided the data into three attained-age strata, such as 25 – <60 years, 60 < 70 years and ≥70 years, and fit separate models within each attained-age stratum. We used counting process methods to account for left truncation in these data, which sets the beginning of exposure for each participant to the age at which they entered the study.26 As it has been shown27 that adjusting for baseline age in attained-age models that account for left–truncation using age at entry has only a negligible effect on HRs (i.e., <0.040), we did not include baseline age as a covariate in the models. For all analyses, we treated BMI as a categorical variable based on federal BMI-defined body weight classifications,28 and those used by Flegal and colleagues.24,25,29 Specifically, underweight (BMI < 18.5 kg m−2), normal weight, the reference category (BMI of 18.5 – <25 kg m−2), overweight (BMI of 25 – <30 kg m−2), obesity grade 1 (BMI of 30 – <35 kg m−2), and obesity grade 2–3 (BMI of ≥35). Covariates in the models included sex and smoking status (as the PRHHP contained only men, smoking status was the only covariate in those models). The PH assumptions were evaluated in models of each database through visual inspection of scaled Schoenfeld residuals.30 The PH assumption for the effect of BMI appeared reasonable within each attained-age stratum. In situations where there was evidence for deviation from PH concerning the effect of sex or smoking, we also fitted additional models, including these factors as time-dependant covariates. In general, this did not significantly alter our estimates for the effect of BMI, and so these results are not presented.
Pooled estimates were computed using a standard weighted average, with the weights given by the inverse of the squared standard error of the study-specific estimates. Data were analyzed using either the SAS System for Windows (release 9.1; SAS Institute Inc., Cary, NC, USA) or the R Statistical Computing Environment.31 Because NHANES III involves a complex sampling design, sample weights were incorporated using SUDAAN (release 10.0.1; Research Triangle Institute, Research Triangle Park, NC, USA) and the survey package for R.32
RESULTS
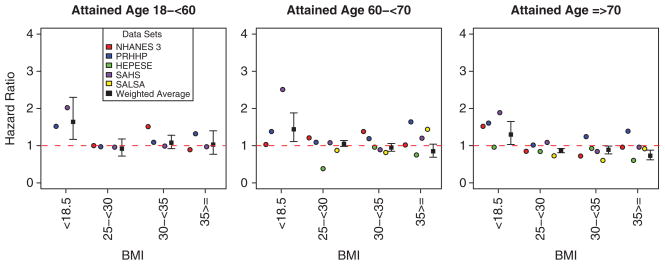
Descriptions of the five data sets used and information on their mortality data are shown in Table 1. Estimated HRs are shown in Table 2 according to BMI category, age group, dataset and a combined estimate, which incorporates the estimates across data sets (see also Figure 1).
Table 2.
HRsa and 95% CIs according to BMI category: attained age group and data set
| BMI | NHANES III | PRHHP | HEPESE | SAHS | SALSA | Combined |
|---|---|---|---|---|---|---|
| Age 18 – <60 years | ||||||
| Underweight (BMI < 18.5 kg m−2) | –b | 1.52* (1.07–2.17) | –c | 2.02b (0.49–8.30) | 1.64* (1.17–2.30) | |
| Normal weight (BMI 18.5 – <25 kg m−2) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (BMI 25 – <30 kg m−2) | 0.83 (0.5–1.38) | 0.97 (0.84–1.12) | –c | 0.96 (0.72–1.28) | 0.73 (0.58–0.93) | |
| Obesity grade 1 (BMI 30 – <35 kg m−2) | 1.51 (0.75–3.02) | 1.09 (0.88–1.34) | –c | 0.99 (0.71–1.40) | 1.08 (0.92–1.28) | |
| Obesity grade 2–3 (BMI≥35 kg m−2) | 0.89 (0.37–2.18) | 1.32 (0.70–2.48) | –c | 0.97 (0.66–1.42) | 1.03 (0.77–1.40) | |
