Abstract
Objective
Prospective memory (PM), which can be understood as the processes involved in realizing a delayed intention, is consistently found to be impaired following a traumatic brain injury (TBI). Although PM can be empirically dissociated from retrospective memory, it inherently involves both a prospective component (i.e., remembering that an action needs to be carried out) and retrospective components (i.e., remembering what action needs to be executed and when). This study utilized a multinomial processing tree (MPT) model to disentangle the prospective (that) and retrospective recognition (when) components underlying PM following moderate-to-severe TBI.
Method
Seventeen participants with moderate to severe TBI and 17 age- and education-matched control participants completed an event-based PM task that was embedded within an ongoing computer-based color-matching task.
Results
The MPT modeling approach revealed a significant group difference in the prospective component, indicating that the control participants allocated greater preparatory attentional resources to the PM task compared to the TBI participants. Participants in the TBI group were also found to be significantly more impaired than controls in the when aspect of the retrospective component.
Conclusions
These findings indicated that the TBI participants had greater difficulty allocating the necessary preparatory attentional resources to the PM task and greater difficulty discriminating between PM targets and non-targets during task execution, despite demonstrating intact post-test recall and/or recognition of the PM tasks and targets.
Keywords: Traumatic brain injury, prospective memory, retrospective memory, multinomial modeling
Failures in prospective remembering, or our ability to remember to carry out an intended action in the future, are a common and disruptive occurrence in our daily lives. Both neurologically healthy individuals and persons with a traumatic brain injury (TBI) have noted the impact of prospective memory (PM) failures in their daily lives to be greater than that of retrospective memory (RM) failures (Mateer, Sohlberg, & Crinean, 1987). While research has consistently demonstrated that PM can be dissociated from RM (e.g., Bisiacchi, 1996; Maylor et al., 2002; Palmer & McDonald, 2000; West & Craik, 2001; West & Krompinger, 2005), it is important to understand that all PM tasks inherently involve both a prospective and retrospective component. The prospective component of PM can be conceptualized as remembering that an action needs to be carried out, while the retrospective component involves remembering what action needs to be executed (e.g., Einstein et al., 1992; Ellis, 1996) and when it must be executed (Smith & Bayen, 2004).
While PM tasks can be classified into three categories – time-based, activity-based, and event-based (Kvavilashvili & Ellis, 1996) – most experimental PM tasks tend to be event-based. Event-based PM tasks are to be performed when a specific external cue is presented (e.g., give a message to a coworker when seeing him/her at work). Several theoretical models exist regarding the mechanisms that underlie event-based PM abilities. The multiprocess view (McDaniel & Einstein, 2000, 2005) posits that prospective remembering can rely on either strategic monitoring processes for cue detection or spontaneous retrieval processes, depending on task-specific factors such as the type of task and cue, the nature of the ongoing activity, and characteristics of the individual. In contrast to the multiprocess view, the preparatory attentional and memory processes (PAM) theory (Smith, 2003, 2008, 2010) asserts that prospective remembering will always require resource demanding preparatory attentional processes that can take the form either of explicit monitoring processes or more subtle processes needed to maintain the intention. The PAM theory argues that these resource-demanding processes are engaged not only during trials involving the PM target, but also those in which the target is not present in order to maintain the intention. According to the PAM theory, these processes make up the prospective component of PM, while the retrospective component is conceptualized as the processes responsible both for recalling the intended action and discriminating between PM targets and nontargets. Thus, the recollection of the intended action refers to the what component of retrospective processes, while discrimination between targets and nontargets comprises the when component, termed the retrospective recognition component of PM (Smith & Bayen, 2004).
Numerous studies examining prospective remembering in various neurologically impaired populations, including individuals with a TBI, have consistently found significant PM impairments (e.g., Fortin, Godbout, & Braun, 2003; Knight, Titov, & Crawford, 2006; Roche, Fleming, & Shum, 2002; Roche, Moody, Szabo, Fleming, & Shum, 2007; Schmitter-Edgecombe & Wright, 2004). In general, many studies tend to assume that observed deficits in PM following TBI are due to impairments in the prospective component of PM (e.g., Fish et al., 2006; Groot et al., 2002; Kinsella et al., 1996; Knight, Harnett, & Titov, 2005; Knight et al., 2006; Mathias & Mansfield, 2005).
Only a handful of studies have attempted to tease apart prospective and retrospective processes underlying PM failures following TBI, generally yielding conflicting findings (e.g., Carlesimo, Casadio, & Caltagirone, 2004; Henry, Phillips, Crawford, Kliegel, Theodorou, & Summers, 2007; Kliegal, Eschen, & Thone-Otto, 2004). For example, Carlesimo and colleagues (2004) utilized a unique scoring method in which performances were scored based on spontaneous task initiation versus accurate recollection of the content of the intended action following failure of spontaneous initiation. The authors found that in addition to the TBI group being significantly less accurate in spontaneously initiating the PM task compared to controls, they were also significantly worse at accurately recalling the content of the intention, suggesting deficits in both the prospective and retrospective components of prospective remembering. In contrast, Kliegal and colleagues (2004) attempted to tease apart the prospective and retrospective components in a sample of healthy older adults and individuals with severe TBI by differentially assessing intention formation, retention, initiation, and execution. While the TBI group demonstrated impaired performances in the intention formation, initiation, and execution phases, the results failed to reveal significant group differences for the intention retention phase. The authors interpreted these findings to suggest that PM deficits following severe TBI were the result of impaired prospective processes underlying PM rather than the result of retrospective memory impairments. However, these authors selected only individuals with a TBI who demonstrated intact retrospective memory abilities for inclusion in the study, as their main purpose was to examine the impact of executive dysfunction on prospective remembering. It is therefore not surprising that, unlike Carlesimo and colleagues (2004), this study failed to reveal an impact of deficient retrospective memory processes underlying prospective remembering. Selectively including only those TBI participants with intact retrospective memory abilities on neuropsychological measures limits the generalizability of these findings, as a vast amount of evidence documents residual deficits in retrospective memory following TBI (e.g., Brooks, 1972, 1975; Crosson, Novack, Trenerry & Craig, 1988; Vakil, 2005).
Still other studies have utilized post-test recall and/or recognition tests of the PM task and targets to examine the retrospective component of PM (e.g., Altgassen, Kliegel, Rendell, Henry, & Zollig, 2008; Cohen, Jaudas, & Gollwitzer, 2008; Jager & Kliegel, 2008; McDaniel et al., 2004). For example, Henry and colleagues (Henry et al., 2007) found that impairments in PM functioning in their TBI sample were likely related to the prospective component of PM, which was assessed using post-test recall of the PM task instructions and target events. However, this method fails to allow for a thorough examination of the retrospective component, as it is only an examination of the what aspect of the retrospective component, to the neglect of the retrospective recognition, or when, aspect (i.e., the ability to discriminate between targets and nontargets). As Smith and Bayen (2006) discuss, recall of the target(s) following the task does not necessarily ensure that the target(s) are recognized during the task. Because recognition failure for items that are successfully recalled at a later time has been demonstrated on other cognitive tasks (Tulving & Thomson, 1973), it is important to develop alternative approaches to separately examine all components underlying prospective remembering.
