Abstract
Hypothermia can terminate epileptiform discharges in vitro and in vivo epilepsy models. Hypothermia is becoming a standard treatment for brain injury in infants with perinatal hypoxic ischemic encephalopathy, and it is gaining ground as a potential treatment in patients with drug resistant epilepsy. However, the exact mechanism of action of cooling the brain tissue is unclear. We have studied the 4-aminopyridine model of epilepsy in mice using single- and dual-patch clamp and perforated multi-electrode array recordings from the hippocampus and cortex. Cooling consistently terminated 4-aminopyridine induced epileptiform-like discharges in hippocampal neurons and increased input resistance that was not mimicked by transient receptor potential channel antagonists. Dual-patch clamp recordings showed significant synchrony between distant CA1 and CA3 pyramidal neurons, but less so between the pyramidal neurons and interneurons. In CA1 and CA3 neurons, hypothermia blocked rhythmic action potential discharges and disrupted their synchrony; however, in interneurons, hypothermia blocked rhythmic discharges without abolishing action potentials. In parallel, multi-electrode array recordings showed that synchronized discharges were disrupted by hypothermia, whereas multi-unit activity was unaffected. The differential effect of cooling on transmitting or secreting γ-aminobutyric acid interneurons might disrupt normal network synchrony, aborting the epileptiform discharges. Moreover, the persistence of action potential firing in interneurons would have additional antiepileptic effects through tonic γ-aminobutyric acid release.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0078-5) contains supplementary material, which is available to authorized users.
Keywords: Hypothermia, Epilepsy, Interneuron, Synchrony, GABA
Introduction
Direct cortical application of therapeutic modalities to treat epilepsy has gained significance in the last few years. This includes direct cortical stimulation, cortical delivery of antiepileptic drugs, and application of hypothermia to the brain. Physiological body temperature is an important determinant of neural functions. Therapeutic hypothermia is becoming a standard treatment for brain injury in infants with perinatal hypoxic ischemic encephalopathy by reducing the risk of apoptosis and early necrosis, cerebral metabolic rate, and the release of nitric oxide and free radicals. Early hypothermia may result in histological and functional improvement in animal models of perinatal brain injury [1]. Changes in temperature have dynamic influences on hippocampal neural activities. It has been established that changes as little as 2° to 3°C in brain temperature affect brain functions and neuronal properties [2, 3]. Therefore, heat-sensitive molecular compounds seem to be essential for brain functions at physiological temperature. Furthermore, hypothermia has been shown to terminate experimentally induced seizure activity of in vitro and in vivo models of epilepsy within seconds, without causing acute or delayed injury to the cooled brain [4–7]. It is suggested by these studies that cooling could represent a new approach to seizure control in intractable focal epilepsies as an alternative to resective surgery, which may result in suboptimal seizure control and possible neurological deficits.
Temperature levels that must be achieved to obtain seizure suppression have also been studied. It has been found that cooling to <24°C is required to reversibly inactivate general neuronal function [7]. Similarly, inhibition of experimental seizure activity requires cooling to 24°C, whereas complete cessation is only obtained at temperatures of 20° to 22°C. Furthermore, by comparing rapid cooling at rates of 2° to 5°C per second to slower cooling at rates of 0.1° to 1°C per second, we have shown that the cooling rate has significant influence on the efficacy of seizure suppression. We have found that very rapid cooling can abort epileptiform discharges with minor drops in temperature irrespective of the final temperature achieved, whereas slow cooling requires a lower range of temperatures (cooling to 21°-22°C caused a 90% reduction in event frequency and cooling to 14° to 15°C caused terminated discharges). Cooling is effective in diverse in vitro epilepsy models and in diverse brain regions (hippocampus, neocortex, dentate granule layer, and entorhinal cortex). Cooling also inhibits synaptic transmission, as reflected by the field excitatory postsynaptic potentials and population spikes, in parallel with its effect on spontaneous epileptiform activity [8]. However, there is a paucity of data on the cellular mechanisms involved in the effects of hypothermia on epileptiform discharges.
Imaging a fluorescent synaptic marker with 2-photon microscopy, Yang et al. [9] have proposed a presynaptic mechanism through reduced transmitter release during rapid cooling. In addition, presence of temperature sensitive ion channels in the neurons may mediate the effects of cooling. In hippocampal cultures, the presence of transient receptor potential channel V (TRPV)-4 that is activated at temperatures within the physiological range (>27°-34°C) has been reported [10].
On the other hand, simultaneous dual recordings in hippocampal pyramidal cells and interneurons have shown a role for alternating activity of these neurons during epileptiform discharges in an in vitro model of epilepsy. It has been reported that in the presence of 4-aminopyridine (4-AP) and decreased magnesium, interneuron activity increased during interictal periods and entered into long-lasting depolarization block during ictal discharges, allowing sustained firing of pyramidal cells [11]. These findings prompted the question of whether hypothermia regulates epileptiform activity by differentially affecting distinct cell types.
Here we report the results of our investigation of mechanisms underlying the effectiveness of hypothermia in an in vitro model of epilepsy. We have examined a number of potential target mechanisms involving TRPV channels, and selective effects on transmitting or receiving γ-aminobutyric acid (GABAergic) interneurons that alter network synchrony in hippocampal and extrahippocampal brain regions.
