Abstract
Aim
The hormonally controlled mobilization and release of fatty acids from adipocytes into the circulation is an important physiological process required for energy homeostasis. While uptake of fatty acids by adipocytes has been suggested to be predominantly protein-mediated, it is unclear whether the efflux of fatty acids also requires membrane proteins.
Methods
We used fluorescent fatty acid efflux assays and colorimetric assays for free fatty acids and glycerol to identify inhibitors with effects on fatty acid efflux, but not lipolysis, in 3T3-L1 adipocytes. We assessed the effect of these inhibitors on a fibroblast-based cell line expressing fatty acid transport protein 1, hormone-sensitive lipase, and perilipin, that presumably lacks adipocyte-speicific proteins for fatty acid efflux.
Results
We identified DIDS as an inhibitor of fatty acid efflux that did not impair lipolysis or the cellular exit of glycerol, but lead to an accumulation of intracellular fatty acids. In contrast, fatty acid efflux by the reconstituted cellular model for fatty acid efflux was responsive to lipolytic stimuli, but insensitive to DIDS inhibition.
Conclusion
We propose that adipocytes specifically express an as yet unidentified DIDS sensitive protein that enhances the efflux of fatty acids and therefore may lead to novel treatment approaches for obesity-related disorders characterized by abnormal lipid fluxes and ectopic triglyceride accumulation.
Keywords: Adipocyte, Fatty acid, Lipid metabolism, Lipolysis
Introduction
The controlled release of free fatty acids (FFAs) from intracellular triglyceride (TAG) stores into the circulation is a predominant feature of white adipose tissue (WAT) and comprises a key component of energy homeostasis. Primarily, WAT lipolysis can be stimulated by factors that activate the adenylyl cyclase-cyclic AMP-protein kinase A (PKA) pathway (Collins et al., 2001), such as β-adrenergic receptor agonists, glucagon, or GLP-1. In contrast, anti-lipolytic factors, including insulin, generally lower cyclic AMP levels and PKA activity. PKA activity is linked to lipolysis through its ability to phosphorylate hormone-sensitive lipase (HSL) (Stralfors and Belfrage, 1983) and the lipid droplet-associated protein perilipin (Egan et al., 1990, Greenberg et al., 1991, Souza et al., 2002). Unphosphorylated perilipin blocks the access of HSL and other lipases, such as adipocyte triglyceride lipase, to their substrates while PKA-dependent phosphorylation of perilipin recruits HSL from the cytosol to the lipid droplet resulting in stimulated lipolysis (Souza et al., 1998, Brasaemle et al., 2000, Martinez-Botas et al., 2000, Tansey et al., 2001). Once FFAs and glycerol are hydrolytically generated within the adipose cell, they are rapidly and efficiently released from the cell. Cellular exit of glycerol has been shown to be mediated by aquaporins (Maeda et al., 2008), however it is currently unknown if any proteins are involved in FFA efflux.
While several proteins that enhance the uptake of FFAs have been identified, evidence in support of a hypothetical FFA efflux system in WAT is more circumstantial, although specific transporters mediate cellular export of other hydrophobic and amphipatic biomolecules. Organic anion transporting polypeptides (OATP/Slc21) are sodium independent facilitative transporters that mediate the uptake and efflux of a plethora of hydrophilic compounds, including drugs, hormones, bile acids, pravastatin, and eicosanoids (Mikkaichi et al., 2004). The ability to transport various prostaglandins, which are oxygenated products of the fatty acid arachidonate, highlights the potential of OATPs to transport fatty acids. Additionally, ATP-binding cassette (ABC) proteins are important for the efflux of several lipids, including phospholipids, bile, and cholesterol (Pohl et al., 2005), and mutations in ABC transporters have been linked to several hereditary lipid disorders, including Tangiers disease, Stargardt syndrome, progressive familial intrahepatic cholestasis and adrenoleukodystrophy (ALD) (Dean, 2005). Of particular relevance in this context are mutations in ABCD1 that cause ALD. ABCD1 deficiency results in the inability to transport very-long chain fatty acid into peroxisomes where they are normally oxidized. This is important for two reasons: first, the transport step from cytoplasm into peroxisomes is topologically comparable to export from the inside to the outside of cells, and second, the transported substrate is a fatty acid. Further observations that support the notion that fatty acid efflux is protein mediated include the finding by Abumrad et al. (Abumrad et al., 1984), that epinephrine could increase the export of FFAs from adipocytes independent of changes in lipolysis.
We attempt to address the topic of FFA export from adipocytes in this study using a pharmacological approach and several commercially available compounds: phloretin, verapamil, glyburide, and 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid (DIDS). Phloretin is known to alter the protein-mediated transport processes of many charged and uncharged substrates including glucose (Fuhrmann et al., 1992), urea (Toon and Solomon, 1987), glycerol, and chloride (Owen et al., 1974). Verapamil, glyburide, and DIDS have all been shown to inhibit various ABC transporters (Prehm and Schumacher, 2004). DIDS is also an inhibitor of anion exchangers and organic anion transport polypeptides (OATP/Slc21), and was shown to potently inhibit Cl− exchangers (IC50 2µM) (Cabantchik and Greger, 1992) and OATP3 (IC50 115µM) (Walters et al., 2000). Here we present evidence that 3T3-L1 adipocytes express an as yet unidentified protein involved in FFA efflux that is sensitive to inhibition by antagonists of various transporters of hydrophobic and amphipatic biomolecules leading to an accumulation of intracellular FFAs without affecting glycerol efflux.
