Abstract
Protease activated receptor-2 (PAR-2) derived cycloxygenase-2 (COX-2) was recently implicated in a cardiac mast cell and fibroblast cross-talk signaling cascade mediating myocardial remodeling secondary to mechanical stress. We designed this study to investigate in vitro assays of isolated adult cardiac fibroblasts to determine whether binding of tryptase to the PAR-2 receptor on cardiac fibroblasts will lead to increased expression of COX-2 and subsequent formation of the arachodonic acid metabolite 15-d-Prostaglandin J2 (15-d-PGJ2). The effects of tryptase (100 mU) and co-incubation with PAR-2 inhibitor peptide sequence FSLLRY-NH2 (10-6M) on proliferation, hydroxyproline concentration, 15-d-PGJ2 formation and PAR-2/COX-2 expression were investigated in fibroblasts isolated from 9 week old SD rats. Tryptase induced a significant increase in fibroproliferation, hydroxyproline, 15-d-PGJ2 formation and PAR-2 expression which were markedly attenuated by FSLLRY. Tryptase-induced changes in cardiac fibroblast function utilize a PAR-2 dependent mechanism.
Keywords: COX-2, Prostaglandin, PAR-2, FSLLRY
Introduction
Recent studies have demonstrated that tryptase, one of the more abundant enzymes contained within mast cells, has modulatory effects on the phenotype of neighboring cells including fibroblasts in the kidney, testis, and lung (Somasundaram et al. 2005; Frungieri et al. 2005; Skrabal et al. 2004; de Almeida et al. 2002; Levi-Schaffer and Piliponsky 2003; Gailit et al. 2001). Tryptase is a ligand for the protease activated receptor-2 (PAR-2) isoform of the protease ligand class of surface receptors (for review see Steinberg 2005). PAR-2 activation, which is upstream of COX-2 expression, has been shown to possess acute cardioprotective effects capable of limiting infarct size (McLean et al. 2002; Jiang et al. 2007). Contrastingly, COX-2 inhibitor prevention studies in a coronary artery ligation model of ischemia and reperfusion as well as in an isoproterenol model of myocardial damage reported less apoptosis, decreased left ventricular (LV) hypertrophy, and reduced interstitial collagen. This findings suggest that COX-2 enzymatic activity plays a deleterious role in cardiac remodeling and function secondary to increased stress and myocardial injury (LaPointe et al. 2004; Abbate et al. 2007; Saeed and Ahmed 2006).
While PAR-2 has a wide tissue distribution, myocardial PAR-2 protein expression has only been documented in whole tissue extract (Ossovskaya and Bunnett 2004; Macfarlane et al. 2001). Additionally, Maree and Fitzgerald (2002) noted that the overall contribution of PAR-2 activation in acute ischemic injury may not be the same as in injury following non ischemic chronic pathologic stimuli, and it is not known if PAR-2 is expressed in adult cardiac fibroblasts. Moreover, a recent study by Papanicolaou et al. (2010) using cardiac specific transgenic mice (COX-2 CKO) demonstrated that the predominant source of COX-2 expression was from the resident non-myocyte cell population. Therefore, it remains to be determined if this mast cell/fibroblast cross talk signaling pathway exists in the myocardium. If it does its mediatory role in the remodeling ventricle has yet to be fully elucidated. Accordingly, we designed this study to integrate in vitro assays of freshly isolated adult cardiac fibroblasts to test the hypothesis that activation via tryptase of the PAR-2 receptor on cardiac fibroblasts will lead to increased expression of COX-2 and subsequent formation of the arachidonic acid metabolite 15-deoxy-Prostaglandin J2 (15-d-PGJ2).