Methods
Hippocampal Slice Preparation
Coronal brain slices (250–300 μm) containing hippocampus and parts of adjacent cortex were prepared from postnatal day 14 to 27 C57BL/6 J mice. In some experiments glutamic acid decarboxylase-green fluorescent protein (GAD-GFP) mice were used to identify GABAergic interneurons Mice were sacrificed by decapitation in agreement with the guidelines of the American Veterinary Medicine Association (AVMA) Panel on Euthanasia and the Georgetown University Animal Care and Use Committee. These slices included the hippocampus, thalamus, entorhinal cortex, and neocortex. After cutting, the slices recovered for 30 minutes at 37°C and were continuously perfused with carbogen-bubbled (5 ml/min) artificial cerebrospinal fluid (aCSF) (in mM): NaCl (120), KCl (3.1), Na2HPO4 (1.25), NaHCO3 (26), dextrose (5.0), MgCl2 (1.0), and CaCl2 (2.0) 305 mOsm, pH 7.4. During experiments, slices were submerged in aCSF at various temperatures. Cells were visualized with an upright microscope (E600FN Nikon; Tokyo Japan) using infrared-differential interference contrast video microscopy. Patch pipettes were filled with the following (in μM): K-gluconate (145), EGTA (1.1), MgATP (5.0), and HEPES-KOH (10) to pH 7.2, 295 mOsm.
Hippocampal Cell Culture
Primary cultures were donated by Daniel T. Pak (Dept. of Pharmacology & Physiology Georgetown University, Washington DC, USA) and were prepared from rat embryos as previously described [12].
Patch-Clamp Recording
Patch electrodes (5–7 MΩ) were pulled (PP – 83; Narishige, Tokyo, Japan) from borosilicate glass capillary (Drummond, Broomall, PA). No fire polishing or Sylgard coating were used. Series resistance (15 MΩ), in whole cell configuration, was compensated and monitored for consistency throughout the experiment. Current and voltage signals at the head stage of the patch-clamp amplifier (Axopatch 1D; Molecular Devices Co., Sunnyvale, CA) were filtered at 2 kHz with a low-pass Bessel filter and digitized at 5 to 10 kHz using a personal computer equipped with Digidata 1322A data acquisition board and pCLAMP 10 software (both from Molecular Devices Co.). Off-line data analysis, curve fitting, and figure preparation were performed with Clampfit 10 software (Molecular Devices). Membrane input resistance was monitored with repetitive injection of a 240 ms-25 pA current pulse every 5 seconds. Patch-clamp recordings were made from 20 CA3 and 17 CA1/CA2 cells from 17 mice; statistical comparisons were performed with paired or independent t test, accordingly.
Simultaneous Dual Recording
An additional patch-clamp amplifier (Axopatch 1D) was used in dual recordings for whole cell voltage and current clamp, juxtacellular loose cell attached, and field recordings. Recordings were carried out from visually identified mouse hippocampal pyramidal cells and interneurons. Hippocampal neurons were visually localized in the CA1/2 and CA3 areas and were identified as pyramidal neurons or GABAergic interneurons on the basis of their anatomical location and action potential firing pattern. Spontaneous epileptiform discharges were recorded extracellularly with glass micropipettes (tip resistance, 2–5 MΩ) filled with aCSF. The field responses consist of field excitatory postsynaptic potentials and population spikes. Spontaneous discharges were recorded from the pyramidal layers of the hippocampal CA1-3 areas. Data from patch-clamp recordings from visually identified cells in CA1/2 and CA3 pyramidal cells and interneurons grouped across different subregions were compared during baseline and various stages of cooling.
Multi-Electrode Array Recording
Simultaneous field recordings through a 60-channel perforated multi-electrode array (MEA) (Multichannel Systems, Reutlingen, Germany) were carried out from the hippocampus and cortex as previously described [13]. Off-line data analysis and figure preparation were performed with MCRack (Multichannel Systems, Reutlingen, Germany) and MEA tools, with routines written for MATLAB [14]. Spike sorting with principal component analysis of single- or multi-unit discharges was done on each channel using Spike2 version 7.04 software (Cambridge Electronic Design Ltd, Cambridge, UK). These discharges of different morphology and amplitude are action potentials arising from one or more neurons. On each channel, these discharges were reviewed off-line based on amplitude and guided by visual inspection to quantify the effect of cooling in different cell types.
Induction of Epileptiform Activity
Spontaneous epileptiform discharges were elicited in CA3 or CA1/2 hippocampal regions by switching to a perfusion medium containing 50 μM 4-AP, according to methods previously described for rat brain slices [15]. In some experiments, the perfusion solution contained reduced amount (0.5 mM) of Mg2 [11, 16].
Drugs
Stock solutions of all drugs (all from Sigma) were diluted in the extracellular medium to the concentrations desired. Drugs were locally applied through a Y tube [17] modified for optimal solution exchange in brain slices [18]. Bicuculline (25 μM), sodium channel blocker tetrodotoxin (TTX, 0.5-1 μM), 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) receptor antagonist NBQX (5 μM), glycine receptor antagonist strychnine (5 μM), γ-aminobutyric acid (GABA) analogue, and selective GABAA receptor agonist Gaboxadol (10 μM), transient receptor potential (TRP) channel agonists menthol and capsaicin (12.5 μM), and antagonists capsazepine (10 μM) and ruthenium red (RR, 0.1, 1, and 10 μM) were applied in some experiments; TRPV4 agonist 4α-phorbol-12,13-didecanoate (4α-PDD, 20 μM, Sigma) was dissolved in ethanol and diluted 1:1000 in aCSF.
