Abstract
Angiogenin (ANG), also known as ribonuclease (RNASE) 5, is a member of the vertebrate-specific, secreted RNASE superfamily. ANG was originally identified as a tumor angiogenic factor, but its biological activity has been extended from inducing angiogenesis to stimulating cell proliferation and more recently, to promoting cell survival. Under growth conditions, ANG is translocated to nucleus where it accumulates in nucleolus and stimulates ribosomal RNA (rRNA) transcription, thus facilitating cell growth and proliferation. Under stress conditions, ANG is accumulated in cytoplasmic compartments and modulates the production of tiRNA, a novel class of small RNA that is derived from tRNA and is induced by stress. tiRNA suppress global protein translation by inhibiting both cap-dependent and -independent translation including that mediated by weak IRESes. However, strong IRES-mediated translation, a mechanism often used by genes involved in pro-survival and anti-apoptosis, is not affected. Thus, ANG-mediated tiRNA reprogram protein translation, save anabolic energy, and promote cell survival. This recently uncovered function of ANG presents a novel mechanism of action in regulating cell growth and survival.
Keywords: Angiogenin, tiRNA, stress response, cell survival
Angiogenin
Angiogenin (ANG) belongs to a gene superfamily that encodes secreted ribonuclease (RNASE) existing only in vertebrates. This family of enzyme was previously known as pancreatic RNASE superfamily because the prototypic member of the family is bovine pancreatic RNASE A (Cho and Zhang, 2006). Upon the completion of human genome sequencing, it became clear that this is the only family of enzymes that is vertebrate-specific. During the 8th International Conferences on RNASES held in Naples, Italy in October 20–22, 2010, Giuseppe D’Alessio of University of Naples recommended the use of the more accurate and informative nomenclature “Vertebrate Secreted RNASES” to replace the old term “Pancreatic RNASES”.
ANG is the 5th member of the human family of RNASES. It encodes a 14 kDa protein consisting of 123 amino acid residues (Strydom et al., 1985). Although ANG is a small molecule, three distinct function sites including a receptor binding site, a nuclear localization sequence (NLS), and a catalytic site have been identified. The loop region from K60 to N68 is the receptor binding site that interacts with a to-be-identified cell surface receptor (Hallahan et al., 1991). Upon binding to the cell surface receptor, ANG is internalized and translocated to the nucleus (Moroianu and Riordan, 1994b). The nuclear translocation process is mediated by an NLS located between M30 to G34 (Moroianu and Riordan, 1994a). The ribonucleolytic activity of ANG, executed by the catalytic triad H13, K40, and H113 (Shapiro and Vallee, 1989), is believed to function in stimulating rRNA transcription after ANG is localized in the nucleus. All three sites are essential for ANG to have angiogenic and growth stimulating activities.
ANG-stimulated rRNA transcription in angiogenesis
As a tumor angiogenic factor, the role of ANG in angiogenesis has been well documented. ANG has been shown to be upregulated in a variety of human cancers (Li and Hu, 2010). Elevated serum level of and/or enhanced tissue expression of ANG have been noticed in all types of solid and blood cancers so far examined. A major mechanism by which ANG induces angiogenesis is related to its activity in stimulating ribosomal RNA (rRNA) transcription (Li and Hu, 2010). ANG has been shown to undergo nuclear translocation in endothelial cells (Moroianu and Riordan, 1994b) where it binds to the promoter region and stimulates rRNA transcription (Xu et al., 2002; Xu et al., 2003). rRNA transcription is the rate-limiting step in ribosome biogenesis (Moss, 2004), a process required for cell growth as well as maintenance and survival as proteins are required for essentially all cellular activities. ANG-stimulated rRNA transcription has been shown to be permissive for other angiogenic factors to induce angiogenesis (Kishimoto et al., 2005). For example, knockdown of ANG expression or inhibition of ANG activity in endothelial cells resulted in a decrease of rRNA transcription and insensitivity to growth stimuli such as VEGF and bFGF (Kishimoto et al., 2005). Compelling evidence indicates that ANG activity is necessary for other angiogenic factors to induce angiogenesis. ANG-induced rRNA transcription appears to be a common down-stream event of tumor angiogenesis (Kishimoto et al., 2005). Thus, ANG inhibitors have been shown to inhibit not only ANG-induced angiogenesis but also those induced by other angiogenic factors including VEGF, FGF, and EGF (Hirukawa et al., 2005). ANG inhibitors would therefore have a profound effect in inhibiting tumor angiogenesis. Indeed, ANG inhibitors such as its siRNA (Ibaragi et al., 2009a), antisense (Olson et al., 2001), monoclonal antibodies (Olson et al., 2002), soluble binding proteins (Olson et al., 1995), enzymatic inhibitors (Kao et al., 2002), and nuclear translocation blockers (Hirukawa et al., 2005; Ibaragi et al., 2009b; Yoshioka et al., 2006) have all been shown to inhibit tumor angiogenesis and cancer progression in various animal models.