Results
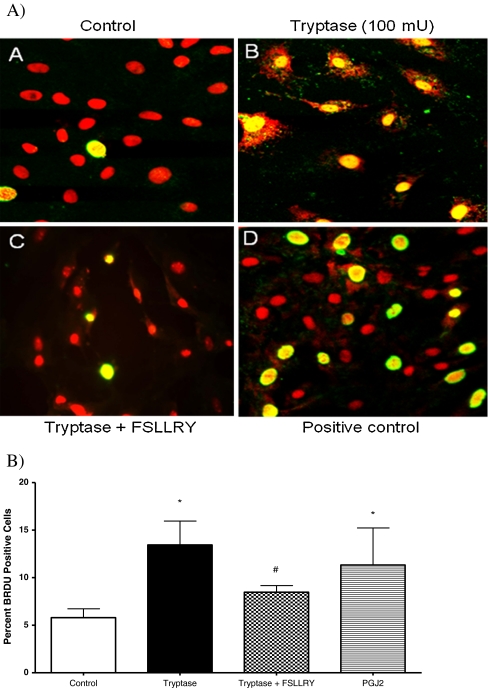
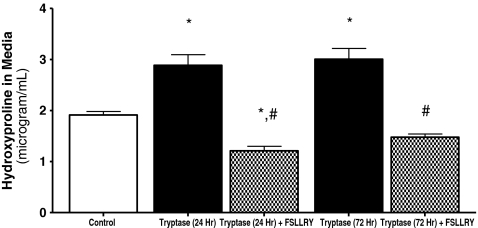
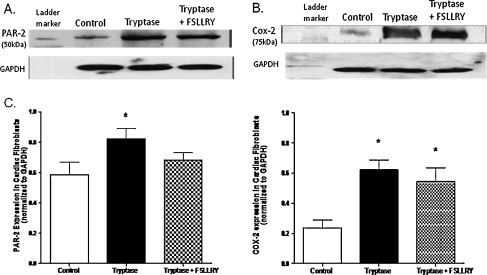
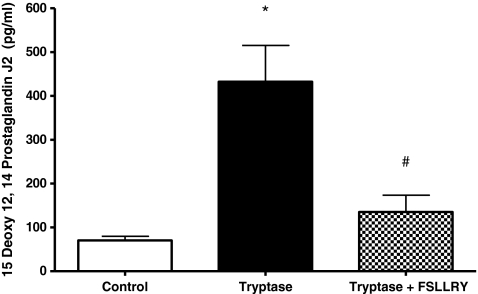
In vitro adult cardiac fibroblast response Experiments were performed to assay the effects of tryptase on cardiac fibroblast proliferation. Fibroblasts were cultured on collagen-coated coverslips and treated with control (1.5% FBS), tryptase (100 mU), tryptase + PAR-2 antagonist peptide sequence (FSLLRY; 10−6 M) or the COX-2 downstream enzymatic product 15-d-PGJ2 (10−6 M) medium, and BrdU incorporation was evaluated. Fibroblasts were simultaneously labeled with picrosirius red and examined by fluorescent microscopy for the detection of collagen. Figure 1 (Panel B) and Fig. 1B illustrate that fibroblast proliferation was increased significantly (∼50%) above control in cultured adult cardiac fibroblasts cells treated with tryptase. However, the presence of FSLLRY significantly inhibited this tryptase effect (Fig. 1 (Panel C) and Fig. 1B), while incubation with 15-d-PGJ2 was used as a positive control (Fig. 1 Panel D). Cell viability was not altered by continued tryptase exposure over 48, 72 or 96 h (97 ± 0.5%, 98 ± 0.4%, or 97 ± 0.9% respectively) relative to control (96 ± 0.4%) when assayed by trypan blue exclusion. Consistent with our findings from BrdU incorporation, we observed a marked 27% increase in cell number at the 48 h time point compared to nonstimulated controls. However, this response was not evident at the longer exposure time points. Next we evaluated the overall concentration of hydroxyproline in the media as an indicator of secreted collagen. Figure 2 indicates that the trypase-induced increase in hydroxyproline concentration in media from cultured fibroblasts at 24 h was sustained for 72 h. Co-incubation with FSLLRY prevented the tryptase-induced increase in hydroxyproline at both time points even to a level significantly less than control at 24 h. Western Blot evaluation of protein extracts from isolated adult cardiac fibroblasts from the second passage demonstrated that PAR-2 was expressed in these cells under non stimulated conditions (Fig. 3a and c). At 72 h, PAR-2 expression was significantly increased by administration of tryptase. This effect was not seen with PAR-2 antagonist peptide sequence co-incubation. Moreover, COX-2 expression, also evident in control cells, was significantly increased in either tryptase or tryptase + FSLLRY treated cells at 72 h (Fig. 3b and c). Lastly, we analyzed 15-d-PGJ2 levels via ELISA in the cultured fibroblast media following 24 h incubation with the same dose of tryptase previously used for the fibroblast proliferation studies. While there was a basal level of 15-d-PGJ2 in the media of unstimulated control adult fibroblasts, there was a significant 4- to 5–fold tryptase-induced increase in the formation of 15-d-PGJ2. The use of a PAR-2 antagonist, FSLLRY, prevented the tryptase-induced increase in 15-d-PGJ2 (Fig. 4).