| Age 60 < 70 years | ||||||
| Underweight (BMI < 18.5 kg m−2) | 1.03 (0.17–6.3) | 1.38* (1.04–1.83) | –b | 2.51b (1.0–6.27) | –b | 1.44* (1.11–1.88) |
| Normal weight (BMI 18.5 – <25 kg m−2) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (BMI 25 – <30 kg m−2) | 1.21 (0.46–3.24) | 1.09 (0.96–1.25) | 0.38* (0.27–0.54) | 1.08 (0.82–1.42) | 0.87 (0.50, 1.53) | 1.05 (0.98–1.14) |
| Obesity grade 1 (BMI 30 – <35 kg m−2) | 1.38 (0.48–3.95) | 1.19 (0.98–1.44) | 0.96 (0.80–1.15) | 0.89 (0.65–1.24) | 0.81 (0.46, 1.46) | 0.95 (0.85–1.06) |
| Obesity grade 2–3 (BMI≥35 kg m−2) | 1.02 (0.34–3.07) | 1.64 (0.94–2.85) | 0.75* (0.61–0.92) | 1.20 (0.85–1.72) | 1.44 (0.80, 2.59) | 0.85 (0.69–1.04) |
| Age≥70 years | ||||||
| Underweight (BMI < 18.5 kg m−2) | 1.52 (0.9–2.54) | 1.60* (1.08–2.37) | 0.95 (0.65–1.40) | 1.88b (0.44–8.04) | –b | 1.30* (1.03–1.65) |
| Normal weight (BMI 18.5 – <25 kg m−2) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (BMI 25 – <30 kg m−2) | 0.85 (0.58–1.26) | 1.01 (0.81–1.26) | 0.84* (0.76–0.93) | 1.08 (0.74–1.59) | 0.72 (0.55, 0.94) | 0.88* (0.81–0.92) |
| Obesity grade 1 (BMI 30 – <35 kg m−2) | 0.72 (0.36–1.42) | 1.24 (0.88–1.73) | 0.92 (0.81–1.05) | 0.84 (0.53–1.32) | 0.60 (0.45, 0.82) | 0.89 (0.80–0.99) |
| Obesity grade 2–3 (BMI≥35 kg m−2) | 0.96 (0.34–2.74) | 1.38 (0.44–4.35) | 0.60* (0.48–0.76) | 0.95 (0.58–1.56) | 0.91 (0.65, 1.21) | 0.73* (0.62–0.88) |
Abbreviations: BMI, body mass index; CI, confidence interval; HEPESE, Hispanic Established Population for Epidemiologic Studies of the Elderly; HR, hazard ratio; NHANES III, Third National Health and Nutrition Examination Survey; PRHHP, Puerto Rico Heart Health Program; SAHS, San Antonio Heart Study; SAHS, San Antonio Heart Study; SALSA, the Sacramento Area Latino Study on Aging.
Adjusted for sex and smoking status (PRHHP comprised of only men).
Underweight category in this age group was comprised of <5% of the total sample.
Hispanic Established Population for Epidemiologic Studies of the Elderly (HEPESE) enrolled only those aged ≥65 years at baseline.
P < 0.05.
Figure 1.
Hazard ratios for mortality in Hispanics by BMI category, age and data set.
As shown in Table 2, underweight (BMI < 18.5 kg m−2) was associated with elevated mortality rate for all attained-age groups in the PRHHP (HRs = 1.38–1.60) and the SAHS (HRs = 1.88–2.51). For the overweight category (BMI 25 – <30 kg m−2) across all attained-age groups in all the data sets, the HR estimates were <1.25. Overweight was associated with decreased mortality rate in HEPESE for those in the attained-age groups of 60 – <70 years and ≥70 years (HRs = 0.38 and 0.84), respectively. Obesity grade 1 (BMI 30 – <35 kg m−2) did not associate with mortality rate (HRs = 0.72–1.51) for any attained-age groups in any of the data sets, whereas obesity grade 2–3 (BMI ≥ 35 kg m−2) associated with decreased mortality in HEPESE for those in the attained-age groups of 60 – <70 years and ≥70 years (HRs = 0.75 and 0.60), respectively. The estimates for the other data sets for obesity grade 2 and 3 are in the order of 0.90–1.6. The combined, weighted estimates confirm that the most elevated HRs (1.3–1.6) were in the underweight category. The combined estimates for the overweight and obesity categories are in the order of 0.7–1.1.