Rapid Cooling and Rewarming
Rapid cooling was induced by switching from the 32o to 34°C perfusion solution to cold (8o to 14°C) aCSF of similar composition, which allowed the brain slice temperature to be reduced to as low as 11°C. However, the majority of experiments were performed at 18o to 22°C. Slice temperature was continuously monitored with a submerged miniature thermistor probe situated within the recording chamber adjacent to the slice (Warner Instruments, Hamden, CT). The temperature response time constant determined by rapidly switching the thermistor probe from 25° to 0°C was 0.5 seconds. Solution from the Y-tube applicator was kept at the same temperature as that in the perfusion bath. During the perforated multi-electrode array (pMEA) experiments, cooling was induced at a rate of 0.1° to 0.5°C per second by switching to cold aCSF to lower the temperature to <20°C, and warming was achieved at a slower rate through an inline heater. Although cooling was performed several times on each slice, only the first cooling experiment was counted and included in this study.
Results
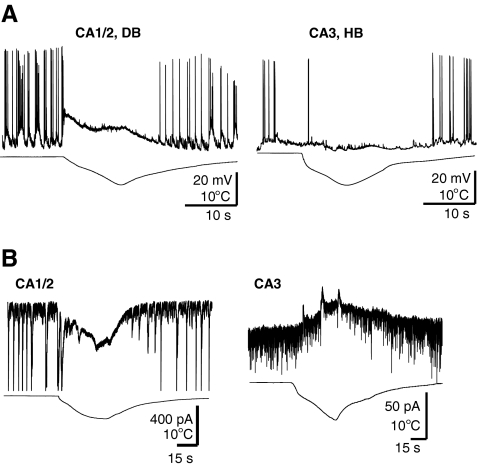
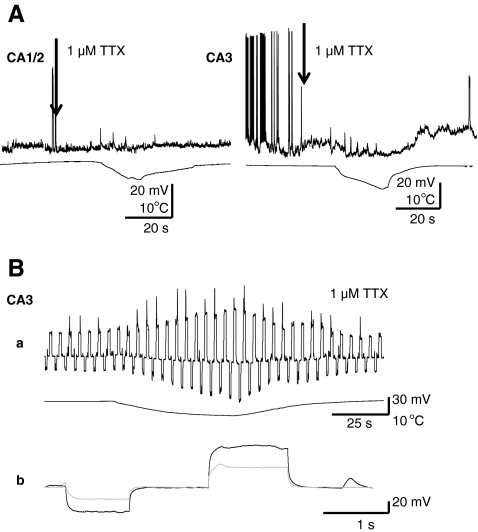
To further investigate the cellular mechanisms of hypothermia in aborting epileptiform discharges, we performed patch-clamp recordings from mouse hippocampal slices in the presence of 4-AP. We recorded from CA1/CA2 and CA3 pyramidal neurons, as well as visually identified interneurons located in the proximity of the pyramidal layer. As shown in the example in Fig. 1A, hypothermia blocked the occurrence of spontaneous action potential discharges induced by 4-AP in pyramidal neurons. In all cell types, hypothermia-induced action potential blockade was accompanied by either depolarization (n = 9/12 in CA1/CA2), hyperpolarization (n = 4/6 in CA3), or depolarization followed by hyperpolarization. Although recording in voltage-clamp membrane depolarization was associated with an inward current in most CA1/CA2 neurons, and hyperpolarization with an outward current in most CA3 neurons (Fig. 1B). Rapid cooling-induced depolarization was eliminated by TTX in both CA1/CA2 neurons and CA3 neurons. However, the more common hyperpolarization in CA3 neurons was TTX insensitive (n = 4) (Fig. 2A). In both cell types studied in the presence of TTX, the effect of cooling was accompanied by remarkable increases in membrane input resistance observed as higher voltages in response to hyperpolarizing and depolarizing current injections (85 ± 15%; n = 5) (Fig. 2B).
Fig. 1.
(A) Opposite effects of rapid cooling on resting membrane potential of CA1/CA2 and CA3 hippocampal neurons. Current clamp recordings in CA3 and CA1/CA2 with depolarization block (DB), or hyperpolarization block (HB). (B) Membrane depolarization was associated with an inward current in CA1/CA2, and hyperpolarization with an outward current in CA3 neurons; voltage clamp; 4-AP 50 μM; hippocampal slice
Fig. 2.