Fig. 1.
A) To determine the effect of tryptase and PGJ2 on isolated adult cardiac fibroblast proliferation, BrdU incorporation was assayed immunocytochemically. Representative photographs of untreated fibroblasts (Panel A) and fibroblasts treated with tryptase (100 mU, Panel B), tryptase + FSRLLY(Panel C), and 15-d-PGJ2 (10−6 M, Panel D), and counter stained with picrosirius red. B) Graphic illustration of the percent of BrdU-positive cells. Data represent five independent experiments.* denotes p ≤ 0.05 vs. control; # denotes p < 0.05 vs. tryptase
Fig. 2.
Collagen deposition from the media of isolated adult cardiac untreated fibroblasts (control) and fibroblasts treated with tryptase (100 mU) or tryptase + FSLLRY for 24 & 72 h was evaluated by hydroxyproline assay. Values are the average of three replicates from four separate hearts and presented as mean ± SEM. * denotes p ≤ 0.05 vs. control. # denotes p ≤ 0.05 vs. tryptase
Fig. 3.
A) Representative Western blot for PAR-2 expression in isolated adult cardiac fibroblasts treated for 24 h with either; control, tryptase (100 mU), or tryptase + FSLLRY (PAR-2 receptor antagonist; 10−6 M) media. B) Representative Western blot for COX-2 expression in isolated adult cardiac fibroblasts treated for 24 h with either; control, tryptase (100 mU), or tryptase + FSLLRY (PAR-2 receptor antagonist; 10−6 M) media. C) Densitometric analysis of PAR-2 and COX-2 expression in control, tryptase, tryptase + FSLLRY treated isolated cardiac fibroblasts. Values normalized to GAPDH expression and expressed as mean ± SEM. * denotes p ≤ 0.05 vs. control
Fig. 4.
ELISA analysis of 15 deoxy 12, 14 Prostaglandin J2 formation in media from isolated adult cardiac untreated fibroblasts (control) and fibroblasts treated for 24 h with tryptase (100 mU) and tryptase + FSLLRY (PAR-2 antagonist, 10−6 M). * denotes p ≤ 0.05 vs. control. # denotes p ≤ 0.05 vs. tryptase
Discussion
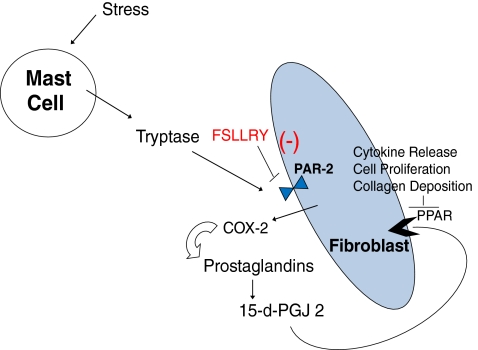
Previous studies have demonstrated that mast cell derived tryptase is a ligand for the protease activated class of surface receptors (PAR) in the heart (Steinberg 2005). The irreversible proteolytic activation of the PAR-2 isoform on fibroblasts stimulates cyclo-oxygenase-2 expression. COX-2 in turn is responsible for the enhanced synthesis of 15-deoxy 12,14 prostaglandin J2 (15-d-PGJ2) among other prostaglandins (i.e. PGI and PGE) (Somasundaram et al. 2005; Frungieri et al. 2005; Levi-Schaffer and Piliponsky 2003; Gailit et al. 2001). It is the PGJ2 arachidonic acid derived molecule that has been shown to act in the kidney, lung, and vasculature as an endogenous ligand activating PPARγ leading to fibroblast proliferation, collagen deposition, and cytokine production (Fig. 5) (Levi-Schaffer and Piliponsky 2003; Frungieri et al. 2002). Recent clinical trials of patients with congestive heart failure demonstrated serious complications associated with the chronic use of certain COX-2 selective inhibitors (i.e. Vioxx and Celecoxib) due to their impact on renal vascular tone (Mukherjee 2008; Hudson et al. 2007). Initially, numerous studies using COX-2 inhibition detailed beneficial structural and functional effects in animal models of ischemia / reperfusion (LaPointe et al. 2004; Abbate et al. 2007; Saeed and Ahmed 2006). Contrastingly, acute ischemic injury studies focused on activation of the upstream inducer of COX-2 expression, PAR-2, demonstrated cardioprotective effects capable of limiting infarct size (McLean et al. 2002; Jiang et al. 2007). However, this response may not be the same under non-ischemic chronic pathologic stimuli (Maree and Fitzgerald 2002). Therefore, this study was undertaken to investigate if this preformed mast cell enzyme/fibroblast surface receptor cross talk signaling pathway exists in the myocardium and its potential mediatory role in the remodeling ventricle.
