Abstract
Delirium is a common yet under-diagnosed syndrome of acute brain dysfunction, which is characterized by inattention, fluctuating mental status, altered level of consciousness, or disorganized thinking. Although our recognition of risk factors for delirium has progressed, our understanding of the underlying pathophysiologic mechanisms remains limited. Improvements in monitoring and assessment for delirium (particularly in the intensive care setting) have resulted in validated and reliable tools such as arousal scales and bedside delirium monitoring instruments. Once delirium is recognized and the modifiable risk factors are addressed, the next step in management (if delirium persists) is often pharmacological intervention. The sedatives, analgesics, and hypnotics most often used in the intensive care unit (ICU) to achieve patient comfort are all too frequently deliriogenic, resulting in a longer duration of ICU and hospital stay, and increased costs. Therefore, identification of safe and efficacious agents to reduce the incidence, duration, and severity of ICU delirium is a hot topic in critical care. Recognizing that there are no medications approved by the Food and Drug Administration (FDA) for the prevention or treatment of delirium, we chose anti-psychotics and alpha-2 agonists as the general pharmacological focus of this article because both were subjects of relatively recent data and ongoing clinical trials. Emerging pharmacological strategies for addressing delirium must be combined with nonpharmacological approaches (such as daily spontaneous awakening trials and spontaneous breathing trials) and early mobility (combined with the increasingly popular approach called: Awakening and Breathing Coordination, Delirium Monitoring, Early Mobility, and Exercise [ABCDE] of critical care) to develop evidence-based approaches that will ensure safer and faster recovery of the sickest patients in our healthcare system.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0102-9) contains supplementary material, which is available to authorized users.
Keywords: Delirium, Critical care, Aging, Mechanical ventilation, Anti-psychotics, Dexmedetomidine
Introduction
“I came awake on the fifth day. My first memory is that of floating up from the ocean bottom, my eyes still waterlogged, and with what felt like scuba gear stuffed in my mouth and throat — I couldn’t speak. As I broke to the surface, I understood that I was still in the ICU at Our Lady, but I heard nothing of what anybody said. She stayed by my side, holding my hand. I craved her touch, fearful I might sink back into the abyss where it was all dark and from which there was no promise of return” [1].
For the last 12 years, our understanding of the epidemiology and assessment of delirium has changed dramatically. Once thought to be an inevitable byproduct of sepsis and severe illness, delirium is now known to be a form of acute brain dysfunction associated with higher mortality [2–4], increased length of hospital stay [5], increased costs [6], and long-term cognitive impairment [7–10]. The development of valid and reliable instruments to assess delirium has resulted in better recognition of delirium [11, 12], although it continues to be under-recognized [13]. The focus of delirium research has been extended to include pharmacological and nonpharmacological strategies to prevent and minimize the duration of delirium. In this review, we will briefly highlight the assessment and risk factors for delirium. We will also focus on the latest work in studying two drug classes: anti-psychotics and alpha-2 agonists (and their use as pharmacotherapy for delirium). Finally, we will describe how pharmacological management of sedation and delirium can be integrated with nonpharmacological therapies, and we will propose a comprehensive “liberation and animation” strategy (i.e., liberating patients from sedation and mechanical ventilation, followed by animating them with early mobility) to improve the length of stay in the intensive care unit (ICU) and hospital, as well as survival and functional status. This approach is now being increasingly referred to as: Awakening and Breathing Coordination, Delirium Monitoring, Early Mobility and Exercise (ABCDE) of critical care, an evidence-based 5-component approach to restructuring the delivery of care in the ICU that we recently described in the medical literature [14, 15], as well as the lay press, such as the Wall Street Journal [16].
Definition, Risk Factors, and Monitoring for Delirium
Delirium is described in the DSM IV-TR as an acute confusional state characterized by fluctuating mental status, inattention, and either altered level of consciousness or disorganized thinking [17]. Delirium is common, particularly in the ICU, where it has been shown to occur in 60% to 80% of ventilated patients [2, 6, 18, 19] and 40% to 60% of nonventilated patients [20, 21]. For the last few decades, delirium has been recognized, yet it has been described with inconsistent terminology, such as ICU syndrome, acute brain dysfunction, acute brain failure, and septic encephalopathy [22], depending on geographic location and specialty training. Delirious patients can present in a number of ways, and the general consensus is to subcategorize the delirium of a patient according to their level of alertness (hyperactive, hypoactive, and mixed delirium) [23]. Although the hyperactive subtype of delirium is the easiest to detect, pure hyperactive delirium (i.e., without hypoactive component) only represents approximately 5% of delirium in the ICU [24]. The hypoactive subtype of delirium is the most common (60%) and the mixed subtype is also frequently found.
Risk factors for the development of delirium in the critical care and postoperative settings have been previously described in the literature [25–30]. Broadly, risk factors can be divided into 3 categories: 1) characteristics of the acute illness itself, 2) patient or host factors, and 3) environmental or iatrogenic factors. There are 3 popular mnemonics for consideration of risk factors of delirium, which include THINK, IWATCHDEATH, and ICUDELIRIUMS (Table 1) (see: http://www.mc.vanderbilt.edu/icudelirium/terminology.html). A couple of very common risk factors are of particular importance. First, in addition to the intrinsic illnesses of patients, such as severe sepsis or congestive heart failure, iatrogenic medications (or the combination of multiple medications) must be considered as a contributing and modifiable factor in the emergence of delirium. Medications notoriously associated with delirium include opiates (especially meperidine), sedatives including benzodiazepines, anticholinergics, antihistamines, antibiotics, corticosteroids, and metoclopramide. Lorazepam and midazolam (benzodiazepines commonly used in the ICU for sedation) were shown to be independent risk factors for transitioning into delirium (odds ratio, 1.2, 95% confidence interval (1.1, 1.4); p = 0.003) [25, 31]. Recently, genetic predisposition to delirium was demonstrated, as the apolipoprotein E4 (APOE4) genotype was found to be a strong predictor of delirium duration after adjusting for age, severity of illness, duration of coma, and admission diagnosis of sepsis, acute respiratory distress syndrome, or pneumonia (odds ratio, 7.32; p = 0.005) [32, 33]. Conflicting reports related to the apolipoprotein E4 (APOE4) genotype [34] reinforce the need to study this association further.
Table 1.
Mnemonics for risk factors, causes, differential diagnosis for delirium
| THINK | ||
| T | = | Toxic situations; congestive heart failure, shock, dehydration, deliriogenic medications (tight titrations), new organ failure |
| H | = | Hypoxemia; also consider giving haloperidol or other antipsychotic? |
| I | = | Infection/sepsis (nosocomial), Immobilization |
| N | = | Nonpharmacological interventions; consider hearing aids, glasses, reorient, sleep protocols, noise control, ambulation |
| K | = | K+ or other electrolyte/metabolic problems |
| ICUDELIRIUMS | ||
| I | = | Iatrogenic exposure; diagnostic procedure, therapeutic intervention |
| C | = | Cognitive impairment; pre-existing dementia, mild cognitive impairment (MCI), or depression |
| U | = | Use of restraints and catheters; re-evaluate use daily |
| D | = | Drugs; use of sedatives (benzodiazepines, opiates), anticholinergic medications, abrupt cessation of smoking or alcohol; withdrawal from chronically used sedatives |
| E | = | Elderly; evaluate patients older than 65 years with greater attention |
| L | = | Laboratory abnormalities; especially hyponatremia, azotemia, hyperbilirubinemia, hypocalcemia, metabolic acidosis |
| I | = | Infection; sepsis, urinary tract or respiratory tract infections |
| R | = | Respiratory; consider respiratory failure, chronic obstructive pulmonary disorder, acute respiratory distress syndrome, pulmonary embolus (PE) |
| I | = | Intracranial perfusion; consider hypertension or hypotension, hemorrhage, stroke, tumor |
| U | = | Urinary/fecal retention, especially in elderly and in postoperative patients |
| M | = | Myocardium; consider myocardial infarction, acute heart failure, arrhythmia |
| S | = | Sleep and sensory deprivation; sleep-cycle disturbance, need for glasses or hearing devices |
| IWATCHDEATH: Differential Diagnosis of Delirium | ||
| Infection | = | HIV, sepsis, pneumonia |
| Withdrawal | = | Alcohol, barbiturates, sedative hypnotic |
| Acute metabolic | = | Acidosis, alkalosis, electrolyte disturbance, hepatic failure, renal failure |
| Trauma | = | Closed head injury, heat stroke, postoperative, severe burns |
| Central nervous | ||
| system | ||
| pathology | = | Abscess, hemorrhage, hydrocephalus, subdural hematoma, seizures, stroke, tumors, vasculitis, encephalitis, meningitis, syphilis |
| Hypoxia | = | Anemia, carbon monoxide poisoning, hypotension, pulmonary/cardiac |
| Deficiencies | = | Vitamin B12, folate, niacin, thiamine |
| Endocrinopathies | = | Hyperadrenocorticism, hypoadrenocorticism; hyperglycemia, hypoglycemia; myxedema, hyperparathyroidism |
| Acute vascular | = | Hypertensive encephalopathy, stroke, arrhythmia, shock |
| Toxins or drugs | = | Prescription drugs, illicit drugs, pesticides, solvents |
| Heavy metals | = | Lead, manganese, mercury |
*http://www.mc.vanderbilt.edu/icudelirium/terminology.html
(Adapted with permission from: Marta Render, MD, Veterans Affairs Inpatient Evaluation Center [IPEC])
The comatose patient is distinguishable from the delirious patient in that the comatose patient does not respond to verbal commands. The delirious patient can be aroused through vocal commands, although they may not be able to follow them. The cardinal feature of delirium is inattention. Other symptoms (such as hallucinations or delusions) can be present and associated with delirium, but they are not required for the diagnosis. Patients with some, but not all, of the clinical symptoms of delirium are described as “subsyndromal,” and their associated outcomes are intermediate between normal and clinically delirious patients [35]. One may think of subsyndromal delirium as an “intermediate state of badness” lying between full delirium and no delirium.