DISCUSSION
Using data from Hispanic respondents from five well-characterized cohort studies of adults aged >18 years who were followed for an average of nearly 13 years, we found that overweight and obesity were not associated with increased mortality rate. The results from the HEPESE, a cohort study that enrolled adults aged ≥65 years at baseline, showed that overweight and obesity were associated with decreased mortality rate. Other investigators33–37 have found similar findings within older populations. In contrast, underweight was associated with increased mortality rate for all attained-age categories for the PRHHP and SAHS data sets, and for the 18 – <60 years attained-age group for NHANES III (although it should be noted that underweight respondents in SAHS and NHANES comprised <5% and 1% of the total sample size, respectively).
The association between underweight and increased mortality rate is consistent with previous BMI-mortality rate studies.38,39 Although we accounted for smoking in the models, we did not omit those who died early in follow-up to address the notion of ‘occult disease’ (i.e., undetectable diseases that might associate with both weight loss and mortality). Cancer-related weight loss tends to occur well after diagnosis,41,42 whereas other diseases (e.g., Alzheimer’s disease, Parkinson’s infections) can produce weight loss before their diagnosis. However, a number of methodological papers39,40 have shown that dropping those who die early in follow-up and/or those with recent weight loss from the analysis does not account for the elevated BMI-mortality rate association observed among the underweight.
Although seemingly paradoxical, our results are also consistent with the findings of numerous studies,43–48 which show a Hispanic-mortality-rate advantage despite the populations’ lower socioeconomic status and increased risk of health conditions such as asthma, diabetes and metabolic syndrome. The reasons for this so-called ‘Hispanic paradox’ are unknown, but do not appear to be satisfactorily explained by methodological factors such as racial and ethnic misclassification on death certificates, or migration and cultural effects pertaining to diet, lifestyle, family structure and social networks.1,44,45–51 Though highly speculative, it is also possible that culturally-driven differences in body image and body attitude52–54 may compel fewer Hispanic adults to attempt weight loss, which raises the possibility that the absence of repeated bouts of weight loss and regain (i.e., weight cycling) may contribute to a reduction in the association of BMI to mortality rate observed in this population. Although not all Hispanic subgroups have experienced similar social integration to mainstream society, the impact of acculturation, resilience and social support continue to be important areas for further study.
Strengths of this study include the use of five well-characterized data sets containing a relatively large number of Hispanic respondents, a consistent analytic approach, use of attained age and stratification to account for confounding and for effect modification by age, and the generation of weighted estimates that combine the HRs derived from the five data sets.