Fig. 5.
Schematic diagram representing the hypothesis for mast cell mediated tryptase activation of PPAR induced remodeling. Release of mast cell derived tryptase binds the PAR-2 receptor leading to formation of COX2 and subsequent formation of 15-d-PGJ2 leading to binding of the nuclear receptor PPAR. [modified from Levi-Schaffer and Piliponsky (2003)]
In vitro adult cardiac fibroblast response to tryptase Although the findings herein are consistent with the work of others, (Frungieri et al. 2005; Levi-Schaffer and Piliponsky 2003; Frungieri et al. 2002) to the best of our knowledge this is the first time PAR-2 expression and function have been documented in isolated adult cardiac fibroblasts. Tryptase was able to induce a marked increase in the fibroblast expression of PAR-2 which was attenuated by co-incubation with the PAR-2 inhibitor peptide sequence FSLLRY. Additionally, the significant increase in tryptase mediated fibroblast proliferation was significantly less in the presence of FSLLRY. Combining these results with the prevention of collagen deposition by the PAR-2 inhibitor sequence peptide strongly indicates that these tryptase-induced changes in cardiac fibroblast phenotype utilize a PAR-2 dependent mechanism. Lastly, the use of PAR-2 antagonism led to a marked reduction in the amount of 15-d-PGJ2 in the media which we originally hypothesized would be due to decreased COX-2 expression (Fig. 5). Additionally, a previous study by Vichai et al. demonstrated a positive feedback signaling loop via prostaglandins in favor of the expression of COX-2 but not COX-1 in mouse lung fibroblasts (Vichai et al. 2005). Specifically, in that study only PGE2 and 15-d-PGJ2 were shown to induce COX-2 mRNA. However, measurement of COX-2 expression (Fig. 4) demonstrated no difference in tryptase induced COX-2 protein levels co-incubated with PAR-2 antagonism. PAR-2 signaling is mediated by several heterotrimeric G-protein pathways (i.e. G alpha(q), G alpha(i), and G alpha(12/13)) (Ricks and Trejo 2009). Previously, Frungieri MB et al. demonstrated that tryptase induced cell proliferation in human non-cardiac fibroblasts was via a Ca++ and cAMP independent but ERK 1/2 dependent mechanism (Frungieri et al. 2005). Whether this pathway is intact and functional in cardiac fibroblasts remains to be determined and should be investigated in future studies.One limitation of this study is the multiple alternate prostaglandin pathways downstream of COX-2. COX-2 is responsible for generating a common precursor molecule which is then modified by several tissue specific isomerases (synthases) that give rise to the various prostaglandins (i.e. PGI, PGE, PGF, and PGD) (Frungieri et al. 2005; Levi-Schaffer and Piliponsky 2003; Gailit et al. 2001). While the role of PGF in cardiac extracellular matrix remodeling remains unknown, several reports have documented anti-fibroproliferative and anti-fibrotic effects by PGI and PGF (Yu et al. 1997; Xiao et al. 2001; Hishikari et al. 2009; Qian et al. 2008). The direct effects of PGD on cardiac fibroblasts remain uncertain, however, PGD serves as the only source for the formation of PGJ as this line of prostaglandins is a dehydration product of PGD and thus is not regulated by an individual synthase.In summary, tryptase stimulated a significant increase in fibroproliferation, collagen deposition, 15-d-PGJ2 formation and PAR-2 expression which was markedly attenuated by PAR-2 receptor antagonist FSLLRY. These results indicate that the tryptase-induced changes in cardiac fibroblast phenotype utilize a PAR-2 dependent mechanism. The presence of such a non myocyte cardiac cell signaling mechanism in the heart creates multiple sites for potential innovative therapeutic strategies.
