Abstract
Background
Colorectal cancer is the most common cancer in Europe. Early diagnosis and treatment gives the patient a chance for complete recovery. Screening colonoscopies in the symptom-free patients are currently performed on a wide scale. The examinations are performed under local anesthesia which does not eliminate all discomfort and pain related to the examination. The aim of this study was to evaluate magnetic scope navigation in screening endoscopic examinations performed to detect early-stage colorectal cancer.
Methods
The study group consisted of 200 patients, aged 40–65 years, who were free from colon cancer symptoms. All patients underwent complete colonoscopy under local anesthesia. The equipment could be fitted with the scope that allows three-dimensional observation of instrument localization in the bowel. The examination was performed by three experienced endoscopists, each of whom performed over 5,000 colonoscopies. The patients were randomized to two groups: those whose equipment did not have 3D navigation (group I) and those whose equipment did have 3D navigation (group II). Each group consisted of 100 cases matched by gender, age, and BMI. The authors compared the duration of introducing instrument to cecum, the pulse rate before the examination and at the time the instrument reached the cecum, and subjective pain evaluation by the patient on the visual analog scale.
Results
Group I consisted of 54 women and 46 men with a mean age of 54.6 years and mean BMI of 27.8 kg/m2, and group II had 58 women and 42 men, mean age of 55.1 years and mean BMI of 26.4 kg/m2. The average time it took for the instrument to reach the cecum was 216s in group I and 181s in group II (P < 0.05). Pain measured on the 10-point VAS scale was 2.44 in group I and 1.85 in group II (P < 0.05). The results showed a significantly shorter time for the instrument to reach the cecum in group II and significantly lower pain intensity during the examination was reported by the group II patients. No significant differences were found in the pulse measurements between the groups (P = 0.5).
Conclusions
3D navigation during colonoscopy decreases the time for the instrument to reach the cecum and lowers pain intensity subjectively reported by the patients. The use of 3D and the possibility to observe instrument localization and maneuvers brings more comfort to the patients.
Keywords: Colorectal cancer, Colonoscopy, Magnetic endoscopic imaging, Pain
Colorectal cancer is the most common cancer in Europe. Early diagnosis together with treatment gives patients a chance for a full recovery. Based on this knowledge, many countries have implemented screening programs allowing early detection in asymptomatic patients. There is a plethora of methods for selecting patients in the high-risk group, as well as many methods of examination [1, 2]. Since 2000, the Polish Ministry of Health has funded and supported the screening program for early detection of colon cancer. The program is aimed toward patients aged 50–65 years, but also those aged 40–49 years who have a first-degree relative with colon cancer. In order to qualify for the screening program, patients are obliged to fill out a questionnaire ruling out all major symptoms of colon cancer. The examination itself is based on total colonoscopy [3]. Our Endoscopy Department has participated in the program since the time of its introduction. The examination is conducted with the patient under local anesthesia with analgesia and sedation. The quality of standard is measured by the percentage of total colonoscopies (i.e., cecal intubation) and adenoma detecting rate, and a 5-point patient satisfaction scale following the procedure. Factors negatively impacting the course of the examination are poor bowel preparation, pain during the examination, and total time to perform the exam [4]. The only variable dependent on the endoscopist is the way the examination is conducted. Maneuvers such as skillful insertion of the endoscope; application of manual abdominal pressure by the endoscopy assistant; rotations, twists, and stiffening of the endoscope; but also timely administration of sedation and analgesia provide comfort and safety during the examination [5]. In order for the examination to be quick and pain-free, the endoscope should be guided by the anatomic position of the colon, not causing overextension of the bowel wall, which is a direct result of excessive insufflation and formation of bowel loops. The loops form in those sections of the bowel that have free mesocolon, i.e., the sigmoid colon and transverse colon. The occurrence of bowel looping varies individually, although they are present in each examination to a certain degree [6]. Continuous straightening of the instrument once a loop has formed and avoiding forceful advancement of the instrument through the bowel comprise the basic skills of technically proper intubation, allowing for shorter total time and lower doses of analgesic medications, thus improving the patient’s tolerance for the procedure. Configuration of the instrument, its location in the bowel, and the topography of its tip can be achieved by fluoroscopy. The downsides to this method are the need for specialized equipment, need for additional staff members, and the risk of radiation to both patient and the endoscopy staff, all consequently increasing the cost of the procedure [7, 8]. Magnetic imaging has become the preferred method of instrument positioning ever since it was introduced in 2002. Accurate anatomical localization and positioning of the endoscope is crucial not only for conducting an effective procedure, but also aiding in more precise localization of pathologies, therefore allowing for easier surgical or pharmacologic treatment [9–11].