ANG-mediated rRNA transcription in cancer cell proliferation
Sustained growth is a hallmark of cancer (Hanahan and Weinberg, 2000). Cancer cells are constantly proliferating and thus require robust ribosome biogenesis to meet the high metabolic demand. Ribosomal biogenesis is a process involving rRNA transcription, processing of the pre-rRNA precursor and assembly of 4 mature rRNA with 79 ribosomal proteins (Nazar, 2004). Genetic, epigenetic, and environmental factors that cause cancers are known to enhance the production of ribosomal proteins (Ruggero and Pandolfi, 2003). However, it was less clear how rRNAs are proportionally increased as they need to be incorporated with ribosomal proteins in an equal molar level to make a functional ribosome. In order to know whether rRNA transcription in cancer cells is modulated by ANG, a series of experiments using prostate cancer as a model has been performed to understand the role of ANG in rRNA transcription, ribosome biogenesis, and cell growth (Ibaragi et al., 2009a; Ibaragi et al., 2009b; Li et al., 2011; Yoshioka et al., 2006). Human ANG is significantly and progressively upregulated in prostate cancer (Katona et al., 2005; Majumder et al., 2003). Mouse Ang is the highest upregulated gene in AKT-induced prostate intraepithelial neoplasia (PIN) in murine prostate-restricted AKT kinase transgenic (MPAKT) mice (Majumder et al., 2003). Knockdown of ANG expression in human prostate cancer cells reduced rRNA transcription and inhibited both in vitro and in vivo growth (Yoshioka et al., 2006). Knockdown of mouse Ang1 in MPAKT mice prevented PIN formation and reversed established PIN as a result of reduced rRNA transcription (Ibaragi et al., 2009a). ANG activity was therefore thought to be a requirement for excessive cancer cell proliferation as it provides the extra rRNA needed to sustain continuous growth. AKT and ANG pathways therefore mediate the production of ribosomal proteins and rRNAs, respectively, and a coordinated action of AKT and ANG allows ribosome biogenesis to take place (Li et al., 2011). Consistently, there is a crosstalk between AKT and ANG pathways (Fig. 1). For example, ANG activates AKT (Trouillon et al., 2010), and activated AKT in turn stimulates nuclear translocation of ANG (Ibaragi et al., 2009b; Kieran et al., 2008). These results clearly demonstrated that ANG-stimulated rRNA transcription is a critical component in prostate cancer progression (Ibaragi et al., 2009a; Yoshioka et al., 2006). The role of ANG in other types of cancer is less understood. However, ANG has also been shown to be involved in proliferation of HeLa cervical cancer cells, MB-435 breast cancer cells, HT-29 colon adenocarcinoma cells, and A432 epidermoid carcinoma cells (Hirukawa et al., 2005; Tsuji et al., 2005). Judging from the universal upregulation of ANG in various types of human cancer, ANG-stimulated rRNA transcription could well be a general requirement for cancer cell proliferation.
Fig. 1.