Material and Methods
Antibodies and reagents
Polyclonal antisera against the C termini of FATP1 and FATP4 were raised as described previously (Doege et al., 2006, Stahl et al., 1999). 14-C oleic acid was obtained from American Radiolabeled Chemicals Inc. (St. Lois, MO). Essentially fatty acid free BSA, forskolin, 3-isobutyl-1-methylxanthine (IBMX), phloretin, DIDS, glyburide, and verapamil were purchased from Sigma.
Cell culture and treatment
3T3-L1 fibroblasts (ATCC) were grown in DMEM containing 10% fetal bovine serum with 2mM L-glutamine and 1% penicillin/streptomycin (DMEM/FBS). A cell differentiation protocol was followed as previously described (Baldini et al., 1992, Stahl et al., 2002). Mature 3T3-L1 adipocytes were used in experiments on days 8–12 of differentiation.
Quencher-based FFA efflux assay
We modified a quencher-based fluorescent FFA uptake assay (Liao et al., 2005) that utilizes a cell-impermeable quencher of extracellular fluorescent fatty acid analogs. 3T3-L1 adipocytes were seeded into black-wall/clear-bottom 96-well plates (Costar) and loaded with 2µM of the fluorescent lipid 4,4-difluoro-5-methyl-4-bora-3a, 4a-diaza-S-indacene-3-dodecanoic acid (C1-BODIPY508/512-C12, Molecular Probes, Inc) bound to 0.1% BSA in HBSS for 30 minutes, followed by a 30 minute pre-incubation with or without the lipolytic stimuli 20µM forskolin and 1mM IBMX, which jointly work to raise cyclic AMP levels and activate PKA. This was replaced with a solution of 200µM quenching agent Q-Red.1 (Molecular Devices), fresh lipolytic stimuli, and 0.1% BSA in HBSS with the indicated inhibitors. Assay plates were immediately read with a fluorescent plate reader (Molecular Devices) utilizing a bottom-read setting.
Colorimetric FFA and glycerol efflux assays
3T3-L1 adipocytes were seeded into 12-well plates and treated with lipolytic stimulation buffer consisting of 20µM forskolin, 200µM IBMX, and 0.1% BSA in HBSS with the indicated inhibitors. Aliquots of the buffer were removed at different time points, centrifuged to pellet cell debris, and the resultant supernatant was tested for the presence of FFAs with the FFA Detection Kit (WAKO) or glycerol with Free Glycerol Reagent (Sigma) per manufacturer’s instructions. Intracellular FFA concentrations were measured using cell lysates with the FFA Detection Kit.
Radiolabeled FFA assay
It was necessary to substitute colorimetric FFA and glycerol efflux assays with radiolabled assays for all experiments using DIDS as we discovered that DIDS inhibits the bacterial long-chain acyl-coA synthetase (LACS) utilized by the colorimetric assays. 3T3-L1 adipocytes were seeded into 12-well plates and incubated for one hour in a buffer of 2µM C14-oleate and 200µM oleate bound to 0.1%BSA in HBSS. Next, cells were washed three times with 0.1% BSA in HBSS and treated with lipolytic stimulation consisting of 20µM forskolin, 200µM IBMX, and 0.1% BSA in HBSS, with or without the addition of DIDS. Aliquots of the buffer were removed at different time points and centrifuged to pellet cell debris. Radioactivity in the supernatant was quantified in a liquid scintillation counter (Packard).
In vitro TAG hydrolase activity assay
We assessed the ability of 400µM glyburide and 400µM phloretin to inhibit lipase activity as described in (Duncan et al., 2008). Reactions proceeded for 10 minutes in the presence of 100uM 3H-triolein before fatty acids were extracted and their radioactivity quantified.
Fluorescent-activated cell sorter (FACS)-based FFA efflux assay
HFP cells were lipid loaded overnight with 120µM each palmitate and oleate and 2µM C1-BODIPY508/512-C12 bound to 1.1% BSA. Cells were washed, trypsinized, and diluted in DMEM with 0.1% BSA and centrifuged for 4 minutes at 1200rpm at 37° Celsius. The supernatant was aspirated and pelleted cells were resuspended in either a control a solution of 0.1% BSA in HBSS, or 0.1% BSA in HBSS with the addition of 20µM forskolin and 1mM IBMX, or 1µg/mL insulin. Cells were sorted via FACS after a 30-minute incubation period.