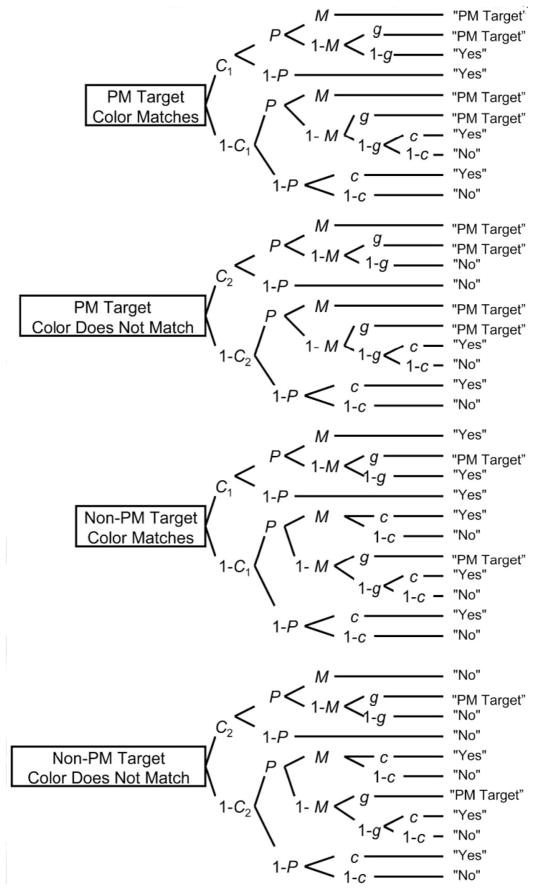
One approach to more thoroughly examining all of the processes underlying PM is through the use of statistical modeling. Smith and Bayen (2004) introduced an event-based PM multinomial processing tree (MPT) model based on the PAM theory as a means to differentially examine the retrospective recognition component (i.e., discriminate between targets and non-targets during task execution) and the prospective component (i.e., remembering that something needs to be done). The model and a diagram of the processing tree from Smith and Bayen (2006) are detailed in Figure 1. As Smith and Bayen (2004, 2006) describe, the model is set to estimate four free parameters: (1) parameter P represents the prospective component, or the likelihood of engaging in preparatory attentional processes; (2) parameter M represents the recognition memory aspect of the retrospective component, or the likelihood of accurately discriminating between the target and non-target words during task execution; (3) parameter C1 represents the likelihood of correctly detecting a match on the ongoing activity (i.e., a color-matching task); (4) parameter C2 represents the likelihood of accurately detecting a non-match trial on the ongoing activity. The experimental task consists of four different trial types (PM targets on match trials, PM targets on non-match trials, non-PM targets on match trials, and non-PM targets on non-match trials) and three response options for each trial (Yes, No, or PM Target).
Figure 1.
Multinomial model of event-based prospective memory. Taken from “The source of adult age differences in event-based prospective memory: A multinomial modeling approach” by R. E. Smith and U. J. Bayen, 2006, Journal of Experimental Psychology: Learning, Memory, and Cognition, 32(3), p. 634. PM = prospective memory; P = probability of engaging in preparatory attentional processes; M = probability to discriminating between targets and non-targets; g = probability of guessing that a color matches; C1 = probability of detecting a color match; C2 = probability of detecting that a color does not match.
The use of the MPT model has been validated in several studies using neurologically intact individuals (e.g., Horn, Bayen, Smith, & Boywitt, in press; Smith & Bayen, 2004, 2005). In one set of experiments, Smith and Bayen (2004) manipulated several variables that were hypothesized to primarily affect either the prospective or retrospective recognition components of PM, including the importance of the ongoing or PM task, the distinctiveness of the PM targets, and the time available for encoding PM targets. As predicted by the PAM theory, MPT modeling results revealed significantly greater preparatory attentional processing in conditions emphasizing the importance of the PM task (rather than the ongoing activity) and semantically similar target-nontargets (rather than semantically distinct target-nontargets). In contrast, manipulating target encoding time significantly affected retrospective recognition processes but not preparatory attentional processes (Smith & Bayen, 2004). The findings from this set of experiments, as well as their later experiments (e.g., Horn et al., in press; Smith & Bayen, 2005, 2006; Smith, Bayen & Martin, 2010), demonstrate that the MPT model is a viable method of disentangling the effects of the prospective and retrospective recognition components underlying prospective memory performance.
The aim of the current study was to disentangle the influence of processes that underlie the prospective component, or remembering that an action needs to be taken, from the retrospective recognition processes of remembering when the action needs to be executed (i.e., discriminating between targets and non-targets during task execution) in individuals who have suffered moderate-to-severe TBI. We controlled the what aspect of the retrospective component by including only those participants who were able to successfully encode the targets and able to recall or recognize the targets and action at post-test assessment. Disentangling these processes can allow for evaluating the extent to which each component contributes to residual PM failures commonly observed following TBI. Similar to the experimental paradigm utilized by Smith and Bayen (2006), this study employed an event-based PM task embedded within an ongoing color-matching task. We utilized both traditional methods of data analysis, as well as an MPT modeling approach to differentially examine the prospective and retrospective recognition components.
Based upon prior research (e.g., Carlesimo et al., 2004; Kliegal et al., 2004; Schmitter-Edgecombe & Wright, 2004; Shum, Valentine, & Cutmore, 1999), we expected that TBI participants would display greater overall PM deficits compared to healthy controls as indicated by fewer PM responses. Furthermore, previous research indicates that individuals with a history of TBI experience deficits in resource-demanding, strategic cognitive processes (e.g., Schmitter-Edgecombe & Beglinger, 2001; Schmitter-Edgecombe, Marks, & Fahy, 1993; Schmitter-Edgecombe & Rogers, 1997; Vakil, Blachstein, & Hoofien, 1991), thus, possible differences in the strategic processes involved in PM were of particular interest. Therefore, the PM task used in this study was selected because both the PAM theory and the multiprocess view predict that this task would involve non-automatic processes. Given the previous research, it was hypothesized that analyses using the MPT model would reveal that individuals with TBI will also show significantly reduced likelihood of engaging in preparatory attentional processes as compared to healthy controls. Furthermore, we hypothesized that if PM deficits following TBI are primarily due to impairments in the prospective component, as much of the literature discussed above suggests, then a significant effect should not be found within the retrospective recognition component of PM.
Method
Participants
A total of 23 individuals with moderate-to-severe TBI participated in this study. Participants with a TBI were recruited using several methods. Individuals whom had previously taken part in TBI studies conducted by our research lab were invited to participate in the current study. Study participants were also recruited from local brain injury survivors’ support groups, local community day treatment programs, and via a brief article in a regional brain injury survivors’ newsletter. Control participants were primarily recruited via word-of-mouth, recruitment from undergraduate psychology classes, and internet postings/advertisements. All participants received a brief report on their current cognitive functioning and were entered into a drawing to win a monetary prize as compensation for participating in the study. Written informed consent was obtained from all participants and protocol approval was obtained from the Institutional Review Board at Washington State University.
All participants were required to learn the PM target words and the PM action prior to starting the experimental trials, and only those participants who were able to accurately recall or recognize all six target words and recall the PM task at the end of testing were included in the final analyses. Of the original 23 individuals, three participants were excluded from analyses because they were unable to successfully encode the six PM target words prior to the PM block of the color-matching trials. Furthermore, two participants were excluded because of the inability to understand task instructions, and one participant was excluded after medical records revealed that the injury was primarily related to seizure and hematoma rather than TBI. This resulted in a remaining sample of 17 participants (8 males, 9 females) with moderate-to-severe TBI in the experimental group. The comparison sample consisted of 17 neurologically healthy matched control participants (8 males, 9 females). Demographic comparisons indicated that the two groups were well matched in age (TBI: range = 18–56 yrs, M = 34.41, SD = 11.48; control: range = 18–52 yrs, M = 33.47, SD = 10.65), t(32) = 0.25, p > .05, and education level (TBI: range = 12–20 yrs, M = 15.76, SD = 2.22; control: range = 12–20 yrs, M = 15.76, SD = 2.01), t(32) = 0.001, p > .05.
