Abstract
Objective
To study the phosphorylation of STAT1 in HLA-B27-transfected human monocytic cells and the role of signaling molecules PKR and p38 in STAT1 phosphorylation.
Methods
U937 human monocytic cell transfectants stably expressing wild type HLA-B27 or mutated HLA-B27 heavy chains (HC) with amino acid substitutions in the B pocket were prepared. Mock transfected cells were prepared using the antibiotic resistance vectors (pSV2neo or RSV5neo) alone. PMA differentiated cells were stimulated with LPS or infected with S. enteritidis. Western blotting and flow cytometry were used to detect the phosphorylation and expression levels of STAT1 protein. Specific inhibitors were added in cell culture to study the role of PKR and p38 on STAT1 phosphorylation.
Results
STAT1 is constitutively highly phosphorylated on tyrosine 701 residue in HLA-B27 positive monocytic cells when compared to control cells, even prior to stimulation with LPS or bacteria. This phenotype is associated with the expression of HLA-B27 HCs that misfold. In addition, phosphorylation of STAT1 is dependent on PKR.
Conclusion
Our results show that STAT1 tyrosine 701 is constitutively highly phosphorylated in HLA-B27 expressing monocyte-macrophage cell line. Since phosphorylation of tyrosine 701 on STAT1 is sufficient to induce interferon-dependent genes, constitutive activity of this phosphorylation site may lead to overexpression of interferon-dependent genes, as well as other STAT1-dependent genes, in HLA-B27 monocyte-macrophages. Our results offer a mechanism by which B27 expression alone, without any external trigger, is potentially capable of inducing activation of STAT1, a critical regulator of the inflammatory response.
INTRODUCTION
Reactive arthritis (ReA) is an inflammatory joint disease belonging to the systemic inflammatory diseases called spondyloarthropathies (SpA). ReA may occur after infection with certain gram negative bacteria like Salmonella and Yersinia. Triggering bacteria, or their components, can persist for an abnormally long time in patients suffering from ReA (1). The development and severity of ReA is strongly associated with the tissue antigen HLA-B27. Although the exact mechanism for emergence of ReA and the role of HLA-B27 in the development of the disease are still unclear, several findings suggest that the tendency of HLA-B27 heavy chains (HCs) to misfold may play an important role in disease pathogenesis (2).
We have studied the role of HLA-B27 in host cells encountering ReA-triggering bacteria in vitro and found that elimination of Salmonella enteritidis is impaired in HLA-B27-transfected cell line as compared to control cells (3). In addition, HLA-B27 expressing cells are more permissive for intracellular replication of S. enteritidis; furthermore this phenotype seems to be dependent on HLA-B27 misfolding (4). Extended studies showed that the p38 pathway regulating intracellular replication of Salmonella is dysregulated in U937 cells expressing misfolding HLA-B27 molecules (5).
Our recent studies indicate that double-stranded RNA activated kinase (PKR), which is an ER stress responsive kinase, is overexpressed but hypophosphorylated in HLA-B27 expressing U937 cells (Sahlberg et al, manuscript submitted for publication). PKR is capable of forming complexes with important regulators of the inflammatory response, like signal transducer and activator of transcription 1 (STAT1) (6). The ability of PKR to control phosphorylation of STAT1 has also been reported (7, 8).
In this study we found that LPS and Salmonella infection induced phosphorylation of STAT1 tyrosine 701 in all U937 transfectants. Interestingly, we observed a strong constitutive phosphorylation in STAT1 tyrosine 701 in HLA-B27 expressing cells, even prior to stimulation. In addition, the increased phosphorylation of tyrosine 701 observed in HLA-B27 expressing cells is dependent on PKR activity.
MATERIALS AND METHODS
Cell lines and transfections
The human monocytic cell line U937 was obtained from American Type Culture Collection (ATCC, Rockville, MD). The U937 cell line expresses the HLA class I alleles A3, A26, B18, B51, Cw1, and Cw3. Cells were cotransfected with HLA-B*2705 genomic DNA (B27g) (9) or with mutated HLA-B*2705 (4) and pSV2neo vector (to confer resistance to Geneticin [G-418]) as described previously (3). In the mutated form of HLA-B*2705 (B27E45M), the methionine is changed to glutamic acid at position 45 (4). For mock, cells were transfected with pSV2neo (Mock 1) alone. Complementary DNA (cDNA) of HLA-B*2705 (B27cDNA) cloned into the RSV5neo vector was transfected as described earlier (3). Transfectants made with RSV5neo alone are referred as ‘Mock 2’. All transfectants were stable and selected for G-418 resistance and for surface expression of the transfected molecule (4).