HFP cell generation
HFP cells were generated by first stably transfecting NIH 3T3 fibroblasts with an expression vector for hormone-sensitive lipase (HSL) and a construct for concurrent expression of murine FATP1 and perilipin. The dual expression construct was based on pBudCE 4.1 (Invitrogen). HFP cells were loaded overnight with 120uM each palmitate and oleate and 2uM C1-BODIPY 508/512-C12 bound to 1.1% BSA. Cells with the highest lipid content were isolated by FACS and expanded in tissue culture.
HFP cell lipid loading media
5.46µM solutions of palmitate and oleate were prepared in water and 2.5% 1N NaOH, respectively, and heated at 70° C. Following fatty acid solubilization, the solutions were combined and 750µL of this mixture was rapidly pipetted into 2.5mL of PBS and 5% BSA. The solution was filter sterilized and 800µL was added to 25mL of DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% BSA to create a 120µM solution of each fatty acid for overnight lipid loading.
Statistics
All data are reported as mean ± SD. All results are representative of a series of experiments and n refers to the number of wells, lysates or suspensions of cells used per experiment unless otherwise noted. Statistical significance was assessed by one-way ANOVA, with p-values less than 0.05 considered significant. Linear regression, IC50 and all other statistics were analyzed using Prism version 5.0 (Graphpad Software, La Jolla, CA).
Results
Identification of FFA efflux inhibitors
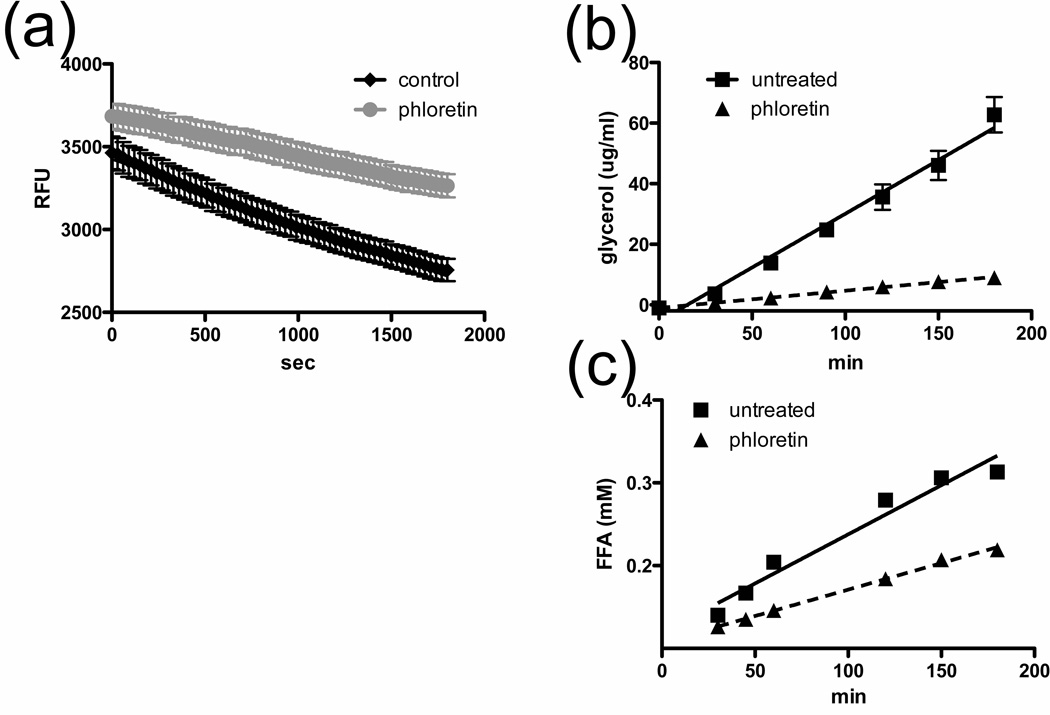
To examine the possibility that adipocytes express an as yet unidentified fatty acid exporter, we performed pharmacological tests using several commercially available compounds with known inhibitory effects on transporters of hydrophobic or amphipatic biomolecules: (phloretin, verapamil, glyburide, and DIDS). We first investigated the effects of phloretin, a broad inhibitor of many cellular transport processes, on FFA efflux from 3T3-L1 adipocytes with a novel quencher-based fluorescent FFA efflux assay. We determined the real-time loss in fluorescence from C1-BODIPY508/512-C12-loaded adipocytes following treatment with a lipolytic stimulus (Fig. 1a). The addition of 500µM phloretin reduces the FFA efflux rate by ~50% from 29 RFU/min to 14 RFU/min during the linear phase, supporting our notion that FFA efflux occurs via a protein-mediated process rather than by passive diffusion.