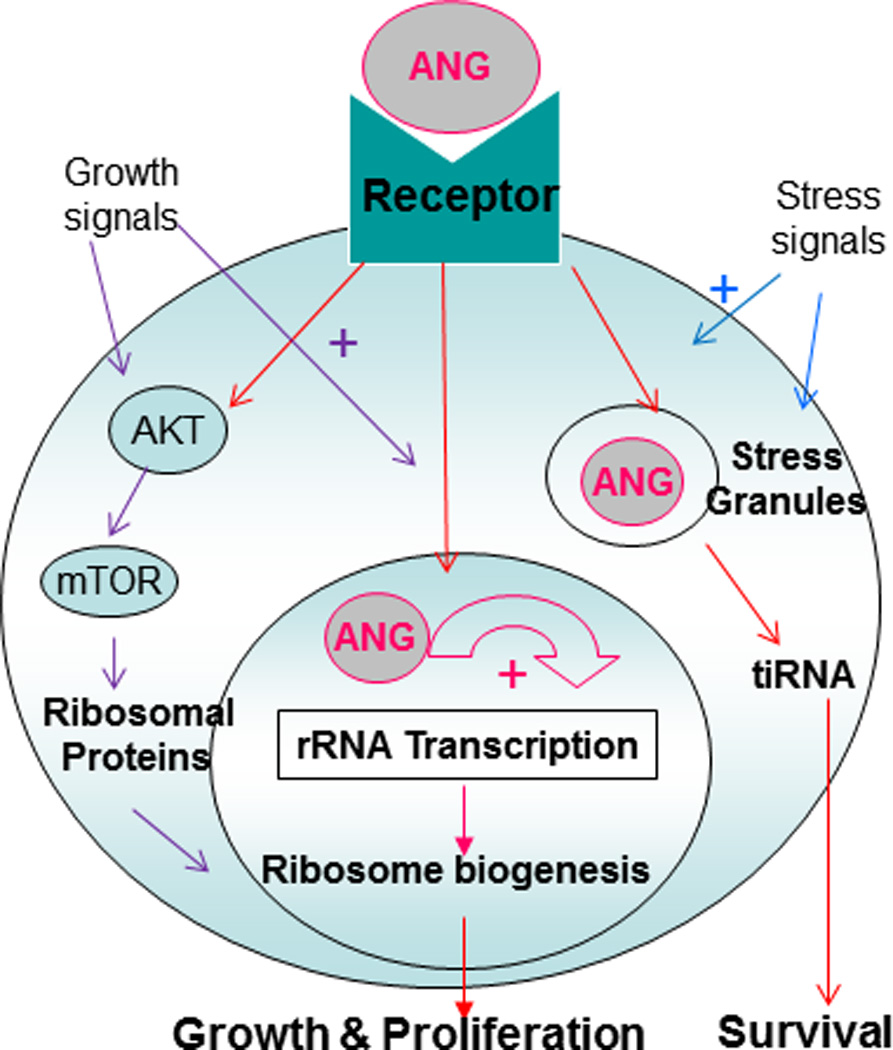
Mechanism of action of ANG. Growth signals stimulate nuclear translocation of ANG, whereas stress signals direct ANG to stress granules. Both pathways are mediated by a cell surface receptor that remains to be identified. Nuclear ANG stimulates rRNA transcription, enabling ribosome biogenesis and therefore cell growth and proliferation. Under stress conditions, ANG is not translocated into the nucleus but is rather accumulated in cytoplasmic compartments such as stress granules where it mediates the production of tiRNA, reprograming protein translation and promoting survival.
These results extended the role of ANG from inducing tumor angiogenesis to directly stimulating cancer cell proliferation. ANG seems to play a double role in cancer progression by stimulating rRNA transcription in both endothelial cells and cancer cell. ANG inhibitors, including siRNA and nuclear translocation blockers, have been shown to inhibit tumor growth in animal models by inhibiting both angiogenesis and cancer cell proliferation (Ibaragi et al., 2009b; Yoshioka et al., 2006).
ANG is responsible for stress-induced tRNA cleavage
A series of recently publications has further extended the biological activity of ANG from enabling cell growth and proliferation to sustaining survival under adverse conditions (Emara et al., 2010; Fu et al., 2009; Ivanov et al., 2011; Yamasaki et al., 2009). In an effort to identify liver-specific miRNA, Xiaofen Zheng laboratory at Beijing Institute of Radiation Medicine cloned and profiled small RNAs (<200 nt) from fetal liver, and was somewhat surprised that 85 of the total 205 clones they sequenced were from tRNA (Fu et al., 2005). More unexpectedly, these tRNA fragments were seemingly produced from mature tRNA by a specific cleavage at the anti-codon loop. Therefore, these small tRNA fragments were named tRNA halves. It took them a couple of years to figure out that ANG is the endoribonuclease responsible for this cleavage (Fu et al., 2009). In vitro, ANG was able to cleave tRNA at the anti-codon region to produce exactly the same tRNA halves as those isolated from tissues. In vivo, ANG level was positively correlated with the production of tRNA halves. For example, overexpression of ANG enhanced the production of tRNA halves, whereas knockdown of ANG expression decreased cellular level of tRNA halves. Another interesting finding was that the production of tRNA halves was induced by various stresses including heat shock, hypothermia, hypoxia, and radiation (Fu et al., 2009). Stress-induced cleavage of tRNA has been shown in Tetrahymena thermophila as a starvation response to promote survival (Lee and Collins, 2005). Although this particular study did not show whether ANG expression was concomitantly enhanced under these stress conditions (Fu et al., 2009), ANG has already been known, as an acute-phase protein (Verselis et al., 1999), to be upregulated in hypoxia (Hartmann et al., 1999; Nakamura et al., 2006). Thus, a possible role of ANG in stress response and in cell survival of adverse conditions could be envisioned from this study.
