Abstract
The barrier integrity of the corneal endothelium, which is conferred by its tight and adherens junctions, is critical for the maintenance of deturgescence of the corneal stroma. Although characteristically leaky, the barrier integrity restricts fluid leakage into the stroma such that the rate of leak does not exceed the rate of the endothelial active fluid transport directed toward the aqueous humor. At a molecular level, the barrier integrity is influenced by the actin cytoskeleton and microtubules, which are coupled to tight and adherens junctions via a variety of linker proteins. Since the cytoskeleton is affected by Rho family small GTPases and p38 MAP kinase, among others, many pathophysiological stimuli induce plasticity to the cytoskeleton and thereby elicit dynamic regulation of the barrier integrity. This review presents an overview of the impact of several bioactive factors on the barrier integrity of the corneal endothelium through altered actin cytoskeleton and/or disassembly of microtubules. The main focus is on the effect of TNF-α (tumor necrosis factor-α) which is a pro-inflammatory molecule found in the intraocular milieu during allograft rejection and anterior uveitis. This cytokine elicits acute activation of p38 MAP kinase, induces disassembly of microtubules, disrupts the peri-junctional actomyosin ring, and concomitantly breaks down the barrier integrity. These effects of TNF-α could be inhibited by stabilizing the microtubules, co-treating with a selective p38 MAP kinase inhibitor, and elevating intracellular cAMP via A2B receptors or direct exposure to forskolin. Overall, the corneal edema following a potential breakdown of the endothelial barrier integrity can be rescued pharmacologically by inhibiting specific cell-signaling mechanisms.
Keywords: Cornea, Endothelium, Tight Junctions, Actomyosin Contraction, TNF-α, Microtubules, p38 MAP kinase
Introduction
The loss of corneal transparency due to decompensated endothelium is a major indication for corneal transplantation (e.g., Fuch’s dystrophy), of which there are ~ 40,000 annually in USA. Other indications include bullous keratopathy, keratoconus, a variety of corneal dystrophies, and chemical burn. Even after transplantation, survival of the endothelium is a major concern (Armitage et al., 2003; George and Larkin, 2004; Niederkorn, 2007). The central endothelial cell loss of the donor cornea over the first post-operative year exceeds 30% despite the use of immunosuppressive agents. Therefore, understanding the basic mechanisms of the endothelial dysfunction in response to pro-inflammatory mediators, which are released secondary to the immune response during allograft rejection, is of prime importance. The focus of this review is on the endothelial dysfunction in response to TNF-α, which can be found in the cornea and aqueous humor during allograft rejection (Rayner et al., 2000; Rayner et al., 2001) and anterior uveitis. Our goal is to identify a class of small molecular weight inhibitors, which can be applied topically to directly treat endothelial dysfunction during allograft rejection and uveitis.
The Barrier Function of the Corneal Endothelium
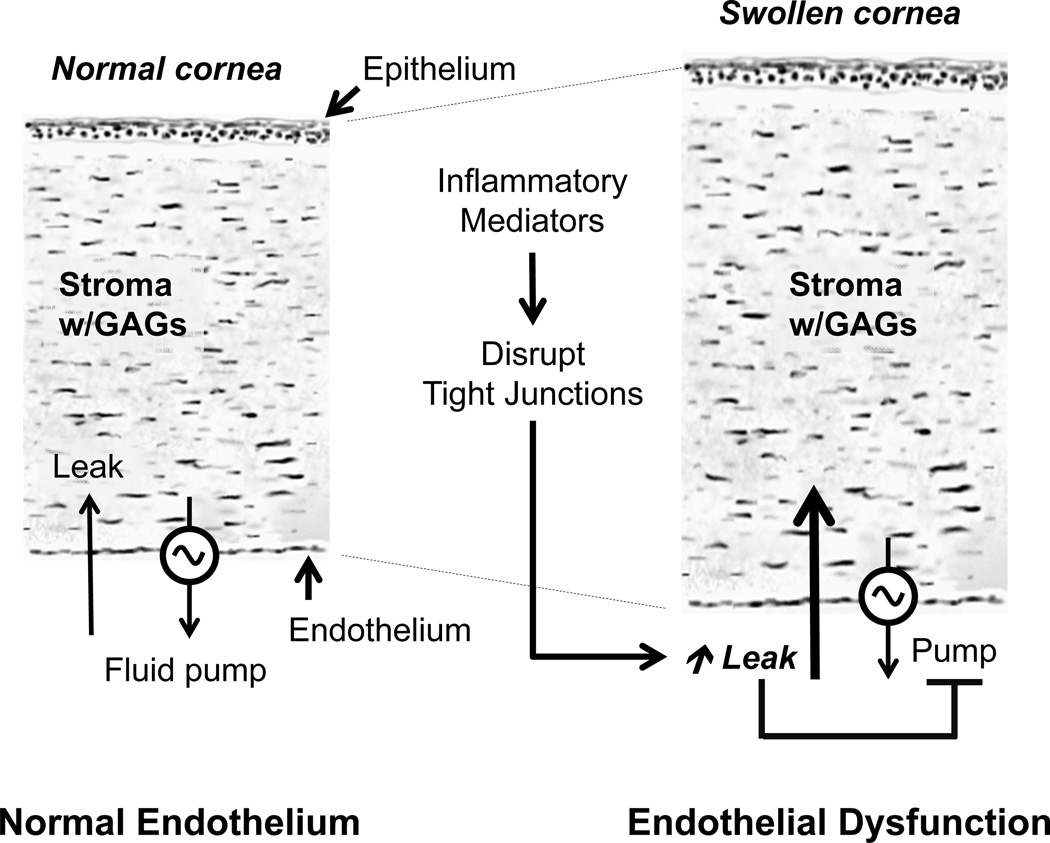
The endothelium, an epithelial interface between the anterior chamber and the corneal stroma, is a monolayer of cells that forms a regular hexagonal mosaic at the posterior surface of the cornea. Its main function is to preserve stromal deturgescence, which is a prerequisite for corneal transparency. The principal challenge for maintaining deturgescence is the swelling pressure associated with the hydrophilic glycosaminoglycans (GAGs) in the stroma. This negative pressure induces a continuous fluid leak into the stroma from the anterior chamber. Although the endothelium (trans-endothelial electrical resistance, or TER, ~ 30 Ω.cm2) is leaky (Noske et al., 1994; Fischbarg et al., 2006), its tight junctions (TJs) restrict the rate of leakage and thereby oppose potential corneal edema. This role of the endothelium, referred to as the barrier function, is dependent on the integrity of its tight and adherens junctions (a.k.a., barrier integrity). In a healthy cornea, the fluid leak is counter-balanced by an active fluid pump mechanism that is also associated with the endothelium. The overall mechanism, which constitutes the Pump-Leak hypothesis as first propounded by Maurice (Maurice; Riley, 1985; Edelhauser, 2006), is shown schematically in Fig. 1.