Recognition and management of delirium in critical care have progressed rapidly during the last decade due to clinical tools that have proven valid, reliable, and easy to replicate. The Society of Critical Care Medicine (SCCM) has recommended two validated tools to monitor for delirium in the ICU [36]. One clinically validated system is the Intensive Care Delirium Screening Checklist (ICDSC) [37]. This screening checklist, based on DSM criteria and delirium features, evaluates patients on an 8-point scale, including altered level of consciousness, inattention, disorientation, psychomotor changes, sleep/wake cycle disturbances, and symptom fluctuation. Patients with greater than 4 points on the scale are said to be delirious, allowing for dichotomous measurements and analysis, with a sensitivity of 99% and specificity of 64% [37]. In 2001, the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) was shown to have high sensitivity (93–100%), high specificity (98–100%), and high inter-rater reliability (kappa = 0.96) [11]. In multiple other investigations since that time, the sensitivity and specificity of the CAM-ICU in high severity of illness and in patients on mechanical ventilation have been repeatedly shown to be high [4, 38–41]. In lower severity of illness states, situations of infrequent in-servicing or re-calibration of staff, or non-ICU settings, the specificity has remained high (>90%), although the sensitivity may be sacrificed by use of this instrument (shown to be near 50% in some studies) [42, 43]. In such circumstances, it is best to spend longer than just the average 1 minute that it takes to do the CAM-ICU, and, instead to generate higher sensitivity, it should be considered necessary to invest upward of 10 minutes with the evaluation, as is often done with other instruments, such as the full CAM or DRS-R98 [44, 45]. The CAM-ICU is used in combination with an “arousal scale,” such as the Riker Sedation-Agitation Scale (SAS) [46, 47], or the Richmond Agitation-Sedation Score (RASS) [48, 49] to create a “sister-instrument” 2-step approach to the examination of consciousness, summarized as a CAM-ICU flowsheet or algorithm that has a number of advantages [12]:
This algorithm provides a framework for assessing sedation and delirium that can be performed rapidly, in less than 2 minutes.
This protocol has its greatest usefulness in tracking changes in the neurological status of the patient with the duration of time, and by comparing the current RASS or SAS score alongside the stated target RASS or SAS score for adjusting potent sedative and narcotic medications.
Use of delirium assessment tools, such as the CAM-ICU, have been shown to result in shorter duration and lower doses of drugs, such as haloperidol used in the ICU [50].
The assessment can be performed by all members of the treatment team (i.e., nurses, technicians, Doctors of Pharmacy, and physicians alike).
This sedation and delirium assessment has been successfully implemented and replicated in medical, surgical, trauma, burn, cardiovascular, neurological, and pediatric ICU settings across medical centers [42, 51–53].
The RASS and CAM-ICU assessments have been used in multiple high-profile randomized prospective trials [18, 54, 55]. The CAM-ICU instrument, which has been translated into many languages, can be accessed online at: http://www.mc.vanderbilt.edu/icudelirium/docs/CAM_ICU_flowsheet.pdf.
By combining an arousal scale and a delirium monitoring tool, the managing team can develop a “brain road map” that asks the following questions for each patient: 1) Where is the patient going? 2) Where is the patient currently? and 3) How did the patient get to their present state? These questions can then be answered with 4 simple indicators:
Target the RASS. Where is the patient going?
Determine the actual RASS. Where is the patient now?
Determine the CAM-ICU. Where is the patient in thought content now?
Assess the current drugs. How did the patient get to his or her present state?
Using a standardized, systematic approach on a daily basis to follow this critical “sixth vital sign” [56] can assist the treatment team in tracking the progress of the patient with the duration of time.
The Pathophysiology of Delirium, Biomarkers, and the Role of Neuroimaging
Despite improved diagnosis and multiple potential treatments, the pathophysiological mechanisms of delirium remain unclear [57, 58]. Hypotheses generated outside the ICU posit that delirium results from a decrease in acetylcholine and increase in dopamine [59, 60]. Other hypotheses include hypoxia, neurotransmitter imbalance [61], inflammation (related to the systemic inflammatory response syndrome or sepsis), aberrant stress responses [58], and toxic or metabolic derangements resulting in encephalopathy.
The distinction between delirium and septic encephalopathy is uncertain at this time, as some argue that sepsis-associated encephalopathy is a different pathophysiological process that may overlap with clinical signs of delirium [62]. Many consider septic encephalopathy a common type of delirium that is rooted in the underlying pathophysiological underpinnings of the septic state (systemic inflammatory and coagulopathic disturbances). The reality is that most septic patients will develop acute brain injury that is likely due to the underlying acute disease coupled with iatrogenic medications and comorbid features, such as hypoxemia and hypoperfusion. An important teaching point is that CAM-ICU or ICDSC, or any other delirium tool, reports the positive or negative features of delirium only, and not the etiology. Once the diagnosis is made, it is then incumbent on the ICU team to consider the differential diagnosis and to make appropriate treatment plans, which often do not include starting a new medication, but rather entail stopping potentially offending medications and using a full armamentarium of nonpharmacological decisions to help the patient recover from the global brain dysfunction that is manifesting itself as delirium.
Two emerging areas of research that may contribute to our understanding of the pathophysiology of delirium include biomarkers and neuroimaging. A recent systematic review of biomarkers in cerebrospinal fluid [63] found no consistent evidence yet of biomarkers associated with delirium; however, implicated derangements include elevated serotonin metabolites, lactate, cortisol (particularly after cardiac surgery) [64], interleukins such as interleukin-6 [65], cortisol [65], brain-specific protein, S100-β, and elevated acetylcholinesterase. Other serum biomarkers that have shown promise include procalcitonin and C-reactive protein [66]. This remains an exciting area of research, as the establishment of biomarkers associated with delirium could aid in both understanding the pathophysiology of delirium and also predicting those patients most at risk for the development of delirium. Another under-studied area of research is neuroimaging. Currently neuroimaging in a delirious patient is mainly conducted in the clinical arena to rule out macro-injury, such as hemorrhage or stroke. Research efforts can incorporate newer techniques, such as functional magnetic resonance imaging, diffusion tensor imaging, arterial spin labeling, and even positron emission tomography, and single-photon emission computed tomographic scanning [67]. The majority of studies have shown nonspecific or diffuse changes in time, such as cortical atrophy, ventricular enlargement, and white matter hyperintensities [68]. These preliminary data support the hypothesis that the majority of patients in the ICU who are suffering from delirium do not undergo macro-injury, but rather micro-injury that is difficult, if not impossible, to capture early using computed tomography or magnetic resonance imaging. This hypothesis remains to be proven and requires further investigation.
Pharmacological Treatment of Delirium
Anti-Psychotics
Haloperidol (used by 75–80% of intensivists) and atypical anti-psychotics (used by 35–40% of intensivists) have emerged as the standard pharmacological treatments for delirium in the ICU [69–71]. This is due in large part to the recommendations of the Society of Critical Care Medicine for the treatment of delirium in 2002 [36]. Whereas there is some evidence suggesting haloperidol may reduce delirium duration [72] and mortality [73], an overall dearth of data is emphasized in systematic reviews [74–77] concluding: 1) “there are no published double-blind, randomized, placebo-controlled trials to establish [the] efficacy or safety of any anti-psychotic medication in the management of delirium. Further study with well-designed clinical trials is required in this area” [75]; 2) “Better designed and larger studies evaluating adding anti-psychotic agents to nonpharmacological treatments are needed” [74]; 3) “Small studies of limited scope require further evidence before translation into specific recommendations for delirium treatment” [76].
Pharmacology of Anti-Psychotics
The main mechanisms of action of haloperidol are thought to be antagonism at cortical dopamine (D2) receptors [78–81], nigrostriatal pathway D2 blockade, and disinhibition of acetylcholine (i.e., acetylcholine increase) [60]. As a butyrophenone-derived anti-psychotic, haloperidol binds with a high affinity at D2 receptors, relatively low affinity at D1 receptors, and it exhibits little adrenergic or muscarinic activity compared to lower potency neuroleptics. Positron emission tomographic studies have shown that even at low doses (2–4 mg/day), haloperidol induces D2 receptor occupancies (53–74%) in a therapeutic range, suggesting that low doses can be used to avoid side effects observed with high doses [80]. The proposed anti-inflammatory effects of haloperidol may also help mitigate organ dysfunction in critical illness [73, 82]. Atypical anti-psychotics are distinguished from the neuroleptic anti-psychotics, such as haloperidol, due to their reduced affinity for dopamine receptors, particularly D2 receptors, and they also have a wider variety of reported affinities for other central nervous system receptors, including serotonin, adrenergic, and muscarinic receptors [79, 83]. For example, an atypical anti-psychotic named ziprasidone is an antagonist at D2, H1, α1, 5-HT2A, 5-HT2C, and 5-HT1D and an agonist at 5-HT1A receptors [79, 81, 83, 84]. A high 5-HT2A/D2 receptor affinity ratio, which is a particularly strong characteristic of ziprasidone, has been correlated with lower propensity for extrapyramidal symptoms (EPS) and may be advantageous in treating the negative symptoms so prevalent in older hypoactive delirious patients [24, 85]. The strikingly divergent receptor affinities of these two classes of anti-psychotics are remarkable, considering the fact that both anti-psychotics are used, based primarily on anecdotal evidence, in widely varying rates around the world for treatment of delirium.