In the recent years several research reports evaluating magnetic imaging during colonoscopy have been published. The majority of authors report that the technique is beneficial in those cases where colonoscopy is performed by a less experienced endoscopist, or during endoscopy training [8, 9, 11–14].
The aim of this study was to assess the value of magnetic imaging as an aid to colonoscopy conducted by experienced endoscopists.
Materials and method
In this study we used the magnetic imaging system for endoscopic navigation manufactured by Olympus, which was introduced to the market for testing purposes in the spring of 2010. It was an addendum to previously acquired colonoscopes for our practice. Data were collected prospectively from currently ongoing colonoscopies, but the elaboration of the findings and their analysis were done in retrospect.
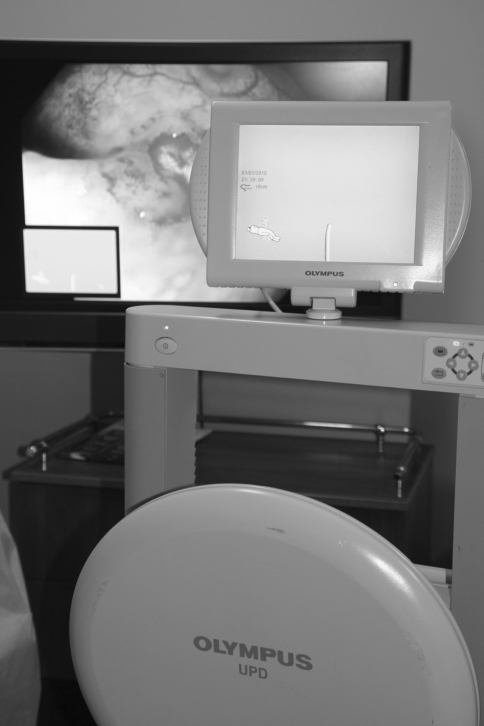
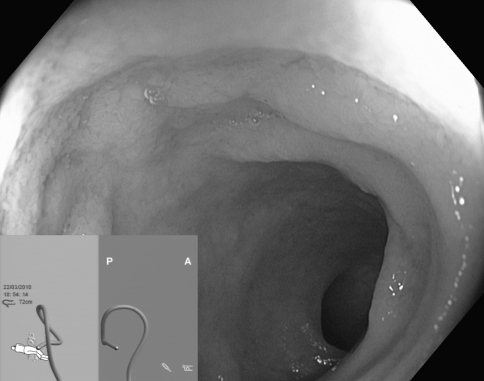
The setup for magnetic endoscope imaging comprises three basic elements: the graphics processor, the endoscope, and the signal receiver (Fig. 1). Our endoscopy unit used three Olympus CF-H180DL instruments that are based on high-definition technology HDTV 1080i. Positioned at regular intervals within the endoscope, along its entire length, are 12 magnetic coils that constitute a generator, each generating a pulsed low-voltage magnetic field. The generator is connected to the endoscope through an attachment within it, made just for that purpose. The magnetic signal is collected by a signal receiver external to the patient, which is then converted electronically to a 3D image on the screen. The effect of spatial imaging is achieved through adequate gray-scale shading, in addition to anteroposterior and lateral projections (Fig. 2). The image appears on the external screen or in a small window on the main screen, next to the endoscopic picture (Fig. 3). Supplementing the system is the independent external magnetic marker held by the endoscopy assistant. The marker enables topographic localization of the tip of the instrument as well as the exact location of abdominal pressure application.