Fig. 1. Effect of phloretin on glycerol and FFA efflux.
a. Intracellular fluorescence of 3T3-L1 adipocytes treated with 0uM (black) or 500uM phloretin (grey) detected after 30-min incubation with forskolin/IBMX. Phloretin was added at the beginning of the assay. n=6 Colorimetric assays to determine glycerol (b) and FFA (c) efflux over time from forskolin/IMBX stimulated 3T3-L1 adipocytes in the presence of 0 µM (squares) or 500µM phloretin (triangles). Lines indicate linear regression through data points to calculate efflux rates. n=3
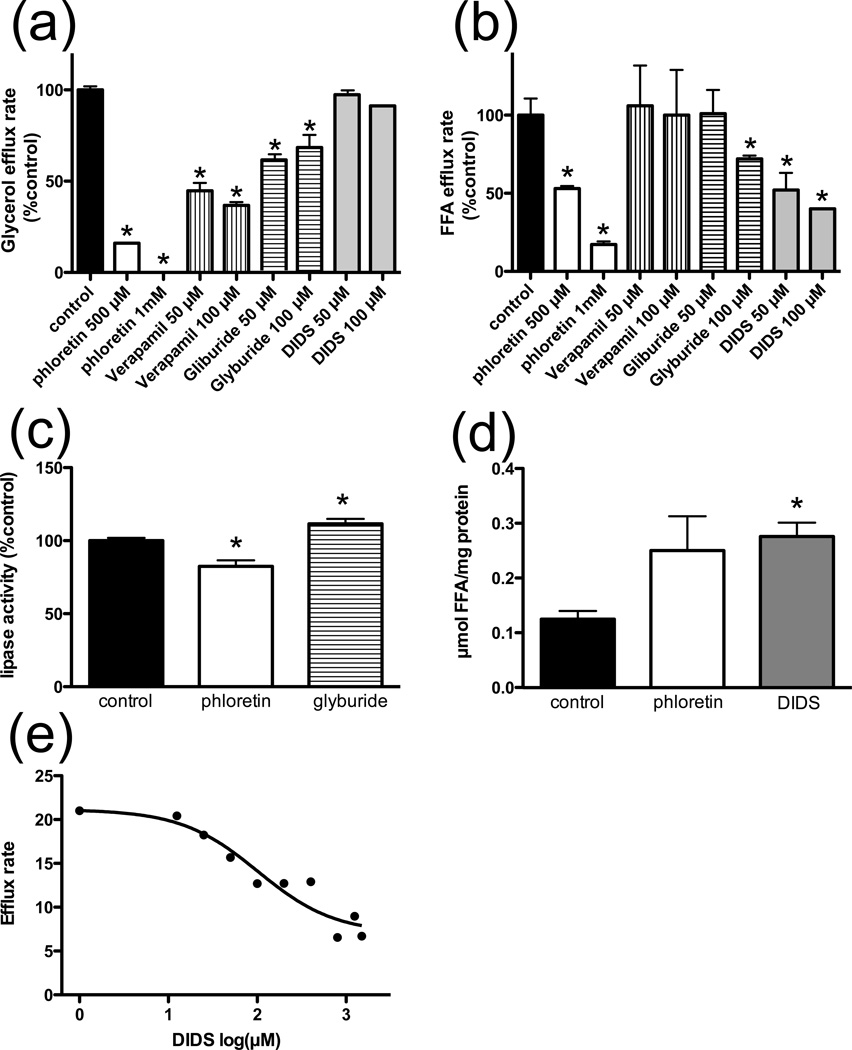
We sought to identify inhibitors with distinct effects on FFA efflux versus lipolysis using colorimetric assays for glycerol and FFAs. A time course with 3T3-L1 adipocytes treated with phloretin demonstrates that both FFA and glycerol efflux are linear over the recorded time frame (Fig. 1b–c). Treatment with 0.5mM or 1mM phloretin significantly reduces both glycerol and FFA release from adipocytes (Fig. 2a–b). At 50µM and 100µM concentrations, verapamil reduces glycerol release by 55.3% and 63.2%, respectively, but neither concentration has an effect on the release of FFAs (Fig. 2a–b). 100µM glyburide treatment results in a reduction of both glycerol and FFA release at 31.6% and 28%, respectively (Fig. 2a–b). Inhibition of glycerol release concomitant with inhibition of FFA release, as seen with phloretin and glyburide treatment, could be due either to inhibition of lipolysis or simultaneous inhibition of both transport processes. To distinguish between these two possibilities, we performed an in vitro TAG hydrolase activity assay and found that while phloretin treatment results in a 17.6% reduction in lipolysis, glyburide has no inhibitory effect and in fact increases lipolysis by 11.5% (Fig. 2c). Thus glyburide most likely has separate inhibitory effects on FFA and glycerol release. DIDS inhibits enzyme-based colorimetric FFA assays that rely on bacterial LACS, so all FFA and glycerol efflux experiments with DIDS utilized radiolabeled tracers. At 50uM and 100uM concentrations, DIDS inhibits FFA release by 48% and 60%, respectively (Fig. 2b), without affecting glycerol release (Fig. 2a). Titration of DIDS shows an apparent IC50 of 102 µM for inhibition of FFA efflux from forskolin/IBMX stimulated 3T3-L1 adipocytes (Fig. 2e). If DIDS inhibits a FFA export protein, one would expect to see an increase in intracellular FFAs in treated cells. Indeed, following lipolytic stimulation, we detect a 2.2-fold and 2-fold increase in intracellular FFA levels in DIDS or phloretin treated cells, respectively (Fig. 2d). Glyburide and DIDS have dose-dependent inhibitory effects on FFA efflux, but not lipolysis, which suggests that these inhibitors act specifically on a FFA exporter. Furthermore, propidium iodide toxicity assays following each experiment showed no toxic effects by glyburide and DIDS on experimental cells over the recorded time frame.