Figure 1. Role of barrier integrity in the regulation of stromal deturgescence.
Panel A: Fluid leakage into the stroma occurs through the paracellular pathway and is driven by a hydraulic gradient equivalent to the swelling pressure of the stroma (~ 50 mm Hg at normal stromal thickness). The paracellular pathway of the endothelium is characterized by a low transendothelial electrical resistance (TER < 30 Ω.cm2), and, hence, the endothelium forms a leaky epithelial interface at the stroma. The rate of leakage, however, is counter-balanced by the active fluid pump activity of the endothelium. Thus, the endothelial barrier integrity and fluid pump activity together maintain stromal deturgescence. Adapted from a previous review (Srinivas, 2010). Panel B: Panel B: When the barrier integrity breaks down, the pump mechanism cannot cope with the leak, and hence, stromal edema would be induced. Furthermore, when the tight junctions are abrogated, the fluid pump mechanism cannot continue since the local osmotic gradients generated by ion transport are dissipated by futile solute back-flux. The cytokines, such as TNF-α, that are implicated in allograft rejection may also directly reduce fluid transport activity by modulating the ion transport mechanisms.
The fluid pump activity of the endothelium, similar to that of other fluid transporting epithelia, is dependent on the ion transport mechanisms, expressed strategically at its basolateral and apical membranes (Bonanno, 2003). These mechanisms elicit a net movement of Na+ and HCO3−/Cl− from the stroma into the aqueous humor. Based on the theory of solute-solvent coupling (Diamond and Bossert, 1967), it is envisaged that the net ionic movement sets up local osmotic gradients across the apical membrane, leading to vectorial fluid movement into the anterior chamber. In order for this transcellular fluid movement to be efficacious, the integrity of the TJs is crucial (Srinivas, 2010) from two points of view. First, the TJs must prevent dissipation of the ionic gradients set up by the ion transport mechanisms by blocking trivial back flux through the paracellular space (referred to as the “gate” function of the TJs). Second, TJs must also limit the lateral diffusion of the integral membrane proteins, including those of the ion transport mechanisms. This so-called “fence” function of the TJs, though a slow process, is critical for maintaining the apical-basal polarity of the transport proteins in the endothelium. In the absence of polarized ion transport, vectorial ionic movement by the endothelium would not be possible. Overall, the barrier integrity of the corneal endothelium is indispensable for maintaining stromal deturgescence and, hence, corneal transparency.
In the following, recent findings on the barrier integrity of the corneal endothelium vis-à-vis cell signaling linked to actomyosin contraction (Satpathy et al., 2004; Srinivas et al., 2004; Satpathy et al., 2005; Srinivas et al., 2006; Jalimarada et al., 2009; Ramachandran and Srinivas, 2009) are reviewed first. Next, trans-endothelial electrical resistance (TER) based on electrical cell-substrate impedance sensing (ECIS) as a measure of barrier integrity (Ramachandran and Srinivas, 2009) is discussed briefly. Specifically, our findings have demonstrated that ECIS enables measurements of TER with high sensitivity and in real-time. Finally, recent work on the (TNF-α)-induced loss of barrier integrity in the bovine corneal endothelium (Shivanna and Srinivas, 2009; Shivanna et al., 2010; Shivanna and Srinivas, 2010) is reviewed.
Regulation of the Barrier Integrity by Cortical Actin Cytoskeleton
The TJs in the corneal endothelium offer weak resistance to paracellular permeability of solutes and water (Srinivas, 2010). Even slight damage to the TJs is sufficient to precipitate stromal swelling. A typical TJ consists of transmembrane proteins bound to cytoplasmic plaques, which are fused to the cortical actin cytoskeleton through a variety of linker proteins. The paracellular pathway is occluded by homophilic interactions of the transmembrane proteins of the TJs (occludins, claudins, and junctional adhesion molecule). Cell-cell proximity, achieved by the tethering forces at the TJs is essential for interactions between the transmembrane proteins. Contraction of the cortical actin cytoskeleton generates a centripetal force opposing the tethering forces at the TJs, leading to a breakdown of the cell-cell apposition and barrier integrity. This form of cytoskeletal regulation of the barrier integrity is known in vascular endothelium and also in certain epithelial monolayers (Garcia et al., 1995; Dudek and Garcia, 2001; Mehta and Malik, 2006).