Fig. 1.
System of endoscopic navigation
Fig. 2.
Alpha loop of the sigmoid colon
Fig. 3.
Colonoscope being advanced to small intestine
This electromagnetic equipment is classified as BF-type electrical medical equipment, meaning that it cannot be used in places where there are strong electromagnetic fields, in enclosed spaces with high concentrations of O2 or N2O, nor in pregnant women and patients with pacemakers.
Included in the study were patients aged 40–65 years, who came for the screening procedure on their own initiative. Besides age, other exclusion criteria were symptoms of colon cancer, such as bleeding unrelated to hemorrhoids, changes in bowel movement regularity, and unexplained weight loss. Also excluded from the study were patients who already had had a colonoscopy.
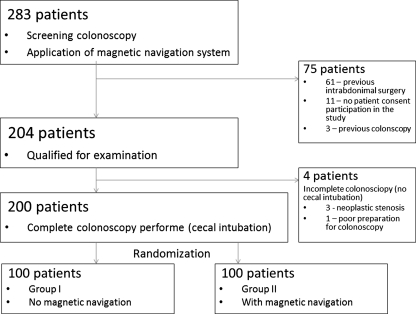
Bowel preparation for colonoscopy was solely oral ingestion of liquid propulsive agents, i.e., 420 g of macrogolum in 4 l of water, taken in four doses every 6 h the day before the colonoscopy. The colonoscopy was performed in an outpatient setting. Two hundred eighty-three colonoscopies with magnetic navigation were performed between February and April 2010 as part of a national colon cancer screening program. Seventy-five patients were excluded. Of those, 61 had a history of intra-abdominal surgery, 3 had a previous colonoscopy, and 11 did not give their consent for participation in the study (Fig. 4). Two hundred patients in total were included in the study, randomized into two groups: group I with magnetic imaging-guided colonoscopy and group II without it. In cases where total colonoscopy was not performed, the next patient who qualified was placed in that same group. This was the case with four patients. All colonoscopies were conducted with the patient under local anesthesia by using 2% lignocaine gel to coat the instrument. The examinations were performed without sedation, since its use would not allow the trial to be conducted. The instruments used in all colonoscopies were from the Olympus series 180. No CO2 was used for bowel insufflation. Three experienced endoscopists conducted the procedures, each having performed over 5,000 colonoscopies independently, intubating the cecum 97% of the time. Assisting during the colonoscopies were experienced endoscopy nurses, each having participated in over 2,000 of them.
Fig. 4.
Diagram of patients’ selections
Patients were initially placed on their left side, whereas the endoscopic technique depended on the endoscopist’s personal preference and experience. During the course of the procedure, maneuvers such as manual abdominal pressure, repositioning of the patient, and instrument rotations, twists, stiffening, and straightening were applied where needed. Data collected relating to the patient were age, gender, height, weight, and BMI. Other factors evaluated were the degree of bowel preparation, total time to reach the cecum, pulse, and pain. Cecum intubation was considered to be attained when ileocecal valve and appendiceal entrance were properly identified. Pulse was recorded by pulse oximetry before, each minute for the duration of the colonoscopy, and 15 min after the colonoscopy. Subjective pain sensation was assessed on a 0–10 point visual analog scale (VAS), with 0 being no pain and 10 being the worst pain imaginable. Pain assessment was recorded immediately after colonoscopy and 15 min later.
There were no conflicts of interest involving the endoscopy unit staff, authors, or the institution in which the study was conducted.
Statistics
The materials acquired in this study were systematized and analyzed and a distribution of variables was established. Since the analyzed parameters do not have normal distribution, nonparametric tests were applied in the analysis. Quality variables were compared using the independent test χ2. For comparison of quantity variables, the Mann–Whitney test was used in two groups. Comparison of quantity data in more than two groups was done using the Kruskal–Wallis test. Materiality threshold was established at P ≤ 0.05.
