Abstract
Purpose
This analysis from an observational study of clinical practice describes the impact of febrile neutropenia (FN) on chemotherapy delivery and hospitalizations.
Methods
Adults with diffuse large B-cell lymphoma (DLBCL) scheduled to receive ≥3 cycles of 2- or 3-weekly CHOP with rituximab (R-CHOP-14/21) were eligible. Primary outcome was incidence of FN.
Results
FN data were available for 409 patients receiving R-CHOP-14 and 702 patients receiving R-CHOP-21. FN incidence was R-CHOP-14, 20% (81/409) and R-CHOP-21, 19% (133/702). Rates of primary prophylaxis with granulocyte-colony stimulating factor were R-CHOP-14, 84% (345/409) and R-CHOP-21, 36% (252/702). A large number of patients experienced their first FN episode in cycle 1 (R-CHOP-14, 24/81 [30%]; R-CHOP-21, 63/133 [47%]). Multiple risk factors (≥2) for FN were more frequent in patients experiencing FN than in patients not experiencing FN (R-CHOP-14, 60/81 [74%] versus 179/328 [55%]; R-CHOP-21, 98/133 [74%] versus 339/569 [60%]). A similar trend was observed for unplanned hospitalizations (R-CHOP-14, 63/81 [78%] versus 68/328 [21%]; R-CHOP-21, 105/133 [79%] versus 100/569 [18%]). Achievement of chemotherapy relative dose intensity ≥90% was lower among patients experiencing FN than in patients not experiencing FN (R-CHOP-14, 30/81 [37%] versus 234/328 [71%]; R-CHOP-21, 83/133 [62%] versus 434/569 [76%]).
Conclusions
In patients with DLBCL treated with R-CHOP-14 or R-CHOP-21, patients with an event of FN were more likely to experience suboptimal chemotherapy delivery and increased incidence of unplanned hospitalizations than those without FN. FN-related hospitalizations are likely to impact chemotherapy delivery and to incur substantial costs.
Keywords: Diffuse large B-cell lymphoma, R-CHOP, Febrile neutropenia, Chemotherapy dose intensity, G-CSF, Prophylaxis
Introduction
In patients with aggressive non-Hodgkin lymphoma (NHL), reduced chemotherapy delivery has been associated with impaired outcomes [20]. Survival may be significantly decreased when such patients receive 3-weekly CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone; CHOP-21) chemotherapy delivered at less than 90% of the planned relative dose intensity (RDI) [3, 15]. Moreover, due to myelotoxicity, patients receiving CHOP regimens often experience febrile neutropenia (FN), which can impede and reduce chemotherapy delivery, particularly if hospitalization is required [16].
International guidelines recommend primary prophylaxis with granulocyte-colony stimulating factor (PP G-CSF) for patients scheduled to receive CHOP-21 and rituximab (R-CHOP-21) who are assessed as being at high risk (≥20%) of experiencing FN and to allow effective delivery of 2-weekly regimens such as R-CHOP-14 [1, 18]. The myelotoxic potential of the planned chemotherapy regimen and known patient risk factors for FN, such as older age (≥65 years), advanced disease, poor performance status, bone marrow involvement, baseline hemoglobin <12 g/dL and co-morbidities, should be considered when assessing FN risk for individual patients [1, 12, 14, 18]. This analysis from an observational study of European and Australian clinical practice describes the impact of FN on chemotherapy delivery and hospitalizations in patients with diffuse large B-cell lymphoma (DLBCL) receiving R-CHOP regimens.
Methods
IMPACT NHL is an international, retrospective and prospective, observational, multicenter study conducted to evaluate FN risk assessment and G-CSF prophylaxis use in clinical practice among patients with NHL receiving CHOP. The study enrolled adults (≥18 years) with any histological type of NHL, for whom at least three cycles of CHOP-14 or CHOP-21, with or without rituximab, were planned. The design of this study has been described elsewhere [7, 17].