(A) Tetrodotoxin (TTX) blocks the effect of cooling in CA1/CA2 but not in CA3 neurons. Rapid cooling-induced depolarization was eliminated by 1 μM TTX in CA1/CA2 neurons. In contrast, most CA3 neurons responded to cooling with TTX insensitive hyperpolarizations. (B) In a CA3 cell cooling resulted in increased membrane input resistance (a), as shown by increased voltages in response to continuous hyperpolarizing and depolarizing current injections (b); hippocampal slice
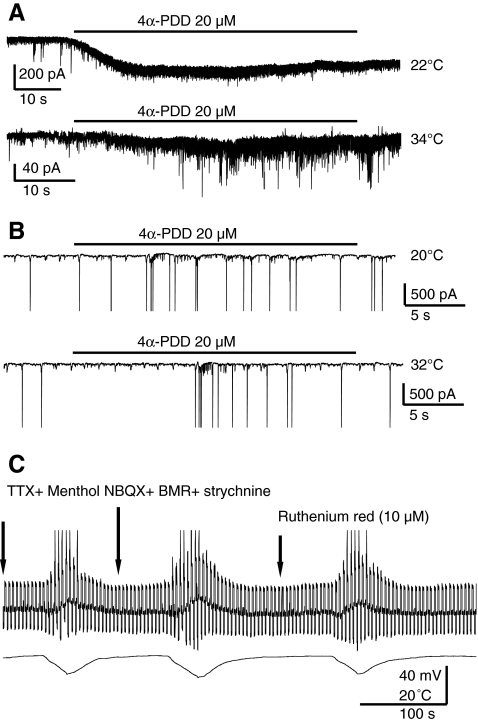
The increase in input resistance suggested the hypothesis that closure of temperature sensitive TRPV4 cation channels activated at warm temperatures (>30°C) might underlie the action of cooling. Indeed, it has been previously reported [10] that 4α-PDD (a TRPV4 agonist) activates currents in hippocampal neurons in primary cultures. Therefore, we compared the effects of this agonist between primary hippocampal cultures and slices. As shown in Fig. 3A, 4α-PDD (20 μM) induced a significantly smaller inward current in hippocampal neurons in cell culture at 34°C (40 ± 7 pA) than at 22°C (240 ± 55pA) (n = 3; p < 0.05), but it failed to induce currents in CA3 pyramidal neurons in slices (n = 3) at both 32°C and 20°C (Fig. 3B). Similar results were found during recording from CA3 neurons, voltage clamped at −60 mV at 32°C in the presence of menthol, the TRPM8 channel selective agonist, which failed to change resting membrane potential, input resistance, and the depolarizing effect of cooling (Fig. 3C). We also tested the TRPV1 agonist capsaicin and the antagonist capsazepine, both of which failed to activate any currents in CA3 neurons voltage clamped at −60 mV at 32°C (n = 3). Finally, we investigated the action of RR, which blocks several TRPV-mediated responses [19, 20]. Because RR has been reported to induce large inward currents due to a significant calcium-mediated presynaptic release caused by the action of RR at presynaptic ryanodine receptors [21], in these experiments we used a cocktail of postsynaptic channel blockers (NBQX, bicuculline, and strychnine). In the presence of this cocktail, RR at 3 different concentrations (0.1, 1, and 10 μM) failed to alter membrane resistance or to block the action of cooling in CA3 neurons voltage clamped at −60 mV at 32°C (Fig. 3C).
Fig. 3.
(A) Hippocampal neurons in culture at DIV21 voltage clamped at −60 mV. Inward current elicited by 20 μM 4-phorbol-12,13-didecanoate (4α-PDD), a transient receptor potential channel V4 agonist at distinct temperatures. The inward current was more robust at 22°C, but the increase in spontaneous postsynaptic current frequency was stronger at 34°C. These effects were seen in 4 of 6 neurons in cultures at DIV21, but were never observed in 4 CA1 or 11 CA3 neurons in at least 4 slices in 2 mice at P24 and P16, respectively. (B) 4α-PDD failed to induce currents in CA3 pyramidal neurons at both 32°C and 20°C; hippocampal slice. (C) The transient receptor potential cation channel subfamily M member 8 (TRPM8) agonist menthol failed to change resting membrane potential (thick line), input resistance (steps in opposite directions), and the effect of cooling (increased step sizes) in a CA3 neuron in current clamp. The combination of tetrodotoxin (TTX) (0.5 μM), NBQX (5 μM), bicuculline (BMR) (25 μM), and strychnine (5 μM), with or without ruthenium red (10 μM) was also ineffective; hippocampal slice
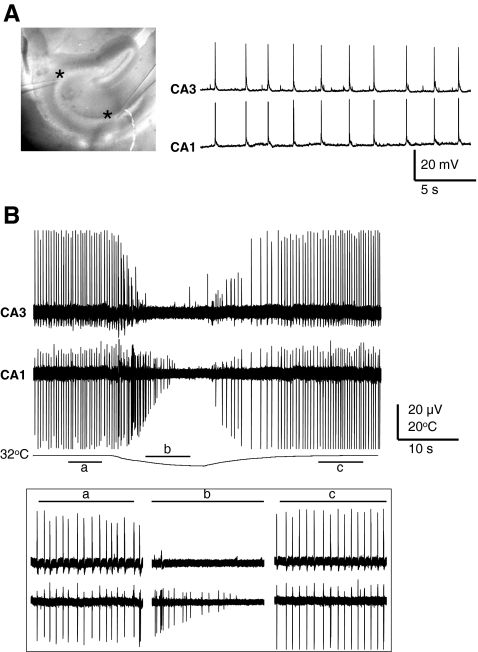
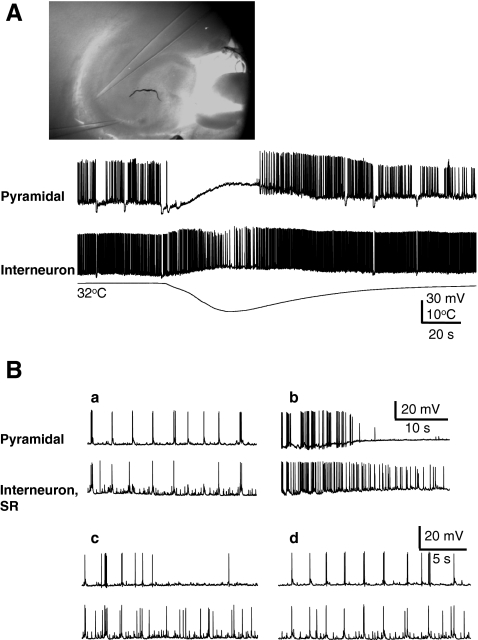
Because these experiments failed to show the involvement of a temperature sensitive ion channel as the underlying mechanism for disruption of the epileptiform discharges during cooling, we considered a network effect as a plausible mechanism. We hypothesized that cell specific responses to cooling might underlie the interruption of synchrony in hippocampal networks. Therefore, we used simultaneous intracellular and extracellular recordings from hippocampal neurons in acute slices using dual-patch clamp and field recordings (n = 18). The baseline recordings in the presence of 4-AP showed a significant degree of synchrony between the pyramidal cells, as recorded simultaneously in CA1 and CA3 with intracellular (Fig. 4A) and extracellular (Fig. 4B) recordings (n = 5). As shown in Fig. 4B cooling, reversibly, disrupted this synchrony. As further shown in Fig. 5A, whereas rapid cooling resulted in suppression of the spontaneous discharges in a CA3 pyramidal cell, interneurons located at the border of the CA3 pyramidal layer only showed decreased activity without complete cessation. This response to cooling was consistently observed in 10 similar pairs and was compared with those obtained through the pMEA recording, as reported as follows. Baseline recordings showed a significant synchrony of action potential firing between pyramidal neurons and interneurons in 40% of pairs as illustrated in Fig. 5Ba recorded simultaneously from a CA1 pyramidal neuron and an interneuron located in the stratum radiatum. In all cases, cooling had a remarkably different effect on the pyramidal cell and interneurons. In pyramidal neurons, cooling produced a brief increase in action potential firing followed by quiescence, whereas, in the interneurons the action potential firing was maintained throughout (Fig. 5Bb). Desynchronized action potential firing was maintained for some time after rewarming (Fig. 5Bc). Eventually the firing pattern of both cells fully recovered (Fig. 5Bd).