Results
Two hundred four patients were included in the study. Of that number, total colonoscopy was not achieved in four of them. In three of those, the reason was a stricture caused by the cancer infiltrate, and in one, a poorly decontaminated bowel. Analysis was done on 200 patients who had total colonoscopy. Group I included 54 females (mean age = 53.7) and 46 males (mean age = 55.6), who underwent colonoscopy without the magnetic imaging system guidance. Group II included 58 females (mean age = 55.9) and 42 males (mean age = 54.1), who underwent colonoscopy with the magnetic imaging guidance system. There was no statistical difference in the age (P = 0.57). BMI was calculated for each patient. The mean BMIs of group I (27.8) and group II (26.44) were comparable (Table 1).
Table 1.
Group comparison
| Gender | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| Group I | 54F/46 M | 41–65 (avg = 54.57) | 169.15 | 79.7 | 19–49 (avg = 27.8) |
| Group II | 58F/42 M | 40–65 (avg = 55.14) | 167.47 | 74.4 | 18–38 (avg = 26.44) |
| P = 0.57 | P = 0.54 | P = 0.18 | P = 0.05 | P = 0.05 |
avg Average
Total time required to reach the cecum was evaluated. In group II the mean total time was 181 s (min 55–max 405 s); specifically, in females it was 185 s and in males 174 s (P = 0.0017). The mean total time to reach the cecum in group I was 216 s (min 50–max 420 s) (Table 2).
Table 2.
Cecum intubation time in both groups
| Group | Gender | n | Cecum intubation time (s) | |||
|---|---|---|---|---|---|---|
| Min | Max | Avg | SD | |||
| I (w/o navigation) | Female | 54 | 80 | 420 | 208 | 73.21 |
| Male | 46 | 50 | 411 | 226 | 74.01 | |
| Together | 100 | 50 | 420 | 216 | 73.72 | |
| II (w/ navigation) | Female | 58 | 60 | 376 | 185 | 78.20 |
| Male | 42 | 55 | 405 | 174 | 90.99 | |
| Together | 100 | 55 | 405 | 181 | 83.54 | |
| Together | 200 | 50 | 420 | 198 | 80.57 | |
The mean total time in the group II was shorter for both genders compared to the mean total time in the group I; for females 185 versus 208 s and for males 174 versus 226 s, respectively. A time difference to reach the cecum in both genders in both groups was not established (P = 0.68); for group I, P = 0.24, and for group II, P = 0.52.
Pain sensation recorded during colonoscopies was evaluated based on both subjective and objective criteria.
Objective pain evaluation was based on pulse readings without analgesia and sedation. Mean pulse value for both groups prior to, at each minute of, and 15 min after colonoscopy did not differ in either group and for either females or males (Table 3).
Table 3.
Pulse rate readings in specific groups
| Group | Gender | n | Pulse | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to exam | 1 min | 2 min | 3 min | 4 min | 5 min | 6 min | End of exam | 15 min after end | |||
| I (w/o navigation) | Female | 54 | 80 | 75 | 78 | 76 | 76 | 77 | 82 | 74 | 69 |
| Male | 46 | 78 | 74 | 77 | 76 | 75 | 75 | 73 | 74 | 71 | |
| Together | 100 | (58–98) | (55–91) | (57–93) | (55–99) | (60–90) | (62–87) | (71–83) | (56–88) | (55–95) | |
| II (w/ navigation) | Female | 58 | 83 | 77 | 78 | 79 | 78 | 76 | 75 | 76 | 71 |
| Male | 42 | 82 | 76 | 75 | 76 | 75 | 75 | 73 | 70 | 67 | |
| Together | 100 | (58–105) | (55–100) | (57–102) | (61–93) | (61–94) | (72–80) | (69–80) | (56–96) | (55–90) | |
| Together | 200 | P = 0.59 | P = 0.51 | ||||||||
Subjective pain evaluation was based on a 10-point visual analog scale. The minimum and maximum values were 0 and 7, respectively. No difference in pain evaluation was observed between the genders in either of the groups. In group I, without the navigation, the pain was estimated to be 2.79, and 15 min after the completion of the colonoscopy, the pain estimation was 2.44. In group II, with the navigation, the respective values were estimated at 2.05 and 1.85. Significant difference in pain estimation was observed when comparing both groups (P < 0.0001 for the value at the end of the colonoscopy and P < 0.007 15 min later) (Table 4).