Haloperidol is administered intravenously or intramuscularly in the critical care setting [86]. Both methods have high bioavailability (~100%). Haloperidol is extensively protein-bound (free fraction in human plasma, 7.5–11.6%) and rapidly distributed throughout the body (Vd 9.5–21.7 L/kg). The mean half-life of haloperidol is 21 hours. It is extensively metabolized by the liver, with only 1% excreted unchanged in urine. Both CYP3A4 and CYP2D6 hepatic enzymes have been implicated in haloperidol metabolism. Haloperidol metabolism can be affected by other drugs extensively metabolized by CYP3A4, such as carbamazepine, phenobarbital, rifampin, and quinidine [86], although these medications are not typically used in the critical care setting.
Haloperidol and Delirium
Haloperidol began to gain popularity for use in the intensive care setting in the early 1990s when Riker et al. [87] demonstrated that continuous infusion of haloperidol could successfully treat agitation while reducing requirements for sedatives and nursing attention. The evidence for haloperidol became stronger in 2005, when Milbrandt et al. [73] published a retrospective cohort analysis of 989 patients, which found that mechanically ventilated patients who received haloperidol had significantly lower hospital mortality compared with those who never received haloperidol (p = 0.004), suggesting a survival benefit. Another study by Kalisvaart et al. [72] tested the efficacy of low-dose haloperidol in elderly hip surgery patients that were at risk for delirium. This study found no efficacy in reducing the prevalence of postoperative delirium, but it did demonstrate a positive effect on the duration and severity of delirium, with reduction in the mean number of hospital days (p = 0.001).
Most recently, Wang et al. [88] tested whether low-dose prophylactic intravenous administration of haloperidol could reduce the incidence of postoperative delirium. Four hundred fifty-seven patients admitted to the ICU after noncardiac surgery were included in this randomized, double-blind, placebo-controlled trial and followed for 7 days after surgery. The incidence of postoperative delirium in the haloperidol group was 15.3% compared to 23.2% in the control group (p = 0.031). The intervention was well-tolerated, as no drug-related side effects were reported.
Atypical Anti-Psychotics
Beginning in 2004, a number of trials were published comparing the safety and efficacy of atypical anti-psychotics to haloperidol. The logic was simple: atypical anti-psychotics may be as efficacious for delirium and were associated with less EPS or side effects compared with haloperidol and other neuroleptic anti-psychotics [75, 76]. Skrobik et al. [89] demonstrated that olanzapine was a safe alternative to haloperidol for delirious patients in the ICU with similar clinical improvement and less EPS. Similar results have been demonstrated in smaller, non-placebo-controlled studies with amisulpride [90], quetiapine [90, 91], and risperidone [92, 93]. Recently, a prospective, single-blind, randomized controlled trial compared the efficacy and safety of haloperidol, olanzapine, and risperidone in the treatment of delirium with 20 to 23 patients in each arm of the study [94]. It showed that risperidone and olanzapine were as efficacious as haloperidol, with significant improvement in DRS-R98 severity scores and lower mini-mental status exam (MMSE) scores in all three groups. No difference was found between the three groups in terms of delirium reduction or side effects.
In 2010, two prospective, multicenter, randomized, double-blind, placebo-controlled pilot studies were published that laid the groundwork for future work. In a pilot study of 36 ICU patients, Devlin et al. [95] demonstrated that quetiapine added to haloperidol, administered as needed, resulted in a reduced duration of delirium (p = 0.006), less agitation, and greater rate of transfer to home or rehabilitation. Patients treated with quetiapine also required fewer days of haloperidol as needed (p = 0.05). Another pilot study, published by Girard et al. [96] called Modifying the Incidence of Delirium (MIND) trial, sought to demonstrate the feasibility of conducting a placebo-controlled trial with both haloperidol and ziprasidone as the prophylaxis/treatment of ICU delirium. Although the trial was designed to mainly demonstrate feasibility and not powered for efficacy, it laid the groundwork for a future phase III trial to compare anti-psychotics versus a placebo for ICU delirium, a trial now funded by the National Institutes of Health and currently underway.
Safety Profile of Anti-Psychotics
In early studies, the adverse effects of haloperidol appeared to exceed those of atypical anti-psychotics [97, 98], but recent data on the adverse effects of both typical and atypical anti-psychotics, such as EPS, akathisia, neuroleptic malignant syndrome, tardive dyskinesia, glucose and cholesterol abnormalities, cardiac dysrhythmias, and venous thromboembolism, suggest the varying side effects of these medications counterbalance one another [99–108]. In general, the safety profiles of typical and atypical anti-psychotics for older patients (especially the demented elderly) have been called into question, regardless of class [108–115].
Anti-psychotics pose a “cross-class” risk of torsades de pointes mediated by the prolongation of the corrected QT (QTc) interval of some patients [102, 116–123]. Recent reports of sudden cardiac death [92, 102, 110–114] underscore the need to conduct further placebo-controlled clinical trials, because these agents are being used rampantly in the ICU [69, 70]. Haloperidol has the lowest ratio of cardiac death among typical and atypical agents [121, 124].
Future Directions Related to Anti-Psychotics and Delirium
In the future, it is imperative that the safety and efficacy of both haloperidol and the atypical anti-psychotics be determined in a placebo-controlled prospective trial. Justification for a placebo-controlled design rests on 4 distinct and firm issues. First, most delirium patients in the ICU can be described as the hypoactive subtype (especially in older patients), which goes unrecognized and thus untreated 65% to 75% of the time in standard practice [13, 125]. Even though delirium monitoring rates in the ICU are 33% and rising [69, 70], most delirium in critically ill patients is not recognized or treated, functionally receiving “placebo” in practice. Second, there are no approved medications by the FDA for the treatment of delirium. Other than the pilot trials previously mentioned [95, 96], there are no placebo-controlled trials of any medications to treat delirium in these high-risk patients (e.g., older medical/surgical ICU patients). Third, it is possible that typical and atypical anti-psychotics could induce harm. Thus, they must be compared to a placebo, lest we persist in ignorance regarding a fundamental piece of medical knowledge. Fourth, and perhaps most importantly, the definitive study must include a placebo comparison to account for spontaneous improvements in delirium.
Alpha-2 Agonists: Clonidine and Dexmedetomidine
Increasingly, the sedatives and hypnotics most commonly used in the ICU have demonstrated potentially deliriogenic effects. As a result, there has been great interest in finding alternative means for sedation and analgesia, and the alpha-2 agonists have shown great promise as a safe pharmacotherapeutic approach. Clonidine has been used for more than 35 years to treat hypertension and withdrawal from alcohol, cocaine, and opioids. Dexmedetomidine is a relatively new alpha-2 agonist that was approved by the FDA in 1999. We will review the pharmacology of these drugs, as well as the growing body of evidence that dexmedetomidine may be a safe and efficacious complement to other sedatives and hypnotics while reducing the incidence and duration of delirium.
Pharmacology of Alpha-2 Agonists
Gregoretti et al. [126] describes the alpha-2 receptor as “the body’s most important presynaptic receptor.” This presynaptic activation results in reduced norepinephrine release, inducing sympatholysis [127]. The alpha-2 adrenergic receptor differs in its pharmacological properties compared to the other adrenergic receptors (alpha-1, beta-1, beta-2). The alpha-2 receptor can be further broken into 3 subtypes: 1) alpha-2a, 2) alpha-2b, and 3) alpha-2c (and these subtypes help explain the different downstream effects). Alpha-2a agonism has been associated with sympatholysis, hypnosis, sedation, analgesia, and general neuroprotective effects [127, 128]. Alpha-2 receptors demonstrate greatest density in the locus ceruleus, as well as the intermediolateral cell column and substantia gelatinosa of the spinal cord. Alpha-2b agonism promotes analgesic effects in the spinal cord, as well as central shivering, whereas alpha-2c has been implicated in post-traumatic stress disorder and physiologic drug withdrawal response [129]. The alpha-2 receptors are G-protein coupled receptors that modulate transmembrane ion channel activity. The differing pharmacodynamic effects of the alpha-2 receptors, therefore, have been attributed to their differing receptor subtypes, various effector mechanisms, and locations within the brain and other organs. The neurospecificity of dexmedetomidine for the alpha-2 receptor is greater than that of clonidine, with a 1620:1 alpha-2/alpha-1 binding ratio [127]. Both clonidine and dexmedetomidine include an imidazole ring moiety that interacts with imidazole receptors in the ventrolateral medulla. This may explain the resulting reduced central adrenergic activity, vasodilatory changes, and bradycardia [126].