Fig. 2. Modification of cellular efflux.
Glycerol (a) and FFA (b) efflux rates from forskolin/IMBX stimulated 3T3-L1 adipocytes in the presence of phloretin, verapamil, glyburide (n=8), or DIDS (n=3) at the indicated concentrations.
c. 3H triolein hydrolysis assay in the presence of 400µM phloretin or glyburide. n=3 cell lysates
d. Intracellular FFA concentration in control, phloretin (500µM) or DIDS (250µM) treated, forsklolin/IBMX stimulated 3T3-L1 adipocytes. n=6
e. Inhibition of 14C-oleate efflux from 3T3-L1 adipocytes by DIDS 30-minutes after initial lipolytic stimulation. Nonlinear fit of data indicates an IC50 of 102µM.
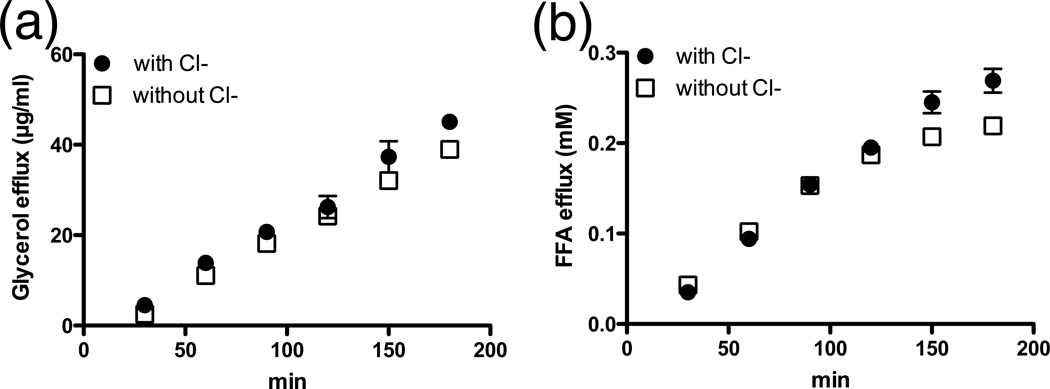
FFA efflux and glycerol release by adipocytes are not dependent on chloride
Since many transport processes of organic anions are coupled to chloride uptake, we examined the dependency of FFA efflux on this substrate using colorimetric assays and found that a lack of chloride in the extracellular medium has no significant effect on FFA and glycerol efflux (Fig. 3). While not completely precluding the possibility, these results argue strongly against the involvement of an anion exchanger in FFA export.
Fig. 3. Effect of chloride on glycerol and FFA efflux.
Glycerol (a.) and FFA (b.) efflux time course from forskolin/IBMX stimulated 3T3-L1 adipocytes with or without the presence of chloride. n=3
A reconstituted model for cellular FFA efflux
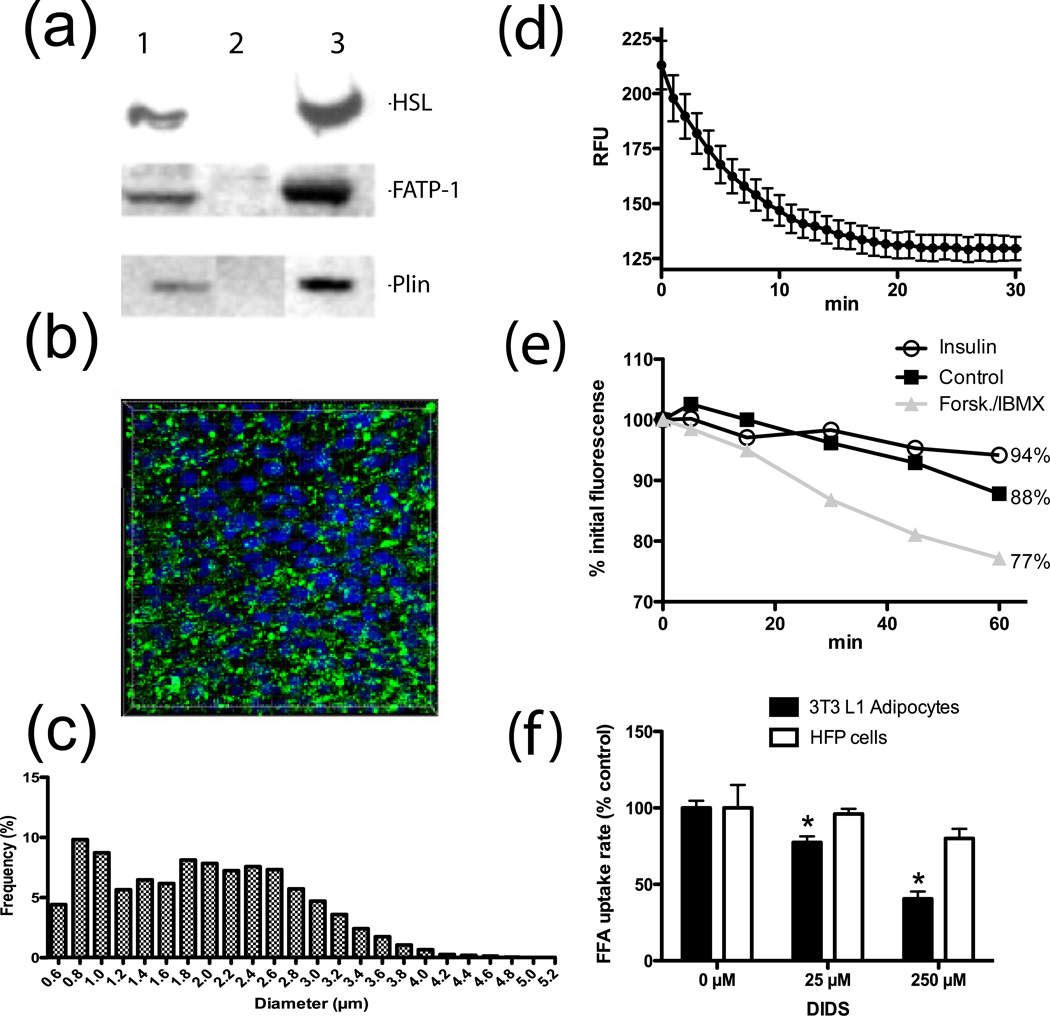
To test whether adipocytes possess additional protein components for efficient FFA efflux, we established a reconstituted cellular model for FFA efflux. To this end, we created a NIH 3T3 fibroblast cell line that can readily load and remobilize stored fatty acids in response to lipolytic stimuli. This cell line is based on the stable overexpression of hormone sensitive lipase (HSL), fatty acid transport protein 1 (FATP/Slc27A1), and perilipin, to facilitate lipolysis, FFA loading, and lipid droplet formation, respectively (Fig. 4a). The resultant cell line (HFP cells) accumulates lipid droplets with a median diameter of 1.9 µm following overnight lipid loading of 2µM C1-BODIPY508/512-C12 in the presence of 120µM each oleate and palmitate (Fig. 4b–c). We performed a quencher-based FFA efflux assay to determine the real-time FFA efflux kinetics of lipid loaded HFP cells treated with lipolytic stimuli (Fig. 4d) and saw an initial robust release of FFAs that leveled off after 20 minutes.