Table 4.
Pain assessment during exam on visual analog scale (VAS)
| Group | Gender | n | VAS at end of exam | VAS 15 min after end of exam | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Avg | SD | Min | Max | Avg | SD | |||
| I (w/o navigation) | Female | 54 | 0 | 5 | 2.80 | 1.25 | 0 | 6 | 2.52 | 1.66 |
| Male | 46 | 1 | 6 | 2.78 | 1.13 | 0 | 6 | 2.35 | 1.57 | |
| Both | 100 | 0 | 6 | 2.79 | 1.19 | 0 | 6 | 2.44 | 1.61 | |
| II (w/ navigation) | Female | 58 | 1 | 6 | 2.14 | 1.12 | 0 | 6 | 1.97 | 1.45 |
| Male | 42 | 0 | 7 | 1.92 | 1.33 | 0 | 5 | 1.69 | 1.51 | |
| Together | 100 | 0 | 7 | 2.05 | 1.21 | 0 | 6 | 1.85 | 1.47 | |
| Together | 200 | P < 0.0001 | P = 0.007 | |||||||
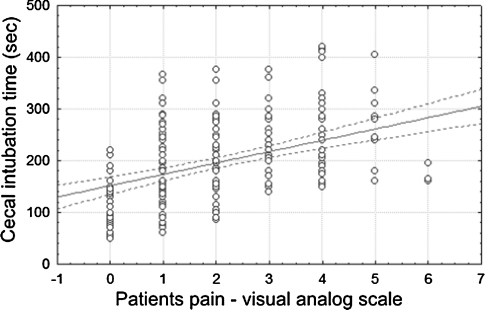
It was concluded that BMI had no impact on total time to cecum intubation (P = 0.88). On the other hand, it was observed that in patients in whom the cecum was intubated faster, the pain reported was lower (P < 0.05). This was observed in both groups (Fig. 5).
Fig. 5.
Correlation between the time of cecal intubation and patient pain
Discussion
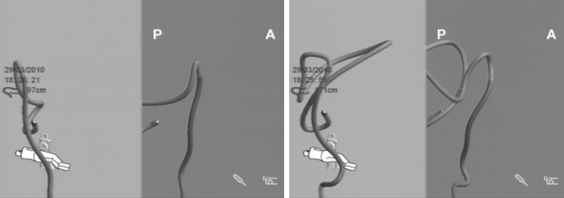
Colonoscopy is one of the most sensitive and specific methods of early colon cancer detection. While there are many screening methods for colon cancer, colonoscopy is considered to be the most precise. Compared to the fecal occult blood test, the cost of colonoscopy is significantly higher; however, it is sufficient to have it done every several years. In order to reach the true value of colonoscopy, cecal intubation is fundamental because it allows for inspection of the entire colon [1, 3, 15]. Together with the adenoma detection rate, the percentage of cecal intubation is considered to be the fundamental criterion for the evaluation of the quality of colonoscopy. The experienced endoscopist intubates the cecum in over 95% of all colonoscopies, although 10–20% of those are reported to be technically difficult, often requiring different maneuvers, and in most cases analgesia with sedation (Fig. 6 ) [10]. In order to increase patient tolerance for the examination, most institutions rely on analgesia with sedation. Increasing the dosage of administered medications allows performance of total colonoscopy with greater ease in difficult cases, as well as in those cases performed by less experienced endoscopists [13, 16]. No analgesia or sedation was used in this study because their use could have changed the patients’ tolerance to the examination, which in turn would affect the results of the study.
Fig. 6.