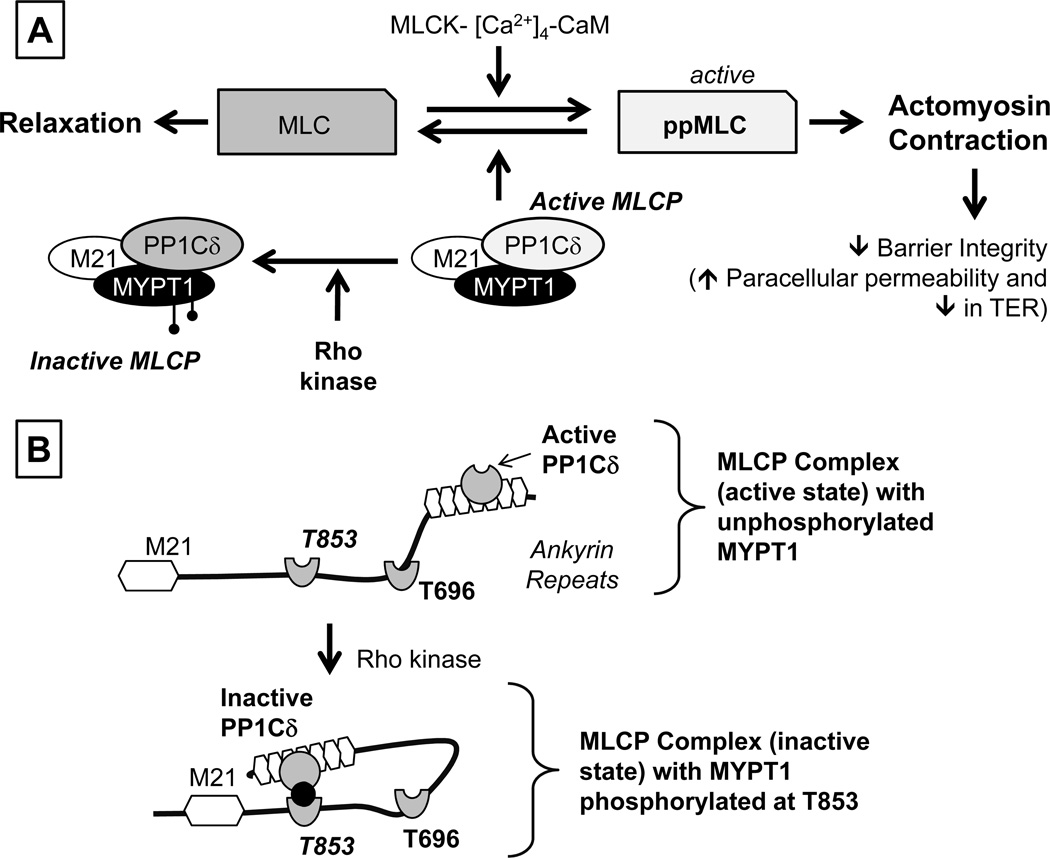
The actomyosin contraction is regulated by phosphorylation of the regulatory light chain of Myosin II (referred to as Myosin Light Chain or MLC). The extent of MLC phosphorylation is determined by two opposing pathways: MLCK (MLC Kinase)-driven phosphorylation and MLCP (MLC Phosphatase)-driven dephosphorylation (summarized in Fig. 2) (Somlyo and Somlyo, 2003; Srinivas, 2010; Ramachandran et al., 2011). MLCK is the dedicated kinase for phosphorylating MLC and is activated when bound to the Ca2+-calmodulin complex. MLC is dephosphorylated by MLCP, a heterotrimeric complex consisting of PP1Cδ (the catalytic subunit of MLCP), a myosin-binding subunit (MYPT1; 130 kDa), and a small subunit (M21; 21 kDa) of unknown function. Phosphorylation of MYPT1 by Rho kinase (at T696 and T853; Fig. 2B) inhibits the phosphatase activity of PP1Cδ. PKC (Protein Kinase C) inactivates MLCP through phosphorylation of CPI-17 (PKC-activated 17 kDa inhibitor protein of type 1 phosphatase;17 kDa), which is known to inactivate PP1Cδ (not shown in Fig. 2) (Somlyo and Somlyo, 2003; Srinivas, 2010; Ramachandran et al., 2011). Overall, the activation of Rho kinase and/or PKC results in actomyosin contraction. In contrast to Rho kinase and PKC, Protein kinase A (PKA) opposes actomyosin contraction. Specifically, RhoA is phosphorylated by (PKA) at Ser-188 and this prevents dissociation of RhoA-GDI (RhoA-GDP-dissociation inhibitor) from RhoA-GDP and thereby curtails activation of RhoA (Ramachandran et al., 2011).
Figure 2. Overview of signaling pathways underlying actomyosin contraction.
Panel A: Myosin light chain (MLC) phosphorylation is catalyzed by Ca2+-Calmodulin-dependent MLC kinase (MLCK). The expression of both endothelial and smooth muscle isoforms are seen in the corneal endothelium. MLC phosphorylation promotes actomyosin interaction, leading to increased cellular contractility. MLCK activity is opposed by MLC phosphatase (MLCP), which catalyzes dephosphorylation of ppMLC. MLCP is a heterotrimeric complex consisting of MYPT1 (a regulatory subunit), PP1Cδ (protein phosphatase 1; catalytic subunit δ-isoform), and M21 (function unknown). Rho kinase, a RhoA effector, phosphorylates MYPT1, thereby inhibiting PP1Cδ. Panel B: Autoinhibition of MLCP: The activity of PP1Cδ is inhibited by ROCK through phosphorylation of MYPT1 at T853 or T696. Specifically, the substrate site of PP1Cδ is accessible when neither T696 nor T853 is unphosphorylated. However, when MYPT1 is phosphorylated at T696 or T853 (phosphorylation of T853 is shown here), the phosphorylated residues interact with the active site of PP1Cδ and suppress the phosphatase activity. Adapted from our previous report (Ramachandran et al., 2011).
A number of studies have shown that increased MLC phosphorylation, while in general induces actomyosin contraction throughout the cell, significantly affects the contraction of the pool of actin cytoskeleton at the apical junction complex (referred to as the peri-junctional actomyosin ring or PAMR) and is responsible for the breakdown of the barrier integrity (Srinivas, 2010). We have found that thrombin activates RhoA–Rho kinase axis through G12/13-coupled PAR-1 (Protease-activated receptor-1) receptors in bovine corneal endothelial cells (Fig. 3) (Satpathy et al., 2004; Srinivas et al., 2004; Satpathy et al., 2005). The resulting increase in MLC phosphorylation leads to a disruption of the PAMR with a concomitant breakdown of the barrier integrity. In two subsequent studies (Srinivas et al., 2004; Satpathy et al., 2005), we demonstrated that the thrombin-induced MLC phosphorylation and the breakdown in the barrier integrity can be suppressed by elevated cAMP, which inhibits activation of RhoA (Ramachandran et al., 2008; Ramachandran et al., 2011). We have also investigated the Ca2+- and PKC-dependent mechanisms involved in MLC phosphorylation upon activation of H1 receptors (Srinivas et al., 2006), which are not known to activate RhoA. These experiments showed that the histamine-induced MLC phosphorylation and the resulting loss of endothelial barrier integrity can be suppressed by ML-7 (a selective MLCK inhibitor) and a PKC inhibitor, indicating a role of MLCK and PKC (Srinivas et al., 2006). These findings are consistent with the cell signaling in the regulation of actomyosin contraction and the breakdown of the barrier integrity as depicted in Fig. 2A.
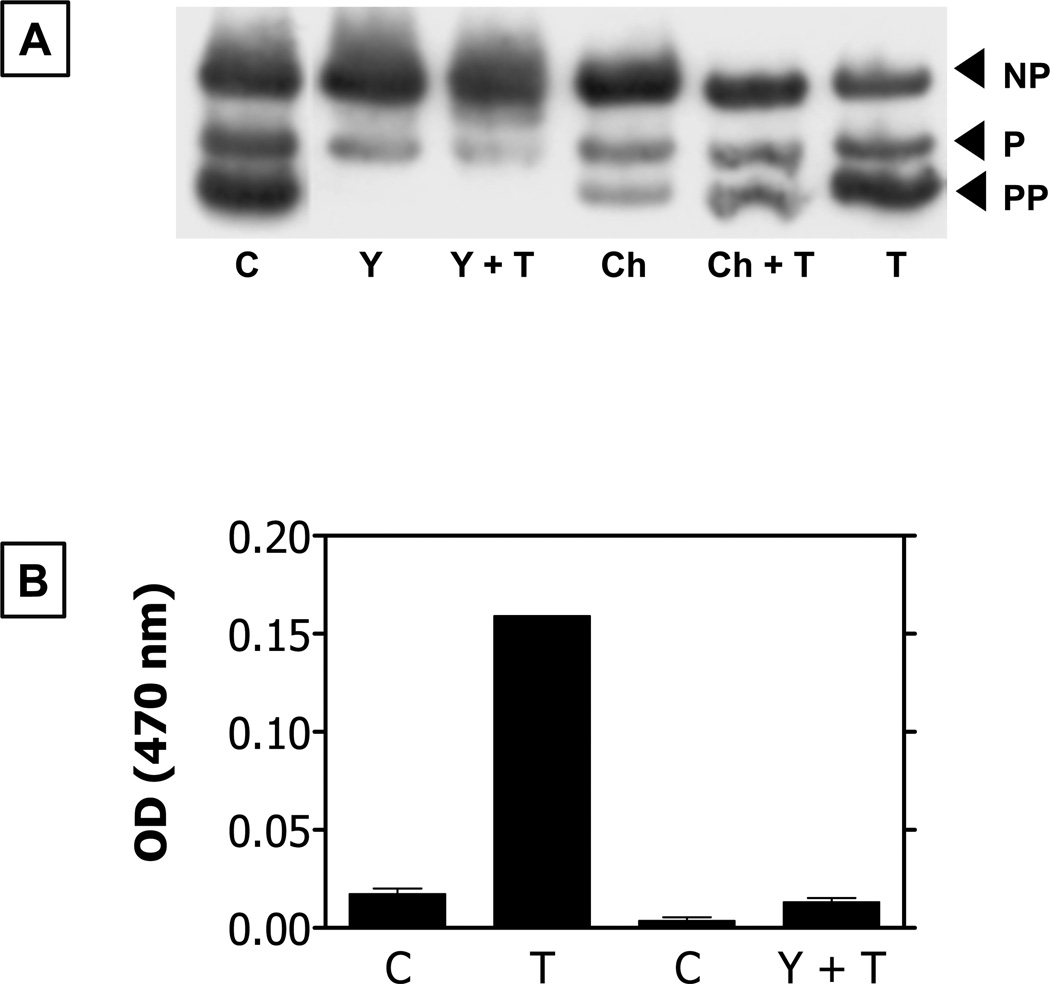
Figure 3. Response of bovine corneal endothelial cells to activation of PAR-1 receptors by thrombin.