Dexmedetomidine has a quick intravenous onset of action of approximately 15 minutes, with peak concentrations within 1 h of starting continuous infusion [127]. The drug is highly bound to protein in the serum, 6% free fraction, with steady state volume of distribution Vd 1.33 L/kg. It is hepatically cleared via glucoronide conjugation and the Cytochrome P450 pathway.
Dexmedetomidine and Delirium
Dexmedetomidine is an effective hypnotic, sedative, and analgesic agent with minimal respiratory depression. It is unique in that it exhibits “cooperative sedation,” allowing arousal while maintaining deep levels of sedation [127]. In addition to protective respiratory effects, dexmedetomidine seems to mimic natural sleep better than the gamma-amino butyric acid (GABA)-ergic agents. This is attributed to its effect in the locus ceruleus where stimulation of alpha-2 receptors leads to inhibition of noradrenergic neurons and disinhibition of GABA-ergic neurons in the ventrolateral preoptic nucleus [130]. Finally, dexmedetomidine may have cardioprotective and neuroprotective effects (through decreased cerebral blood flow) [131], particularly in the perioperative setting, although at this point the various effects of dexmedetomidine through alpha-2 agonism remain poorly understood and require further elucidation.
For the last decade, dexmedetomidine has emerged as a sedative-analgesic agent that may potentially reduce the rate of delirium in the critical care setting. Recent clinical trials have compared this newer medication to those currently used as mainstays in the ICU with promising results. Our present analysis focuses on dexmedetomidine rather than clonidine, given that more clinical data is available on dexmedetomidine. However, we recognize that there is interest across the world in the use of clonidine in the perioperative and critical care setting [126].
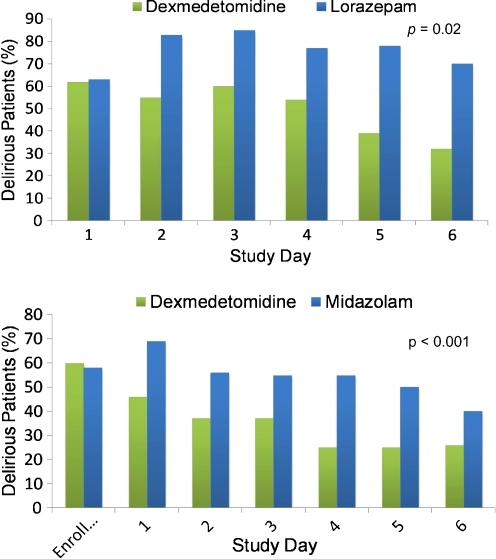
In 2007, Pandharipande et al. [18] published the Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) randomized controlled trial comparing dexmedetomidine with lorazepam for sedation greater than 24 h (the original amount of time [24 h] as indicated for dexmedetomidine approved by the FDA). There were 106 mechanically ventilated patients who were enrolled in the investigation, and target sedation was achieved using the RASS and CAM-ICU. This was the first randomized controlled trial to show that acute brain dysfunction could be reduced through the choice of a sedative. Sedation with dexmedetomidine resulted in more days alive without delirium or coma (p = 0.01) and lower prevalence of coma (p < 0.001) than sedation with lorazepam. A subgroup analysis of the database from the study, which was planned a priori, demonstrated that sedation with dexmedetomidine reduced the daily risk of delirium as well (p = 0.02) [132] (Fig. 1a), and this effect was most prominent in patients with severe sepsis.
Fig. 1.
(a) A priori subgroup analysis of the Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) randomized controlled trial. Compared to sedation with lorazepam, sedation with dexmedetomidine resulted in a markedly reduced daily risk of delirium (p = 0.02). As noted in this article, this relationship was even more pronounced in patients with severe sepsis. Note that both groups start with 60% prevalence of delirium in keeping with most cohorts of mechanically ventilated patients, and then there is a large difference in delirium prevalence throughout the remainder of the study period. Adapted from Pandharipande, et al. [132]. (b) In the Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial comparing dexmedetomidine to midazolam for sedation of mechanically ventilated patients, the prevalence of delirium in dexmedetomidine-treated patients was significantly less than midazolam-treated patients (54% vs 76.6%; p < 0.001). As in (a), both groups started with 60% prevalence of delirium and then showed a large difference in delirium prevalence throughout next week. These (a) and (b) pose the unanswered question as to whether or not it is the avoidance of a gamma-amino butyric acid (GABA)-agonist or the receipt of an alpha-2 agonist that resulted in such marked delirium reduction. Adapted from Riker, et al. [55]
The Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial [55] compared dexmedetomidine to midazolam, a commonly used short-acting benzodiazepine. This study enrolled 375 mechanically ventilated patients at 68 centers. The primary endpoint was percentage of time within the target RASS range, and no difference was observed, again supporting that in a very sick population of mechanically ventilated patients, dexmedetomidine was as effective as traditional approaches at hitting target sedation goals. Perhaps the most interesting finding of the double-blinded SEDCOM study was that the prevalence of delirium in dexmedetomidine-treated patients (while starting balanced at enrollment rates of 60%) was significantly lower within 1 day than in those treated with midazolam (54% vs 76.6%; p < 0.001) (Fig. 1b), and this relationship was consistent throughout a week of study drug administration. These 2 figures (1a and 1b) pose the question, as yet unanswered, as to whether or not it is the avoidance of a GABA-agonist or the receipt of an alpha-2 agonist that resulted in such marked delirium reduction. From a safety perspective, the risk:benefit ratio is important to consider, and this is informed by the fact that those treated with dexmedetomidine were more likely to develop bradycardia (p < 0.001), although the number requiring treatment for bradycardia was not significant.
The DEXCOM study by Shehabi et al. [133] compared dexmedetomidine with morphine-based therapy in 306 patients after cardiac surgery. The primary outcome of the study was prevalence of delirium measured via the CAM-ICU. Although the incidence of delirium between the 2 groups was not statistically significant, the duration of delirium was 3 days fewer in those receiving dexmedetomidine versus morphine (p = 0.0317). Dexmedetomidine-treated patients also experienced less hypotension, required less epinephrine, and were more likely to be extubated earlier. Again, the incidence of bradycardia in those receiving dexmedetomidine was higher than those receiving morphine (p = 0.006).
Ruokonen et al. [134] compared dexmedetomidine with the standard care of propofol or midazolam for long-term sedation during mechanical ventilation. This was done with a noninferiority, double-blind, multicenter study of 85 patients in medical and surgical ICUs expected to have ICU stays greater than 48 h and requiring sedation for greater than 24 h. Dexmedetomidine was found to be comparable to standard care for light sedation, and possibly less suited for patients requiring deeper sedation. Post hoc analyses suggested shorter duration of mechanical ventilation in those receiving dexmedetomidine (p = 0.025). Overall, dexmedetomidine-treated patients were more communicable and more arousable, which reflects on an underappreciated aspect of patient care that ought to be more commonly discussed and focused on when studying different sedation regimens and approaches.
The Acute Neurological ICU Sedation Trial (ANIST) by Mirski et al. [135] compared the use of dexmedetomidine and propofol in sedating patients without compromising arousal or cognition. This small, randomized, double-blind trial included both brain-injured and non-brain-injured intubated patients. Use of dexmedetomidine was associated with improved Adapted Cognitive Exam scores compared with propofol by 19.2 points (p < 0.001), supporting that dexmedetomidine may allow for improved mental engagement (as seen in Ruokonen et al.’s [134] study mentioned previously).
Finally, 1 small open-label trial by Reade et al. [136] compared dexmedetomidine with haloperidol for the treatment of agitated/hyperactive delirium while facilitating extubation. Dexmedetomidine was found to significantly shorten the median time to extubation (p = 0.016), decrease ICU length of stay (p = 0.004), and reduce by half the proportion of time patients required propofol while intubated (p = 0.05).
After reviewing the results of randomized clinical trials (2 alone of which were from the Journal of the American Medical Association, which included nearly 500 patients), the question remains: What is the best explanation of why dexmedetomidine was found associated with improved or reduced delirium rates and increased the days free of acute brain dysfunction? Also, in what manner should these results contribute to a change in clinical practice? Perhaps the difference in rates of delirium were due merely to intensivists achieving sedation goals with dexmedetomidine better than in the past rather than the choice of agent. This theory will be better understood as data on propofol and delirium become available. In the meantime, 2 other theories possibly explain these findings. First, perhaps it was the avoidance of more deliriogenic agents, such as benzodiazepines and opioids that resulted in reduced delirium rates with dexmedetomidine rather than a protective effect of dexmedetomidine against delirium. An alternative hypothesis was that sedation with dexmedetomidine results in more natural sleep physiology compared to GABA-ergic agents. Sleep deprivation, which is common in the ICU setting, has been associated with delirium and similar clinical manifestations [137, 138]. In addition, the action of dexmedetomidine in promoting its sedative effect has been linked to sleep-promoting neural pathways [139]. Clearly much work remains to be done in better understanding these underlying mechanisms and clinical outcomes.
Safety Profiles Related to Dexmedetomidine and Clonidine
The widespread use of clonidine as an anti-hypertensive is limited by its well-known propensity to cause rebound hypertension and agitation. Cessation of dexmedetomidine does not appear to be associated with rebound withdrawal; nevertheless, it was initially approved by the FDA for short-term use (<24 h) due to the concern for rebound effects. As previously noted in multiple trials, dexmedetomidine is known to cause hypotension and bradycardia in some patients. Dexmedetomidine should be used with caution in patients with a history of cardiac disease, overt congestive heart failure, advanced liver disease, or patients on sympatholytic or cholinergic agents [127]. More studies are needed to further characterize the risk factors for developing bradycardia in patients receiving dexmedetomidine to guide clinical decision-making.