The present analysis included a subgroup of patients with DLBCL who received R-CHOP-14 or R-CHOP-21 and for whom FN data were available. In NHL, DLBCL represents the most common histological tumor type routinely treated with R-CHOP regimens.
FN was defined as:
A single oral temperature ≥38.3°C or
A temperature ≥38.0°C for ≥1 h with a neutrophil count of ≤0.5 × 109/L or a neutrophil count ≤1.0 × 109/L which was predicted to fall below 0.5 × 109/L
The proportion of patients experiencing FN during all chemotherapy cycles and during cycle 1 was summarized by R-CHOP regimen. In this report, patients who experienced FN will be referred to as the “FN” group, and patients who did not experience FN referred to as the “No FN” group.
G-CSF use was also summarized by R-CHOP regimen. PP was defined as initiation of G-CSF within days 1–7 of chemotherapy cycle 1 (or within days 1–11 if chemotherapy was dosed beyond day 7). Secondary prophylaxis (SP) was defined as per PP, but with G-CSF initiated in cycles 2 or later.
The proportion of patients experiencing the following outcome measures was summarized by the R-CHOP regimen and FN group:
Chemotherapy dose reductions ≥10% (in cyclophosphamide or doxorubicin) in any cycle
Dose delays >3 days (for patients who received at least two chemotherapy cycles)
Proportion of patients achieving chemotherapy RDI ≥ 90% during all cycles
Unplanned hospitalizations
RDI was defined as the actual dose intensity achieved by a patient relative to their planned dose intensity for their intended chemotherapy schedule (i.e., R-CHOP-14 or R-CHOP-21) and was calculated as follows: RDI (%) = actual dose intensity / planned dose intensity; actual dose intensity = actual dose received for agent (mg/m2) / actual total length of chemotherapy for all agents (days), planned dose intensity = planned total dose for agent (mg/m2) / planned length of chemotherapy for all agents (days). Vincristine, prednisone, and rituximab were excluded from the calculation of dose reductions and RDI as the dose intensities delivered for these agents are unlikely to be affected by neutropenia. An unplanned hospitalization was defined as any unplanned hospitalization involving an overnight stay.
The 95% exact binomial confidence interval (CI) for the incidence of chemotherapy delays, dose reductions, RDI, and unplanned hospitalizations was calculated for each treatment group. All comparisons between treatment groups were descriptive only; no formal hypothesis testing was planned.
Results
In total, 1,111 patients with DLBCL treated between 2005 and 2008, and for whom FN data were available, were included in this analysis. Of these, 409 (37%) received R-CHOP-14 and 702 (63%) received R-CHOP-21.
Six cycles of chemotherapy were planned in half of all patients (R-CHOP-14, 222/409 [54%]; R-CHOP-21, 348/702 [50%]); eight cycles were planned in one third of patients (R-CHOP-14, 131/409 [32%]; R-CHOP-21: 259/702 [38%]); three or four cycles were planned in 13% of patients (R-CHOP-14, 55/409; R-CHOP-21, 88/702). Most patients completed their planned chemotherapy (R-CHOP-14, 357/409 [87%]; R-CHOP-21, 603/702 [86%]). Patients receiving R-CHOP-14 tended to be younger than those receiving R-CHOP-21 and were less likely to have poor Eastern Cooperative Oncology Group performance status scores (Table 1).
Table 1.