Colonoscope configuration in the bowel before and after straightening and stiffening
Colonoscopy is technically more difficult in elderly, thin females and following abdominal and pelvic surgery [14, 16]. The value of the new system of navigation in colonoscopy, as well as patient tolerance to it, excludes conducting the examination with analgesia and sedation because the quality of assessment of the examination would be unreliable. It was observed that shortening the total time for cecal intubation increases patient tolerance for the examination [4, 5, 9, 11]. This applies to all patients who had colonoscopy without sedation. Shortening the examination time is possible only when the endoscope is advanced through the bowel unobstructed. In order to achieve that, it is crucial to avoid looping of the bowel, which most often occurs in the sigmoid colon. A number of maneuvers are used for this purpose, such as manually applying pressure on the abdomen wall, retracting and stiffening the endoscope, and repositioning of the patient. Those maneuvers are effective in only 52% of the cases [11]. Knowing when to apply which maneuver requires experience as well as a system of navigation that enables the endoscopist to see the position of the endoscope in the bowel at any time during the examination. Historically, fluoroscopy was used for this purpose, disregarding the health risk for both the patient and endoscopy staff. The new navigation method has been developing gradually since the time of the first publication on the topic in 1993 by Bladen [7], slowly becoming the standard in colonoscopy [17]. Many authors concluded that the method is less valuable in everyday practice, but more valuable for less experienced endoscopists or for training purposes.
In this study, all colonoscopies were conducted by experienced endoscopists, reaching the cecum 95% of the time without sedation. Selecting such endoscopists eliminates possible factors related to the endoscopy staff which negatively impact patients’ evaluation. The instruments used in this study are of the newest generation, which is why this element of the study was eliminated from the analysis.
It was concluded that the navigation identifies loop formation of the bowel before the patient starts reporting pain related to it, and before the endoscopist starts feeling resistance while advancing the instrument. This facilitates faster cecum intubation, while patients report pain less frequently [5, 11, 16, 18].
According to Heigh et al. [9], the frequency of loop formation occurs in 91% of patients. This is why the magnetic system of navigation facilitates cecum intubation in 89% of the cases, requiring on average the use of four different maneuvers during the examination.
Hoff et al. [16] compared the frequency of cecum intubation and complaints reported by the patients during colonoscopy with and without endoscopic navigation. Colonoscopies were conducted by both experienced and inexperienced endoscopists. Without sedation but with endoscopic navigation, the cecum intubation rate grew from 74 to 90%. Fewer pain reports were observed only in those cases in which colonoscopy was conducted by an experienced endoscopist.
Another crucial element of endoscopic navigation is the possibility of preliminary localization of pathologies in the colon [14]. This is particularly essential now in the era of laparoscopic surgery, where intraoperative localization of pathologies is often impossible.
In conclusion, adding magnetic navigation to endoscopic examinations is one of the directions in which endoscopy will develop in the future. It requires the use of special endoscopes and special setup; however, its advantages it seem to outweigh those potentially negative aspects. Thanks to the application of this new method, colonoscopy is simpler and faster, and patient tolerance for it is better.