Almost at the same time, Paul Anderson laboratory of Brigham and Women’s Hospital reached the same conclusion from their study of stress-induced silencing of protein translation. In mammalian cells, stress-induced phosphorylation of eIF2α inhibits global protein synthesis to conserve anabolic energy for the repair of stress-induced damage (Yamasaki and Anderson, 2008). However, stress-induced translational silence is also observed in cells expressing a nonphosphorylatable eIF2α mutant (S51A) (McEwen et al., 2005), indicating the existence of a phosphor-eIF2α-independent pathway of translational control. A series of elegant experiments demonstrated that this pathway is mediated by a subset of tRNA fragments produced by a specific cleavage at the anticodon loop (Yamasaki et al., 2009). Sequence analyses indicated that they are identical to the tRNA halves identified by Zheng laboratory (Fu et al., 2009). Without knowing Zheng’s results, these tRNA fragments were named tiRNA, standing for tRNA-derived, stress-induced small RNAs, to reflect the fact that they are derived from tRNA and that they are induced by stress (Yamasaki et al., 2009). Similarly, ANG was found to be the ribonuclease that is responsible for this specific cleavage. The enzymatically inactive ANG variant (H13A) lost the ability to mediate the production of tiRNA, indicating that the ribonucleolytic activity of ANG is important. Consistently, overexpression of ribonuclease inhibitor (RI) completely abolished the tiRNA-producing activity of ANG. However, a mere ribonucleolytic activity is inadequate to produce tiRNA. For example, RNASE A and RNASE4, the other two members of this superfamily which are enzymatically more active than ANG, failed to produce any tiRNA (Emara et al., 2010). P112L ANG variant, which is found in amyotrophic lateral sclerosis (ALS) patients (Wu et al., 2007) and which is incapable of nuclear localization, was unable to mediate tiRNA production, indicating that nuclear translocation of ANG, thus the cell surface receptor of ANG, is required for ANG to cleave tRNA in vivo. This is consistent with the finding that exogenously added ANG to cultured cells was able to mediate tiRNA production (Emara et al., 2010; Fu et al., 2009).
Role of ANG-mediated tiRNA in reprograming protein translation
A potential role of ANG in cell survival was first implicated by the finding that ANG-mediated tiRNA is able to stimulate the formation of stress granules (SGs) (Emara et al., 2010). SGs are cytoplasmic foci where untranslated mRNPs are transiently stored. Formation of SGs is an important mechanism of cell survival under adverse conditions. In response to environmental and genetic stresses, mammalian cells reprogram protein translation by suppressing global protein expression but selectively enhancing expression of genes involved in pro-survival and anti-apoptosis (Yamasaki and Anderson, 2008). This process is initiated by phosphor-eIF2α-induced translational repression and is facilitated by sequestration of untranslated mRNAs into SGs. The finding that ANG-mediated tiRNA was able to trigger the formation of SGs indicated that ANG plays an active role in stress response of the cells and may promote cell survival via this mechanism. Indeed, ANG is a neuroprotective factor that prevents neuronal death induced by various stresses (Kieran et al., 2008; Sebastia et al., 2009; Subramanian et al., 2008). Loss-of-function mutations in the coding region of ANG gene have recently been found in ALS patients (Greenway et al., 2006). Systemic administration of wild type ANG protein slowed down motor neuron degeneration in SOD1G93A mice that develop ALS-like symptoms (Kieran et al., 2008). The findings that tiRNA induced SG formation and that ANG potentiated both oxidative stress (sodium arsenite)- and xenobiotic (pateamine)-induced SGs (Emara et al., 2010) not only shed light on the mechanism by which ANG mutations predispose ALS but also provided a rationale for further development of ANG protein therapy in ALS treatment.