Fig. 4. HFP cells are a reconstituted cellular model for FFA efflux.
a. Western blot of HFP (lane 1), 3T3-L1 fibroblast (lane 2), and 3T3-L1 adipocyte (lane 3) lysates probed for stable expression of hormone sensitive lipase (HSL), FATP1, and perilipin (Plin).
b. 3D reconstruction of confocal images of HFP cells following overnight lipid loading in the presence of fluorescent fatty acids (green); nuclei are shown in blue.
c. Analysis of lipid droplet diameter based on 3D reconstruction shown in B. n=5200 lipid droplets
d. Intracellular fluorescence of HFP cells incubated overnight with lipids and C1-BODIPY-C12 stimulated with forskolin/IBMX at time=0. n=4
e. Mean fluorescene of HFP cell populations loaded overnight with lipids and C1-BODIPY-C12 and stimulated for 30min with insulin, forskolin/IBMX, or nothing. n=4
f. Dose-dependent effect of DIDS on FFA efflux rate from forskolin/IBMX stimulated HFP cells and 3T3-L1 adipocytes. n=3
A FACS-based assay was employed as a secondary method to detect FFA efflux from HFP cells, where the decrease in side scatter (granularity) correlates with loss of lipid content. We utilized the FACS-based FFA efflux assay to measure the hormonal control of FFA release by HFP cells. The resultant data reflects the known inhibitory effect of insulin and stimulatory effect of forskolin and IBMX on lipolysis in adipocytes (Fig. 4e). We found that basal FFA efflux causes a 12% reduction in initial fluorescence and insulin or forskolin/IBMX treatments result in a 6% and 23% reduction, respectively. Thus HFP cells constitute a minimal, but sufficient cellular model for FFA efflux because they load FFAs and demonstrate hormonal regulation of FFA efflux. We reasoned that FFA efflux from HFP cells should be dominated by passive diffusion, as these cells are likely to lack the adipocyte-specific protein(s) necessary for efficient FFA export. To support this notion, we compared the DIDS effect on FFA release from HFP cells and adipocytes and found that DIDS treatment causes a dose-dependent reduction in efflux in adipocytes, but not HFP cells (Fig. 4f). This result strongly suggests that DIDS affects an as yet unidentified FFA transporter required for efficient FFA efflux present in adipocytes, but not fibroblasts.