Severity of TBI was defined by the Glasgow Coma Scale (GCS; Teasdale & Jennet, 1974) score, length of loss of consciousness (LOC), length of posttraumatic amnesia (PTA), neuroimaging findings, and/or neurosurgery. In those cases where medical records were unattainable (n = 9) or the depth and/or duration of coma were unclear from medical records (n = 1), participant and/or significant other reports of LOC and PTA were used to estimate severity. Participants were considered to have suffered a severe TBI if they experienced a depth of coma (as measured by the Glasgow Coma Scale) of 8 or less or coma duration of greater than 48 hours (n = 11). Moderate TBI was defined by a GCS score of 9 – 12 or higher if accompanied by positive neuroimaging findings and/or neurosurgery (n = 6; Dennis et al., 2001; Fletcher, et al., 1990; Taylor et al., 2002; Williams, Levin, & Eisenberg, 1990). Retrospective PTA was assessed by having participants with TBI recall their memories (i.e., those that they could actively recall rather than what they had been told) after the injury in chronological order until the examiner was satisfied that normal continuous memory was being described (King et al., 1997). Eighty-eight percent of participants reported a PTA estimate of greater than one day, with 73% of those reporting duration of PTA greater than five days.
Cause of injury for a majority of the TBI participants (n = 11) was a motor vehicle accident, while the remaining injuries were the result of a fall of 10 feet or greater (n = 3), a bicycle accident (n = 2), or an airplane accident (n = 1). To rule out developmental effects, TBI participants were at least 15 years of age at the time of injury and less than 55 years of age at time of initial testing. Because we were interested in the residual effects of TBI on PM performance, all TBI participants were assessed at least one year post-injury (range 1–27 years). Eighty-two percent were three or more years post-injury at the time of participation, and 29% of those were more than 10 years post-injury. Other exclusion criteria included: a prior history of non-TBI-related neurological disorders (e.g., stroke, attention-deficit hyperactivity disorder, etc.); a prior history of treatment for substance abuse; a prior history of moderate-to-severe TBI; a Snellen ratio of less than .50 (measured at a distance of 45 cm); a reading or comprehension impairment; a visual field deficit that would impair viewing of a computer screen; color blindness that would inhibit discrimination between the experimental stimuli; any medical condition that precluded ability to participate in neuropsychological testing (e.g., dementia, aphasia); and an impairment in ability to respond with an upper limb during assessment.
Materials
Questionnaire Measures
Following recruitment, participants received by mail a packet of questionnaires to be completed prior to the testing appointment. This packet included the following questionnaires:
Prospective-Retrospective Memory Questionnaire ([PRMQ] Smith, Della Sala, Logie, & Maylor, 2000)
This self-report measure is a brief 16-item questionnaire that yields independent subscale scores for prospective and retrospective functioning in daily life.
Dysexecutive Questionnaire ([DEX] Wilson et al., 1996)
This self-report measure is a brief, 20-item questionnaire about executive-based behavioral changes and is designed to measure various aspects of executive deficits (e.g., perseveration, distractibility, decision-making, impulsivity, etc.).
Performance-Based Neuropsychological Measures
To characterize the TBI population, participants were administered a battery of performance-based neuropsychological tests. The following measures were individually administered to each participant:
Shipley Institute of Living Scale – Vocabulary Subtest (Shipley, 1940)
This scale is a self-administered test intended to estimate general intellectual functioning in adults. The Vocabulary subtest consists of 40 multiple-choice vocabulary items in which participants choose a word closest in meaning to a target word from among four options. The score from the Vocabulary subtest was used to derive an estimate of each participant’s verbal intellectual abilities.
Repeatable Battery for the Assessment of Neuropsychological Status – Form A (RBANS; Randolph, Tierney, Mohr, & Chase, 1998)
This test battery is intended to be a brief (approximately 30 minutes) but comprehensive screening measure of performance in various areas of cognitive functioning. It produces scores within five indices: (1) immediate memory; (2) visuospatial/constructional; (3) language; (4) attention; (5) and delayed memory.
Trail Making Test (TMT; Reitan, 1992)
This test, which involves two forms (TMT-A and TMT-B), is commonly used to examine attention, processing speed, and executive functioning (i.e., sequencing and cognitive flexibility). The score on each form represents the amount of time the individuals take to complete the task. Part A is commonly used to measure processing speed and visual tracking, while Part B is often used to measure aspects of executive functioning.
Delis-Kaplan Executive Function System – Design Fluency Subtest (D-KEFS; Delis, Kaplan, & Kramer, 2001)
This individually-administered test battery is designed to measure various types of executive functions. The Design Fluency subtest is comprised of three parts requiring individuals to connect a varied number of dots to create as many unique designs as possible within a given time limit. It is intended to assess planning and flexibility in thinking in a visuospatial modality.
Experimental Prospective Memory Test
Similar to Smith and Bayen (2006), we administered an event-based PM task embedded within an ongoing color-matching task in order to examine PM functioning.
Ongoing Color-Matching Task
The materials for the ongoing color-matching task with the embedded PM targets were adapted from Smith and Bayen (2006) for use in the current study. Five colors were selected for this task: blue, red, green, yellow, and white. Colored rectangles (1.5 × 1.3 in.) were individually displayed in the center of a black computer screen. Eighteen-point font words were also individually displayed in one of the above colors in the center of the black computer screen.
Prospective Memory Task
As described in Smith and Bayen (2004, 2006), this portion of the experiment was developed by randomly selecting 124 medium-frequency words from the Kucera and Francis (1967) norms. From these, two sets of six words were chosen as prospective memory targets. The remaining 112 words were randomly assigned to one of two filler word lists to be used for the ongoing color-matching task. This resulted in two 6-item target word lists and two 56-item filler word lists, with four possible combinations. These combinations were counterbalanced across participants so that each list served equally often as the baseline and experimental blocks.
Procedure
General Procedure
The full testing session lasted between approximately 150 – 180 minutes. Each session began with a brief neuropsychological intake to obtain demographic information, followed by testing. The experimental prospective memory test was embedded within the neuropsychological test battery. Rest breaks were offered to each participant as needed.
Experimental Prospective Memory Test Procedures
Procedures for the experimental PM paradigm closely modeled those used by Smith and Bayen (2004, 2006). The experiment protocol was programmed and administered using EPrime ® (Schneider, Eschman, & Zuccolotto, 2002). Instructions for the ongoing color-matching task were displayed on the computer screen and emphasized both speed and accuracy. As part of each trial, four colored rectangles were individually displayed in the center of a black computer screen for 500 ms each. A blank screen appeared for 250 ms in between the presentation of each colored rectangle. Following the final rectangle and blank screen, a word was displayed in lowercase letters. In half of the trials, the word was displayed in one of the four colors presented in the preceding rectangles (match trials) and in the other half, the word was displayed in a color different from any of the preceding rectangle colors (non-match trials). This study utilized a response box with five horizontally-lined response keys to collect response data. The middle three keys of the response box were labeled “1”, “2”, and “3”, which were used for making responses in this study. For right-handed participants, the “1” key corresponded to a “yes” response, the “2” key corresponded to a “no” response, and the “3” key was designated for the PM response key. For left-handed participants, the “3” key corresponded to a “yes” response, the “2” key corresponded to a “no” response, and the “1” key was designated for the PM response key. Participants were required to press the “yes” key (“1” or “3”) with their index finger on the response box for match trials and the “no” key (“2) with their middle finger for non-match trials. Different instructions were presented depending on the handedness of the participant. For match trials, the color of the word was randomly selected amongst the four preceding rectangles, and the order of match and non-match trials were randomized with the constraint that no more than three match or non-match trials in a row occurred. Following a response, a screen appeared which instructed participants to press the spacebar in order to progress to the next trial, which allowed for participants to control the pace at which they completed the experiment.