Panel A: MLC phosphorylation in response to thrombin is inhibited by Y-27632 (Rho kinase inhibitor) and Chelerythrin (a PKC inhibitor). C: Control (Untreated cells). T: Thrombin; NP: Non-phosphorylated; P: Mono-Phosphorylated; PP: Di-phosphorylated; Y: Y-27632; Ch: Chelerythrin. Panel B: Bar graph showing permeability to Horseradish peroxidase (HRP; ~44 kDa). Increase in optical density at 470 nm signifies increase in permeability to HRP. Results summarized from our previous report (Satpathy et al., 2004).
Apart from the direct impact of the actin cytoskeleton, we have also found that there is significant cross-talk between actin cytoskeleton and microtubules (Jalimarada et al., 2009). Specifically, deliberate microtubule disassembly by exposure to nocodazole led to activation of RhoA, consequent disruption of the PAMR, and loss of the barrier integrity. These studies also emphasize a strong role for actin cytoskeleton along the locus of the apical junctional complex in the regulation of the corneal endothelial barrier integrity. Taken together, our results also provide insights into how other bioactive factors found in the aqueous humor may influence the barrier function of the endothelium and alter stromal hydration control in a dynamic manner.
Breakdown and Formation of the Tight Junctions in the Endothelium
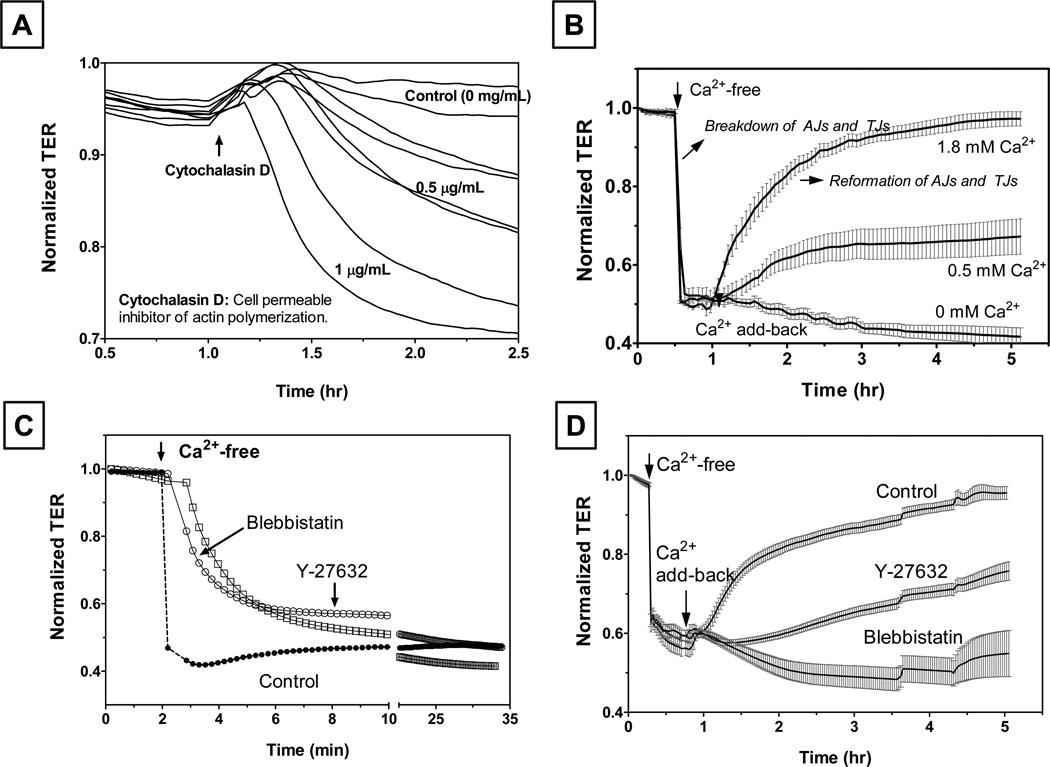
In contrast to the indirect influence of enhanced actomyosin contraction, cell loss presents a direct threat to the barrier integrity of the corneal endothelium as it is not regenerative in humans (Joyce, 2003). While loss of endothelial cells occurs constantly during aging, it is pronounced during Fuchs’ dystrophy, following allograft rejection (Srinivas, 2010), and in response to iatrogenic injury (e.g., phacoemulsification). In situations where endothelial monolayer sustains loss of cells, understanding the factors likely to impact on the reassembly of cell-cell junctions and reestablishing the barrier integrity following cell spreading in an effort to cover the denuded area is crucial. In this context, we recently investigated the influence of actin cytoskeleton on the dynamics of disassembly and reassembly of the AJs (adherens junctions) and TJs upon extracellular Ca2+ depletion and Ca2+ add-back, respectively (Ramachandran and Srinivas, 2009). To follow the temporal course of the integrity of the AJs and TJs during Ca2+ switch, we measured TER (trans-endothelial electrical resistance) using electric cell-substrate impedance sensing (ECIS; using ECIS1600R, Applied Biophysics, Inc., Troy, NY). ECIS allows rapid changes in TER to be measured in real time (Srinivas, 2010). For ECIS, cells are grown to confluence on small, gelatin-coated gold electrodes. A small AC current is applied across the cells, and the impedance for the current flow is then measured by a lock-in amplifier at ~ 0.1 Hz. The resistive component of the impedance for the current flow represents TER since the plasma membrane resistance is large (Tiruppathi et al., 1992). Fig. 4A shows the dynamics of TER in response to cytochalasin D, which is known to break down the barrier integrity in other epithelia. In this experiment, electrodes were inoculated with bovine corneal endothelial cells and grown to grow to confluence. TER reached a steady state in ~ 18 hrs and then cells were exposed to cytochalasin D. As an actin polymerization inhibitor, cytochalasin D breaks up the PAMR, and as a result, barrier integrity is lost. Fig. 4B shows the response to the Ca2+ switch protocol. Disassembly of the AJs, and as a consequence TJs, was induced by replacing Ringers containing 2 mM Ca2+ with Ca2+-free Ringers (containing 2 mM EGTA). Subsequently, reassembly of the AJs, and thus resealing of the TJs, was promoted by exposing to Ca2+-rich Ringers (2 mM Ca2+) on the same cells. As expected, exposure to the Ca2+-free medium led to precipitous loss in the TER (Fig. 4B), and subsequent repletion of Ca2+ led to a recovery of the TER (Fig. 4B). These results from our recent study (Ramachandran and Srinivas, 2009) not only show that ECIS-based TER measurements reflect the status of the barrier integrity but also quantify the dynamics of reformation of the AJs and TJs. By Western blot analysis, we have also shown evidence that Ca2+ depletion results in activation of RhoA following disengagement of cadherins (i.e., breakdown of AJs) (Ramachandran and Srinivas, 2009). This activation is likely to have contributed toward acceleration of the breakdown of the TJs since pre-exposure to Y-27632 (a selective Rho kinase inhibitor) and blebbistatin (a selective Myosin II ATPase inhibitor) inhibited the response to Ca2+ depletion (Fig. 4C). Interestingly, actomyosin contraction is also essential for the reformation of the AJs and TJs, as shown in Fig. 4D. These results establish a role for actomyosin contraction in the reformation of AJs and TJs following cell spreading, which is induced by focal loss of endothelial cells.
Figure 4. Reformation of TJs and AJs in bovine corneal endothelial monolayers assessed by trans-endothelial electrical resistance (TER).