The duration and dose of dexmedetomidine, as well as the safety profile, must be considered. First of all, the FDA package for this medication is limited by data derived from 24-h administration studies that only included a limited dose. In the recent ICU studies, this dose and duration were increased in conjunction with FDA oversight (maximum dose doubled and duration not limited to 24 h, but more realistically up to 5 days in 1 study or for the duration of mechanical ventilation in another) [18, 55]. Those doses showed no new toxicities or safety concerns, but confirmed the risk of bradycardia, which was a significant finding in both the Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) and the SEDCOM studies. In general, when a patient develops clinically significant bradycardia while on dexmedetomidine, the drug should be stopped, and its short half-life usually renders the bradycardia short-lived. Sometimes we restart the drug at a lower dose or abandon its use in the patient altogether.
Conclusions Related to Alpha-2 Agonists
There is mounting evidence that dexmedetomidine may be an acceptable alternative to opioids and GABA-ergic medications in addressing the sedative, analgesic, and hypnotic needs of critically ill patients in the ICU. One benefit of dexmedetomidine is the lack of respiratory depression, even at high doses. Bradycardia and hypotension are the two side effects that must be considered.
Costs associated with the ICU can be significant and should be considered in the management of delirium and choice of sedation. When studies have matched dexmedetomidine to other sedative agents and compared length of ICU stay, costs of mechanical ventilation, and drug acquisition costs, dexmedetomidine was not found to be associated with significantly increased total cost [18], and in one study it was associated with decreased total ICU costs when used for long-term sedation compared with midazolam [140].
It must be emphasized, we are not suggesting that dexmedetomidine is the solution for all patients in the ICU, or that dexmedetomidine is a “better” drug than any others used in the ICU setting. Rather, alpha-2 agonists, such as dexmedetomidine may play a complementary role as part of a comprehensive strategy to address the complex needs of the critically ill patient. This is particularly true for patients in whom opioids and benzodiazepines may present additional risk factors for the development or exacerbation of delirium [21, 31, 141].
Other Pharmacological Treatments for Delirium
Other pharmacological strategies to addressing delirium have shown no advantage to date. Of particular disappointment are the acetylcholinesterase inhibitors, such as donezepil and rivastigmine [142]. This theory was understandably based on the popular cholinergic hypothesis of delirium and the observation that anticholinergic medications can readily worsen the delirious patient. Unfortunately, these medications, which have some usefulness in the treatment of dementia, have not panned out for the delirious elderly. In 2010, van Eijk et al. [143], in a multicenter, double-blind, placebo-controlled randomized trial, it was demonstrated that rivastigmine added to usual care with haloperidol did not decrease duration of delirium. In fact, the trial was stopped early due to futility and the concern of increased duration of delirium (p = 0.06) and increased mortality (p = 0.07) in the rivastigmine intervention group.
Intriguingly, statins have been suggested as a potential new therapeutic in the treatment of delirium [144]. This hypothesis is based on the observation that delirium is associated with a proinflammatory state resulting in microglia activation, ischemic/hemorrhagic lesions, neuronal apoptosis, and interruption of the blood-brain barrier [144]. Statins have been shown in mice models to favor a switch toward an anti-inflammatory phenotype that may encourage neuronal healing rather than damage [145, 146], and thereby decrease the long-term effects of cognitive dysfunction. This observation correlates with clinical observations by Katznelson et al. [147] that preoperative use of statins in cardiac surgery patients was associated with a protective effect against delirium, reducing the odds of delirium by as much as 46%. Considerable further research remains to be done; nevertheless, this represents the need to understand how clinical observations correlate with basic science research so that novel therapeutic strategies can be developed.
Nonpharmacological Treatment of Delirium
Understanding the role of drugs, such as the anti-psychotics (both haloperidol and the atypicals) and the alpha-2 agonists, such as dexmedetomidine, is key to developing a comprehensive strategy to preventing, reducing severity, and decreasing the duration of delirium whenever possible. The role of pharmacotherapy must be part of a broader strategy to accomplish this goal. Therefore, developing an evidence-based approach that includes both pharmacological and nonpharmacological strategies will be critical to addressing this multifactorial syndrome.
A number of multicomponent programs for addressing delirium in settings other than the ICU have shown promise. For instance, the Hospital Elder Life Program [148] trained interdisciplinary teams to recognize 6 delirium factors: 1) orientation, 2) therapeutic activities, 3) early mobilization, 4) vision/hearing optimization, 5) oral volume repletion, and 6) sleep enhancement. This program has been implemented in many centers with success in improving quality of care for the elderly. Other interventional programs that recognize and address the multifactorial nature of delirium have shown similar success in reducing delirium [149, 150].
Liberation and Animation: The ABCDEs of Critical Care
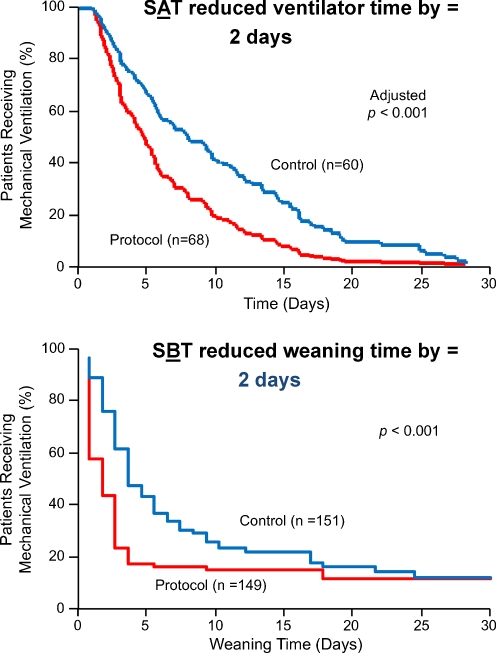
In the ICU, progress in decreasing prevalence and duration of delirium has been linked to advances in sedation practices and management of mechanical ventilation. In 2000, Kress et al. [151] recognized the problems associated with continuous sedation, such as longer duration of mechanical ventilation, longer stay in the ICU and hospital, and limited physical and neurological examination. They proposed a daily interruption of administering sedatives to allow the patient to “wake up.” This “spontaneous awakening trial” (SAT) strategy decreased duration of mechanical ventilation by 2 days (p = 0.004) (Fig. 2a) and length of stay in the ICU by 3.5 days without increasing long-term post-traumatic stress symptoms, an early concern after publication [152]. In 1996, a similar methodology by Ely et al. [153] was found to promote earlier discontinuation of mechanical ventilation and decrease duration of mechanical ventilation by instituting daily “spontaneous breathing trials” (SBTs), leading to a decrease in weaning time also by 2 days (p = 0.003) (Fig. 2b).
Fig. 2.
(a) Two investigations formed the basis for the Awakening and Breathing Controlled (ABC) trial. The first step in modern “weaning” programs is step A: spontaneous awakening trials (SATs), a daily interruption of sedatives and narcotics, which in this investigation reduced time on mechanical ventilation by 2 days. Adapted from Kress JP, et al. [151]. (b) The second step in modern day “weaning” protocols is step B: spontaneous breathing trials (SBTs), a daily interruption of the provision of ventilator support, such as turning the settings to continuous positive airway pressure (CPAP), was shown in this investigation to reduce time on mechanical ventilation by 2 days. Adapted from Ely EW, et al. [153]
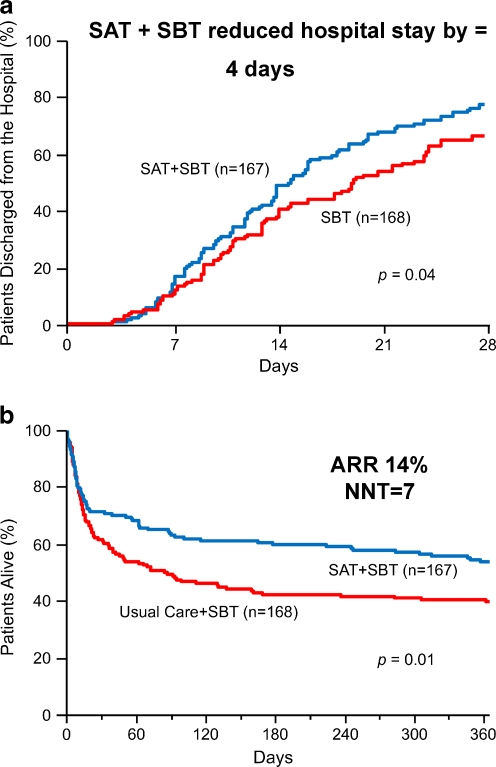
In 2008, the Awakening and Breathing Controlled (ABC) trial [54], a multicenter, randomized controlled trial put these 2 components of care together (SATs followed by SBTs). The ABC trial demonstrated for the first time that a protocol pairing nursing-delivered targeted sedation plus SATs with respiratory therapy-delivered SBTs in mechanically ventilated patients resulted in more days breathing without assistance (p = 0.02), a decrease in ICU stay by 4 days (p = 0.01) (Fig. 3a), and earlier discharge from the hospital also by 4 days (p = 0.04) compared to SBT plus usual care and similarly patient-targeted sedation without daily mandated SATs. The only potential safety hazard found was that there were more self-extubations in the intervention group than in the control group (p = 0.03), but the number of patients requiring reintubation after self-extubation was similar (0.47). Thus, the patients were right. Remarkably, patients in the intervention group were 14% less likely to die in the following year (p = 0.01) (Fig. 3b), and 1 life was saved for every 7 patients treated in the intervention group (NNT = 7.4), the first intervention studying the removal of support, such as sedation and mechanical ventilation to demonstrate a survival benefit.