Baseline demographics and disease characteristics
| R-CHOP-14 (N = 409) | R-CHOP-21 (N = 702) | |||||
|---|---|---|---|---|---|---|
| FN | No FN | Overall | FN | No FN | Overalla | |
| N = 81 | N = 328 | N = 409 | N = 133 | N = 569 | N = 704 | |
| Age in years, mean ± SD | 61.1 ± 13.1 | 57.7 ± 15.0 | 58.4 ± 14.7 | 65.2 ± 11.8 | 62.0 ± 14.2 | 62.6 ± 13.8 |
| Age ≥65 years, n (%) | 39 (48) | 129 (39) | 168 (41) | 85 (64) | 275 (48) | 361 (51) |
| Female, n (%) | 42 (52) | 129 (39) | 171 (42) | 70 (53) | 265 (45) | 327 (46) |
| BMI in kg/m2, mean ± SD | 25.9 ± 4.4b | 25.4 ± 4.9c | 25.5 ± 4.8 | 25.5 ± 4.9 | 25.7 ± 4.4d | 25.7 ± 4.5 |
| ECOG performance status, n (%) | ||||||
| 0, 1 | 68 (84) | 304 (93) | 372 (91) | 100 (75) | 489 (86) | 591 (84) |
| 2, 3, 4 | 13 (16) | 18 (5) | 31 (8) | 24 (18) | 48 (8) | 72 (10) |
| Missing | 0 (0) | 6 (2) | 6 (1) | 9 (7) | 32 (6) | 41 (6) |
| Ann Arbor stage, n (%) | ||||||
| III | 22 (27) | 73 (22) | 95 (23) | 20 (15) | 117 (21) | 138 (20) |
| IV | 40 (49) | 108 (33) | 148 (36) | 57 (43) | 180 (32) | 237 (34) |
| IPI, n (%) | ||||||
| Low | 14 (17) | 110 (34) | 124 (30) | 26 (20) | 184 (32) | 211 (30) |
| Intermediate | 44 (54) | 166 (51) | 210 (51) | 60 (45) | 223 (39) | 284 (40) |
| High | 18 (22) | 18 (5) | 36 (9) | 26 (20) | 62 (11) | 88 (13) |
| Missing | 5 (6) | 34 (10) | 39 (10) | 21 (16) | 100 (18) | 121 (17) |
| Bone marrow involvement, n (%) | 14 (17) | 41 (13) | 55 (13) | 26 (20) | 83 (15) | 109 (15) |
| History of co-morbidities, n (%) | 48 (59) | 141 (43) | 189 (46) | 80 (60) | 292 (52) | 375 (53) |
| No prior treatmente, n (%) | 72 (89) | 299 (91) | 371 (91) | 122 (92) | 510 (90) | 634 (90) |
| Number of risk factors for FNf, n (%) | ||||||
| 0 | 5 (6) | 54 (16) | 59 (14) | 10 (8) | 93 (16) | 104 (15) |
| 1 | 16 (20) | 95 (29) | 111 (27) | 25 (19) | 137 (24) | 162 (23) |
| 2 | 23 (28) | 84 (26) | 107 (26) | 22 (17) | 148 (26) | 170 (24) |
| 3 | 14 (17) | 63 (19) | 77 (19) | 36 (27) | 102(18) | 139 (20) |
| ≥4 | 23 (28) | 32 (10) | 55 (13) | 40 (30) | 89 (16) | 129 (18) |
Known risk factors for FN are italicized
FN febrile neutropenia, BMI body mass index, ECOG Eastern Cooperative Oncology Group, IPI International Prognostic Index
aIncludes two patients for whom FN data were unavailable and who are excluded from other analyses presented
b n = 80
c n = 327
d n = 566
eNo chemotherapy, radiotherapy, or other prior treatment
fRisk factors are age ≥65 years; Ann Arbor stage III or IV; ECOG performance status ≥2; current or continuing cardiovascular, respiratory, renal, or hepatic/biliary co-morbidities; baseline serum albumin <35 g/L; baseline hemoglobin <12 g/dL; bone marrow involvement
PP G-CSF was administered in 84% (345/409) of patients receiving R-CHOP-14 and in 36% (252/702) of patients receiving R-CHOP-21. Approximately two thirds of these patients received pegfilgrastim (R-CHOP-14, 226/345 [66%]; R-CHOP-21, 174/252 [69%]). The remainder received daily G-CSF, with a mean (±SD) of five daily G-CSF doses per cycle [R-CHOP-14, 5.23 ± 2.73; R-CHOP-21, 5.25 ± 1.65]. SP G-CSF was administered in 14% (56/409) of patients receiving R-CHOP-14 and in 29% (203/702) of patients receiving R-CHOP-21.