Panel A: Loss of barrier integrity in response to cytochalasin D. At the up-arrow shown at about 1 hr after reaching the steady state, cells were exposed to cytochalasin D. As expected, cytochalasin D led to a decrease in the normalized resistance in a dose-dependent manner. Panel B: Breakdown and reassembly of apical junctions during Ca2+ Switch: When cells were exposed to Ca2+-free Ringers (with 2 mM EGTA), a precipitous fall in TER consistent with the breakdown of AJs is noticed. Ca2+ add-back led to a gradual recovery of TER. The rate and extent of recovery was dependent on the level of extracellular Ca2+. Panel C: Disassembly of AJs is opposed by inhibition of actomyosin contraction. Cells, pretreated with Y-27632 (Rho kinase inhibitor) or blebbistatin (Myosin II ATPase inhibitor) for 10 min, were exposed Ca2+-free Ringers. The rate of decrease in TER was smaller compared to the rate observed in the absence of Y-27632 and blebbistatin. Panel D: Reformation of AJs and TJs is also opposed by loss of actomyosin contraction. When cells were pre-exposed to Y-27632 or blebbistatin, recovery of TER in response to Ca2+ addition was inhibited. These results, adapted from our previous report (Ramachandran and Srinivas, 2009), suggest a role for actomyosin contraction in the disassembly and reformation of tight junctions.
Involvement of TNF-α in the Corneal Allograft Rejection and Anterior Uveitis
The corneal endothelium is a direct target of intraocular inflammation during anterior uveitis (bystander effect) and allograft rejection following corneal transplantation. Anterior uveitis involves cellular infiltration (neutrophils, CD4+ T cells), an increase in protein permeability (i.e., due to breakdown of blood-aqueous barrier), upregulation of cytokines (e.g., TNF-α, IL-1β, and IFN-γ) and chemokines (e.g., MCP-1; monocyte chemotactic protein-1) in the aqueous humor and uveal tract ((El-Shabrawi and Hermann, 2002; Johnsen-Soriano et al., 2010). Autoimmune diseases, bacterial/viral infections, and chemical and metabolic injuries are known to induce uveitis. In addition to the use of topical/systemic immunosuppressive drugs, treatment targeted to the corneal endothelium would be useful to prevent corneal edema and consequent loss of acute vision. As noted earlier, allograft rejection is also relatively frequent in high-risk transplantation (George and Larkin, 2004). As with anterior uveitis, TNF-α is also implicated in the corneal endothelial failure during allograft rejection; the transcriptional activity of the cytokine has been detected, and TNF-α levels are also significantly elevated in the aqueous humor and the serum (Rayner et al., 2000; Rayner et al., 2001). The cytokine is presumed to be released mainly from the circulating cells of the immune system, which infiltrate the anterior chamber during rejection. In consistence with the inflammatory load in the aqueous humor, the corneal endothelial cells show significant elevation in the expression of a variety of adhesion molecules that potentiate firm adhesion and transmigration of leukocytes into the stroma. These include intercellular adhesion molecule-1 (ICAM-1), E-selectin (endothelial leukocyte adhesion molecule-1), and vascular cell adhesion molecule-1 (VCAM-1). Finally, in situations of both allograft rejection and anterior uveitis, anti-TNF-α therapy has been found to be efficacious, indicating the relevance of the cytokine in the corresponding pathophysiology.
A number of studies have demonstrated regulation of barrier integrity by cytokines, leading to a clear understanding of the etiology of a number of pathological conditions (Capaldo and Nusrat, 2009). In addition to their pro-inflammatory role (e.g., through the expression of cell adhesion molecules), cytokines break down the barrier integrity through distinct mechanisms of cell signaling independent of apoptosis (Bruewer et al., 2003). Given the prominent role of pro-inflammatory cytokines in many pathological situations, impact of TNF-α in the breakdown of the barrier integrity of a variety of endothelial and epithelial monolayers has been examined (Capaldo and Nusrat, 2009). A previous study by Watsky et al. has shown that TNF-α breaks down the barrier integrity of the corneal endothelium (Watsky et al., 1996). However, the molecular mechanisms in the corneal endothelium remain unexplored. In other epithelia, the cytokine is known to break down the barrier integrity concomitant with disruption of the PAMR, formation of stress fibers, and disassembly of microtubules (Goldblum et al., 1993; Watsky et al., 1996; Petrache et al., 2003; Ye et al., 2006). Despite the formation of stress fibers, inhibitors of the actomyosin contraction (e.g., Y-27632-mediated inhibition of Rho kinase) have not been efficacious in the corneal endothelium (unpublished data). These observations led us to focus on other mechanisms, especially those involving p38 MAP kinase (Shivanna and Srinivas, 2009; Shivanna et al., 2010; Shivanna and Srinivas, 2010).