Fig. 3.
(a) The Awakening and Breathing Controlled (ABC) trial reduced intensive care unit stay (not shown) and hospital length of stay for mechanically ventilated patients by 4 days when daily spontaneous awakening trials (SATs) were paired with daily spontaneous breathing trials (SBTs) compared to the control group with sedation per usual care plus daily SBT. Adapted from Girard TD, et al. [54]. (b) The ABC trial was the first randomized controlled trial of any “weaning” component of critical care (i.e., the “back end of critical care”) to demonstrate that pairing spontaneous awakening trials (SATs) with spontaneous breathing trials (SBTs) increased survival, and the number needed to treat to save 1 life was 7. Adapted from Girard TD, et al. [54]
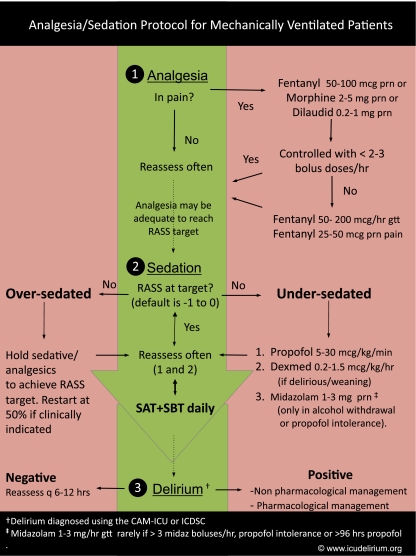
Integrating all of these evidence-based approaches has resulted in the proposed “Liberation and Animation” strategy [154]. Liberation refers to reducing the harmful effects of sedative exposure through the use of target-based sedation protocols, SATs, and the proper choice of sedative, as well as liberation from mechanical ventilation and the ICU. Animation refers to early mobilization, which was shown by Schweickert et al. [155] to reduce delirium and improve long-term cognitive outcomes. This strategy can be achieved by implementing the ABCDE bundle [14, 15]: that is, awakening and breathing trial coordination; choice of sedatives and analgesics; daily delirium monitoring; and early mobilization and exercise. Examples of analgesia and sedation protocols that incorporate routine sedation and delirium monitoring with responsible choice of sedatives and analgesics are shown in Fig. 4 and on the ICU delirium website (see: http://www.mc.vanderbilt.edu/icudelirium/docs/WakeUpAndBreathe.pdf).
Fig. 4.
Analgesia/sedation protocol for mechanically ventilated patients. This is an example of an evidence-based protocol that combines pharmacological strategies with spontaneous awakening and breathing trials, and also leads to a delirium protocol (not shown). Adapted from: http://www.mc.vanderbilt.edu/icudelirium/docs/Sedation_protocol.pdf
Long-Term Sequelae of Delirium
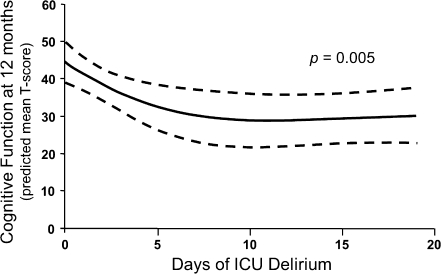
The presence of delirium, particularly in mechanically ventilated patients, has been associated with later development of post-traumatic stress disorder [156, 157], long-term cognitive impairment [7, 8], and increased mortality at 6 months [2] after hospital stay. In 2010, Girard et al. [9] demonstrated in a prospective cohort study that the duration of delirium, which is potentially modifiable, was independently associated with long-term cognitive impairment (Fig. 5). The importance of the duration of delirium has been subsequently confirmed in studies by van den Boogaard et al. [10], Shehabi et al. [158], and Pisani et al. [3]; these studies showed that the duration of delirium was associated with increased 1-year mortality (hazard ratio, 1.10; 95% confidence interval, 1.02–1.18). In closing, it is important to remember that the duration of delirium is potentially a point of intervention. By implementing prudent, evidence-based protocols that combine targeted pharmacological strategies with daily awakening and breathing trials, critical care physicians and nurses may make a significant impact on the quality of life for their patients after hospitalization.
Fig. 5.
Delirium and long-term cognitive outcomes. These data show that delirium duration was an independent predictor of neuropsychological function at 12 months, such that for every additional day of delirium up to approximately 12 days, the cognitive function of this cohort was worse when measured 1 year after ICU care (p = 0.005). Adapted from Girard TD, et al. [9]
Six Observations from an ICU Physician Following Her ICU Delirium Episode
Get the patient out of bed. Tethers have always contributed to my delirium, be it monitors for the heart, monitors for breathing and oxygen saturation, intravenously, or a bladder catheter, it does not seem to matter. The tethers are confining and remind me of how little control I have over anything, even things as simple as bodily functions. Getting me out of bed has always made me feel like the team and I are working together, not against each other. This simple action helps ground me in the environment and helps my mind, which seems to work overtime to understand the stimuli that bombard it. Seeing people in their environments helps keep me from connecting the dots in the wrong direction.
Methods to restrain patients, either using bindings or drugs that slow the brain should only be used as a last resort and the family should know about it. These are dangerous treatments, with side effects ranging from loss of feeling in the distribution of a nerve that lost oxygen while being bound, to getting tangled in restraints, to falls, and probably worst, paradoxical reactions. For me, the intramuscular injection of an anti-psychotic actually worsened my delirium, just as Ativan (lorazepam) is known to do the same, often for elderly patients. Physical restraints also provoke patients. Also be aware of the effect of tethers on other usual practices. Should a patient in 4-point restraints really be percussed (bounced carelessly around a bed for 15 minutes), or are there other alternatives that might have the same effect, but improve delirium (like sitting in a chair)? The family should request alternatives to drugs and restraints, and set a schedule for a patient, including a nap time. Turn lights on during the day and off at night. Set times for physical therapy, times for eating, times for bathing, and times for visiting, and stick with the schedule. Have family members present to remind the patient not to “pick” at their intravenous line or other monitors. Often patients will be less paranoid with the family.
Do not deny the patient their experience. Do not treat the patient as a child. As clinicians, we often work overtime to convince the patient that whatever is being seen is not there. When I have lost trust with the team caring for me, their perpetual efforts to reground me was “proof” that I could not trust anyone. This has not been an unusual experience. The most helpful conversation I have ever had was with a physician who acknowledged that I was in a “dark” place and that she did not “know what that place was, but that it was a bad experience for me.” Immediately, I believed she knew I was suffering and that she would not do anything to make it worse. I trusted this physician, and we had a longstanding relationship. But family members who are present and who can talk with a patient in this same manner may be of comfort to many.
Have more patience than the patient. Be compassionate and don’t shout; not all delirious patients are hearing impaired. We cannot expect when we confront patients with delirium that the patient will have the same physical and emotional strength that we have as caregivers. We should not blame patients for their behavior when they do not have the cognitive processing available to change their behavior. When we, as caregivers, are confronted by the shouts of the patient, we should speak with calm, quiet voices. Use a gentle touch, when possible. We should hold ourselves accountable to de-escalate a situation.
Be aware of the long-term consequences of delirium. To date, the visual hallucinations I have witnessed when delirious are among the most prominent visual memories within that “cache” of my brain. Post-traumatic stress disorder is common among those who have experienced delirium, and these patients should be offered counseling services when it is appropriate. Because of the long-term consequences of delirium, we should be cognizant of patients with chronic illness who must return to the ICUs. These patients may be hesitant to return to the hospital, even if it is medically necessary, because they are afraid. In the ICU, if a patient experienced delirium in the past, it is very important to give notice to the care of patient by minimizing drugs that contribute to delirium, and it is important that these patients are surrounded by people that do not threaten them.
-
Surround the patient with familiarity. Break visiting hour rules to allow family to be with the patient. The patient may feel like someone is there to help protect them or speak out for them when they cannot. A familiar schedule will help the patient become acclimated to day and night, and also to what is to be expected. Knowing what to expect gives some control back to a vulnerable patient who feels like everything is happening in a haphazard fashion. Families who are in hospital systems with limited visiting hours should ask that these rules be broken during the period of time that the patient is delirious.
(Personal communication from Alison Clay, MD, Duke University.)