Materials and methods
Animal welfare All experiments were performed using 9 week old (200–250 g) male Sprague-Dawley rats housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and were approved by our Institution’s Animal Care and Use Committee. Anesthesia for the experimental procedure was affected by sodium pentobarbital (50 mg/kg, i.p.).
Experimental design
Experimental groups Treatment groups consisted of: Control (1.5% FBS), Tryptase (100 mU; Promega,), Tryptase + PAR-2 antagonist peptide sequence (FSLLRY; 10−6 M) or 15-deoxy –Δ12, 14 –PGJ 2 (PGJ2, 10−6 M) from Cayman Chemical, Ann Arbor, MI. The selective PAR-2 antagonist (H-Phe-Ser-Leu-Leu-Arg-Tyr-NH2) (Al-Ani et al. 2002; Wilson et al. 2005), FSLLRY-amide (Peptides International, Louisville, KY), was dissolved in dH2O. Drug treated control studies were also performed. However, no differences were observed relative to control values (data not shown).
Cardiac fibroblast collagen synthesis Cardiac fibroblasts were obtained from adult male Sprague-Dawley rats (n = 5). Briefly, hearts were minced and digested with Liberase 3 (Roche) and fibroblast purified by selective attachment to plastic culture ware. The fibroblasts were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% neonatal bovine serum and 5% fetal calf serum, with media replacement every other day. All fibroblasts were used in passage number 2, since adult fibroblasts have been shown to maintain their phenotype through the initial 5 passages (Burgess et al. 2002; Baudino et al. 2006). One million fibroblasts were seeded on 100 mm plastic culture plates. Cells were allowed to adhere to the plate in DMEM with 10% neonatal bovine serum and 5% fetal calf serum for 24 h, before being rinsed with Mosconas Salt Solution. Media was replaced with DMEM-F12 with no serum for 24 h and rinsed again with Mosconas Salt Solution then replaced with DMEM containing 1.5% fetal bovine serum and 100 mU/mL of recombinant tryptase (Promega®) and incubated for 24 & 72 h. At the completion of the experimental period, media was collected and snap frozen. Fibroblast collagen synthesis in response to tryptase was determined by hydroxyproline analysis of collected media as described by Edwards and O’Brien (1980). Briefly, 6N HCL was added to all samples in a 1:1 ratio and the samples maintained at 108°C overnight before filtering and vacuum centrifugation. The samples were then reconstituted in citrate buffer (0.2 M) and reacted with chloramine-T for 20 min, followed by incubation with aldehyde for another 20 min, before cooling in an ice-water bath. Absorbance was read at 550 nm and compared to hydroxyproline standards ranging from 0 to 10 μg/mL.
Fibroblast proliferation Fibroblasts were isolated as described above and grown on coverslips until 50–60% confluent for proliferation studies. The cells were then incubated with tryptase and 5-Bromo-2′deoxy-uridine (BrdU, Roche) labeling reagent added to the medium under sterile conditions for the final 4 h of the 24 h treatment period. The culture medium was then aspirated and the cells rinsed 3 times with washing buffer before fixation with ethanol/glycine for 1 h at −20°C. Anti-BrdU mouse IgG antibody was added to the cells and incubated for 1 h at 37°C. Following washing, FITC-conjugated anti-mouse secondary antibody was added to the cells for 1 h at 37°C. Propidium Iodide (Molecular Probes™) served as a nuclear marker of all cells. The cells were mounted and examined for BrdU incorporation using a Biorad MRC-1024 confocal laser-scanning microscope at 400X magnification. Data are presented as the number of BrdU-positive cells relative to the total number of cells (% labeled). Fibroblasts were simultaneously labeled with picrosirius red and examined by fluorescent microscopy for the detection of collagen. Fifteen random fields were quantified from each of five pairs of coverslips treated with control (1.5% FBS), tryptase (100 mU; Promega,), tryptase + PAR-2 antagonist peptide sequence (FSRLLY; 10−6 M) or 15-deoxy –Δ12, 14 –PGJ 2 (PGJ2, 10–6 M) from Cayman Chemical, Ann Arbor, MI.