Response to TNF-α
Disassembly of Microtubules
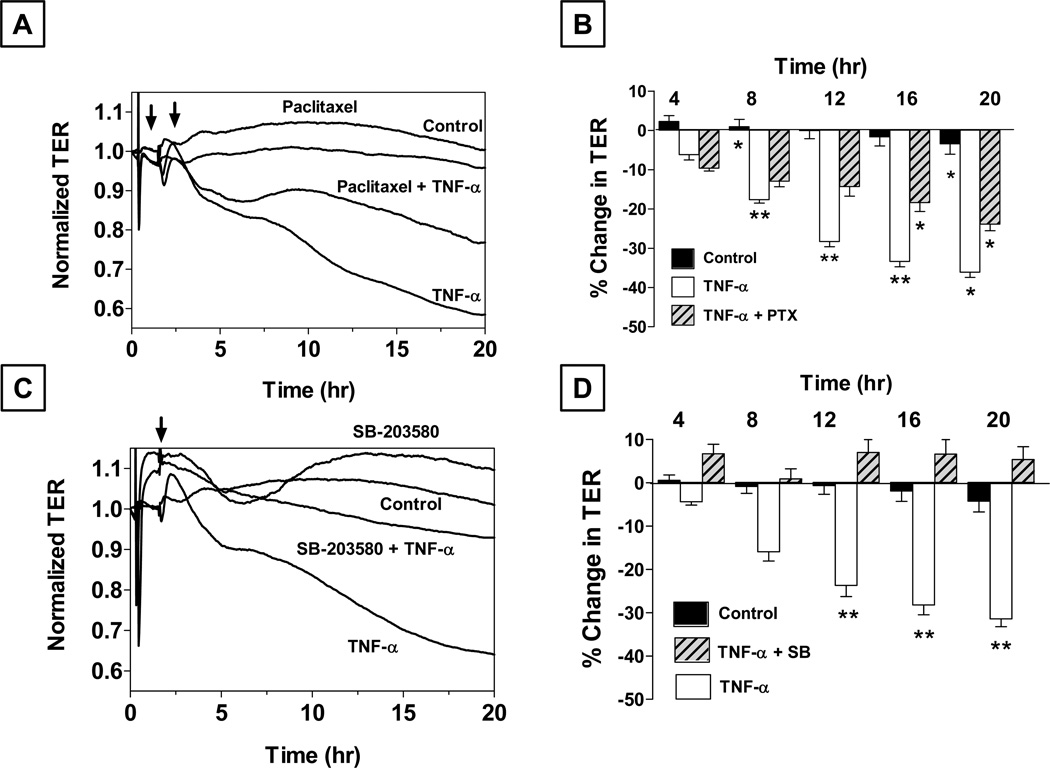
Among the known pathophysiologic stresses, oxidative stress and exposure to cytokines (e.g., TNF-α, TGF-β) and proteases (e.g., thrombin) are known to cause disassembly of microtubules (Petrache et al., 2003; Birukova et al., 2004; Birukova et al., 2005). Since we had previously shown that microtubule disassembly breaks down the barrier integrity of the corneal endothelium (Jalimarada et al., 2009), we first examined the effect of TNF-α on microtubules (Shivanna and Srinivas, 2009). As expected, exposure to the cytokine led to disassembly of the microtubules and also caused disruption of the PAMR. As shown in Fig. 5A, exposure to TNF-α caused a slow decline in TER over 20 hrs. A similar exposure to cells grown on porous culture inserts led to a significant increase in the permeability to FITC dextran (10 kDa). These changes, which indicate a loss of the barrier integrity, were also reflected by dislocation of ZO-1 at the cell border and disassembly of cadherins (Shivanna and Srinivas, 2009). To confirm these responses, we challenged cells with the cytokine after stabilizing the microtubules using paclitaxel. As shown in Figs. 5A and 5B, paclitaxel induced microtubule stabilization and suppressed the response to TNF-α (Shivanna and Srinivas, 2009).
Figure 5. Role of microtubules and p38 MAP kinase in the (TNF-α)-induced response in bovine corneal endothelial monolayers.
Panels A and B: Cells were pretreated with 10 µM paclitaxel (PTX) for 1 hr with or without 20 ng/ml TNF-α Paclitaxel attenuates the decrease in TER by TNF-α. Panel B shows bar graph of experiments similar to that shown in Panel A. The % reduction in TER induced by TNF-α is significantly greater than control beyond 8 hrs. Note: TNF-α + PTX: TNF-α + Paclitaxel. Error bars represent the SEM (n = 6). *Indicates significant difference from the control. C vs. TNF-α: p < 0.001. Paclitaxel significantly opposes (TNF-α)-induced reduction in TER beyond 12 hrs. **Indicates significant difference in TNF-α vs. TNF-α + PTX: p < 0.001. Summarized from our previous publication (Shivanna and Srinivas, 2009). Panels C and D: Pretreatment with SB-203580 (a selective p38 MAP kinase inhibitor; 10 µM) attenuates the decrease in TER by TNF-α. Panel D shows a bar graph of experiments similar to that shown in Panel C. Note: TNF-α + SB: TNF-α + SB-203580. Error bars represent the SEM (n > 5). **Significantly different than TNF-α, p < 0.001. Results summarized from our previous report (Shivanna et al., 2010).
Activation of p38 MAP kinase
The (TNF-α)-induced disassembly of microtubules indicated a role for p38 MAP kinase in the cytokine response. Previous studies with vascular endothelial cells have demonstrated p38 MAP kinase-dependent reactive oxygen species (ROS) production and activation of Hsp27 (heat shock protein 27) (Mehta and Malik, 2006). While ROS is known to cause microtubule disassembly, activation of Hsp27 is associated with the disruption of the actin cytoskeleton. In this context, we investigated whether the (TNF-α)-induced activation of p38 MAP kinase is involved in the microtubule disassembly, disruption of the PAMR, and breakdown of the barrier integrity (Shivanna et al., 2010). We first observed that exposure to the cytokine results in a transient activation of p38 MAP kinase, as determined by Western blot analysis. Accordingly, exposure to SB-203580 (a selective inhibitor of p38 MAP kinase) suppressed the loss in TER (Fig. 5C and 5D) and opposed the impact of the cytokine on the actin cytoskeleton and microtubules (Shivanna et al., 2010). These results, taken together, suggest that TNF-α breaks down the endothelial barrier integrity via acute activation of p38 MAP kinase.