This study has certain limitations. First, the data sets we used were limited primarily to Mexican Americans and Puerto Rican respondents. As the Hispanic population varies markedly on variables such as socioeconomic status, and demographic and lifestyle characteristics, such as age structure, fertility, diet, and social and family networks,43–51 our results may not be representative of the Hispanic population as a whole. A related issue concerns the fact that data from the PRHHP were collected during a considerably different time period (1965–1980) than the other data sets. This introduces heterogeneity that is not accounted for in the statistical models (however, omitting the estimates derived from the PRHHP does not substantially alter the estimates). Moreover, age was available only in 4-year categories in PRHHP, which caused interval censoring of our end points in these data. We are not aware of any methods designed for handling interval censoring in Cox models stratified according to the attained age with left truncation. We acknowledge that our approach to handling this imprecision may have yielded underestimated standard errors of parameter estimates. However, we do not believe that this biased the parameter estimates. Finally, it is important to note that, consistent with most obesity-mortality studies, we generated our HRs based on the National Heart, Lung and Blood Institute (NHLBI) BMI-defined obesity categories. Although this allows for direct comparisons with estimates derived from other cohorts24,25,29, it raises the possibility that we may have obtained substantially different HRs if we had chosen other BMI-defined categories, particularly a different reference category. However, as a sensitivity analysis, we generated additional sets of HRs using the BMI-defined reference category of 23 – <25 kg m−2 (data not shown). On the whole, the HRs generated were consistent with those derived with the NHLBI reference category of 18.5 – <25. The exceptions were the larger HRs in the 18.5 – <23 kg m−2 category obtained in the 60 – <70 years attained-age category in NHANES III and the HEPESE, as well as in the combined estimates for the 18 – <60 years and 60 – <70 years attained-age categories. These results are not surprising as they likely reflect the slight elevation in risk on the left end of the U-shaped curve typically seen in BMI-mortality analyses. There were also two slightly elevated HRs in the 25 – <30 range (i.e., for HEPESE and combined estimates for the 60 – <70 years attained-age category, 1.32 and 1.17, respectively). Similarly, this likely reflects a lowering of the mortality rate in the reference category by removing individuals in the 18.5 – <23 range, individuals who tend to display an elevated mortality risk. However, given the modest magnitude of the HRs, the issue of multiple testing, confidence intervals that were wider than those obtained from the NHLBI-defined reference category, and the lack of a clear dose–response relationship between BMI >25 kg m−2 and MR, the results of the sensitivity analysis confirm that there is no clear evidence that overweight and obesity associate with elevated mortality rate among Hispanic adults.
In total, we offer BMI-mortality rate estimates that are restricted only to Hispanic respondents. Overweight and obesity do not appear to associate with elevated mortality rate among Hispanic adults. Our findings have significant public health implications as we continue to examine the pathogenic role of excess body weight in diverse populations. Although Hispanics have higher rates of obesity and diabetes, the life expectancy for Hispanics is 2.5 years longer than the life expectancy for non-Hispanic Whites and almost 8 years longer than non-Hispanic Blacks.1 Moreover, on average, Hispanics have lower median family incomes, higher poverty rate and are less likely to have a college education compared with non-Hispanic Whites; yet these are characteristics associated with better health and longer life.46–51 Thus, our findings support the need for more research to better understand the predictors of healthy long life, and how clinicians might use the results from well-characterized studies to counsel patients of diverse background about healthy weight and its relationship to chronic disease prevention, healthy life and health care expenditure.
Acknowledgments
This project was partially supported by NIH Grants, R21DK077959, T32HL072757, AG12975 and DK60753. We gratefully thank the investigators who provided us with data.
Footnotes
CONFLICT OF INTEREST
DBA has received grants, honoraria, donations, and consulting fees from numerous food, beverage, pharmaceutical companies, and other commercial, government and nonprofit entities with interests in obesity. The remaining authors declare no conflict of interest.