Overall, 20% (81/409) of patients receiving R-CHOP-14 and 19% of patients receiving R-CHOP-21 (133/702) experienced FN at any time during the study. Median duration of FN episode was 4.0 days for both regimens (R-CHOP-14, range 1–20 days; R-CHOP-21, range 1–21 days). In the R-CHOP-14 group, approximately one third of patients with FN experienced their first episode in cycle 1 (24/81 [30%]). In the R-CHOP-21 group, approximately half of the patients with FN experienced their first episode in cycle 1 (63/133 [47%]).
Baseline demographics and disease characteristics are summarized by R-CHOP regimen and FN group in Table 1. As expected, patients who experienced FN generally exhibited more risk factors for FN.
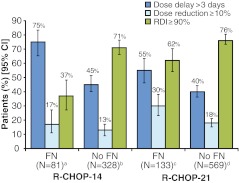
Chemotherapy dose delays and dose reductions are presented in Fig. 1. Delays of >3 days were more frequent in patients who experienced FN than in patients who did not experience FN for both R-CHOP-14 (75% [95% CI 65–83%] versus 45% [40–51%]) and R-CHOP-21 (55% [46–63%] versus 40% [36–44%]). Dose reductions were similar between FN and no FN patients in the R-CHOP-14 group, but differences were more prominent in the R-CHOP-21 group (30% [23–38%] versus 18% [15–21%]). Consequently, fewer patients experiencing FN achieved RDI ≥90%. In the R-CHOP-14 group, only 37% (27–48%) of patients who experienced FN achieved RDI ≥90%; in the R-CHOP-21 group, RDI ≥90% was achieved by 62% (54–70%) of patients who experienced FN. In contrast, for both regimens, RDI ≥90% was achieved by over 70% of patients who did not experience FN (R-CHOP-14, 71% [66–76%]; R-CHOP-21, 76% [73–80%]).
Fig. 1.
Dose delays of >3 days in patients who received at least two chemotherapy cycles, dose reductions of ≥10% in cyclophosphamide or doxorubicin in any cycle, and patients achieving RDI ≥90%. For dose delays, superscript a, N = 80; superscript b, N = 325; superscript c, N = 128; superscript d, N = 563
For both R-CHOP regimens, unplanned hospitalizations occurred in approximately 80% of patients who experienced FN and 20% of patients who did not experience FN (R-CHOP-14—63/81, 78% [95% CI 68–86%] versus 68/328, 21% [17–26%]; R-CHOP-21—105/133, 79% [71–85%] versus 100/569, 18% [15–21%]). Among patients experiencing FN, mean (±SD) duration of hospitalization was 12.9 (±16.5) days for patients receiving R-CHOP-14 and 9.6 (±16.5) days for those receiving R-CHOP-21. Mean duration of hospitalization was approximately 10 days for patients not experiencing FN (R-CHOP-14, 10.4 [±13.2] days; R-CHOP-21, 9.8 [±9.1] days). Febrile neutropenia/neutropenic event was the most common reason for unplanned hospitalizations among patients who experienced FN (R-CHOP-14, 75 of 118 hospitalizations [64%]; R-CHOP-21, 132/192 [69%]). Nonhematological adverse event was the most common reason among patients who did not experience FN (R-CHOP-14, 37/82 [45%]; R-CHOP-21, 51/132 [39%]).
Discussion
Patients with DLBCL are routinely treated with R-CHOP. This myelotoxic regimen often results in FN, unplanned hospitalizations, and impaired chemotherapy delivery [16]. This analysis from a large observational study provides additional data regarding the impact of FN on chemotherapy delivery and hospitalizations among patients with DLBCL receiving R-CHOP-14 or R-CHOP-21.