The cells were maintained in supplemented RPMI 1640 medium (4). The expression of transfected HLA molecules was confirmed by FACScan flow cytometry (BD Immunocytometry Systems, San Jose, CA) each time the new batch of cells was thawed for use. Cells were stained with fluorescein isothiocyanate-conjugated anti-human HLA-B27 monoclonal antibody (mAb) (clone FD705-9EIEI0; One Lambda, Canoga Park, CA) as described previously (4). The level of HLA-B27 expression was comparable in all HLA-B27 transfected cells as shown earlier (4), and comparable to HLA-B51, one of the MHC class I molecules endogenously expressed by U937 cells. The expression levels corresponded to those physiologically expressed on the cell surface of peripheral blood monocytes (3).
LPS stimulation
Cells were diluted to a concentration of 1.0 × 106/ml and seeded in 25 cm2 tissue culture flasks (Greiner Bio One, Frickenhausen, Germany). For differentiation to adherent macrophages, the cells were incubated with 10 ng/ml phorbol myristate acetate (PMA; Sigma-Aldrich, St Louis, MO) for 24 hours. Cells were stimulated with S. enteritidis LPS (Sigma-Aldrich) as described earlier (10)
Infection with S. enteritidis
The strain of S. enteritidis used was a stool isolate from a patient with Salmonella-triggered ReA. Prior to infection the cells were treated as described above and infected with S. enteritidis as described previously (4).
Preparation of cell extracts
After LPS stimulation or infection, samples were harvested and washed twice with ice-cold PBS. Samples were prepared as described earlier (10).
Western blot analysis
Western blots were performed using rabbit polyclonal antibodies STAT1, phospho-STAT1 Tyr701 and phospho-STAT1 Ser727 (Cell Signaling Technology, Danvers, MA). Rat monoclonal antibody Hsc70 (Stressgen Bioreagents, Ann Arbor, MI) was used as a loading control. To quantify the intensity of the bands, the densitometric analysis was performed by MCID 5+ image analysis software. Relative intensity (RI) of the bands were calculated as intensity of the phosphorylated band divided by the intensity of loading control and proportioned to the first band of each lane. Two isoforms of STAT1, α and β, were analysed together as one band.
Inhibition assays
The PKR inhibitor and a negative control for PKR inhibitor (10 μM, Calbiochem, Darmstadt, Germany) or p38 MAPK inhibitor SB202190 and negative control for p38 inhibitor SB202474 (10 μM, Calbiochem) were added 15 minutes before LPS. The assay was continued as in LPS stimulation.
Immunofluorescence staining and flow cytometry
PMA-maturated and LPS-stimulated cells were fixed with 1,5-3 % formaldehyde and permeabilized with 100 % ice cold methanol. For staining Alexa 647-labeled phospho-specific mAb to STAT1 tyrosine 701 (Becton Dickinson Biosciences, San Jose, CA) was used. Samples were analysed by FACSCalibur flow cytometry (BD Biosciences). The statistical analysis was performed using Student’s two-tailed t-test.
RESULTS
To study whether HLA-B27 has an influence on STAT1 regulation, we used U937 cells transfected either with genomic or cDNA for HLA-B27 (B27g or B27cDNA, respectively) To investigate whether the modulatory effects of HLA-B27 on STAT1 regulation are caused by misfolding HLA-B27 HCs, we used cells expressing mutated HLA-B27 molecule (B27E45M). In these cells methionine at position 45 in the B pocket, a region of the peptide-binding groove is substituted for glutamic acid. It has been shown that glutamic acid at this position is critical for the aberrant behaviour of B27 molecule, and that substitution with methionine dramatically enhances folding and prevents misfolding (2, 4).