Western blot analysis SDS-denatured myocardial protein extract samples were subjected to electrophoresis in 10% (w/v) polyacrylamide gels. Briefly, nitrocellulose membranes containing the material transferred from the polyacrylamide gel were incubated with 3% (w/v) gelatin in 0.02 M Tris, pH 7.4, 0.5 M NaCl (TBS) for two 30-minperiods to block any nonspecific reactivity. Appropriate dilutions of monoclonal or polyclonal antibody (COX-2 and PAR-2, Abcam®, Cambridge, MA and Santa Cruz® respectively) was applied and incubated overnight at room temperature. Excess antibody was removed by sequentially washing the membranes in TBS containing 3% gelatin and then incubated with alkaline phosphatase-conjugated goat anti-mouse IgG at room temperature for 2 h. After the second antibody step, membranes were sequentially washed and developed with enhanced chemiluminescense (Amersham) and exposure to hyperfilm (Kodak). The membrane was then stripped and reprobed for GAPDH. Once normalized in this fashion, the differences in protein expression from hearts belonging to each group were averaged.
Enzyme-linked immunosorbent assay 15-deoxy-delta 12, 14-Prostaglandin J2 levels in media from the cultured fibroblasts were analyzed using commercially available kits (Stressgen, Ann Arbor, MN). Briefly, precoated wells were treated with assay buffer and 100 μl of extracted protein sample for 1 h, and washed 5 times with wash buffer. Wells were then incubated with appropriate monoclonal antibody, washed again followed by HRP conjugated antibody and read by spectrophotometry at 650 nm, according to manufacturer’s specifications.
Statistical analysis Statistical analyses were performed with Graphpad Prism 5.0 (GraphPad Software, Inc. San Diego, CA). All grouped data are expressed as means ± SEM. Grouped data comparisons were made by one-way analysis of variance with intergroup comparisons analyzed using Bonferroni post-hoc testing. Statistical significance was taken to be p ≤ 0.05.
Acknowledgments
We are grateful to Andrew Barnes and Rebecca Nolan for their technical assistance.
Disclosure We have no conflicts of interest to declare in regards to relationships (inclusive of but not limited to, employment by an industrial concern, ownership of stock, membership on a standing advisory council or committee, being on the board of directors, or being publicly associated with the company or its products) with pharmaceutical companies, biomedical device manufacturers, or other corporations whose products or services related to the subject matter of this article.
Author contribution DBM conceived of the study, and participated in its design and coordination, and performed the statistical analysis and drafted the manuscript. JM carried out the cell culture studies, immunohistochemistry and performed statistical analysis. JSJ was involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Funding sources This study was supported by NIH Heart, Lung and Blood Institute Grant RO1-HL-62228 (jsj), University of South Carolina Magellan Award (dbm) and University of South Carolina Center of Biomedical Research Excellence (COBRE) Cardiovascular Diseases (dbm).
References
- Abbate A, Salloum FN, Ockaili RA, Fowler AA, III, Biondi-Zoccai GG, Straino S, et al. Improvement of cardiac function with parecoxib, a cyclo-oxygenase-2 inhibitor, in a rat model of ischemic heart failure. J Cardiovasc Pharmacol. 2007;49:416–418. doi: 10.1097/FJC.0b013e31804a5e50. [DOI] [PubMed] [Google Scholar]
- Al-Ani B, Saifeddine M, Wijesuriya SJ, Hollenberg MD. Modified proteinase-activated receptor-1 and −2 derived peptides inhibit proteinase-activated receptor-2 activation by trypsin. J Pharmacol Exp Ther. 2002;300:702–708. doi: 10.1124/jpet.300.2.702. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc Pathol. 2002;11:78–87. doi: 10.1016/S1054-8807(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Almeida A, Mustin D, Forman MF, Brower GL, Janicki JS, Carver W. Effects of mast cells on the behavior of isolated heart fibroblasts: modulation of collagen remodeling and gene expression. J Cell Physiol. 2002;191:51–59. doi: 10.1002/jcp.10071. [DOI] [PubMed] [Google Scholar]