In this study of clinical practice, around 20% of patients receiving R-CHOP-14 and R-CHOP-21 experienced FN. Furthermore, many patients experienced FN during cycle 1, a finding consistent with previously published data. In a recent retrospective Spanish study of FN management among cancer patients treated with cytotoxic chemotherapy, almost half of all FN events occurred in cycle 1 [13]. Similarly, US studies have reported cycle 1 FN rates of 28–58% among NHL patients [2, 4, 5, 12, 19]. This high incidence of cycle 1 FN suggests that secondary prophylaxis G-CSF may not be the best approach for managing chemotherapy-induced FN and provides evidence that FN management could be improved in patients treated with R-CHOP.
While over half of patients receiving R-CHOP-21 exhibited multiple risk factors for FN, only 36% of patients receiving this regimen received PP G-CSF. Moreover, a small number of patients experiencing FN exhibited none of the known risk factors, suggesting that additional, as yet unidentified, risk factors for FN may exist. Notably, only 84% of patients receiving R-CHOP-14 received PP G-CSF, despite international guidelines recommending PP G-CSF to support dose dense regimens [1, 18]. For both R-CHOP-14 and R-CHOP-21, the demographics and baseline disease characteristics of patients in the FN and no FN subgroups were markedly different, with patients experiencing FN more likely to have multiple risk factors for FN. This suggests that assessing known risk factors for FN before the first chemotherapy cycle may help clinicians better identify patients at high risk of FN.
Patients experiencing FN were more likely to receive suboptimal chemotherapy RDI and to require unplanned hospitalization. Such reduced chemotherapy delivery has been associated with impaired outcomes and significantly decreased survival in patients with NHL [16, 20]. Furthermore, FN-related hospitalizations incur high economic costs, with duration of stay an important factor [8–11, 13]. In the current analysis, mean stays of 9–13 days were observed, consistent with mean stays of 10.3 and 10.7 days observed in patients with lymphoma and NHL [8, 13]. Two recent studies of the burden of FN management in Spain and France reported mean medical costs of €4,514 and €4,931 per FN-related hospitalization [6, 13]. Attention to G-CSF PP in accordance with international guidelines may help to prevent FN, with the cost of growth factors being at least partially offset by reduced hospitalization. In addition to the economic burden, FN-related hospitalizations are associated with overall inpatient mortality rates of 8–14%, with mortality rates of 21–40% observed among patients with documented infections [8, 10].
Our findings are strengthened by the fact that this was a large population representative of clinical practice. However, since no data regarding the timing of chemotherapy delivery and hospitalizations in relation to FN episodes are available, a causal link between FN and these outcomes could not be established in this study. In conclusion, this analysis demonstrates that in patients with DLBCL treated with R-CHOP-14 or R-CHOP-21, patients with an event of FN were more likely to experience suboptimal chemotherapy delivery and increased unplanned hospitalizations than those without FN.
Acknowledgments
This study was sponsored by Amgen (Europe) GmbH, Zug, Switzerland. The authors would like to thank Claire Desborough, who provided medical writing assistance on behalf of Amgen Ltd, UK.