STAT1 tyrosine 701 phosphorylation is enhanced in HLA-B27 expressing cells
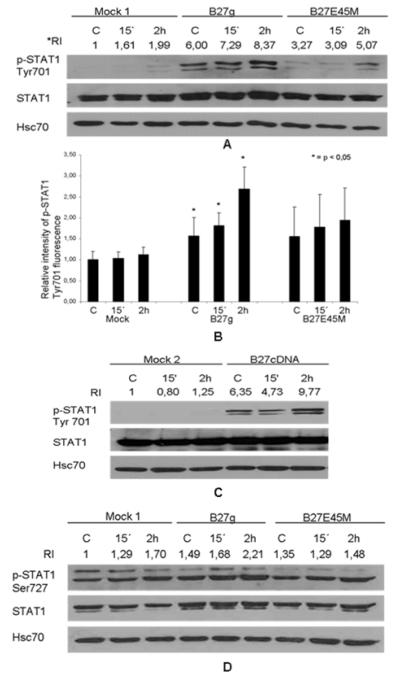
As shown in Figure 1A, LPS stimulation increased the phosphorylation of tyrosine 701 by 2 hours in all transfectants (Mock 1, B27g and B27E45M). Interestingly, tyrosine 701 was found to be strongly phosphorylated in B27g transfectants even prior to stimulation, whereas only a modest activity was observed in B27E45M cells and no detectable activity in Mock 1 cells. Flow cytometry was used to confirm the data obtained from western blot. The median intensity of p-STAT1 tyrosine 701 fluorescence measured in B27g expressing cells was significantly stronger than in Mock 1 and B27E45M expressing cells, even prior to LPS stimulation. In addition the difference of fluorescence intensity between Mock 1 and B27g expressing cells was statistically significant (p < 0,05) (Figure 1B). To even further confirm that expression of wild type B27 leads to increased phosphorylation, a completely distinct transfected cell line, B27cDNA, was examined (Figure 1C). A strong constitutive phosphorylation of tyrosine 701 was observed in B27cDNA transfectants when compared with Mock 2, and LPS stimulation increased the phosphorylation.
Figure 1.
STAT1 tyrosine 701 phosphorylation is enhanced in LPS stimulated HLA-B27-transfected cells compared to mock and mutated HLA-B27 (B27E45M) cells. U937 cells were transfected with genomic clones of HLA-B27 (B27g), mutated HLA-B27 clones (B27E45M) or with vector (Mock 1) for A, B and D, and with cDNA encoding HLA-B27 (B27cDNA) or with vector alone (Mock 2) for C. Cells were PMA-maturated, stimulated with LPS and collected 15 min or 2 hours after stimulation. A, Phosphorylation of STAT1 tyrosine 701 studied by Western blot method with p-STAT1 Tyr701 antibody. B, Cells subjected to flow cytometry analysis and STAT1 tyrosine phosphorylation detected with conjugated p-STAT1 Tyr701 antibody. Values are proportioned to Mock 1 control and are the mean and SD of 3 independent experiments. C, Phosphorylation of tyrosine 701 analysed by western blot with p-STAT1 Tyr701 antibody. D, Phosphorylation of serine 727 analysed by western blot with p-STAT1 Ser727 antibody. Western blots were stripped and reprobed with STAT1 antibody and Hsc70 antibody as loading controls. Double band seen in blots (A, C and D) represents α and β isoforms of STAT1. Figures are representative of 3 independent experiments. *RI = relative intensity of the bands, see material and methods.
Next we examined the activity of another important phosphorylation site of STAT1, serine 727. As shown in Figure 1D, Serine 727 was phosphorylated prior to LPS stimulation in all transfectants. The phosphorylation was modestly stronger in B27g transfectants compared to Mock 1 and B27E45M transfectants. LPS stimulation led to increased phosphorylation of serine 727 at 2 hours in all transfectants (Figure 1D).