Conclusion
Delirium remains an important form of acute brain dysfunction that is under-recognized in ICUs around the world. Recognition of delirium has improved through the development of arousal scales and ICU tools, such as the ICDSC and CAM-ICU. Recognition of the underlying etiology and reduction of risk factors for the development of delirium remain the first step in management; the next step often is pharmacological treatment. Haloperidol is a neuroleptic anti-psychotic widely used in the treatment of delirium, although evidence for its use is anecdotal and not yet truly proven with high levels of scientific rigor. Atypical anti-psychotics gained popularity due to their purported reduced association with EPS. However, their risk of corrected QT (QTc) prolongation and arrhythmia, along with metabolic disturbances, do not guarantee a thoroughly safer profile than haloperidol, and randomized, placebo-controlled trials are needed to definitively demonstrate the safety and efficacy of typical and atypical anti-psychotics. The alpha-2 agonists, such as dexmedetomidine, have gained popularity in their use due to decreased respiratory suppression and recent trials demonstrating reduced delirium prevalence as compared with GABA-ergic drugs (e.g., benzodiazepines). Patients receiving dexmedetomidine should be closely followed for bradycardia and hypotension. The choice of pharmacotherapy should be understood as part of a larger multicomponent approach to managing the critically ill patient. Future evidence-based strategies to minimize duration of mechanical ventilation and duration of delirium, while assuring patient comfort and safety, should be based on spontaneous awakening and breathing trials, daily delirium monitoring, and early mobilization (the ABCDE bundle).
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
This work was supported by Dr. Ely from the National Institute of Health grants (AG-027472-01A5 and AG035117-01A1) and the VA Geriatric Research Education and Clinical Center (GRECC).
Required Author Forms Disclosure forms provided by the authors are available with the online version of this article.
Disclosures Dr. Ely has received grants and honoraria from Hospira, grants from Eli Lilly, and consults for Cumberland and Masimo. Both Drs. Hipp and Ely would like to acknowledge the constant support of Dr. Dana Hipp and Dr. Kim Ely as they pursued this manuscript and ongoing research in the field of delirium in the ICU.
References
- 1.Verghese A. Cutting for Stone. First Vintage Books Edition. New York: Vintage Books; 2009. [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Ely EW, Smith DS, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: Impact on long-term health related quality of life and cognitive functioning. Crit Care Med 2012;40(1):112-8. [DOI] [PubMed]
- 11.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 12.Guenther U, Popp J, Koecher L, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010;25:144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35:1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasilevskis EE, Ely EW, Speroff T, Pun B, Boehme L, Dittus R. Reducing Iatrogenic risks. ICU-acquired delirium and weakness — crossing the quality chasm. Chest 2010;138:1224-1233. [DOI] [PMC free article] [PubMed]
- 15.Vasilevskis EE, Pandharipande PP, Girard TD, Ely EW. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38:S683–S691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landro L. Changing intensive care to improve life afterwards. The Wall Street Journal, Feb. 15, 2011. Available at: http://online.wsj.com/article/SB10001424052748704081604576144321242020948.html. Accessed 7 November, 2011.
- 17.Diagnostic and statistical manual of mental disorders, 4th edit. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 18.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 19.Pun B, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132:624–636. doi: 10.1378/chest.06-1795. [DOI] [PubMed] [Google Scholar]
- 20.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisani MA, Murphy TE, Araujo KL, Slattum P, Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morandi A, Pandharipande P, Trabucchi M, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med. 2008;34:1907–1915. doi: 10.1007/s00134-008-1177-6. [DOI] [PubMed] [Google Scholar]
- 23.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33:1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 25.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for intensive care delirium: a systematic review. Intensive Crit Care Nurs. 2008;24:98–107. doi: 10.1016/j.iccn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 28.Pisani MA, Murphy TE, Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 29.Yildizeli B, Ozyurtkan MO, Batirel HF, Kuscu K, Bekiroglu N, Yuksel M. Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg. 2005;79:1004–1009. doi: 10.1016/j.athoracsur.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12(Suppl 3):S3. doi: 10.1186/cc6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 33.Munster BC, Korevaar JC, Zwinderman AH, Leeflang MM, Rooij SE. The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry. 2009;17:856–862. doi: 10.1097/JGP.0b013e3181ab8c84. [DOI] [PubMed] [Google Scholar]
- 34.Munster BC, Korevaar JC, Rooij SE, Levi M, Zwinderman AH. The association between delirium and the apolipoprotein E epsilon4 allele in the elderly. Psychiatr Genet. 2007;17:261–266. doi: 10.1097/YPG.0b013e3280c8efd4. [DOI] [PubMed] [Google Scholar]
- 35.Ouimet S, Riker R, Bergeon N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33:1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 36.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 38.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Guenther U, Popp J, Koecher L, et al. Validity and Reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2009;25:144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Tobar E, Romero C, Galleguillos T, et al. Confusion assessment method for diagnosing delirium in ICU patients (CAM-ICU): cultural adaptation and validation of the Spanish version. Med Intensiva. 2010;43:14–21. doi: 10.1016/j.medin.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Luetz A, Heymann A, Radtke F, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–418. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]
- 42.Eijk MM, Boogaard M, Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184:340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 43.Neufeld KJ, Hayat MJ, Coughlin JM, et al. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics. 2011;52:133–140. doi: 10.1016/j.psym.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 45.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of Delirium Rating Scale Revised-98: comparison to the delirium rating scale and cognitive test for delirium. J of Neuropsychiatry and Clin Neurosciences. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 46.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 47.Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the sedation-agitation scale with the bispectral index and visual analog scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27:853–858. doi: 10.1007/s001340100912. [DOI] [PubMed] [Google Scholar]
- 48.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 49.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 50.van den BM, Pickkers P, van der HH, Roodbol G, van AT, Schoonhoven L. Implementation of a delirium assessment tool in the ICU can influence haloperidol use. Crit Care 2009;13:R131. [DOI] [PMC free article] [PubMed]
- 51.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: a report from two medical centers. Crit Care Med. 2005;33:1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 52.Soja SL, Pandharipande PP, Fleming SB, et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Med. 2008;34:1263–1268. doi: 10.1007/s00134-008-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith HA, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit (pCAM-ICU) Crit Care Med. 2011;39:150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 55.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty JH, Rudolph J, Shay K, et al. Delirium is a serious and under-recognized problem: why assessment of mental status should be the sixth vital sign. J Am Med Dir Assoc. 2007;8:273–275. doi: 10.1016/j.jamda.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008;24:45–65. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Maclullich AM, Ferguson KJ, Miller T, Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 60.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandharipande PP, Morandi A, Adams JR, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–1892. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zampieri FG, Park M, Machado FS, Azevedo LC. Sepsis-associated encephalopathy: not just delirium. Clinics (Sao Paulo) 2011;66:1825–1831. doi: 10.1590/S1807-59322011001000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall RJ, Shenkin SD, Maclullich AM. A Systematic literature review of cerebrospinal fluid biomarkers in delirium. Dement Geriatr Cogn Disord. 2011;32:79–93. doi: 10.1159/000330757. [DOI] [PubMed] [Google Scholar]
- 64.Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care. 2010;14:R238. doi: 10.1186/cc9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 66.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:R78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM. Neuroimaging studies of delirium: A systematic review. J Psychosom Res. 2008;65:239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Morandi A, Gunther ML, Vasilevskis EE, et al. Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont ) 2010;7:28–33. [PMC free article] [PubMed] [Google Scholar]
- 69.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 70.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37:825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mac Sweeney R, Barber V, Page V, et al. A national survey of the management of delirium in UK intensive care units. QJM 2010;103:243-251. [DOI] [PubMed]
- 72.Kalisvaart KJ, Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 73.Milbrandt EB, Kersten A, Kong L, et al. Haloperidol use is associated with lower hospital mortality in mechanically ventilated patients. Crit Care Med. 2005;33:226–229. doi: 10.1097/01.ccm.0000150743.16005.9a. [DOI] [PubMed] [Google Scholar]
- 74.Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40:1966–1973. doi: 10.1345/aph.1H241. [DOI] [PubMed] [Google Scholar]
- 75.Seitz DP, Gill SS, Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21. doi: 10.4088/jcp.v68n0102. [DOI] [PubMed] [Google Scholar]
- 76.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev 2007;(2):CD005594. [DOI] [PubMed]
- 77.Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults–a systematic evidence review. J Gen Intern Med. 2009;24:848–853. doi: 10.1007/s11606-009-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolkin A, Brodie JD, Barouche F, et al. Dopamine receptor occupancy and plasma haloperidol levels. Arch Gen Psychiatry. 1989;46:482–484. doi: 10.1001/archpsyc.1989.01810050096021. [DOI] [PubMed] [Google Scholar]
- 79.Bishara D, Taylor D. Upcoming agents for the treatment of schizophrenia: mechanism of action, efficacy and tolerability. Drugs. 2008;68:2269–2292. doi: 10.2165/0003495-200868160-00002. [DOI] [PubMed] [Google Scholar]
- 80.Kapur S, Remington G, Jones C, et al. High levels of dopamine d2 receptor occupancy with low-dose haloperidol treatment: a pet study. Am J Psychiatry. 1996;153:948–950. doi: 10.1176/ajp.153.7.948. [DOI] [PubMed] [Google Scholar]