The mechanism by which tiRNA inhibits protein translation was elucidated by a recent study also from Anderson laboratory (Ivanov et al., 2011). Among the two tRNA halves produced by ANG, only the 5’ halves (5’-tiRNAs) were able to inhibit protein translation. The 3’ halves (3’-tiRNAs) had no activities. Moreover, not all 5’-tiRNAs are active. Only a subset of 5’-tiRNAs such as 5’-tiRNAAla and 5’-tiRNACyc that contain a 5’-oligo-G motif (four to five consecutive guanine residues) are able to inhibit protein translation. The reason for this structure requirement is because this oligo-G motif is required for the tiRNA to inhibit the binding of eIF4G/A to uncapped mRNA and of eIF4E/G/A to m7G-capped mRNA. The strength of eIF4G:RNA interaction is a critical factor to determine whether or not its translation initiation is inhibited by tiRNA. For example, translation mediated by WT EMCV-IRES (UA6) that has a strong interaction with eIF4G was not inhibited by tiRNA, whereas those mediated by a mutant IRES (UA7) that has a weaker interaction with eIF4G were inhibited by tiRNA (Ivanov et al., 2011). The finding that tiRNA does not inhibit IRES-mediated translation is of significant interest and importance as IRES is often used for translation of mRNAs that are involved in pro-survival and anti-apoptosis. For example, The majority of the 51 so far reported human cellular IRES are from the genes involved in anti-apoptosis such as BAG-1 (Coldwell et al., 2001), Bcl-2 (Sherrill et al., 2004), Hiap2 (Warnakulasuriyarachchi et al., 2004), and XIAP (Holcik et al., 1999); angiogenesis such as HIF-1α (Lang et al., 2002), VEGF (Stein et al., 1998), Tie2 (Park et al., 2005), FGF-1 (Martineau et al., 2004), FGF-2 (Bonnal et al., 2003) and IGF1R (Giraud et al., 2001); and cell survival such as c-Myc (Stoneley et al., 1998), c-Myb (Mitchell et al., 2005), Runx1 (Pozner et al., 2000), and RunX1T1 (Mitchell et al., 2005). Thus, while ANG-mediated tiRNAs suppress global protein translation, they may selectively enhance the translation of a subset of mRNAs involved in anti-apoptosis thereby promoting cell survival under adverse conditions. Consistently, ANG has been shown to upregulate the expression of Bcl-2 at both the mRNA and protein level (Li et al., 2010). The enhancement to Bcl-2 protein (4.6-fold) is more significant than that to Bcl-2 mRNA (2.6-fold), suggesting that ANG stimulates both Bcl-2 transcription and translation (Li et al., 2010).
Summary
ANG is an old angiogenic molecule identified from tumor conditioned medium purely based on an in vivo angiogenesis assay (Fett et al., 1985). However, the biological role of ANG should not be limited to induction of angiogenesis as suggested by its widespread expression in all human organs and tissues (Weiner et al., 1987). The finding that ANG is able to directly stimulate cancer cell proliferation marked the first functional expansion of this molecule (Hirukawa et al., 2005; Tsuji et al., 2005; Yoshioka et al., 2006). The findings that ANG mediates stress-induced production of tiRNA further expanded the role of ANG from cell growth to cell survival (Emara et al., 2010; Fu et al., 2009; Ivanov et al., 2011; Yamasaki et al., 2009). Stress-induced cleavage of tRNA is a phenomenon first described as a starvation response in Tetrahymena thermophila (Lee and Collins, 2005), and later observed in bacteria (Haiser et al., 2008), fungi (Jochl et al., 2008), and most recently in mammalian cells (Thompson et al., 2008). tiRNAs reprogram protein translation, save anabolic energy, and enhance damage repair and promote survival. Cellular stresses inflicted by environmental and genetic factors are an underlying mechanism for both cancers and neurodegenerative diseases, the two pathological conditions where ANG has been shown to play a role. For example, hypoxic and oxidative stresses are a common etiology for cancer. Accumulation of misfolded protein aggregates that increases ER stress is a hallmark of neurodegenerative diseases.