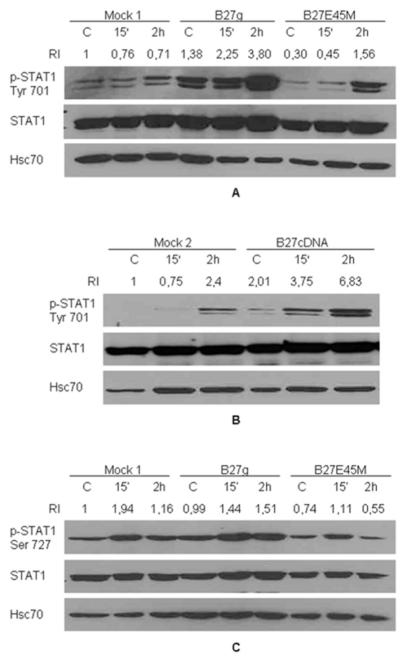
Next we wanted to find out if phosphorylation of STAT1 tyrosine 701 is also enhanced in HLA-B27 expressing cells exposed to Salmonella infection. As shown in Figures 2A and 2B, bacteria induced a more dramatic increase in tyrosine 701 phosphorylation in all transfectants when compared to LPS stimulated cells and phosphorylation of tyrosine 701 was detected prior to infection. Phosphorylation of serine 727 was slightly increased in B27g cells when compared to Mock 1 and mutated B27E45M cells, even prior to infection (Figure 2C). The resolution of STAT1 isoforms, α and β, varies from experiment to another, probably due to methodological variability.
Figure 2.
STAT1 tyrosine 701 phosphorylation is enhanced in Salmonella enteritidis infected HLA-B27-transfected cells compared to mock cells and mutated HLA-B27 (B27E45M) cells. U937 cells were transfected with genomic clones of HLA-B27 (B27g), mutated HLA-B27 clones (B27E45M) or with vector (Mock 1) for A and C and with cDNA encoding HLA-B27 (B27cDNA) or with vector alone (Mock 2) for B. PMA-maturated U937 cells were infected with S. enteritidis and collected 15 min or 2 hours after washing excess bacteria away. A, Phosphorylation of STAT1 tyrosine 701 studied by Western blot method with p-STAT1 Tyr701 antibody. B, Phosphorylation of tyrosine 701 analysed by western blot with p-STAT1 Tyr701 antibody. C, Phosphorylation of serine 727 was analysed by western blot with p-STAT1 Ser727 antibody. Blots were stripped and reprobed with STAT1 antibody and Hsc70 antibody as loading controls. Double band seen in blots (A, B and C) represents α and β isoforms of STAT1. Figures are representative of 3 independent experiments.
PKR activity is required for constitutive STAT1 tyrosine 701 phosphorylation in HLA-B27 expressing cells
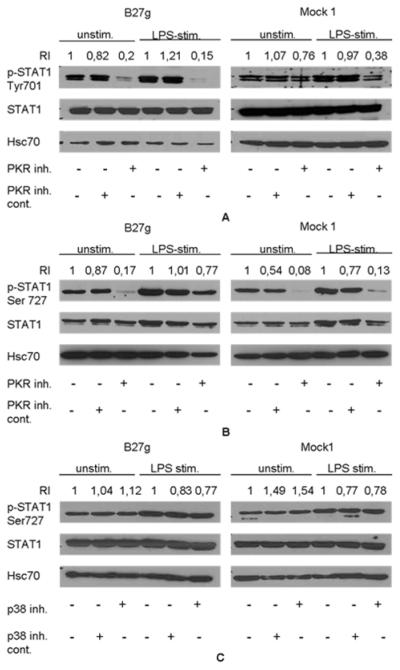
Since PKR is known to regulate STAT1 activation (7, 8), we studied how inhibition of PKR affects STAT1 phosphorylation. Interestingly, phosphorylation of STAT1 tyrosine 701 was dramatically decreased in B27g cells in both LPS and unstimulated cells due to PKR-inhibition (Figure 3A). In Mock 1 cells the PKR inhibitor had only a small influence on spontaneous tyrosine 701 phosphorylation in unstimulated cells, but the usual LPS-induced increase in phosphorylation (Figure 1A) was prevented (Figure 3A). PKR-inhibitor had also an effect on STAT1 serine 727 phosphorylation (Figure 3B). In both B27g and Mock 1 transfected cells, the inhibition of PKR prevented serine 727 phosphorylation almost completely in unstimulated cells. Interestingly, in B27g transfected cells the phosphorylation stayed relatively high after PKR inhibition although in Mock 1 cells it was effectively prevented (Figure 3B).