Participants completed one set of 12 practice trials, and no practice trial sets had to be repeated as all participants included in the final analyses demonstrated understanding of the task. This was then followed by the first block of 62 color-matching trials, which did not include instructions for the embedded PM task. This non-PM baseline block was used to compare performances on the ongoing task alone versus the ongoing task with an embedded PM task. At the end of this baseline block, participants were provided with PM task instructions and each of the six PM target words on the computer screen.
The PM task instructions informed participants that they must press the third key (“3” or “1”) with their ring finger whenever one of six target words appears in the color-matching task. The instructions were neutral in regard to the importance of either the PM task or the ongoing task in that participants were not told that one task was more important than the other. Furthermore, they were not provided with specific instruction on whether they should execute the PM task before, instead of, or after responding to the ongoing task (Smith & Bayen, 2006). Prospective memory instructions sometimes request that participants make the prospective memory response after making the ongoing task response (e.g., Smith et al., 2007; Experiment 3). Because prospective memory tasks outside of the laboratory often involve interrupting ongoing activities, the current instructions are likely to provide a better analog to real word prospective memory tasks (Smith & Bayen, 2004). Furthermore, Smith and Bayen (2004) demonstrated that instructing participants to make the prospective memory response after the ongoing task response did not affect prospective memory performance, nor the model parameters, relative to instructions that did not require that the prospective memory response be made after an ongoing task response. In the current study, when participants made a PM task response, the PM response was made instead of an ongoing task response.
After receiving the PM task instructions, all six target words were presented simultaneously on the computer screen. Participants were allowed as much time as they needed to study the PM target words, and were told to inform the examiner when they were finished studying the words. The examiner then lowered the computer screen and initiated a 30 s delay in which the participants were asked to count backward by fours starting with a given number to prevent target word rehearsal and maintenance. Participants were then asked to recall the target words in any order to ensure that the words had been adequately encoded. When any target items failed to be recalled, the participant was again shown the list of PM target words and again allowed to study the list for as long as needed. This procedure was repeated until the participant was able to successfully recall all six target words twice in a row. Although it did not reach statistical significance, the TBI group (M = 2.76, SD = 1.98) required more repetitions to encode the six target words compared to the control group (M = 1.94, SD = 1.20), t(32) = 1.47, p = .15, (1 – β) = 0.291, d = .50.
Prior to the start of the PM block of trials, a 10-minute delay period occurred in which the participants completed a fine motor skills task. Following the 10-minute delay, participants returned to the ongoing color-matching task without being reminded of the PM task instructions. Target items appeared on trials 10, 20, 30, 40, 50, and 60, and the order of the target words was randomized for each participant. Following the PM block of trials, participants were asked to recall the six target words. If a participant failed to recall any of the target words, a recognition trial was provided (TBI: n = 10; control: n = 4). They were also asked to recall the PM task instructions, which was followed by a recognition trial in the case that a participant failed to accurately recall the task (TBI: n = 1; control: n = 0). As previously described, only those participants who were able to successfully encode the six target words, accurately recall or recognize all six target words and accurately recall or recognize the PM task at the end of testing were included in the final analyses. Each block of the experimental PM task lasted approximately 5–8 minutes.
Results
Traditional data analyses were conducted using IBM SPSS Statistics© software.
Neuropsychological Measures
Clinical data on cognitive functioning collected at the time of study participation were analyzed to further explore our sample. As can be seen in Table 1, the TBI group performed significantly poorer than the control group on the RBANS indices of Immediate Memory, t(32) = −2.66, p = .01, Attention, t(32) = −3.03, p < .01, and Delayed Memory, t(32) = −2.14, p < .05. Consistent with the neuropsychological data, in comparison to controls, TBI participants also self-reported significantly greater impairments in everyday prospective memory abilities, t(32) = 4.35, p < .001, retrospective memory abilities, t(32) = 3.50, p < .001, and executive abilities (DEX total), t(32) = 4.11, p < .001 (see Table 1) on questionnaire measures.
Table 1.
Demographic data, performance-based neuropsychological variables, and self-reported questionnaire data for TBI (n = 17) and control (n = 17) groups.
| Variables or test | TBIs | Controls | d | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Demographic Variables | |||||
| Age (years) | 34.41 | 11.48 | 33.47 | 10.65 | .08 |
| Education (years) | 15.76 | 2.22 | 15.76 | 2.02 | .00 |
| Neuropsychological Tests | |||||
| RBANS Indices | |||||
| Immediate Memory | 91.59 | 12.79 | 103.29 | 12.90 | .91** |
| Attention | 90.18 | 16.53 | 106.76 | 15.34 | 1.04** |
| Delayed Memory | 90.65 | 17.68 | 101.47 | 8.98 | .77* |
| 100.76 | 19.20 | 100.63 | 10.16 | .01 | |
| Visuospatial/Construction Language | 92.53 | 19.21 | 99.50 | 16.03 | .39 |
| D-KEFS Design Fluency | |||||
| Composite Scorea | 11.12 | 3.06 | 13.06 | 2.70 | .67 |
| Trail Making Test – Part Ab | 27.71 | 9.93 | 25.59 | 10.54 | .21 |
| Trail Making Test – Part Bb | 65.59 | 46.19 | 58.00 | 33.65 | .19 |
| Questionnaires | |||||
| PRMQ Prospective Scalec | 26.12 | 7.57 | 16.35 | 5.34 | 1.49** |
| PRMQ Retrospective Scalec | 21.18 | 6.51 | 14.00 | 5.41 | 1.20** |
| DEX Totalc | 28.18 | 10.61 | 14.06 | 9.37 | 1.41** |
Notes. TBI = Traumatic brain injury; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; D-KEFS = Delis-Kaplan Executive Functions System; PRMQ = Prospective-Retrospective Memory Questionnaire; DEX = Dysexecutive Questionnaire; PM = prospective memory.
Scaled score.
Time in seconds.
Raw scores.
p < .05.
p < .01.
Experimental Task
Given the range of ages in the TBI and control groups, prior to analyzing the experimental task data, we checked to see whether age was correlated with any of the experimental PM measures. Because no significant correlations were found with age for either group and the TBI and control groups were well-matched on age, this factor was not considered as a variable in the analyses. A summary table of the experimental task data is provided in Table 2.
Table 2.