Based on these recent findings, a new mode of action of ANG in mediating cell proliferation and survival can be proposed (Fig. 1). ANG has three types of target cells (endothelial cells, cancer cells, and motor neurons). These cells respond to ANG but exhibit some difference probably due to the difference in ANG receptor expression. Endothelial cells are the first type of responsive cells that have been used extensively for studying ANG biology. The activity of ANG in endothelial cells is strictly dependent on the cell density in vitro and proliferating status in vivo (Hu et al., 1997). ANG receptor is expressed only in sparsely cultured endothelial cells, and in sprouting neovessels in vivo (Hu et al., 1997). Cancer cells are the second type of ANG responsive cells. ANG undergoes nuclear translocation in cancer cells in a cell density-independent manner because of the constitutive expression of ANG receptor (Tsuji et al., 2005). Constitutive nuclear translocation of ANG in cancer cells is a driving force for cancer progression (Ibaragi et al., 2009a; Ibaragi et al., 2009b; Yoshioka et al., 2006). Motor neurons are the third type of ANG responsive cells. ANG is strongly expressed in the spinal cord both during development and in adulthood (Wu et al., 2007). Loss-of-function mutations in the coding region of ANG have been found in ALS patients (Greenway et al., 2006) and ANG has been shown to enhance motor neuron survival (Kieran et al., 2008). Both growth and survival activities of ANG seem to be mediated by the same receptor. Under growth conditions, the receptor mediates internalization of ANG that is subsequently translocated to the nucleus. Upon reaching the nucleus, ANG binds to rDNA promoter and stimulates rRNA transcription. Under stress conditions, the receptor mediates the translocation of ANG into cytoplasmic compartments, most likely SG, where it may enhance tiRNA production and promote survival. Full elucidation of the mechanism of action of ANG in regulating growth and survival would need identification of ANG receptor and its signaling pathways. However, despite extensive search and research by many laboratories worldwide over the past two decades, the identity of such a receptor remains elusive. It is expected that the functional extension of the biological activity of ANG and expansion of its target cells will provide an alternative means to facilitate the identification and characterization of the long overdue ANG receptor. ANG research has entered a very exciting period. Great discoveries are anticipated in the coming years.
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant numbers: R01NS065237, R01CA105241
Literature Cited
- Bonnal S, Schaeffer C, Creancier L, Clamens S, Moine H, Prats AC, Vagner S. A single internal ribosome entry site containing a G quartet RNA structure drives fibroblast growth factor 2 gene expression at four alternative translation initiation codons. J Biol Chem. 2003;278(41):39330–39336. doi: 10.1074/jbc.M305580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Zhang J. Ancient expansion of the ribonuclease A superfamily revealed by genomic analysis of placental and marsupial mammals. Gene. 2006;373:116–125. doi: 10.1016/j.gene.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Coldwell MJ, deSchoolmeester ML, Fraser GA, Pickering BM, Packham G, Willis AE. The p36 isoform of BAG-1 is translated by internal ribosome entry following heat shock. Oncogene. 2001;20(30):4095–4100. doi: 10.1038/sj.onc.1204547. [DOI] [PubMed] [Google Scholar]
- Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285(14):10959–10968. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;283(2):437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Fu H, Tie Y, Xu C, Zhang Z, Zhu J, Shi Y, Jiang H, Sun Z, Zheng X. Identification of human fetal liver miRNAs by a novel method. FEBS Lett. 2005;579(17):3849–3854. doi: 10.1016/j.febslet.2005.05.064. [DOI] [PubMed] [Google Scholar]
- Giraud S, Greco A, Brink M, Diaz JJ, Delafontaine P. Translation initiation of the insulin-like growth factor I receptor mRNA is mediated by an internal ribosome entry site. J Biol Chem. 2001;276(8):5668–5675. doi: 10.1074/jbc.M005928200. [DOI] [PubMed] [Google Scholar]
- Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, Patterson V, Swingler R, Kieran D, Prehn J, Morrison KE, Green A, Acharya KR, Brown RH, Jr, Hardiman O. ANG mutations segregate with familial and 'sporadic' amyotrophic lateral sclerosis. Nat Genet. 2006;38(4):411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Karginov FV, Hannon GJ, Elliot MA. Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic Acids Res. 2008;36(3):732–741. doi: 10.1093/nar/gkm1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan TW, Shapiro R, Vallee BL. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991;88(6):2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Kunz M, Kostlin S, Gillitzer R, Toksoy A, Brocker EB, Klein CE. Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Research. 1999;59(7):1578–1583. [PubMed] [Google Scholar]
- Hirukawa S, Olson KA, Tsuji T, Hu GF. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clin Cancer Res. 2005;11(24 Pt 1):8745–8752. doi: 10.1158/1078-0432.CCR-05-1495. [DOI] [PubMed] [Google Scholar]
- Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1(3):190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc Natl Acad Sci U S A. 1997;94(6):2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaragi S, Yoshioka N, Kishikawa H, Hu JK, Sadow PM, Li M, Hu G-f. Angiogenin-stimulated Ribosomal RNA Transcription Is Essential for Initiation and Survival of AKT-induced Prostate Intraepithelial Neoplasia. Mol Cancer Res. 2009a;7(3):415–424. doi: 10.1158/1541-7786.MCR-08-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaragi S, Yoshioka N, Li S, Hu MG, Hirukawa S, Sadow PM, Hu G-f. Neamine inhibits prostate cancer growth by suppressing angiogenin-mediated ribosomal RNA transcription. Clin Cancer Res. 2009b;15(6):1981–1988. doi: 10.1158/1078-0432.CCR-08-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Mol Cell. 2011;43(4):613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochl C, Rederstorff M, Hertel J, Stadler PF, Hofacker IL, Schrettl M, Haas H, Huttenhofer A. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res. 2008;36(8):2677–2689. doi: 10.1093/nar/gkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RY, Jenkins JL, Olson KA, Key ME, Fett JW, Shapiro R. A small-molecule inhibitor of the ribonucleolytic activity of human angiogenin that possesses antitumor activity. Proc Natl Acad Sci U S A. 2002;99(15):10066–10071. [Google Scholar]
- Katona TM, Neubauer BL, Iversen PW, Zhang S, Baldridge LA, Cheng L. Elevated expression of angiogenin in prostate cancer and its precursors. Clin Cancer Res. 2005;11(23):8358–8363. doi: 10.1158/1078-0432.CCR-05-0962. [DOI] [PubMed] [Google Scholar]
- Kieran D, Sebastia J, Greenway MJ, King MA, Connaughton D, Concannon CG, Fenner B, Hardiman O, Prehn JH. Control of motoneuron survival by angiogenin. J Neurosci. 2008;28(52):14056–14061. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13(5):1792–1801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Collins K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J Biol Chem. 2005;208(52):42744–42749. doi: 10.1074/jbc.M510356200. [DOI] [PubMed] [Google Scholar]
- Li S, Hu GF. Angiogenin-mediated rRNA transcription in cancer and neurodegeneration. Int J Biochem Mol Biol. 2010;1(1):26–35. [PMC free article] [PubMed] [Google Scholar]
- Li S, Ibaragi S, Hu GF. Angiogenin as a molecular target for the treatment of prostate cancer. Curr Cancer Ther Rev. 2011;7(2):83–90. doi: 10.2174/1573394711107020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yu W, Kishikawa H, Hu GF. Angiogenin prevents serum withdrawal-induced apoptosis of P19 embryonal carcinoma cells. FEBS J. 2010;277(17):3575–3587. doi: 10.1111/j.1742-4658.2010.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, Loda M, Sellers WR. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100(13):7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau Y, Le Bec C, Monbrun L, Allo V, Chiu IM, Danos O, Moine H, Prats H, Prats AC. Internal ribosome entry site structural motifs conserved among mammalian fibroblast growth factor 1 alternatively spliced mRNAs. Mol Cell Biol. 2004;24(17):7622–7635. doi: 10.1128/MCB.24.17.7622-7635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen E, Kedersha N, Song B, Scheuner D, Gilks N, Han A, Chen JJ, Anderson P, Kaufman RJ. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem. 2005;280(17):16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Spriggs KA, Bushell M, Evans JR, Stoneley M, Le Quesne JP, Spriggs RV, Willis AE. Identification of a motif that mediates polypyrimidine tract-binding protein-dependent internal ribosome entry. Genes Dev. 2005;19(13):1556–1571. doi: 10.1101/gad.339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Identification of the nucleolar targeting signal of human angiogenin. Biochem Biophys Res Commun. 1994a;203(3):1765–1772. doi: 10.1006/bbrc.1994.2391. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994b;91(5):1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T. At the crossroads of growth control; making ribosomal RNA. Curr Opin Genet Dev. 2004;14(2):210–217. doi: 10.1016/j.gde.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yamabe H, Osawa H, Nakamura N, Shimada M, Kumasaka R, Murakami R, Fujita T, Osanai T, Okumura K. Hypoxic conditions stimulate the production of angiogenin and vascular endothelial growth factor by human renal proximal tubular epithelial cells in culture. Nephrol Dial Transplant. 2006;21(6):1489–1495. doi: 10.1093/ndt/gfl041. [DOI] [PubMed] [Google Scholar]