Fig. 4.
(A, left and right) Baseline dual current clamp, and (B) dual simultaneous extracellular recordings show a significant degree of synchrony between the CA1 and CA3 pyramidal neurons. Cooling reversibly disrupts synchronicity between pyramidal neurons (inset; a, baseline; b during cooling; c after rerwarming); asterisks show the recording sites in CA1 and CA3; hippocampal slice; 4-AP 50 μM
Fig. 5.
(A) Cooling differentially affects firing rates of pyramidal neurons and interneurons; 4-aminopyridine (4-AP) 50 μM, dual current clamp recordings. (B) Dual current clamp recordings from a CA1 pyramidal neuron and a stratum radiatum (SR) interneuron in the presence of 4-AP at 32°C illustrates the synchronous network activation of both neurons before cooling (a). Remarkably different effects of cooling between the 2 neurons, with maintained action potential firing in the interneuron (b). Recordings from these neuron during the first 2 minutes after rewarming at 32°C (c) that illustrate a temporarily desynchronized action potential firing, which recovers 2 minutes after cooling (d). Note that occasionally a temporary increase in pyramidal cell firing rate, as shown in panel (b), was also observed. Green GAD gfp, P15; hippocampal slice
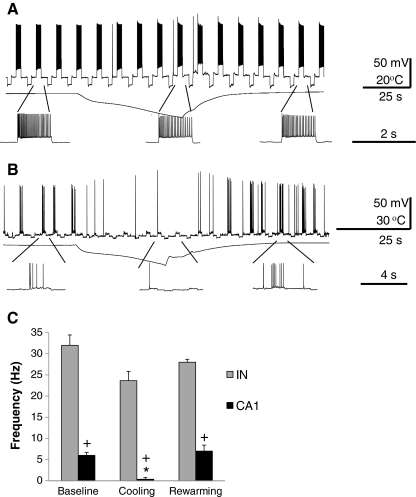
As 4-AP may affect the firing properties of neurons, we tested if the differential cooling effect between principal neurons and interneurons could be observed in the absence of 4-AP. However, because we did not observe spontaneous firing in the absence of 4-AP, we used repetitive depolarizing pulses of equal amplitude to evoke action potential firing in interneurons (Fig. 6A) and pyramidal neurons (Fig. 6B). As summarized in the histogram in Fig. 6C, the baseline firing rate was significantly higher in the interneurons than in the pyramidal cells (32 ± 3.4 Hz; 6 ±1 Hz; p = 0.003); cooling significantly decreased the firing rate in pyramidal cells (p = 0.013) in the absence of 4-AP, but did not significantly decrease the interneuron firing rate.
Fig. 6.
(A) Repetitive depolarizing pulses of equal amplitude evoke action potentials in a putative interneuron (IN), and (B) pyramidal CA1 neuron voltage clamped at −60 mV, both in the absence of 4-aminopyridine (4-AP). Magnified segments are shown below each trace for 3 significant time periods: before, during, and after cooling. (C) Summary histogram of action potential firing rates from interneurons (n = 3) and pyramidal neurons (n = 3) recorded at 3 distinct time periods. Similar to the response recorded in the presence of 4-AP, there is a significant decrease in pyramidal cell action potential during cooling, but no significant drop in interneuron firing rate. (*) Indicates significant change compared to baseline frequency (p = 0.013); (+) indicates significant difference compared to interneuron action potential frequency (p = 0.003); hippocampal slice
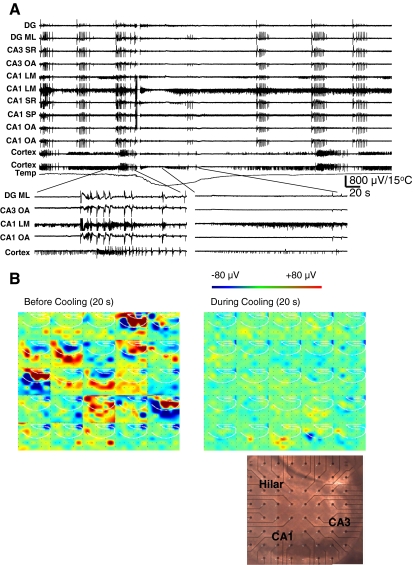
To gain a more comprehensive view of network synchrony during 4-AP-induced epileptiform discharges, we used the pMEA to simultaneously record local field potentials from selected electrodes located in distinct hippocampal and cortical regions (8 slices, 4 mice) using a previously described method [13]. These recordings showed synchronized runs of interictal discharges in the presence of 4-AP. In contrast, cortical electrodes displayed ictal-like continuous discharges that were not synchronized with those in the hippocampal regions. Furthermore, long bursts of multi-unit firing were recorded from individual electrodes in cortex and hippocampus that were not synchronous with the 4-AP-induced epileptiform discharges. Interestingly, cooling had a differential effect on the epileptiform and multi-unit discharges in the cortex and hippocampus. Cooling abolished the interictal activity in the hippocampus and the ictal-like cortical activity, whereas continuous firing multi-unit activity was unaffected (Fig. 7A). We also analyzed the spontaneous 4-AP-induced activity from the entire pMEA before and during cooling by using a color-coded voltages map (Fig. 7B). As previously reported [13], the spontaneous activity originates from the hilus/CA3 area. After cooling, there is complete suppression of spontaneous discharges across the entire pMEA.