References
- 1.Arias E. United States life tables by Hispanic origin. National Center for Health Statistics. Vital Health Stat. 2010;2:152. [PubMed] [Google Scholar]
- 2.Population Division, US Census Bureau. Annual estimates of the population by sex, race and Hispanic or Latino origin for the United States: 1 April 2000–1 July 2004 (NC-EST2004-03) 2005. [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the US, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93 (11 Suppl 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 6.de Gonzalez AB, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 9.Hunninghake GM, Weiss ST, Celedon JC. Asthma in Hispanics. Am J Respir Crit Care Med. 2006;173:143–163. doi: 10.1164/rccm.200508-1232SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahluwalia IB, Mack KA, Murphy W, Mokdad AH, Bales VS. State-specific prevalence of selected chronic disease-related characteristics: Behavioral Risk Factor Surveillance System, 2001. Morb Mortal Wkly Rep. 2003;52:1–80. [PubMed] [Google Scholar]
- 11.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analysis of 57 prospective studies. Lancet. 2009;373:362–369. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality Follow-Up through 2006: Matching Methodology. Office of Analysis and Epidemiology; Hyattsville, MD: May, 2009. [Google Scholar]
- 14.Garcia-Palmieri MR, Feliberti M, Costas R, Jr, Colón AA, Cruz-Vidal M, Cortés-Alicea M, et al. An epidemiological study on coronary heart disease in Puerto Rico: the Puerto Rico Heart Health Program. Biol Asoc Med PR. 1969;61:174–179. [PubMed] [Google Scholar]
- 15.Garcia-Palmieri MR, Costas R, Jr, Cruz-Vidal M, Sorlie PD, Havlik RJ. Increased physical activity: a protective factor against heart attacks in Puerto Rico. Am J Cardiol. 1982;50:749–755. doi: 10.1016/0002-9149(82)91229-2. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Palmieri MR, Sorlie P, Costas R, Havlik R. An apparent inverse relationship between serum cholesterol and cancer mortality in Puerto Rico. Am J Epidemiol. 1981;114:29–40. doi: 10.1093/oxfordjournals.aje.a113171. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Palmieri MR, Sorlie PD, Havlik RJ, Costas R, Jr, Cruz-Vidal M. Urban-rural differences in 12 year coronary heart disease mortality: the Puerto Rico Heart Health Program. J Clin Epidemiol. 1988;41:285–292. doi: 10.1016/0895-4356(88)90133-3. [DOI] [PubMed] [Google Scholar]
- 18.Black SA, Ray LA, Angel RJ, Espino DV, Miranda M, Markides KS, editors. Resource Book of the Hispanic Established Population for the Epidemiological Study of the Elderly. National Archive of Computerized on Aging; Ann Arbor: 2005. [Google Scholar]
- 19.Markides KS, Rudkin L, Angel RJ, Espino DV. Health status of Hispanic elderly in the United States. In: Martin LG, Soldo BJ, Foote K, editors. Racial and Ethnic Differences in Late Life Health in the United States. National Academy Press; Washington DC: 1997. [Google Scholar]
- 20.Stern MP, Rosenthal M, Haffner SM, Hazuda HP, Franco LJ. Sex differences in the effect of sociocultural status on diabetes and cardiovascular risk factors in Mexican-Americans: the San Antonio Heart Study. Am J Epidemiol. 1984;120:834–851. doi: 10.1093/oxfordjournals.aje.a113956. [DOI] [PubMed] [Google Scholar]
- 21.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jaqust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 22.Thiébaut A, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 23.Korn E, Graubard B, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 24.Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 25.Flegal K, Graubard B, Williamson D, Gail M. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol. 2007;166:975–982. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- 26.Anderson PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 27.Gail M, Graubard B, Williamson D, Flegal K. Comments on ‘Choice of time scale and its effect on significance of predictors in longitudinal studies’. Stat Med. 2009;28:1315–1317. doi: 10.1002/sim.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute. [(accessed 25 August 2010).];Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. http://www.nhlbi.nih.gov/guidelines/obesity.