- Nazar RN. Ribosomal RNA processing and ribosome biogenesis in eukaryotes. IUBMB Life. 2004;56(8):457–465. doi: 10.1080/15216540400010867. [DOI] [PubMed] [Google Scholar]
- Olson KA, Byers HR, Key ME, Fett JW. Prevention of human prostate tumor metastasis in athymic mice by antisense targeting of human angiogenin. Clin Cancer Res. 2001;7(11):3598–3605. [PubMed] [Google Scholar]
- Olson KA, Byers HR, Key ME, Fett JW. Inhibition of prostate carcinoma establishment and metastatic growth in mice by an antiangiogenin monoclonal antibody. Int J Cancer. 2002;98(6):923–929. doi: 10.1002/ijc.10282. [DOI] [PubMed] [Google Scholar]
- Olson KA, Fett JW, French TC, Key ME, Vallee BL. Angiogenin antagonists prevent tumor growth in vivo. Proc Natl Acad Sci U S A. 1995;92(2):442–446. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Lee JM, Blais JD, Bell JC, Pelletier J. Internal translation initiation mediated by the angiogenic factor Tie2. J Biol Chem. 2005;280(22):20945–20953. doi: 10.1074/jbc.M412744200. [DOI] [PubMed] [Google Scholar]
- Pozner A, Goldenberg D, Negreanu V, Le SY, Elroy-Stein O, Levanon D, Groner Y. Transcription-coupled translation control of AML1/RUNX1 is mediated by cap- and internal ribosome entry site-dependent mechanisms. Mol Cell Biol. 2002;20(7):2297–2307. doi: 10.1128/mcb.20.7.2297-2307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3(3):179–192. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, Fitzpatrick SF, Taylor CT, Prehn JH. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16(9):1238–1247. doi: 10.1038/cdd.2009.52. [DOI] [PubMed] [Google Scholar]
- Shapiro R, Vallee BL. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem. 2004;279(28):29066–29074. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18(6):3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneley M, Paulin FE, Le Quesne JP, Chappell SA, Willis AE. C-Myc 5' untranslated region contains an internal ribosome entry segment. Oncogene. 1998;16(3):423–428. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- Strydom DJ, Fett JW, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Crabtree B, Acharya KR. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum Mol Genet. 2008;17(1):130–149. doi: 10.1093/hmg/ddm290. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14(10):2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillon R, Kang DK, Park H, Chang SI, O'Hare D. Angiogenin induces nitric oxide synthesis in endothelial cells through PI-3 and Akt kinases. Biochemistry. 2010;49(15):3282–3288. doi: 10.1021/bi902122w. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005;65(4):1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Verselis SJ, Olson KA, Fett JW. Regulation of angiogenin expression in human HepG2 hepatoma cells by mediators of the acute-phase response. Biochem Biophys Res Commun. 1999;259(1):178–184. doi: 10.1006/bbrc.1999.0744. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriyarachchi D, Cerquozzi S, Cheung HH, Holcik M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J Biol Chem. 2004;279(17):17148–17157. doi: 10.1074/jbc.M308737200. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Weiner LH, Swain JL. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987;237(4812):280–282. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]
- Wu D, Yu W, Kishikawa H, Folkerth RD, Iafrate AJ, Shen Y, Xin W, Sims K, Hu GF. Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis. Ann Neurol. 2007 doi: 10.1002/ana.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZP, Tsuji T, Riordan JF, Hu GF. The nuclear function of angiogenin in endothelial cells is related to rRNA production. Biochem Biophys Res Commun. 2002;294(2):287–292. doi: 10.1016/S0006-291X(02)00479-5. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry. 2003;42(1):121–128. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Curr Opin Cell Biol. 2008;20(2):222–226. doi: 10.1016/j.ceb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185(1):35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci USA. 2006;103(39):14519–14524. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]