Fig. 7.
(A) Local field potentials recorded from selected perforated multi-electrode array (pMEA) electrodes located in distinct hippocampal regions showing synchronized runs of interictal discharges. In contrast, cortical electrodes display long bursts of multi-unit firing that are not synchronized with the hippocampal discharges. Cooling decreases the interictal activity in hippocampus, alters cortical activity, but has less effect on multi-unit discharges in cortex, and even less in the CA1 stratum lacunosum moleculare (LM) electrode. (B) Montage of 25 panels of color-coded voltages illustrating the spontaneous 4-aminopyridine-induced activity from the entire pMEA recording before (left) and during (right) cooling to <20°C for a 20-second interval illustrated side-by-side for the selected columns of pMEA electrodes versus time displaying 1 frame every 800 ms. With cooling, there is complete suppression of spontaneous discharges. Picture of a hippocampal coronal slice from a P14 mouse placed over pMEA electrodes is shown indicating the origins of spontaneous activity as hilus and CA3 areas. DG = dentate granular cell; ML = molecular layer; OA = stratum oriens/alveus; SP = stratum pyramidale; SR = stratum radiatum; slice recording
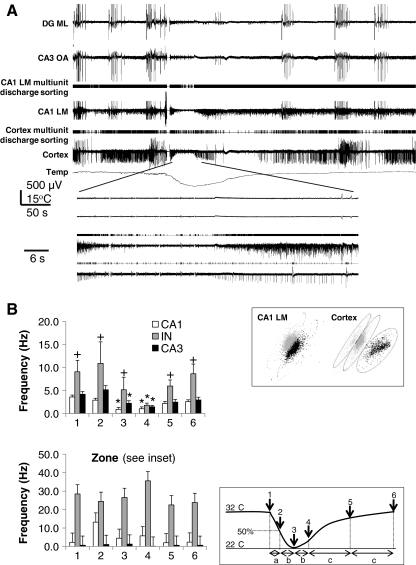
As cooling has differential effects on interneurons and pyramidal neurons and preserved multi-unit firing in the pMEA recording, we quantified the effect of cooling in different cell types. We analyzed spike sorted multi-unit activity recorded by pMEA electrodes located in CA1 and CA3 areas, as in the example in Fig. 8A. Units in these areas were grouped according to their location in the CA3 or CA1 subregions. In addition, units with basal frequencies higher than 10 Hz pooled from both areas were identified as putative interneurons. We also quantified the firing rates of visually identified CA pyramidal cells and interneurons recorded through patch clamp and grouped across different subregions during baseline and the various stages of cooling (Fig. 8B, inset). The averaged spike frequency values for cells studied with patch recording (Fig. 8B, top) showed a significant drop between baseline and peak hypothermia (zone 3) in CA1 (p = 0.016) and more significantly in CA3 pyramidal neurons (p = 0.0001), that lasted beyond the peak hypothermia through zone 4, whereas in the interneurons, the change in frequency was not significantly different at the peak hypothermia and was decreased only in zone 4 (p < 0.017). Interestingly, the averaged spike frequency value before and after cooling was always higher for interneurons than CA1/2 and CA3 pyramidal neurons (Fig. 8, top).
Fig. 8.
(A) Selected cortical and hippocampal perforated multi-electrode array (pMEA) electrodes show the effect of cooling on synchronized runs of interictal discharges along with the results of spike sorting. Multi-unit discharges acquired from CA1 stratum lacunosum moleculare (LM) and cortex were sorted by principal component analysis. In contrast to the other electrodes, 2 distinct units recorded in CA1 LM and 3 units in the cortical electrode (top inset) are minimally affected by cooling. (B) Action potential frequency during cooling obtained through cell attached recordings from visually identified neurons in hippocampal slices (top) as in Fig. 5, compared to those derived from principal component analysis of multi-unit activity recorded from individual electrodes on pMEA (bottom). (*) Indicates significant changes compared to baseline frequency in zone 1 (p < 0.01); (+) indicates significant differences compared to the CA1 and CA3 pyramidal cells action potential frequencies (p < 0.03). Data are shown as frequency measured at the end of distinct time zones, in which zone 1 reflects the averaged frequency for 30 to 100 seconds before the start of cooling, zones 2 and 3 reflecting the averaged first and second half of cooling rise time, zones 4 and 5 for the first and second (slower) phase of recovery from peak cooling, and zone 6 as recovery baseline (bottom inset); slice recording
These results were then compared to the results from spike sorting of single- or multi-unit activity recorded through pMEA electrodes located in CA1 and CA3 areas. Units with basal frequencies higher than 5 Hz pooled from both areas were identified as putative interneurons. The putative interneurons showed higher baseline frequency rates of multi-unit discharges recorded on the pMEA (29 ± 24 Hz), compared to the direct recordings from visually identified interneurons (9.1 ± 2.5 Hz). The multi-unit discharge frequency increased toward the peak of the cooling effect, although this change was not statistically significant at different time zones (Fig. 8B, bottom).