Accuracy and reaction time (RT) summary data for TBI (n = 17) and control (n = 17) groups.
| Variables | TBIs | Controls | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| PM Task Performance | ||||
| Proportion correct | 0.41 | 0.40 | 0.61 | 0.38 |
| Ongoing Task Performance | ||||
| Accuracy | ||||
| Block 1 Match Trials | 0.91 | 0.10 | 0.93 | 0.07 |
| Block 1 Non-match Trials | 0.85 | 0.18 | 0.95 | 0.06 |
| Block 2 Match Trials | 0.82 | 0.11 | 0.87 | 0.12 |
| Block 2 Non-match Trials | 0.87 | 0.14 | 0.93 | 0.08 |
| Reaction Time | ||||
| Block 1 Match Trials | 1274.19 | 401.34 | 1096.42 | 283.94 |
| Block 1 Non-match Trials | 1439.42 | 523.98 | 1136.35 | 376.08 |
| Block 2 Match Trials | 1769.11 | 579.60 | 1701.74 | 496.95 |
| Block 2 Non-match Trials | 1902.67 | 666.36 | 1776.41 | 590.16 |
Notes. TBI = traumatic brain injury; PM = prospective memory; M = mean; SD = standard deviation. Ongoing task response time and accuracy reported here is determined after excluding the target trials and two trials following each target trial (see text for additional details).
Prospective Memory Task Performance
Given that prior research has consistently found reduced PM performance following TBI (e.g., Carlesimo et al., 2004; Knight et al., 2005; Knight et al., 2006), we used a one-tailed t-test to test our a-priori prediction that the TBI group would respond to a smaller proportion of PM targets than the control group. The predicted decrease in the proportion of correct prospective memory responses in the TBI group (M = 0.41, SD = 0.40) relative to the control group (M = 0.61, SD = 0.38) approached significance, t(32) = −1.46, p = .07, and showed a medium effect size, d = .51.
Ongoing Color-Matching Task
For color-matching task analyses, the PM target trials and two trials following the appearance of each target in the experimental block were excluded in order to avoid finding an artificial cost associated with PM responses. Similarly, we removed the baseline trials that were in the same position as those that were removed from the experimental block.
Accuracy
The analyses of ongoing task performance accuracy began with an examination of baseline performance in Block 1 of the color-matching task in a Group (TBI vs. control) X Trial Type (match vs. non-match) ANOVA. The effect of trial type was not significant, F<1, p > .36, and the two variables did not interact, F(1,32) = 2.73, MSE = .009, p > .10. We therefore collapsed over trial type for the remaining analyses of accuracy. Because there was a trend towards group differences on baseline accuracy, F(1,32)= 3.29, MSE = .009, p < .08, with higher accuracy in the control group (M = .94, SEM = .01) than in the TBI group (M = .88, SEM = .03), we examined the effects of Block on accuracy rate (baseline Block 1 vs. experimental Block 2) separately for each group. In the case of the control group, the decline in accuracy from Block 1 (M = .94, SEM = .01) to Block 2 (M = .90, SEM = .02) was significant, F(1,16) = 4.34, MSE = .002, p = .05, ηp2 = .21, indicating a cost to accuracy performance on the ongoing task for the control group. Although accuracy was numerically reduced from Block 1 (M = .88, SEM = .03) to Block 2 for the TBI group (M = .84, SEM = .02), the effect of Block was not significant in the TBI group, F(1,16) = 2.28, MSE = .006, p > .15. Previous PM experiments using the color-matching task have sometimes found a cost to performance on measures of ongoing task accuracy (e.g., Smith & Bayen, 2006, Experiment 1; Smith et al., 2010), but an accuracy cost is not always demonstrated (e.g., Smith et al., 2007). In contrast, a cost has been consistently demonstrated on reaction times using this type of task (Smith & Bayen, 2004, 2006; Smith et al., 2007, 2010) and we turn to the RT data next.
Reaction Times (RT)
For the RT analysis, response times at or above 2.5 standard deviations from the participant’s mean response for each trial type were considered outliers and removed from analysis. Only accurate trials were included in the RT analyses. A Group (TBI vs. control) X Trial Type (match vs. non-match) ANOVA was conducted to examine baseline RTs (Block 1), revealing a significant main effect of Trial Type, F(1,32) = 6.02, MSE = 29731, p = .02, ηp2 = .16. Baseline RTs were longer for non-match trials (M = 1288, SE = 81) than for match trials (M = 1185, SE = 61). As with accuracy, the main effect of group on baseline RT approached significance, F(1,32) = 3.29, MSE = 299111, p < .08, with faster reaction times for control participants (M = 1119, SEM = 75) than for the TBI group (M = 1356, SEM = 109). The two variables did not interact, F(1,32) = 2.24, p > .14.
Because baseline RTs differed as a function of trial type and the group difference approached significance, complicating comparisons of the two conditions, the effect of adding the PM task in Block 2 was evaluated separately for each group and for each trial type. Relative to RTs in Block 1 (M = 1096, SEM = 69 for match trials; M = 1136, SEM = 91 for non-match trials), the control group had significantly longer RTs in block 2 for both match trials (M = 1702, SEM = 121), F(1,16) = 42.36, MSE = 73525, p < .001, ηp2 = .73, and non-match trials (M = 1776, SEM = 143), F(1,16) = 32.98, MSE = 105600, p < .001, ηp2 = .67. Similarly, the TBI group showed a significant increase in RT from Block 1 to Block 2 for both match trials (Block 1: M = 1274, SEM = 97; Block 2: M = 1769, SEM = 141), F(1,16) = 17.78, MSE = 117074, p = .001, ηp2 = .53, and for non-match trials (Block 1: M = 1439, SEM = 127; Block 2: M = 1903, SEM = 162), F(1,16) = 7.73, MSE = 236142, p = .01, ηp2 = .33. The slower RTs in Block 2 indicate a cost to ongoing task performance for both groups, consistent with the PAM theory’s proposal that resources are allocated away from the ongoing task for preparing to make the PM response prior to the occurrence of the target events (Smith, 2003, 2008, 2010)2.
The extent of RT slowing or cost in Block 2 may be interpreted as indicating the extent to which a participant engages in preparatory attentional processing. Given that the effect sizes for the comparison of Block 1 and Block 2 were medium to large for the control group (i.e., .73 and .67) and small to medium for the TBI group (i.e., .53 and .33), this may point to differences between the two groups in the extent of preparatory attentional processing as a possible explanation for the group difference trend in observed PM performance. However, as noted above, when baseline differences exist, a clear interpretation of the relative cost is not possible, and this is especially true when the dependent measure is nonlinear in scale, as is the case for RTs (see Smith & Bayen, 2006 and Smith et al., 2010, for discussion of baseline differences as a function of age). In addition to the problem of unequal baselines, RTs can be influenced by factors other than the extent of preparatory attentional processing, such as differential difficulty of the target recognition aspect of the task. Therefore, interpreting the extent of cost to performance in different groups as a direct indicator of the extent of preparatory attentional processing can be problematic (Smith, 2010). The limitations to interpreting the ongoing task highlight the advantages of applying the multinomial model for measuring the contribution of preparatory attentional processing and retrospective recognition to prospective memory performance in the two different groups.
Multinomial Process Tree Modeling
As previously mentioned, the MPT model used in the current study was originally developed and validated by Smith and Bayen (2004, 2005; Horn et al., in press). The model has been used successfully to investigate differences between populations when baseline ongoing task performance complicates interpretation (Smith & Bayen, 2006; Smith, Bayen, & Martin, 2010). The response frequencies utilized in this model can be found in Table 3.
Table 3.