Discussion
While several proteins are linked to FFA uptake in adipocytes, the mechanism of FFA export from adipocytes remains unknown. To address this issue, we developed assays and a cell line for FFA efflux quantification and generated evidence to suggest that adipocytes express an as yet unidentified fatty acid exporter. We demonstrated that DIDS and glyburide block FFA export without affecting lipolysis. DIDS is of particular interest because it has a dose-dependent inhibitory effect on FFA efflux from adipocytes, without affecting glycerol release. Further, we created a minimal reconstituted cellular model (HFP cells) to measure hormonally regulated FFA efflux. Importantly, while HFP cells faithfully replicate hormonal effects of lipolysis, FFA efflux from HFP cells is not susceptible to inhibition by DIDS. This is in contrast to the dose-dependent inhibitory effect by DIDS on adipocyte FFA efflux and argues for the existence of an adipocyte FFA efflux protein.
Analogous transport systems in adipocytes should be examined for potential involvement in FFA efflux. Flip-flop transport of FFAs across the plasma membrane is bidirectional by nature, however the majority of FFA uptake by adipocytes has been shown to occur via a rapid, saturable, substrate-specific, and hormonally regulated mechanism indicative of protein-mediated processes (Black and DiRusso, 2003, Bonen et al., 2003, Stahl, 2004). While the major proteins involved in FFA uptake by adipocytes are CD36, acyl-CoA synthetase 1, and fatty acid transport protein 1 (FATP1/Slc27A1) (Ibrahimi and Abumrad, 2002, Schaffer and Lodish, 1994, Must et al., 1999), there is little evidence to suggest that these transporters could act bidirectionally. FATP1 is not a probable candidate for a FFA exporter because deletion of FATP1, the predominant FATP expressed by adipocytes, results in decreased uptake of FFAs and unimpaired efflux (Wu et al., 2006). CD36 is a scavenger receptor thought to respond to the accumulation of FFAs at the plasma membrane, however CD36 null animals display no obvious defects in FFA release from adipocytes as their serum levels are actually elevated (Febbraio et al., 1999). It is also unlikely that LACSs are involved in FFA efflux because their mode of action, trapping FFAs via activation to acyl-CoA, is unidirectional (Ellis et al., 2010). Analogous FFA efflux transport systems in cardiomyocytes (Carley et al., 2010) and hepatocytes (Sorrentino, et al. 1991, Sorrentino, et al. 1994), may also provide insight to the adipocyte FFA efflux mechanism. Further studies are required to determine if the proposed FFA exporter is unique to adipose tissue or also found in heart and liver.
Given the evidence that DIDS and glyburide inhibit FFA efflux, one could speculate that the proposed FFA exporter is either part of a novel family or previously identified group of proteins inhibitable by these compounds. Thus, when anion exchangers are excluded because FFA efflux appears to occur via a chloride-independent process, the most conceivable identity for the proposed FFA efflux protein(s) is an OATP or ABC transporter. Alternatively, FFA efflux may be dependent on plasma membrane lipid rafts such as caveolae. Caveolin is the structural protein component of caveolae, which are small flask-shaped invaginations of the plasma membrane. Caveolae may be involved in fatty acid transport as caveolin has been shown to bind fatty acids (Trigatti et al., 1999). More importantly, caveolin 1 knockout mice display defects in lipid mobilization and fail to increase serum FFA levels in response to a cold challenge (Cohen et al., 2005), supporting a role for caveolin in lipolysis and/or FFA efflux. However, it is currently unclear if and how DIDS could affect caveolin, its ability to bind FFAs, or the structure of caveolae. Further characterization with more specific inhibitors could be useful in narrowing this set of potential FFA exporters.
While we accumulated evidence that FFA export from adipocytes is protein-mediated and have some notion of the types of proteins involved in this process, future progress relies on a more detailed strategy for identifying the proposed plasma membrane FFA efflux transporter. HFP cells will be useful for expression cloning gain-of-function studies, as HFP cells that demonstrate high levels of efflux after the introduction of adipocyte cDNAs can be selected for further analysis. DIDS can aid in the discovery of novel proteins when performing secondary assays with clones of interest as a tool for distinguishing inhibition of FFA efflux from inhibition of lipolysis or glycerol efflux. Additionally, a candidate gene or genome wide loss-of-function approach based on RNA interference in 3T3-L1 adipocytes could be used to identify proteins required for efficient FFA efflux. This would be particularly useful for evaluating caveolin 1 and specific members of the OATP and ABC transport families for their involvement in FFA efflux.
During fasting, the majority of circulating FFAs in serum are derived from the breakdown of stored triacylglycerides in adipose tissue. Chronically elevated serum FFA levels have been linked to obesity associated pathologies including insulin resistance (Bray, 2004), hepatosteatosis (Marchesini et al., 2001), and pancreatic β-cell lipotoxicity (Lee et al., 1994). Elucidating the mechanisms and proteins involved with FFA efflux from adipocytes could have important implications for the treatment of obesity-related disorders characterized by abnormal lipid fluxes. Particularly, sequestering FFAs within adipocytes could be an attractive strategy for alleviating the abnormally high levels of circulating FFAs characteristic of obesity-related conditions. Our evidence supports the notion that FFA efflux by adipocytes is a protein mediated process, which justifies further studies into the identity of this export protein and its presumed role in physiological and pathological conditions.