- Edwards CA, O’Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: possible relevance to human fibrotic disorders. Proc Natl Acad Sci USA. 2002;99:15072–15077. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungieri MB, Albrecht M, Raemsch R, Mayerhofer A. The action of the mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fibroblast proliferation involves activation of the extracellular signal-regulated kinase isoforms 1 and 2 (erk1/2) Cell Signal. 2005;17:525–533. doi: 10.1016/j.cellsig.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Investig Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- Hishikari K, Suzuki J, Ogawa M, Isobe K, Takahashi T, Onishi M, et al. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2009;81:123–132. doi: 10.1093/cvr/cvn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M, Rahme E, Richard H, Pilote L. Risk of congestive heart failure with nonsteroidal anti-inflammatory drugs and selective Cyclooxygenase 2 inhibitors: a class effect? Arthritis Rheum. 2007;57:516–523. doi: 10.1002/art.22614. [DOI] [PubMed] [Google Scholar]
- Jiang R, Zatta A, Kin H, Wang N, Reeves JG, Mykytenko J, et al. PAR-2 activation at the time of reperfusion salvages myocardium via an ERK1/2 pathway in in vivo rat hearts. Am J Physiol Heart Circ Physiol. 2007;293:H2845–H2852. doi: 10.1152/ajpheart.00209.2007. [DOI] [PubMed] [Google Scholar]
- LaPointe MC, Mendez M, Leung A, Tao Z, Yang XP. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol. 2004;286:H1416–H1424. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Piliponsky AM. Tryptase, a novel link between allergic inflammation and fibrosis. Trends Immunol. 2003;24:158–161. doi: 10.1016/S1471-4906(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Maree A, Fitzgerald D. PAR2 is partout and now in the heart. Circ Res. 2002;90:366–368. doi: 10.1161/01.RES.0000012906.85785.6A. [DOI] [PubMed] [Google Scholar]
- McLean PG, Aston D, Sarkar D, Ahluwalia A. Protease-activated receptor-2 activation causes EDHF-like coronary vasodilation: selective preservation in ischemia/reperfusion injury: involvement of lipoxygenase products, VR1 receptors, and C-fibers. Circ Res. 2002;90:465–472. doi: 10.1161/hh0402.105372. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. Nonsteroidal anti-inflammatory drugs and the heart: what is the danger? Congest Heart Fail. 2008;14:75–82. doi: 10.1111/j.1751-7133.2008.07453.x. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, Streicher JM, Ishikawa TO, Herschman H, Wang Y, Walsh K. Preserved heart function and maintained response to cardiac stresses in a genetic model of cardiomyocyte-targeted deficiency of cyclooxygenase-2. J Mol Cell Cardiol. 2010;49:196–209. doi: 10.1016/j.yjmcc.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian JY, Harding P, Liu Y, Shesely E, Yang XP, Lapointe MC. Reduced cardiac remodeling and function in cardiac-specific EP4 receptor knockout mice with myocardial infarction. Hypertension. 2008;51:560–566. doi: 10.1161/HYPERTENSIONAHA.107.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks TK, Trejo J. Phosphorylation of protease-activated receptor-2 differentially regulates desensitization and internalization. J Biol Chem. 2009;284:34444–34457. doi: 10.1074/jbc.M109.048942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed SA, Ahmed S. Anti-ischemic effects of nimesulide, a cyclooxygenase-2 inhibitor on the ischemic model of rabbit induced by isoproterenol. Arch Pharm Res. 2006;29:977–983. doi: 10.1007/BF02969281. [DOI] [PubMed] [Google Scholar]
- Skrabal CA, Thompson LO, Southard RE, Joyce DL, Noon GP, Loebe M, et al. Interaction between isolated human myocardial mast cells and cultured fibroblasts. J Surg Res. 2004;118:66–70. doi: 10.1016/j.jss.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, et al. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–111. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- Vichai V, Suyarnsesthakorn C, Pittayakhajonwut D, Sriklung K, Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm Res. 2005;54:163–172. doi: 10.1007/s00011-004-1338-1. [DOI] [PubMed] [Google Scholar]
- Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, et al. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J. 2005;388:967–972. doi: 10.1042/BJ20041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CY, Hara A, Yuhki K, Fujino T, Ma H, Okada Y, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- Yu H, Gallagher AM, Garfin PM, Printz MP. Prostacyclin release by rat cardiac fibroblasts: inhibition of collagen expression. Hypertension. 1997;30:1047–1053. doi: 10.1161/01.hyp.30.5.1047. [DOI] [PubMed] [Google Scholar]