Response frequencies for the prospective memory (PM) task for TBI and control groups.
| Item Type | Response Type | No | PM Target |
|---|---|---|---|
| Yes | |||
| TBI group | |||
| Target, match | 25 | 6 | 20 |
| Target, nonmatch | 3 | 26 | 22 |
| Nontarget, match | 391 | 81 | 4 |
| Nontarget, nonmatch | 63 | 410 | 3 |
| Control group | |||
| Target, match | 16 | 4 | 31 |
| Target, nonmatch | 1 | 19 | 31 |
| Nontarget, match | 417 | 59 | 0 |
| Nontarget, nonmatch | 33 | 442 | 1 |
Note: The response frequencies reported here include all trials of Block 2 of the ongoing task.
We utilized the HMMTree and Multitree programs to analyze the MPT model data, which was designed to compute parameter estimates, confidence intervals, and goodness-of-fit statistics for MPT models (Moshagen, 2010; Stahl & Klauer, 2007). Based upon a sample size of N = 1054 (17 participants × 62 trials) and a conventional alpha level of .05, the power for detecting moderate (w = .3) deviations from observed data was 1.0, and the power was .74 for detecting small deviations (w =.1).3 Using the goodness-of-fit test statistic G2 for the four free parameters within the individual model, we found the model provided a good fit to the data for both the TBI group, G2(4) = 0.63, and the control group, G2(4) = 1.00, as both values were below the critical value of 9.49 for df = 4.
To examine potential group differences in the likelihood of engaging in preparatory attentional processes, we then set P equal across both groups and evaluated the change in the fit of the model. This yielded a value of G2(1) = 3.86, p < .05, which exceeded the critical value of 3.84 for df = 1 at an α-level of .05, with control participants demonstrating greater likelihood of engaging in preparatory attentional processes (P) as compared to TBI participants. An examination of the effect of group on recognition memory for the PM target events as measured by parameter M yielded a value of G2(1) = 5.04, p = .02, which also exceeded the critical value of 3.84. Control participants were more likely to correctly discriminate between PM targets and non-targets during task execution as compared to TBI participants, despite the fact that any participants who were unable to accurately recall or recognize all six PM target words and the PM task at post-test were excluded from the analyses. Thus, being able to recognize target events when specifically requested to do so on a post-test questionnaire is not an accurate reflection of being able to discriminate between target events and non-target events in the midst of the ongoing task. Following the same procedures for the ongoing task parameters, group was found to significantly affect parameter C2, or the probability of detecting that a color does not match, G2(1) = 11.07, p < .001, as well as parameter C1, or the ability to detect a color match, G2(1) = 4.15, p =.04, with control participants being able to better detect both compared to TBI participants.
Correlational Analyses
Exploratory correlational analyses were conducted to examine potential relationships between the proportion of PM responses and demographic and neuropsychological measures. Data on injury characteristics were also included in the analyses for the TBI group. Because a large number of variables were examined, a more conservative p-value of .01 was used to interpret statistical significance in order to decrease the likelihood of Type I errors. The proportion of PM responses did not significantly correlate with age, r = −.02, or education level, r = −.04. For the TBI group, proportion of PM responses also did not significantly correlate with time since injury, r = −.07, coma duration, r = .25, or length of PTA, r = −.35. Finally, the proportion of PM responses did not significantly correlate with any of the RBANS Indices or any of the other neuropsychological measures, r’s < .44.
Discussion
Prospective memory is an essential feature in our daily lives, enabling us to remember to take medications, attend appointments, pay bills, and manage a variety of other instrumental responsibilities. Because this fundamental cognitive ability is commonly disrupted following TBI, the aim of this study was to better understand the mechanisms underlying this disruption. Specifically, the goal was to disentangle the influence of strategic prospective processes (i.e., remembering that an action needs to be taken) from retrospective recognition processes (i.e., remembering when the action needs to be executed) in PM failures following moderate-to-severe TBI. Data from a computerized event-based PM task were analyzed by both traditional methods of data analysis and the MPT modeling approach.
Traditional methods of analyses supported most of our hypotheses. First, the TBI participants trended toward making fewer PM responses on the event-based PM task than control participants. This finding is consistent with a large body of previous research indicating impaired PM performance following moderate and severe TBI (e.g., Carlesimo et al., 2004; Cockburn, 1996; Henry et al., 2007; Kliegal et al., 2004; Knight et al., 2005; Mathias & Mansfield, 2005; Roche et al., 2002; 2007; Schmitter-Edgecombe & Wright, 2004). Second, both control and TBI participants were significantly slower at responding to both match and non-match trials during the experimental block with the embedded PM task as compared to the baseline block. These findings indicate a cost to ongoing task performance with the addition of the PM task, even on trials in which no targets were present, suggesting that participants in both groups were engaging in preparatory attentional processing. These findings are consistent with both the PAM theory (Smith, 2003, 2008, 2010) and the multiprocess view (McDaniel & Einstein, 2007), as both would predict that the PM task used in the current study was resource-demanding and elicited the engagement of preparatory attentional processes.
A closer examination of the RT data showed that the effect sizes for the difference between the PM block and baseline block were larger for the control participants relative to the TBI participants. This suggests that the control participants employed greater preparatory attentional processes than the TBI participants, supporting our hypothesis that individuals with moderate-to-severe TBI exhibit reduced engagement in preparatory attentional processes. Given that we found a significant discrepancy between groups in performance on attention-based tasks, it is possible that the TBI group had fewer attentional resources to employ for the PM task. Overall, these findings are consistent with previous research indicating that individuals with a TBI experience deficits in resource-demanding, strategic cognitive processes (e.g., Schmitter-Edgecombe & Beglinger, 2001; Schmitter-Edgecombe et al., 1993; Schmitter-Edgecombe & Rogers, 1997; Vakil et al., 1991).
While we may wish to conclude, based upon ongoing task RT data, that the trend of reduced PM performance in our TBI participants was largely the result of reduced allocation of preparatory attentional resources and, thus, differences in the prospective component, as discussed previously, the difference in baseline performance between the two groups prevents clear interpretation of the variations in cost in the two groups. Furthermore, these findings do not reveal the extent to which retrospective recognition processes are impacting PM performance. We used the MPT modeling approach in order to examine these factors without relying on the indirect method of measuring RT. As expected, we found a significant group difference in the prospective parameter (P), indicating that the control participants allocated greater preparatory attentional resources to the PM task compared to the TBI participants. In addition, the MPT results also revealed that participants in the TBI group were significantly more impaired than controls in the retrospective recognition parameter, or parameter M (i.e., the when aspect of the retrospective component). This indicates that despite demonstrating intact retrospective memory for the PM task and target words (i.e., what component) after task completion, participants in the TBI group had significant difficulty with discriminating between targets and non-targets during task execution. It should also be noted that the TBI participants also showed poorer discriminability than controls when detecting both a color match and a color non-match during the ongoing color matching task, suggesting that the poorer retrospective recognition (i.e., when) processes of the TBI participants could be reflective of a broader problem with item discriminability during task execution.