Effect of Elevated cAMP
Elevated cAMP increases barrier integrity in the corneal endothelium. Riley et al. (Riley et al., 1998) demonstrated enhanced stromal hydration control through cAMP-mediated decrease in the paracellular permeability to carboxyfluorescein. In recent studies, we also found that agents that elevate cAMP suppressed the thrombin- and histamine-induced loss of barrier integrity in the corneal endothelium (Srinivas et al., 2004; Srinivas et al., 2006; Srinivas, 2010).
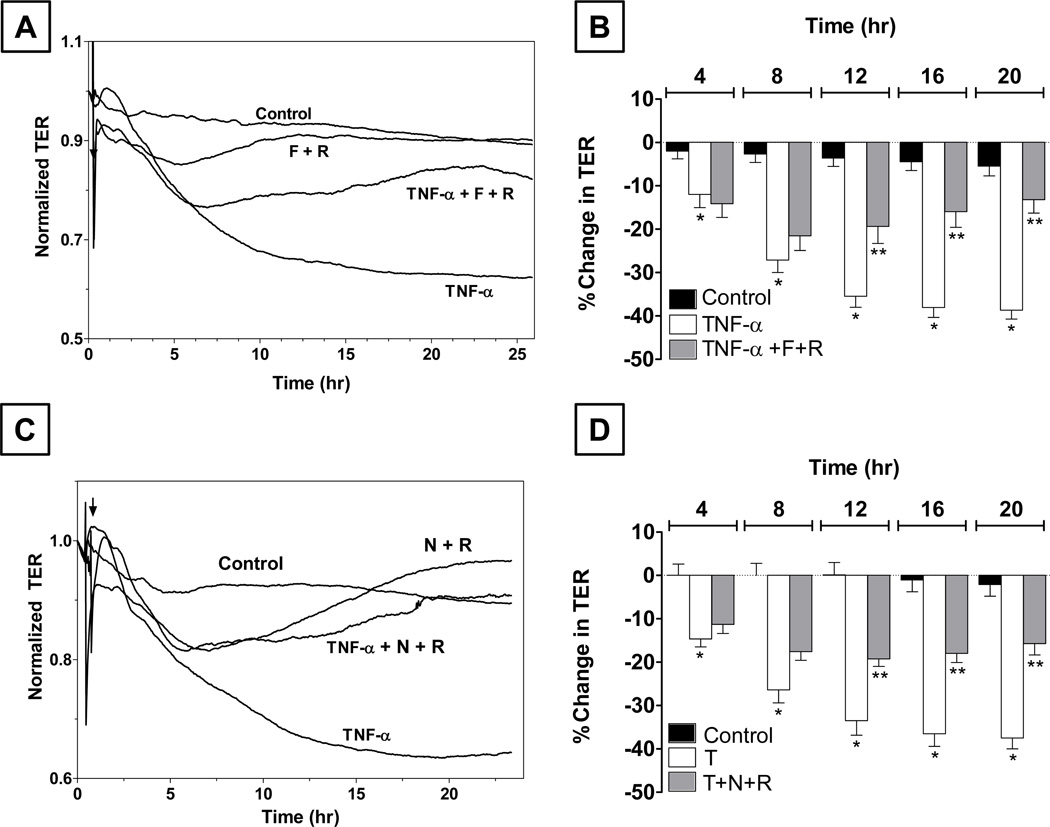
In contrast to these findings, cAMP levels are reportedly reduced in response to TNF-α through an upregulation of cAMP-dependent phosphodiesterases (PDE) activities. Furthermore, there are many bioactive factors, including adrenomedullin and vasoactive intestinal peptide (VIP), in the aqueous humor that have the potential to increase cAMP in the corneal endothelium. As we showed earlier, the endothelium also expresses adenosine-sensitive A2B receptors, which are coupled to Gs G-protein (Srinivas et al., 2004). Given these considerations, we recently examined the effect of elevated cAMP in the corneal endothelium in response to TNF-α (Shivanna and Srinivas, 2010). As shown in Figs. 6A and 6B, we observed that co-treatment with forskolin (a direct activator of adenylate cyclase; 10 µM) and rolipram (a selective inhibitor of a cAMP-dependent PDE4; 50 µM) prevented the (TNF-α)-induced sustained decrease in TER. Similar co-treatments also inhibited the (TNF-α)-induced activation of p38 MAP kinase, disassembly of microtubules, and disruption of the PAMR (Shivanna and Srinivas, 2010). We also confirmed these responses with the use of A2B agonists (e.g., adenosine and NECA) to elevate cAMP as shown in Figs. 6C and 6D. These findings indicate a cross-talk between cAMP and p38 MAP kinase that is yet to be understood.
Figure 6. Effect of elevated cAMP on the (TNF-α)-induced response in bovine corneal endothelial monolayers.
Panel A: Co-treatment with 10 µM forskolin (F) and 50 µM rolipram (R) opposes the decline in TER induced by TNF-α. Panel B: Summary of experiments (n = 6) similar to that shown in Panel A. The % reduction in TER induced by the cytokine is greater than control after 8 hrs of exposure. Co-treatment opposes the (TNF-α)-induced reduction in TER after 12 hrs of exposure. * and ** denote p < 0.001 when comparing the (TNF-α)-treated group with the control group and (TNF-α+F+R)-treated group with the (TNF-α)-treated group, respectively. Panel C: Co-treatment with 20 µM NECA (N) and 50 µM R opposes the decline in TER induced by TNF-α. Panel D: Summary of experiments (n = 6) similar to that shown in Panel C. The % reduction in TER induced by the cytokine is greater than control after 8 hrs of exposure. Co-treatment opposes the (TNF-α)-induced reduction in TER after 12 hrs of exposure. * and ** denote p<0.001 when comparing the (TNF-α)-treated group with the control group and (TNF-α + N+R)-treated group with the (TNF-α)-treated group, respectively. Results summarized from our previous publication (Shivanna and Srinivas, 2010).
Summary and Conclusions
The barrier integrity of the corneal endothelium complements its fluid transport activity by maintaining stromal deturgescence, which is essential for corneal transparency. The barrier integrity, dependent on the TJs, is closely linked to the plasticity of the actin cytoskeleton and microtubules. Extracellular stresses involving cytokines and neurohormonal stimuli mobilize the activity of small GTPases of Rho family and/or stress kinases (e.g., p38 MAP kinase) and, in turn, produce actin remodeling, actomyosin contraction, and/or microtubule disassembly. These changes enable remodeling of the apical junctional complex, leading to changes in the barrier integrity, cell-cell adhesion, and intercellular communication (D'Hondt et al., 2007; Ponsaerts et al., 2008). Therefore, the endothelial barrier integrity is susceptible to extracellular stresses. We have highlighted these aspects with TNF-α, a pro-inflammatory cytokine found in the aqueous humor during allograft rejection and anterior uveitis. Our findings have shown that the response to the cytokine is set forth with an acute activation of p38 MAP kinase, leading to a disassembly of microtubule and breakdown of the barrier integrity. We have also demonstrated that elevated cAMP, which is known to stimulate fluid transport through enhanced ion transport, also opposes the TNF-α response. These results will be helpful in finding potential strategies to rescue, prevent, and overcome loss of barrier integrity of the corneal endothelium.