Acknowledgments
Disclosure
Drs. Bucki, Kulig, Matyja, and Szura have no conflicts of interest or financial ties to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci. 2008;53(12):3049–3054. doi: 10.1007/s10620-008-0286-y. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Wu H, Guan YS. Colonography by CT, MRI and PET/CT combined with conventional colonoscopy in colorectal cancer screening and staging. World J Gastroenterol. 2008;14(6):853–863. doi: 10.3748/wjg.14.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, Tanaka H, Kinjo M, Sakumoto K. Prospective evaluation of factors predicting difficulty and pain during sedation-free colonoscopy. Dis Colon Rectum. 2005;48(6):1295–1300. doi: 10.1007/s10350-004-0940-1. [DOI] [PubMed] [Google Scholar]
- 5.Cataldo PA. Colonoscopy without sedation. Dis Colon Rectum. 1996;39(3):257–261. doi: 10.1007/BF02049463. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh YH, Tseng KC, Chou AL. Patient self-administered abdominal pressure to reduce loop formation during minimally sedated colonoscopy. Dig Dis Sci. 2010;55(5):1429–1433. doi: 10.1007/s10620-009-0876-3. [DOI] [PubMed] [Google Scholar]
- 7.Bladen JS, Anderson AP, Bell GD, Rameh B, Evans B, Heatley DJ. Non-radiological technique for three-dimensional imaging of endoscopes. Lancet. 1993;341(8847):719–722. doi: 10.1016/0140-6736(93)90487-2. [DOI] [PubMed] [Google Scholar]
- 8.Saunders BP, Bell GD, Williams CB, Bladen JS, Anderson AP. First clinical results with a real time, electronic imager as an aid to colonoscopy. Gut. 1995;36(6):913–917. doi: 10.1136/gut.36.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heigh RI, DiBaise JK, Prechel JA, Horn BJ, San Miguel S, Heigh EG, Leighton JA, Edgelow CJ, Fleischer DE. Use of an electromagnetic colonoscope to assess maneuvers associated with cecal intubation. BMC Gastroenterol. 2009;9:24. doi: 10.1186/1471-230X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown GJ, Saunders BP. Advances in colonic imaging: technical improvements in colonoscopy. Eur J Gastroenterol Hepatol. 2005;17(8):785–792. doi: 10.1097/00042737-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Syed G, Shah SG, Saunders BP, Brooker JC, Williams CB. Magnetic imaging of colonoscopy: an audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc. 2000;52(1):1–8. doi: 10.1067/mge.2000.107296. [DOI] [PubMed] [Google Scholar]
- 12.Bell GD, Rowland RS, Rutter M, Abu-Sada M, Dogramadzi S, Allen C. Colonoscopy aided by magnetic 3D imaging: assessing the routine use of a stiffening sigmoid overtube to speed up the procedure. Med Biol Eng Comput. 1999;37(5):605–611. doi: 10.1007/BF02513355. [DOI] [PubMed] [Google Scholar]
- 13.Shah SG, Brooker JC, Thapar C, Williams CB, Saunders BP. Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging. Endoscopy. 2002;34(6):435–440. doi: 10.1055/s-2002-31995. [DOI] [PubMed] [Google Scholar]
- 14.Ambardar S, Arnell TD, Whelan RL, Nihalani A, Forde KA. A preliminary prospective study of the usefulness of a magnetic endoscope locating device during colonoscopy. Surg Endosc. 2005;19(7):897–901. doi: 10.1007/s00464-004-8948-0. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski MF, Regula J. Colorectal cancer screening by colonoscopy—current issues. Digestion. 2007;76(1):20–25. doi: 10.1159/000108390. [DOI] [PubMed] [Google Scholar]
- 16.Hoff G, Bretthauer M, Dahler S, Huppertz-Hauss G, Sauar J, Paulsen J, Seip B, Moritz V. Improvement in caecal intubation rate and pain reduction by using 3-dimensional magnetic imaging for unsedated colonoscopy: a randomized trial of patients referred for colonoscopy. Scand J Gastroenterol. 2007;2(7):885–889. doi: 10.1080/00365520601127125. [DOI] [PubMed] [Google Scholar]
- 17.Jess P, Bulut O, Almasi A, Waaddegaard P. The usefulness of a magnetic endoscope locating device in colonoscopy in daily practice: a prospective case-controlled study. Surg Endosc. 2009;23(6):1353–1355. doi: 10.1007/s00464-008-0179-3. [DOI] [PubMed] [Google Scholar]
- 18.Franciosi JP, Mascarenhas M, Semeao E, Flick J, Kelly J, Mamula P. Randomised controlled trial of paediatric magnetic positioning device assisted colonoscopy: a pilot and feasibility study. Dig Liver Dis. 2009;41(2):123–126. doi: 10.1016/j.dld.2008.06.010. [DOI] [PubMed] [Google Scholar]