The current experiment provides new information regarding the effects of TBI on the cognitive processes that underlie prospective memory and, as the discussion of response times indicates, this study highlights the advantages of the formal modeling approach. However, there are potential limitations to the current study. Given that six participants with a TBI had to be excluded from final analyses due to various factors (e.g., inability to understand instructions), future research will benefit from starting with a larger sample size. In addition, given that three TBI participants had to be excluded from analyses due to their inability to encode the PM targets to criteria, our findings may not generalize to TBI participants who have more severe cognitive impairments which make it difficult to remember the what aspect of a task to be executed at a subsequent point in time. Furthermore, because our participants were self-selected and self-referred rather than having been recruited through a medical setting, selection bias may be a potential confound, which limits the generalizability of our findings. Additionally, our TBI sample consisted of a heterogeneous sample, ranging in age, time since injury, and injury severity, which may have limited our ability to detect subtle group differences. In addition, given that the duration of the testing session was approximately 150–180 minutes, this may have differentially affected the TBI participants’ performances due to greater fatigue. However, differences in fatigue would likely impact RT performances and, as discussed above, control participants demonstrated a greater increase in RT across the testing session (i.e., from Block 1 to Block 2) than TBI participants, suggesting that fatigue likely did not differentially impact the TBI participants. Finally, the use of our specific event-based PM task limits the generalizability of our findings to other types of PM tasks. Future research will need to address these concerns in order to provide a more thorough picture of how prospective and retrospective components contribute to PM impairment following moderate-to-severe TBI.
To the knowledge of these authors, no other study has attempted to understand the impact of the retrospective recognition (i.e., when) processes underlying PM impairment in a TBI population. Given that many studies examining PM following TBI tend to assume that observed deficits are due to impairments in the prospective component of PM (e.g., Cockburn, 1996; Fish et al., 2006; Fortin et al., 2003; Knight et al., 2006; Mathias & Mansfield, 2005; Shum et al., 1999) with no consideration of the ability to recognize PM targets during task execution, it will be important to further examine and replicate this finding in a larger sample. Because impairments in PM can be detrimental to successful rehabilitation following TBI due to the need to remember important activities such as medical appointments and medication regimens, it is important for researchers and clinicians to gain a thorough understanding of the processes and components involved in this unique construct. Gaining a better understanding of PM can allow for clinicians to more effectively address PM impairments in response to TBI, as well as to understand the extent to which survivors of TBI experience residual deficits in PM functioning.
Acknowledgments
This study was partially supported by grant #R01 NS47690 from NINDS to MSE and was completed as partial fulfillment of the first author’s dissertation requirements at the Department of Psychology, Washington State University. Additional support was provided by Grant AG034965 from the National Institute on Aging to RES. We thank the TBI participants and the members of the Head Injury Research Team for their help in collecting and scoring the data and Laura Randol for assistance with manuscript preparation.
Footnotes
All reported effect sizes were calculated with the GPOWER program by Erdfelder, Faul, & Buchner (1996).
The possibility that slower reaction times in Block 2 are due simply to fatigue can be rejected on the basis of previous demonstrations of a significant practice effect in Block 2 when the second block is performed without the PM task (Smith & Bayen, 2004, 2006; Smith et al., 2007).
Power analysis was conducted with GPOWER 3 program by Faul, Erdfleder, Lang, & Buchner (2007).
Contributor Information
Shital P. Pavawalla, Mental Health & Behavioral Sciences, James A. Haley Veterans’ Hospital
Maureen Schmitter-Edgecombe, Department of Psychology, Washington State University
Rebekah E. Smith, Department of Psychology, The University of Texas at San Antonio
References
- Altgassen M, Kliegel M, Rendell P, Henry JD, Zollig J. Prospective memory in schizophrenia: The impact of varying retrospective-memory load. Journal of Clinical and Experimental Neuropsychology. 2008;30(7):777–788. doi: 10.1080/13803390701779552. [DOI] [PubMed] [Google Scholar]
- Bisiacchi P. The neuropsychological approach in the study of prospective memory. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Lawrence Erlbaum Associates, Publishers; Mahwah, NJ: US: 1996. [Google Scholar]
- Bisiacchi PS, Schiff S, Ciccola A, Kliegel M. The role of dual-task and task-switch in prospective memory: Behavioural data and neural correlates. Neuropsychologia. 2009;47:1362–1373. doi: 10.1016/j.neuropsychologia.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Brooks DN. Long and short-term memory in head injured patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1972;39:593–601. doi: 10.1136/jnnp.39.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DN. Memory and head injury. Journal of Nervous and Mental Disease. 1972;155(5):350–355. doi: 10.1097/00005053-197211000-00007. [DOI] [PubMed] [Google Scholar]
- Brooks DN. Wechsler Memory Scale performance and its relationship to brain damage after severe closed head injury. Journal of Neurology, Neurosurgery, and Psychiatry. 1975;39:593–601. doi: 10.1136/jnnp.39.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Casadio P, Caltagirone C. Prospective and retrospective components in the memory for actions to be performed in patients with severe closed-head injury. Journal of the International Neuropsychological Society. 2004;10(5):679–688. doi: 10.1017/S1355617704105079. [DOI] [PubMed] [Google Scholar]
- Cockburn J. Failure of prospective memory after acquired brain damage: Preliminary investigation and suggestions for future directions. Journal of Clinical and Experimental Neuropsychology. 1996;18(2):304–309. doi: 10.1080/01688639608408284. [DOI] [PubMed] [Google Scholar]
- Cohen A, Jaudas A, Gollwitzer PM. Number of cues influences the cost of remembering to remember. Memory & Cognition. 2008;36(1):149–156. doi: 10.3758/mc.36.1.149. [DOI] [PubMed] [Google Scholar]
- Crosson B, Novack TA, Trenerry MR, Craig PL. California Verbal Learning Test (CVLT) performance in severely head-injured and neurologically normal adult males. Journal of Clinical and Experimental Neuropsychology. 1988;10(6):754–768. doi: 10.1080/01688638808402812. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: Harcourt Brace & Company; 2001. [Google Scholar]
- Dennis M, Guger S, Roncadin C, Barnes M, Schachar R. Attentional-inhibitory control and social-behavioral regulation after childhood closed head injury: do biological, developmental, and recovery variables predict outcome? Journal of the International Neuropsychological Society. 2001;7:683–692. doi: 10.1017/s1355617701766040. [DOI] [PubMed] [Google Scholar]
- Einstein GO, Holland LJ, McDaniel MA, Guynn MJ. Age-related deficits in prospective memory: The influence of task complexity. Psychology and Aging. 1992;7(3):471–478. doi: 10.1037//0882-7974.7.3.471. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Manzi M, Cochran B, Baker M. Prospective memory and aging: Forgetting intentions over short delays. Psychology and Aging. 2000;15:671–683. doi: 10.1037//0882-7974.15.4.671. [DOI] [PubMed] [Google Scholar]
- Ellis J. Prospective memory or the realization of delayed intentions: A conceptual framework for research. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Lawrence Erlbaum Associates, Publishers; Mahwah, NJ: US: 1996. [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996;28:1–11. [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fish J, Evans JJ, Nimmo M, Martin E, Kersel D, Bateman A, et al. Rehabilitation of executive dysfunction following brain injury: “Content-free” cueing improves everyday prospective memory performance. Neuropsychologia. 2006;45:1318–1330. doi: 10.1016/j.neuropsychologia.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg H. Behavioral changes after closed head injury in children. Journal of Consulting and Clinical Psychology. 1990;58:93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Fortin S, Godbout L, Braun CMJ. Cognitive structure of executive deficits in frontally lesioned head trauma patients performing activities of daily living. Cortex. 2003;39(2):273–291. doi: 10.1016/s0010-9452(08)70109-6. [DOI] [PubMed] [Google Scholar]
- Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8(5):645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Kliegel M, Theodorou G, Summers F. Traumatic brain injury and prospective memory: Influence of task complexity. Journal of Clinical and Experimental Neuropsychology. 2007;29(5):457–466. doi: 10.1080/13803390600762717. [DOI] [PubMed] [Google Scholar]
- Horn SS, Bayen UJ, Smith RE, Boywitt CD. The multinomial model of prospective memory: Validity of ongoing-task parameters. Experimental Psychology. doi: 10.1027/1618-3169/a000091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager T, Kliegel M. Time-based and event-based prospective memory across adulthood: Underlying mechanisms and differential costs on the ongoing task. The Journal of General Psychology. 2008;135(1):4–22. doi: 10.3200/GENP.135.1.4-22. [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT, Caldwell FE. Measurement of post-traumatic amnesia: how reliable is it? Journal of Neurology, Neurosurgery, and Psychiatry. 1997;62:38–42. doi: 10.1136/jnnp.62.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella G, Murtagh D, Landry A, Homfray K. Everyday memory following traumatic brain injury. Brain Injury. 1996;10(7):499–507. doi: 10.1080/026990596124214. [DOI] [PubMed] [Google Scholar]
- Kliegal M, Eschen A, Thone-Otto AIT. Planning and realization of complex intentions in traumatic brain injury and normal aging. Brain and Cognition. 2004;56:43–54. doi: 10.1016/j.bandc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Knight RG, Harnett M, Titov N. The effects of traumatic brain injury on the predicted and actual performance of a test of prospective remembering. Brain Injury. 2005;19(1):27–38. doi: 10.1080/02699050410001720022. [DOI] [PubMed] [Google Scholar]
- Knight RG, Titov N, Crawford M. The effects of distraction on prospective remembering following traumatic brain injury assessed in a simulated naturalistic environment. Journal of the International Neuropsychological Society. 2006;12:8–16. doi: 10.1017/S1355617706060048. [DOI] [PubMed] [Google Scholar]
- Kučera H, Francis W. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Kvavilashvili L, Ellis J. Varieties of intention: Some distinctions and classifications. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Lawrence Erlbaum Associates, Publishers; Mahwah, NJ: US: 1996. [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney, Australia: Psychology Foundation of Australia; 1995. [Google Scholar]
- Marsh RL, Hicks JL. Event-based prospective memory and executive control of working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1–14. doi: 10.1037//0278-7393.24.2.336. [DOI] [PubMed] [Google Scholar]
- Mateer CA, Sohlberg MM, Crinean J. Focus on clinical research: Perceptions of memory function in individuals with closed-head injury. Journal of Head Trauma Rehabilitation. 1987;2(3):74–84. [Google Scholar]
- Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Injury. 2005;19(4):271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Smith G, Della Sala S, Logie RH. Prospective and retrospective memory in normal aging and dementia: An experimental study. Memory & Cognition. 2002;30(6):871–884. doi: 10.3758/bf03195773. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:5127–5144. [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, Breneiser J. Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(3):605–614. doi: 10.1037/0278-7393.30.3.605. [DOI] [PubMed] [Google Scholar]
- Moshagen M. multiTree: A computer program for the analysis of multinomial processing tree models. Behavior Research Methods. 2010;42:42–54. doi: 10.3758/BRM.42.1.42. [DOI] [PubMed] [Google Scholar]
- Palmer HM, McDonald S. The role of frontal and temporal lobe processes in prospective remembering. Brain and Cognition Special Issue: 9th Annual Rotman Research Inst Conference “Traumatic brain injury: Diagnosis, outcome, & rehabilitation”; March 24–26, 1999; 2000. pp. 103–107. [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Raskin S. Memory for intentions screening test [abstract] Journal of the International Neuropsychological Society. 2004;10(Suppl 1):110. [Google Scholar]
- Reitan RM. Trail making test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. [Google Scholar]
- Roche NL, Fleming JM, Shum DHK. Self-awareness of prospective memory failure in adults with traumatic brain injury. Brain Injury. 2002;16(11):931–945. doi: 10.1080/02699050210138581. [DOI] [PubMed] [Google Scholar]
- Roche NL, Moody A, Szabo K, Fleming JM, Shum DHK. Prospective memory in adults with traumatic brain injury: An analysis of perceived reasons for remembering and forgetting. Neuropsychological Rehabilitation. 2007;17(3):314–334. doi: 10.1080/09602010600831004. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Beglinger L. Acquisition of skilled visual search performance following severe closed-head injury. Journal of the International Neuropsychological Society. 2001;7:615–630. doi: 10.1017/s1355617701755099. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Marks W, Fahy JF. Semantic priming following severe closed-head trauma: Automatic and attentional processes. Neuropsychology. 1993;7:136–148. [Google Scholar]
- Schmitter-Edgecombe M, Rogers WA. Automatic process development following severe closed-head injury. Neuropsychology. 1997;11:296–308. doi: 10.1037//0894-4105.11.2.296. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Wright MJ. Event-based prospective memory following severe closed-head injury. Neuropsychology. 2004;18(2):353–361. doi: 10.1037/0894-4105.18.2.353. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. Journal of Psychology: Interdisciplinary and Applied. 1940;9:371–377. [Google Scholar]
- Shum D, Valentine M, Cutmore T. Performance of individuals with severe long-term traumatic brain injury on time-, event-, and activity-based prospective memory tasks. Journal of Clinical & Experimental Neuropsychology. 1999;21(1):49–58. doi: 10.1076/jcen.21.1.49.943. [DOI] [PubMed] [Google Scholar]
- Smith G, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8(5):311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2003;29(3):347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE. Connecting the past and the future: Attention, memory, and delayed intentions. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. Mahwah, NJ: Erlbaum; 2008. pp. 27–50. [Google Scholar]
- Smith RE. What costs do reveal and moving beyond cost: Reply to Einstein and McDaniel (2010) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1089–1095. doi: 10.1037/a0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ. A multinomial model of event-based prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(4):756–777. doi: 10.1037/0278-7393.30.4.756. [DOI] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ. The effects of working memory resource availability on prospective memory: A formal modeling approach. Experimental Psychology. 2005;52(4):243–256. doi: 10.1027/1618-3169.52.4.243. [DOI] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ. The source of adult age differences in event-based prospective memory: A multinomial modeling approach. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(3):623–635. doi: 10.1037/0278-7393.32.3.623. [DOI] [PubMed] [Google Scholar]
- Smith RE, Bayen UJ, Martin C. The cognitive processes underlying event-based prospective memory in school-age children and young adults: A formal model-based study. Developmental Psychology. 2010;46(1):230–244. doi: 10.1037/a0017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcome after traumatic brain injury in children: Behavior and Achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80(5):352–373. [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: A selected review. Journal of Clinical and Experimental Neuropsychology. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H, Hoofien D. Automatic temporal order judgment: The effect of intentionality of retrieval on closed-head injured patients. Journal of Clinical and Experimental Neuropsychology. 1991;13:291–298. doi: 10.1080/01688639108401044. [DOI] [PubMed] [Google Scholar]
- West R, Craik FIM. Influences on the efficiency of prospective memory in younger and older adults. Psychology and Aging. 2001;16(4):682–696. [PubMed] [Google Scholar]
- West R, Krompinger J. Neural correlates of prospective and retrospective memory. Neuropsychologia. 2005;43:418–433. doi: 10.1016/j.neuropsychologia.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eienberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioral Assessment of the Dysexecutive Syndrome. Thames Valley Test Company; Bury St. Edmonds: 1996. [Google Scholar]