Discussion
In this work, we have attempted to further characterize the effects of hypothermia on neuronal activity and its cellular and network mechanisms. Intracellular current clamp recordings in CA1 and CA3 pyramidal neurons showed that hypothermia induced depolarization or hyperpolarization, or a combination of both, that were accompanied by a blockade of action potential firing. The depolarization was eliminated by TTX in CA1/CA2 neurons. These effects of hypothermia observed with whole cell recordings in mouse hippocampal slices expand what was previously reported in rats with field recordings [9].
It has been hypothesized that a loss of function caused by alterations in sodium channel gating properties during hypothermia could interrupt neuronal depolarization and abort the epileptiform discharges [5, 22]. Indeed, we observed blockade of action potentials in hippocampal pyramidal neurons that was associated with either depolarization or hyperpolarization. However, in the presence of TTX, cooling only caused hyperpolarization associated with an outward current, raising the possibility of closure of a tonically active inward current triggered by warmer temperatures in the recorded neuron. This was further confirmed by remarkable increases in membrane input resistance in response to hyperpolarizing and depolarizing current injections.
As TRPV4 has been shown to be expressed in hippocampal neurons in primary culture [10] and is activated by warm temperatures (>30°C), this cationic channel was an ideal candidate to mediate the effect of cooling. However, its selective agonist 4α-PDD failed to induce currents during cooling in slices, which suggests that the expression of TRPV4 channel is restricted to the culture model. Our results also excluded activation of TRPV1, as agonist capsaicin or antagonist capsazepine failed to activate any currents in CA3 neurons at 32°C.
Similarly, the possible involvement of TRPM8 channels, which are activated by cooling with a threshold of ~25°C [23], was ruled out. The results with RR, which blocks several TRPV-mediated responses [19], but failed to alter cooling responses in hippocampal neurons, provides further evidence against the possible involvement of temperature-sensitive TRP channels in aborting epileptiform discharges.
A common effect of cooling in all neurons tested is an increase in membrane input resistance. Previous studies have reported temperature-dependent increases in input resistance [24, 25]. The underlying mechanisms of thermal-induced changes in resistance are not very clear, but one intriguing suggestion that still awaits demonstration points at the alteration of membrane structure by temperature [25].
The results of dual-patch clamp recordings during cooling at baseline in the presence of 4-AP showed a significant degree of synchrony between distant CA1 and CA3 pyramidal neurons, and to a lesser extent between principal cells and interneurons. The synchrony and rhythmic action potential discharges in CA1 and CA3 pyramidal neurons were disrupted by hypothermia. Cooling also blocked rhythmic discharges in interneurons, but did not abolish action potential firing. This desynchronizing effect would last for the duration of cooling, but was reversible in all cell types on rewarming. The differential effects of cooling on action potential firing between pyramidal neurons and interneurons was not due to the presence of 4-AP, as it was observed on depolarizing current injections even in the absence of 4-AP.
The pMEA recordings revealed independent interictal and ictal-like discharges in the cortex and hippocampus, possibly due to the coronal slicing orientation. Hypothermia reversibly abolishes 4-AP-induced epileptiform discharges in all hippocampal and cortical sites while preserving spontaneous multi-unit firing. Spike sorting of the multi-unit discharges recorded by pMEA showed a consistent number of cells with higher firing rates being differentially affected by cooling. In contrast to the cessation of epileptiform discharges in response to cooling, as seen with local field potentials, putative fast spiking interneurons did not show significant reductions in frequency. This suggests a different threshold between interneurons and pyramidal neurons in response to cooling, as confirmed with patch-clamp recordings.
The underlying mechanisms for the preferential effects of cooling on pyramidal cells versus interneurons might be related to differences in intrinsic action potential firing mechanisms in these neurons, varying thresholds in voltage-gated channels, or differences in synaptic afferents to these cells. However, the differential effects of cooling, seen even in the absence of 4-AP when spontaneous synaptic activity is considerably less pronounced, suggests that the unique spiking properties of interneurons are probably the key. Indeed, several studies [26] have shown that hippocampal interneurons are rich in expression of the NaV1.1 subunit variant of voltage-gated Na2+ channels, whereas pyramidal neurons express NaV1.6 subunit. Although detailed biophysical studies of temperature sensitivity of these distinct channels are not available, point mutations of NaV1.1 channels have been clearly implicated in the pathogenesis of clinical conditions, such as generalized epilepsy with febrile seizures plus [26].
The role of brief high-frequency discharges in synchronizing neuronal activity has been recognized. It has been shown that GABAergic neurons can play both antiepileptic and ictogenic roles in different networks in the brain by synchronizing neural networks [27]. Therefore, this finding raises the possibility that the observed decrease in fast interneuron synchronous discharges on cooling might contribute to the antiepileptic activity of cooling by disturbing the normal synchrony between different cell types and networks in the hippocampus, hence aborting epileptiform discharges within in vitro models of ictogenesis. Furthermore, the persistence of action potential firing in interneurons would have an additional antiepileptic effect through tonic GABA release, and cooling will induce prolongation of decay of phasic inhibitory synaptic current, similar to the effect of benzodiazepines and barbiturates [28].