Conflicts of interest
Ruth Pettengell has received honoraria from Amgen (Europe) GmbH, who sponsored this research, and from Roche. Antonio Salar Silvestre has received consultancy fees from Amgen (Europe) GmbH and Roche. Matthias Schwenkglenks has received research funding from Amgen (Europe) GmbH and has served on advisory boards for Amgen (Europe) GmbH. Ulrich Jaeger has received consultancy fees and research funding from Roche. Zsolt Szabo is an employee of Amgen (Europe) GmbH and holds shares in Amgen Inc. Kate Bendall is a consultant for Amgen (Europe) GmbH and receives consultancy fees from Amgen UK. Hans E. Johnson, Pieternella J. Lugtenburg, Ulrich Dührsen, and Francesca G. Rossi report no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Aapro MS, Bohlius J, Cameron DA, Lago LD, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C. Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;1:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Balducci L, Al-Halawani H, Charu V, Tam J, Shahin S, Dreiling L, Ershler WB. Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist. 2007;12:1416–1424. doi: 10.1634/theoncologist.12-12-1416. [DOI] [PubMed] [Google Scholar]
- 3.Bosly A, Bron D, Van Hoof A, De Bock R, Berneman Z, Ferrant A, Kaufman L, Dauwe M, Verhoef G. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87:277–283. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 4.Case DC, Jr, Desch CE, Kalman LA, Vongkovit P, Mena RR, Fridman M, Allen B. Community-based trial of R-CHOP and maintenance rituximab for intermediate- or high-grade non-Hodgkin lymphoma with first-cycle filgrastim for older patients. Clin Lymphoma Myeloma. 2007;7:354–360. doi: 10.3816/CLM.2007.n.012. [DOI] [PubMed] [Google Scholar]
- 5.Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6:109–118. doi: 10.6004/jnccn.2008.0012. [DOI] [PubMed] [Google Scholar]
- 6.Durand-Zaleski L, Vainchtock A, Bogillot O. L’utilisation de la base nationale PMSI pour determiner le cout d’un cymptome: le cas de la neutropenie febrile. (Use of an administrative database to estimate the economic burden of febrile neutropenia.) Journal D’Economie Medicale. 2007;25:269–280. [Google Scholar]
- 7.Haioun C, Salar A, Pettengell R, Erik Johnsen H, Duehrsen U, Gaia Rossi F, Verhoef G, Schwenkglenks M, Jaeger U, Hamilton L, Pujol B, Lugtenburg PJ. Anemia and erythropoiesis-stimulating agent administration in patients with non-Hodgkin lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone ± rituximab chemotherapy: results from an observational study. Leuk Lymphoma. 2011;52(5):796–803. doi: 10.3109/10428194.2011.557166. [DOI] [PubMed] [Google Scholar]
- 8.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 9.Lathia N, Mittmann N, DeAngelis C, Knowles S, Cheung M, Piliotis E, Shear N, Walker S. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116:742–748. doi: 10.1002/cncr.24773. [DOI] [PubMed] [Google Scholar]
- 10.Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27:381–396. doi: 10.2165/00044011-200727060-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Kuderer N, Greene J, Balducci L. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer. 1998;34:1857–1864. doi: 10.1016/S0959-8049(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44:2069–2076. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 13.Mayordomo JI, Lopez A, Vinolas N, Castellanos J, Pernas S, Domingo Alonso J, Frau A, Layola M, Antonio Gasquet J, Sanchez J. Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr Med Res Opin. 2009;25:2533–2542. doi: 10.1185/03007990903209563. [DOI] [PubMed] [Google Scholar]
- 14.Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M. Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU prospective observational european neutropenia study. Br J Haematol. 2009;144:677–685. doi: 10.1111/j.1365-2141.2008.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87:429–430. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettengell R, Schwenkglenks M, Leonard R, Bosly A, Paridaens R, Constenla M, Szucs TD, Jackisch C. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–1309. doi: 10.1007/s00520-008-0430-4. [DOI] [PubMed] [Google Scholar]
- 17.Salar A, Haioun C, Rossi FG, Duehrsen U, Pettengell R, Johnsen HE, Jaeger U, Verhoef G, Schwenkglenks M, Principe F, Bacon P, Bendall K, Lugtenburg PJ. Febrile neutropenia risk assessment and granulocyte-colony stimulating factor support in patients with diffuse large B cell lymphoma receiving R-CHOP regimens. Blood. 2009;22:107. [Google Scholar]
- 18.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 19.Teegala SR, Zhou X, Huen A, Ji Y, Fayad LE, Romaguera JE, Vadhan-Raj S. Risk factors for neutropenic fever in lymphoma patients receiving chemotherapy. J Clin Oncol. 2007;25:19616. [Google Scholar]
- 20.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;3:221–240. doi: 10.1016/j.critrevonc.2010.02.002. [DOI] [PubMed] [Google Scholar]