- 29.Flegal KM, Grubard BI, Williamson DF. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 30.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 31.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. http://www.R-project.org. [Google Scholar]
- 32.Lumley T. Analysis of complex survey samples. J Stat Software. 2004;9:1–19. [Google Scholar]
- 33.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 34.Fontaine KR, Heo M, Cheskin LJ, Allison DB. Body mass index, smoking, and mortality among older American women. J Wom Health. 1998;7:1257–1261. doi: 10.1089/jwh.1998.7.1257. [DOI] [PubMed] [Google Scholar]
- 35.Brill FA, Giles WH, Keenan NL, Croft JB, Davis DR, Jackson KL, et al. Effect of body mass index on activity limitation and mortality among older women. The National Health Interview Survey, 1986–1990. J Wom Health. 1997;6:435–440. doi: 10.1089/jwh.1997.6.435. [DOI] [PubMed] [Google Scholar]
- 36.Diehr P, Bild DE, Harris TB, Duxbury A, Siscovick D, Rossi M. Body mass index and mortality in nonsmoking older adults: the Cardiovascular Health Study. Am J Pub Health. 1998;88:623–629. doi: 10.2105/ajph.88.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens J. Impact of age on associations between weight and mortality. Nutr Rev. 2000;58:129–137. doi: 10.1111/j.1753-4887.2000.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 38.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- 39.Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 40.Allison DB, Heo M, Flanders DW, Faith MS, Williamson DF. Examination of ‘early mortality exclusion’ as an approach to control for confounding by occult disease in epidemiologic studies of mortality risk factors. Am J Epidemiol. 1997;146:672–680. doi: 10.1093/oxfordjournals.aje.a009334. [DOI] [PubMed] [Google Scholar]
- 41.Lankisch P, Gerzmann M, Gerzmann JF, Lehnick D. Unintentional weight loss: diagnosis and prognosis. The first prospective follow-up study from a secondary referral centre. J Intern Med. 2001;249:41–46. doi: 10.1046/j.1365-2796.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 42.Metalidis C, Knockaert DC, Bobbaers H, Vanderschueren S. Involuntary weight loss. Does a negative baseline evaluation provide adequate reassurance? Eur J Intern Med. 2008;19:345–349. doi: 10.1016/j.ejim.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Sorlie PD, Backlund E, Johnson NJ, Rogot E. Mortality by Hispanic status in the United States. JAMA. 1993;270:2464–2468. [PubMed] [Google Scholar]
- 44.Liao Y, Cooper RS, Cao G, Durazo-Arvizu R, Kaufman JS, Like A, et al. Mortality patterns among adult Hispanics: findings from NHIS, 1986 to 1990. Am J Pub Health. 1998;88:227–232. doi: 10.2105/ajph.88.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–265. [PMC free article] [PubMed] [Google Scholar]
- 46.Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Dem. 2004;41:385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- 47.Patel KV, Eschback K, Ray LA, Markides KS. Evaluation of mortality data for older Mexican-Americans: implications for the Hispanic paradox. Am J Epidemiol. 2004;159:707–715. doi: 10.1093/aje/kwh089. [DOI] [PubMed] [Google Scholar]
- 48.Turra CM, Elo IT. The impact of salmon bias on the Hispanic mortality advantage: new evidence from social security data. Pop Res Policy Rev. 2008;27:515–530. doi: 10.1007/s11113-008-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund EL. The validity of race and hispanic origin reporting on death certificates in the United States. national center for health statistics. Vital Health Stat. 2008;2:148. [PubMed] [Google Scholar]
- 50.Arias E, Eschbach K, Schauman WS, Backlund EL, Sorlie PD. The Hispanic mortality advantage and ethnic misclassification on US death certificates. Am J Pub Health. 2010;100:S171–S177. doi: 10.2105/AJPH.2008.135863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elo IT, Turra CM, Kestenbaum B, Fergusson BR. Mortality among elderly hispanics in the United States: past evidence and new results. Dem. 2004;41:109–128. doi: 10.1353/dem.2004.0001. [DOI] [PubMed] [Google Scholar]
- 52.Powell TM, de Lemos JA, Banks K, Ayers CR, Rohatgi A, Khera A, et al. Body size misperception: a novel determinant in the obesity epidemic. Arch Intern Med. 2010;170:1695–1697. doi: 10.1001/archinternmed.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petti YM, Cowell JM. An assessment of measures of body image, body attitude, acculturation, and weight status among Mexican American women. Am Acad Nurse Pract. 2011;23:84–91. doi: 10.1111/j.1745-7599.2010.00581.x. [DOI] [PubMed] [Google Scholar]
- 54.Kuchler F, Variyam JN. Mistakes were made: misperception as a barrier to reducing overweight. Int J Obes Relat Metab Disord. 2003;27:856–861. doi: 10.1038/sj.ijo.0802293. [DOI] [PubMed] [Google Scholar]