The effect of high temperature in triggering seizures has been known in the clinical setting. The decay, and to a lesser extent, the amplitude of GABAA-mediated inhibitory synaptic currents are very sensitive to temperature changes. Indeed, hyperthermia by decreasing charge transfer at the synapse acts as a convulsant agent [28]. Although the exact ictogenic mechanism in childhood febrile seizures is unclear, it has been associated with temperature-dependent changes in synaptic efficacy consequent to mutations involving the GABAA γ2 subunit [29, 30]. Our findings of distinct effects of cooling on interneurons suggest that the anti-seizure effect of hypothermia is likely to be modulated through GABA receptors. Further studies will help define the subtypes of interneurons that may potentially play a pivotal role in the therapeutic effects of cooling.
Despite accumulating in vitro and in vivo data, the clinical use of implanted cooling devices as a treatment for epilepsy and other neurological disorders is currently hampered by technical problems. In particular, a safe and efficient method for heat transfer and dissipation, as well as an effective method of power consumption seem to be among the major obstacles. With further elucidation of cellular mechanisms involved, it might be plausible to design clinical studies safe enough to test the potential therapeutic effects of cooling in humans.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
This work was supported by funding from the CONACyT of the Mexican Government and the National Science Foundation Partnerships for International Research and Education (A.G.S.), the National Science Foundation Partnerships for International Research and Education (PIRE) grant (S.V., and A. G.S.), and in part by the Luce Foundation (R.D.). The authors do not have any real or perceived conflict of interest. Full conflict of interest disclosure is available in the electronic supplementary material for this article.
References
- 1.Gancia P, Pomero G. Brain cooling therapy. Minerva Pediatr. 2010;62:173–175. [PubMed] [Google Scholar]
- 2.Ritchie JM, Straub RW. The effect of cooling on the size of the action potential of mammalian non-medullated fibres. J Physiol. 1956;134:712–717. doi: 10.1113/jphysiol.1956.sp005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiff SJ, Somjen GG. The effects of temperature on synaptic transmission in hippocampal tissue slices. Brain Res. 1985;345:279–284. doi: 10.1016/0006-8993(85)91004-2. [DOI] [PubMed] [Google Scholar]
- 4.Hill MW, Wong M, Amarakone A, Rothman SM. Rapid cooling aborts seizure-like activity in rodent hippocampal-entorhinal slices. Epilepsia. 2000;41:1241–1248. doi: 10.1111/j.1528-1157.2000.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 5.Motamedi GK, Salazar P, Smith EL, et al. Termination of epileptiform activity by cooling in rat hippocampal slice epilepsy models. Epilepsy Res. 2006;70:200–210. doi: 10.1016/j.eplepsyres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Yang XF, Rothman SM. Focal cooling rapidly terminates experimental neocortical seizures. Ann Neurol. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- 7.Yang XF, Duffy DW, Morley RE, Rothman SM. Neocortical seizure termination by focal cooling: temperature dependence and automated seizure detection. Epilepsia. 2002;43:240–245. doi: 10.1046/j.1528-1157.2002.33301.x. [DOI] [PubMed] [Google Scholar]
- 8.Lomber SG, Payne BR, Horel JA. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 1999;86:179–194. doi: 10.1016/S0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- 9.Yang XF, Ouyang Y, Kennedy BR, Rothman SM. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: observations using 2-photon microscopy. J Physiol. 2005;567:215–224. doi: 10.1113/jphysiol.2005.088948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/S0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Sulser A, Wang J, Motamedi GK, Avoli M, Vicini S, Dzakpasu R. The 4-aminopyridine in vitro epilepsy model analyzed with a perforated multi-electrode array. Neuropharmacology 2011;60:1142–53. [DOI] [PMC free article] [PubMed]
- 14.Egert U, Knott T, Schwarz C, et al. MEA-Tools: an open source toolbox for the analysis of multi-electrode data with MATLAB. J Neurosci Methods 2002 30;117:33–42. [DOI] [PubMed]
- 15.Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- 16.Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by low magnesium in rat entorhinal cortex hippocampal slices. Epilepsia. 2000;41:28–33. doi: 10.1111/j.1528-1157.2000.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 17.Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- 18.Hevers W, Luddens H. Pharmacological heterogeneity of gamma-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology. 2002;42:34–47. doi: 10.1016/S0028-3908(01)00158-7. [DOI] [PubMed] [Google Scholar]
- 19.Moon C. An investigation of the effects of ruthenium red, nitric oxide and endothelin-1 on infrared receptor activity in a crotaline snake. Neuroscience. 2004;124:913–918. doi: 10.1016/j.neuroscience.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/S0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 21.Ma J. Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J Gen Physiol. 1993;102:1031–1056. doi: 10.1085/jgp.102.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen AD. Nonlinear temperature modulation of sodium channel kinetics in GH(3) cells. Biochim Biophys Acta. 2001;1511:391–396. doi: 10.1016/S0005-2736(01)00301-7. [DOI] [PubMed] [Google Scholar]
- 23.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin JD, Boulant JA. Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J Physiol. 1995;488(pt 2):407–418. doi: 10.1113/jphysiol.1995.sp020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payton BW, Bennett MV, Pappas GD. Temperature-dependence of resistance at an electrotonic synapse. Science. 1969;165:594–597. doi: 10.1126/science.165.3893.594. [DOI] [PubMed] [Google Scholar]
- 26.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiruska P, Csicsvari J, Powell AD, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu L, Leung LS. Effects of temperature elevation on neuronal inhibition in hippocampal neurons of immature and mature rats. J Neurosci Res. 2009;87:2773–2785. doi: 10.1002/jnr.22105. [DOI] [PubMed] [Google Scholar]
- 29.Hill EL, Hosie S, Mulligan RS, et al. Temperature elevation increases GABA(A) -mediated cortical inhibition in a mouse model of genetic epilepsy. Epilepsia. 2011;52:179–184. doi: 10.1111/j.1528-1167.2010.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)