- 81.Tauscher J, Kufferle B, Asenbaum S, Tauscher-Wisniewski S, Kasper S. Striatal dopamine-2 receptor occupancy as measured with [123I]iodobenzamide and SPECT predicted the occurrence of EPS in patients treated with atypical antipsychotics and haloperidol. Psychopharmacology (Berl) 2002;162:42–49. doi: 10.1007/s00213-002-1082-6. [DOI] [PubMed] [Google Scholar]
- 82.Song C, Lin A, Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42:157–164. doi: 10.1016/s0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt AW, Lebel LA, Howard HR, Jr, Zorn SH. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;425:197–201. doi: 10.1016/s0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- 84.Cosi C, Waget A, Rollet K, Tesori V, Newman-Tancredi A. Clozapine, ziprasidone and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: role of 5-HT1A receptor activation. Brain Res. 2005;1043:32–41. doi: 10.1016/j.brainres.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 85.Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 2011;11:59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 86.Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet. 1999;37:435–456. doi: 10.2165/00003088-199937060-00001. [DOI] [PubMed] [Google Scholar]
- 87.Riker R, Fraser G, Cox P. Continuous infusion of haloperidol controls agitation in critically ill patients. Crit Care Med. 1994;22:433–440. doi: 10.1097/00003246-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: A randomized controlled trial. Crit Care Med 2011;xx:xx-xx. [DOI] [PubMed]
- 89.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 90.Lee KU, Won WY, Lee HK, et al. Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol. 2005;20:311–314. doi: 10.1097/00004850-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Pae CU, Lee SJ, Lee CU, Lee C, Paik IH. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004;19:125–127. doi: 10.1002/hup.559. [DOI] [PubMed] [Google Scholar]
- 92.Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004;12:437–439. doi: 10.1176/appi.ajgp.12.4.437. [DOI] [PubMed] [Google Scholar]
- 93.Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301. doi: 10.1016/S0033-3182(04)70170-X. [DOI] [PubMed] [Google Scholar]
- 94.Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71:277–281. doi: 10.1016/j.jpsychores.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 95.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 96.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–437. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tran PV, Dellva MA, Tollefson GD, Beasley CM, Jr, Potvin JH, Kiesler GM. Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. J Clin Psychiatry. 1997;58:205–211. doi: 10.4088/jcp.v58n0505. [DOI] [PubMed] [Google Scholar]
- 98.Riker RB, Fraser GL, Richen P. Movement disorders associated with withdrawal from high-dose intravenous haloperidol therapy in delirious icu patients. Chest. 1997;111:1778–1781. doi: 10.1378/chest.111.6.1778. [DOI] [PubMed] [Google Scholar]
- 99.Zornberg GL, Jick H. Antipsychotic drug use and risk of first-time idiopathic venous thromboembolism: a case-control study. Lancet. 2000;356:1219–1223. doi: 10.1016/S0140-6736(00)02784-7. [DOI] [PubMed] [Google Scholar]
- 100.Miyaji S, Yamamoto K, Hoshino S, Yamamoto H, Sakai Y, Miyaoka H. Comparison of the risk of adverse events between risperidone and haloperidol in delirium patients. Psychiatry Clin Neurosci. 2007;61:275–282. doi: 10.1111/j.1440-1819.2007.01655.x. [DOI] [PubMed] [Google Scholar]
- 101.Lee PE, Sykora K, Gill SS, et al. Antipsychotic medications and drug-induced movement disorders other than parkinsonism: a population-based cohort study in older adults. J Am Geriatr Soc. 2005;53:1374–1379. doi: 10.1111/j.1532-5415.2005.53418.x. [DOI] [PubMed] [Google Scholar]
- 102.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nielsen J, Bruhn AM. Atypical neuroleptic malignant syndrome caused by olanzapine. Acta Psychiatr Scand. 2005;112:238–240. doi: 10.1111/j.1600-0447.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 104.Lindenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160:290–296. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- 105.Liperoti R, Pedone C, Lapane KL, Mor V, Bernabei R, Gambassi G. Venous thromboembolism among elderly patients treated with atypical and conventional antipsychotic agents. Arch Intern Med. 2005;165:2677–2682. doi: 10.1001/archinte.165.22.2677. [DOI] [PubMed] [Google Scholar]
- 106.Rochon PA, Stukel TA, Sykora K, et al. Atypical antipsychotics and parkinsonism. Arch Intern Med. 2005;165:1882–1888. doi: 10.1001/archinte.165.16.1882. [DOI] [PubMed] [Google Scholar]
- 107.Rosencheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA. 2003;290:2693–2702. doi: 10.1001/jama.290.20.2693. [DOI] [PubMed] [Google Scholar]
- 108.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 109.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 110.Ballard C, Hanney ML, Theodoulou M, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow up on a randomised placebo-controlled trial. Lancet Neurol. 2009;8:151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 111.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 112.Kuehn BM. FDA warns antipsychotic drugs may be risky for elderly. JAMA. 2005;293:2462. doi: 10.1001/jama.293.20.2462. [DOI] [PubMed] [Google Scholar]
- 113.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 114.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 115.Tyrer P, Kendall T. The spurious advance of antipsychotic drug therapy. Lancet. 2009;373:4–5. doi: 10.1016/S0140-6736(08)61765-1. [DOI] [PubMed] [Google Scholar]
- 116.Liperoti R, Gambassi G, Lapane KL, et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med. 2005;165:696–701. doi: 10.1001/archinte.165.6.696. [DOI] [PubMed] [Google Scholar]
- 117.Glassman AH, Bigger JT. Antipsychotic drugs: prolonged QTc Interval, Torsade de Pointes, and sudded death. Am J Psychiatry. 2001;158:1774–1784. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 118.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58:1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 119.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 120.Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de Pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol. 1998;81:238–240. doi: 10.1016/s0002-9149(97)00888-6. [DOI] [PubMed] [Google Scholar]
- 121.Harrigan EP, Miceli JJ, Anziano R, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- 122.Straus SM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164:1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 123.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 124.Hatta K, Takahashi T, Nakamura H, et al. The association between intravenous haloperidol and the prolonged QT interval. J Clin Psychopharmacol. 2001;21:257–261. doi: 10.1097/00004714-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 125.Eijk MM, Marum RJ, Klijn IA. de WN, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37:1881–1885. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 126.Gregoretti C, Moglia B, Pelosi P, Navalesi P. Clonidine in perioperative medicine and intensive care unit: more than an anti-hypertensive drug. Current Drug Targets. 2009;10:667–686. doi: 10.2174/138945009788982478. [DOI] [PubMed] [Google Scholar]
- 127.Panzer O, Moitra V, Sladen RN. Pharmacology of sedative-analgesic agents: dexmedetomidine, remifentanil, ketamine, volatile anesthetics, and the role of peripheral mu antagonists. Crit Care Clin. 2009;25:451–469. doi: 10.1016/j.ccc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Ma D, Hossain M, Rajakumaraswamy N, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 129.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 130.Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 131.Prielipp RC, Wall MH, Tobin JR, et al. Dexmedetomidine-induced sedation in volunteers decreases regional and global cerebral blood flow. Anesth Analg. 2002;95:1052–1059. doi: 10.1097/00000539-200210000-00048. [DOI] [PubMed] [Google Scholar]
- 132.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (dexmedetomidine compared to morphine-dexcom study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 134.Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35:282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]
- 135.Mirski MA, Lewin JJ, III, Ledroux S, et al. Cognitive improvement during continuous sedation in critically ill, awake and responsive patients: the Acute Neurological ICU Sedation Trial (ANIST) Intensive Care Med. 2010;36:1505–1513. doi: 10.1007/s00134-010-1874-9. [DOI] [PubMed] [Google Scholar]
- 136.Reade MC, O'Sullivan K, Bates S, Goldsmith D, Ainslie WR, Bellomo R. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. 2009;13:R75. doi: 10.1186/cc7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 138.Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: Delirium in ICU patients - importance of sleep deprivation. Crit Care. 2009;13:234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The a2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 140.Dasta JF, Kane-Gill SL, Pencina M, et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38:497–503. doi: 10.1097/CCM.0b013e3181bc81c9. [DOI] [PubMed] [Google Scholar]
- 141.Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109:2365–2373. doi: 10.1002/cncr.22665. [DOI] [PubMed] [Google Scholar]
- 142.Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. [DOI] [PMC free article] [PubMed]
- 143.Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 144.Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP. Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest. 2011;140:580–585. doi: 10.1378/chest.10-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Famer D, Wahlund LO, Crisby M. Rosuvastatin reduces microglia in the brain of wild type and ApoE knockout mice on a high cholesterol diet; implications for prevention of stroke and AD. Biochem Biophys Res Commun. 2010;402:367–372. doi: 10.1016/j.bbrc.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 146.Townsend KP, Shytle DR, Bai Y, et al. Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res. 2004;78:167–176. doi: 10.1002/jnr.20234. [DOI] [PubMed] [Google Scholar]
- 147.Katznelson R, Djaiani GN, Borger MA, et al. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110:67–73. doi: 10.1097/ALN.0b013e318190b4d9. [DOI] [PubMed] [Google Scholar]
- 148.Inouye SK, Baker DI, Fugal P, Bradley EH. Dissemination of the hospital elder life program: implementation, adaptation, and successes. J Am Geriatr Soc. 2006;54:1492–1499. doi: 10.1111/j.1532-5415.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 149.Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53:622–628. doi: 10.1111/j.1532-5415.2005.53210.x. [DOI] [PubMed] [Google Scholar]
- 150.Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52:79–90. doi: 10.1111/j.1365-2648.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- 151.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 152.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 153.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 154.King MS, Render ML, Ely EW, Watson PL. Liberation and animation: strategies to minimize brain dysfunction in critically ill patients. Semin Respir Crit Care Med. 2010;31:87–96. doi: 10.1055/s-0029-1246284. [DOI] [PubMed] [Google Scholar]
- 155.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for posttraumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R28. doi: 10.1186/cc5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11:R27. doi: 10.1186/cc5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care unit patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)