Figure 3.
PKR regulates phosphorylation of STAT1 tyrosine 701 and serine 727. PKR inhibitor decreases phosphorylation of STAT1 tyrosine 701 and serine 727 in LPS stimulated and in unstimulated cells, whereas p38 inhibitor has no effect on serine 727 phosphorylation. HLA-B27 genomic DNA transfected U937 cells (B27g) and Mock 1 cells were treated with PKR or p38 inhibitors (10 μM of each) or with PKR or p38 negative control molecules (10 μM of each) 15 minutes before LPS was added. Cells were harvested 2 hours after LPS stimulation. A, Cells treated with PKR inhibitors and phosphorylation of STAT1 tyrosine 701 studied by Western blot method with p-STAT1 Tyr701 antibody. B, Cells treated with PKR inhibitors and phosphorylation of STAT1 serine 727 studied by Western blot method with p-STAT1 Ser727 antibody. C, Cells were treated with p38 inhibitors and phosphorylation of serine 727 studied by Western blot method with STAT1 Ser727 antibody. Double band seen in blots represents α and β isoforms of STAT1. Blots were stripped and reprobed with STAT1 and Hsc70 antibodies as loading control. RI values are proportioned to first lanes of unstimulated and LPS stimulated bands in each blot.
Since the p38 MAPK pathway is known to participate in serine 727 phosphorylation of STAT1 (11), we used p38 inhibitors to test whether it affects the activation of STAT1. LPS stimulation increased phosphorylation of serine 727 in 2 hours (as in Figure 3B), but the inhibition of p38 activity had no effect on either constitutive or LPS-induced phosphorylation of serine 727 (Figure 3C).
DISCUSSION
In the present study, we examined the phosphorylation of transcription factor STAT1 in cells expressing the MHC class I protein HLA-B27. We found that in HLA-B27 positive monocytic cells tyrosine residue 701 of STAT1 is constitutively highly activated when compared to control cells, even prior to LPS or bacteria stimulation. In addition, this activation is dependent on PKR.
To understand the importance of the modulatory effects induced by B27, we have studied the interaction between ReA-triggering bacteria and the host. Recently, we observed that PKR is overexpresssed but hypophosphorylated in monocytic cells stably expressing HLA-B27 (Sahlberg et al.). This finding prompted us to study the regulation of STAT1 since PKR has been reported to regulate STAT1 activity (7, 8).
STAT1 belongs to a family of signal transducers and activators of transcription and it plays a central role in the induction of IFN-dependent genes. The latent unphosphorylated form of STAT1 resides in the cytoplasm but after tyrosine 701 phosphorylation it dimerizes, translocates to the nucleus and activates gene expression. In addition, phosphorylation of serine 727 residue of STAT1 is required for its maximal transcriptional activity. Type I and II IFNs are known to induce phosphorylation of both tyrosine 701 and serine 727 residues (12, 13). Furthermore, common stress signals stimulate STAT1 serine 727 phosphorylation (11). We observed that B27 expression spontaneously induced constitutive phosphorylation of tyrosine 701 in STAT1. Since activation of this residue is sufficient to induce IFN-dependent genes (14), constitutive activity may lead to overexpression of these genes in HLA-B27 monocyte-macrophages.
PKR is shown to be involved in STAT1 activation. PKR-/- bone-marrow-derived mouse macrophages exhibited defective STAT1 phosphorylation in response to LPS (7), and in mouse osteoblasts, loss of PKR activity induced aberrant accumulation of STAT1 protein (15). Interestingly, we observed that inhibition of PKR activity induced a dramatic decrease in STAT1 tyrosine 701 phosphorylation in B27 positive cells. Although further studies are required to explain these interesting results, these findings support the idea of disturbed PKR signaling in the cells expressing HLA-B27 molecule.
Our findings offer novel insights into understanding the role of HLA-B27 in the pathogenesis of ReA, and possibly AS and other related SpAs. In conclusion, STAT1 plays an important role regulating inflammation in infection and the delicate balance between proinflammatory and anti-inflammatory mediators. Thus, these results suggest a possible mechanism by which B27 expression alone, without any external trigger, is potentially capable of inducing activation of a critical regulator of the inflammatory response.