Highlights.
Barrier integrity of the endothelium is critical for stromal hydration control
Cell signaling, via altered cytoskeleton, regulated barrier integrity.
TNF-alpha breaks down the barrier integrity via activation of p38 MAP kinase
Acknowledgements
I would like to thank all my collaborators and students for their contributions. Research grants from the Office of the Vice President for Research, Indiana University, Bloomington. I thank Dr. Nancy Joyce, Ph.D., for organizing this review series and also for her friendship and encouragement for many years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44(8):3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204(3):934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004;18(15):1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003;22(1):69–94. doi: 10.1016/s1350-9462(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171(11):6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hondt C, Srinivas SP, Vereecke J, Himpens B. Adenosine opposes thrombin-induced inhibition of intercellular calcium wave in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48(4):1518–1527. doi: 10.1167/iovs.06-1062. [DOI] [PubMed] [Google Scholar]
- Diamond JM, Bossert WH. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Garcia JM. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Edelhauser HF. The balance between corneal transparency and edema: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2006;47(5):1754–1767. doi: 10.1167/iovs.05-1139. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi Y, Hermann J. Anti-tumor necrosis factor-alpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology. 2002;109(12):2342–2346. doi: 10.1016/s0161-6420(02)01292-7. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP, Iserovich P, Rubashkin A. The Role of the Tight Junction in Paracellular Fluid Transport across Corneal Endothelium. Electro-osmosis as a Driving Force. J Membr Biol. 2006;210(2):117–130. doi: 10.1007/s00232-005-0850-8. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163(3):510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- George AJ, Larkin DF. Corneal transplantation: the forgotten graft. Am J Transplant. 2004;4(5):678–685. doi: 10.1111/j.1600-6143.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Campbell-Washington J. TNF-alpha induces endothelial cell F-actin depolymerization, new actin synthesis, and barrier dysfunction. Am J Physiol. 1993;264(4 Pt 1):C894–C905. doi: 10.1152/ajpcell.1993.264.4.C894. [DOI] [PubMed] [Google Scholar]
- Jalimarada SS, Shivanna M, Kini V, Mehta D, Srinivas SP. Microtubule disassembly breaks down the barrier integrity of corneal endothelium. Exp Eye Res. 2009;89(3):333–343. doi: 10.1016/j.exer.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen-Soriano S, Sancho-Tello M, Arnal E, Diaz-Llopis M, Navea A, Miranda M, Bosch-Morell F, Romero FJ. Comparison of the acute effects of anti-TNF-alpha drugs on a uveitis experimental model. Ocul Immunol Inflamm. 2010;18(3):208–215. doi: 10.3109/09273940903521964. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22(3):359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Maurice D. The cornea and sclera. San Diego: Academic Press; [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32(12):1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- Noske W, Fromm M, Levarlet B, Kreusel KM, Hirsch M. Tight junctions of the human corneal endothelium: morphological and electrophysiological features. Ger J Ophthalmol. 1994;3(4–5):253–257. [PubMed] [Google Scholar]
- Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28(5):574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- Ponsaerts R, D'Hondt C, Bultynck G, Srinivas SP, Vereecke J, Himpens B. The myosin II ATPase inhibitor blebbistatin prevents thrombin-induced inhibition of intercellular calcium wave propagation in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49(11):4816–4827. doi: 10.1167/iovs.07-1533. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Patil RV, Sharif NA, Srinivas SP. Effect of elevated intracellular cAMP levels on actomyosin contraction in bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(3):1474–1485. doi: 10.1167/iovs.10-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Satpathy M, Mehta D, Srinivas SP. Forskolin induces myosin light chain dephosphorylation in bovine trabecular meshwork cells. Curr Eye Res. 2008;33(2):169–176. doi: 10.1080/02713680701837067. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Srinivas SP. Actomyosin Contraction Regulates Formation and Disassembly of Adherens and Tight Junctions in the Corneal Endothelium. Invest Ophthalmol. 2009 doi: 10.1167/iovs.09-4421. (Accepted, In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner SA, King WJ, Comer RM, Isaacs JD, Hale G, George AJ, Larkin DF. Local bioactive tumour necrosis factor (TNF) in corneal allotransplantation. Clin Exp Immunol. 2000;122(1):109–116. doi: 10.1046/j.1365-2249.2000.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner SA, Larkin DF, George AJ. TNF receptor secretion after ex vivo adenoviral gene transfer to cornea and effect on in vivo graft survival. Invest Ophthalmol Vis Sci. 2001;42(7):1568–1573. [PubMed] [Google Scholar]
- Riley M. Pump and leak in regulation of fluid transport in rabbit cornea. Curr Eye Res. 1985;4(4):371–376. doi: 10.3109/02713688509025150. [DOI] [PubMed] [Google Scholar]
- Riley MV, Winkler BS, Starnes CA, Peters MI, Dang L. Regulation of corneal endothelial barrier function by adenosine, cyclic AMP, and protein kinases. Invest Ophthalmol Vis Sci. 1998;39(11):2076–2084. [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Jin Y, Srinivas SP. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp Eye Res. 2005;81(2):183–192. doi: 10.1016/j.exer.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79(4):477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Shivanna M, Rajashekhar G, Srinivas SP. Barrier dysfunction of the corneal endothelium in response to TNF-alpha: role of p38 MAP kinase. Invest Ophthalmol Vis Sci. 2010;51(3):1575–1582. doi: 10.1167/iovs.09-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna M, Srinivas SP. Microtubule stabilization opposes the (TNF-alpha)-induced loss in the barrier integrity of corneal endothelium. Exp Eye Res. 2009;89(6):950–959. doi: 10.1016/j.exer.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna M, Srinivas SP. Elevated cAMP opposes (TNF-alpha)-induced loss in the barrier integrity of corneal endothelium. Mol Vis. 2010;16:1781–1790. [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Srinivas SP. Dynamic regulation of barrier integrity of the corneal endothelium. Optom Vis Sci. 2010;87(4):E239–E254. doi: 10.1097/OPX.0b013e3181d39464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas SP, Satpathy M, Gallagher P, Lariviere E, Van Driessche W. Adenosine induces dephosphorylation of myosin II regulatory light chain in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79(4):543–551. doi: 10.1016/j.exer.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Srinivas SP, Satpathy M, Guo Y, Anandan V. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47(9):4011–4018. doi: 10.1167/iovs.05-1127. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89(17):7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsky MA, Guan Z, Ragsdale DN. Effect of tumor necrosis factor alpha on rabbit corneal endothelial permeability. Invest Ophthalmol Vis Sci. 1996;37(9):1924–1929. [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]