Acknowledgements
This work was supported by ADA (grant 7-04-CD-14) and NIH/NIDDK (grants R56DK066336/R01DK066336) to A.S and in part by the Department of Veterans Affairs (Office of Research and Development, Medical Research Service) by NIH (grant R01AG028098) to F.B.K. We sincerely thank Robin Duncan and Maryam Ahmadian from the Department of Nutritional Sciences and Toxicology at UC Berkeley for assistance with the TAG hydrolase assays and Bingfang Huan and Sukanta Bhattacharyya from Molecular Devices for providing the Q-Red.1 quenching agent.
Abbreviations
- ABCD1
ATP-binding cassette transporter D1
- BSA
bovine serum albumin
- DIDS
4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid
- FATP
fatty acid transport protein
- FFA
free fatty acid
- HBSS
Hank’s Buffered Salt Solution
- HFP
hormone sensitive lipase-FATP1-perilipin
- HSL
hormone sensitive lipase
- IBMX
3-isobutyl-1-methylxanthine
- LACS
long-chain acyl-coA synthetase
- RFU
relative fluorescent units
- OATP
organic anion transport protein
- TAG
triacylglyceride
- WAT
white adipose tissue
Footnotes
Conflict of interest:
The authors have no conflict of interest to disclose.
References
- Abumrad NA, Park JH, Park CR. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984;259:8945–8953. [PubMed] [Google Scholar]
- Baldini G, Hohl T, Lin HY, Lodish HF. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci U S A. 1992;89:5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Benton CR, Campbell SE, Chabowski A, Clarke DC, Han XX, Glatz JF, Luiken JJ. Plasmalemmal fatty acid transport is regulated in heart and skeletal muscle by contraction, insulin and leptin, and in obesity and diabetes. Acta Physiol Scand. 2003;178:347–356. doi: 10.1046/j.1365-201X.2003.01157.x. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992;262:C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Schubert W, Brasaemle DL, Scherer PE, Lisanti MP. Caveolin-1 expression is essential for proper nonshivering thermogenesis in brown adipose tissue. Diabetes. 2005;54:679–686. doi: 10.2337/diabetes.54.3.679. [DOI] [PubMed] [Google Scholar]
- Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. doi: 10.1016/S0076-6879(05)00024-8. [DOI] [PubMed] [Google Scholar]
- Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Londos C. Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and beta-adrenergic stimulation. J Biol Chem. 1990;265:18769–18775. [PubMed] [Google Scholar]
- Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins S, Muoio D, Coleman R. Adipose Acyl-CoA Synthetase-1 Directs Fatty Acids toward beta-Oxidation and Is Required for Cold Thermogenesis. Cell Metabolism. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- Fuhrmann GF, Dernedde S, Frenking G. Phloretin Keto-Enol-Tautomerism and Inhibition of Glucose-Transport in Human Erythrocytes (Including Effects of Phloretin on Anion Transport) Biochimica Et Biophysica Acta. 1992;1110:105–111. doi: 10.1016/0005-2736(92)90300-b. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Curr Opin Clin Nutr Metab Care. 2002;5:139–145. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Sportsman R, Harris J, Stahl A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res. 2005;46:597–602. doi: 10.1194/jlr.D400023-JLR200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T. The organic anion transporter (OATP) family. Drug Metab Pharmacokinet. 2004;19:171–179. doi: 10.2133/dmpk.19.171. [DOI] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Owen JD, Steggall M, Eyring EM. Effect of Phloretin on Red-Cell Nonelectrolyte Permeability. Journal of Membrane Biology. 1974;19:79–92. doi: 10.1007/BF01869971. [DOI] [PubMed] [Google Scholar]
- Pohl A, Devaux PF, Herrmann A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Prehm P, Schumacher U. Inhibition of hyaluronan export from human fibroblasts by inhibitors of multidrug resistance transporters. Biochem Pharmacol. 2004;68:1401–1410. doi: 10.1016/j.bcp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, Greenberg AS. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor a to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem. 1998;273:24665–24669. doi: 10.1074/jbc.273.38.24665. [DOI] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2:477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Stralfors P, Belfrage P. Phosphorylation of hormone-sensitive lipase by cyclic AMP-dependent protein kinase. J Biol Chem. 1983;258:15146–15152. [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng C-X, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon MR, Solomon AK. Modulation of water and urea transport in human red cells: effects of pH and phloretin. J Membr Biol. 1987;99:157–164. doi: 10.1007/BF01995696. [DOI] [PubMed] [Google Scholar]
- Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 1999;255:34–39. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- Walters HC, Craddock AL, Fusegawa H, Willingham MC, Dawson PA. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2000;279:G1188–G1200. doi: 10.1152/ajpgi.2000.279.6.G1188. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26:3455–3467. doi: 10.1128/MCB.26.9.3455-3467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]