ACKNOWLEDGMENTS
We thank Dr. Joel D. Taurog for providing the genomic DNA of HLA-B27, Dr. Beatrice Carreno for HLA-B27 cDNA and David T. Y. Yu for HLA-B27 cDNA transfectants. Tuula Rantasalo and Tuija Turjas are warmly thanked for their technical assistance.
Supported by grants from the Academy of Finland, the Sigrid Jusélius Foundation, Finnish Cultural Foundation and National Institutes of Health (NIH) grants R01-AR-46177 and R01-AR-48372.
REFERENCES
- 1.Vahamiko S, Penttinen MA, Granfors K. Aetiology and pathogenesis of reactive arthritis: role of non-antigen-presenting effects of HLA-B27. Arthritis Res Ther. 2005;7:136–41. doi: 10.1186/ar1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colbert RA, DeLay ML, Klenk EI, Layh-Schmitt G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233:181–202. doi: 10.1111/j.0105-2896.2009.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laitio P, Virtala M, Salmi M, Pelliniemi LJ, Yu DT, Granfors K. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur J Immunol. 1997;27:1331–8. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- 4.Penttinen MA, Heiskanen KM, Mohapatra R, DeLay ML, Colbert RA, Sistonen L, et al. Enhanced intracellular replication of Salmonella enteritidis in HLA-B27-expressing human monocytic cells: dependency on glutamic acid at position 45 in the B pocket of HLA-B27. Arthritis Rheum. 2004;50:2255–63. doi: 10.1002/art.20336. [DOI] [PubMed] [Google Scholar]
- 5.Sahlberg AS, Penttinen MA, Heiskanen KM, Colbert RA, Sistonen L, Granfors K. Evidence that the p38 MAP kinase pathway is dysregulated in HLA-B27-expressing human monocytic cells: correlation with HLA-B27 misfolding. Arthritis Rheum. 2007;56:2652–62. doi: 10.1002/art.22746. [DOI] [PubMed] [Google Scholar]
- 6.Wong AH, Tam NW, Yang YL, Cuddihy AR, Li S, Kirchhoff S, et al. Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. Embo J. 1997;16:1291–304. doi: 10.1093/emboj/16.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004;428:341–5. doi: 10.1038/nature02405. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Raven JF, Baltzis D, Kazemi S, Brunet DV, Hatzoglou M, et al. The catalytic activity of the eukaryotic initiation factor-2alpha kinase PKR is required to negatively regulate Stat1 and Stat3 via activation of the T-cell protein-tyrosine phosphatase. J Biol Chem. 2006;281:9439–49. doi: 10.1074/jbc.M504977200. [DOI] [PubMed] [Google Scholar]
- 9.Taurog JD, Lowen L, Forman J, Hammer RE. HLA-B27 in inbred and non-inbred transgenic mice. Cell surface expression and recognition as an alloantigen in the absence of human beta 2-microglobulin. J Immunol. 1988;141:4020–3. [PubMed] [Google Scholar]
- 10.Penttinen MA, Holmberg CI, Sistonen L, Granfors K. HLA-B27 modulates nuclear factor kappaB activation in human monocytic cells exposed to lipopolysaccharide. Arthritis Rheum. 2002;46:2172–80. doi: 10.1002/art.10557. [DOI] [PubMed] [Google Scholar]
- 11.Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, et al. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc Natl Acad Sci U S A. 1999;96:13956–61. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su L, David M. Distinct mechanisms of STAT phosphorylation via the interferon-alpha/beta receptor. Selective inhibition of STAT3 and STAT5 by piceatannol. J Biol Chem. 2000;275:12661–6. doi: 10.1074/jbc.275.17.12661. [DOI] [PubMed] [Google Scholar]
- 13.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 14.Wen Z, Zhong Z, Darnell JE., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Okamura H, Amorim BR, Hinode D, Yoshida H, Haneji T. PKR-mediated degradation of STAT1 regulates osteoblast differentiation. Exp Cell Res. 2009 doi: 10.1016/j.yexcr.2009.02.003. [DOI] [PubMed] [Google Scholar]